Abstract
Patients with antineutrophil cytoplasmic antibodies (ANCAs) frequently develop severe vasculitis and glomerulonephritis. Although ANCAs, particularly antimyeloperoxidase (anti-MPO), have been shown to promote leukocyte adhesion in postcapillary venules, their ability to promote adhesion in the glomerular vasculature is less clear. We used intravital microscopy to examine glomerular leukocyte adhesion induced by anti-MPO. In mice pretreated with LPS, 50 μg anti-MPO induced LFA-1–dependent adhesion in glomeruli. In concert with this finding, in mice pretreated with LPS, more than 80% of circulating neutrophils bound anti-MPO within 5 minutes of intravenous administration. However, even in the absence of LPS, more than 40% of circulating neutrophils bound anti-MPO in vivo, a response not seen in MPO−/− mice. In addition, a higher dose of anti-MPO (200 μg) induced robust glomerular leukocyte adhesion in the absence of LPS. The latter response was β2-integrin independent, instead requiring the α4-integrin, which was up-regulated on neutrophils in response to anti-MPO. These data indicate that anti-MPO antibodies bind to circulating neutrophils, and can induce glomerular leukocyte adhesion via multiple pathways. Lower doses induce adhesion only after an infection-related stimulus, whereas higher doses are capable of inducing responses in the absence of an additional inflammatory stimulus, via alternative adhesion mechanisms.
Introduction
Systemic vasculitis is commonly associated with the presence of circulating antineutrophil cytoplasmic antibodies (ANCAs). In this condition, renal disease and crescentic glomerulonephritis are major causes of morbidity.1 The most common antigens recognized by ANCAs are the neutrophil granule proteins myeloperoxidase (MPO) and proteinase-3 (Pr3).2,3 A growing body of evidence now implicates ANCAs as playing key roles in the pathogenesis of these diseases.4,5 Neutrophil-dependent glomerulonephritis can be induced by transfer of ANCA-like, anti-MPO antibodies, and augmented by administration of LPS.4,6 The capacity of anti-MPO antibodies to activate neutrophils, leading to oxidant production and release of granule contents, is believed to underlie their pathogenic capabilities.7,8 However, recent studies indicate that the ability of ANCAs to promote neutrophil adhesion in the microvasculature is a key effector step leading to glomerular and vascular disease.5,9
Evidence supporting a proadhesive role for ANCAs comes from both in vitro and in vivo studies. In a flow chamber adhesion assay, treatment of human neutrophils with ANCAs has been shown to promote conversion from rolling to adhesion.10-12 Antihuman MPO antibodies have also been shown to enhance chemokine-induced neutrophil adhesion and transmigration in mesenteric postcapillary venules in rats.9 Similarly, passive transfer of antibodies against murine MPO has been found to increase cytokine-induced leukocyte adhesion in murine cremasteric postcapillary venules.13 Despite these studies, whether comparable adhesion mechanisms also apply in glomerular capillaries is unclear. Indeed, adhesion molecule–independent “trapping” of ANCA-activated neutrophils has been proposed as a putative mechanism of ANCA-associated glomerular neutrophil accumulation.13,14
This issue is critical in that the mechanisms of leukocyte adhesion in glomeruli are likely to be different from those at work in postcapillary venules and in vitro flow chamber systems. Using glomerular intravital microscopy, we have observed that the process whereby leukocytes undergo adhesion in inflamed glomerular capillaries is distinct from that which occurs in postcapillary venules, in that it can occur in the absence of rolling, while retaining a role for P-selectin.15 Using direct visualization of the glomerulus following in situ immune complex deposition, we found that leukocytes underwent recruitment via rapid arrest, using both P-selectin/PSGL-1 and β2-integrin/ICAM-1 pathways.15 This demonstrated that leukocyte recruitment remained an adhesion molecule–dependent event, rather than a process of physical “trapping.” Whether anti-MPO induces glomerular adhesion via a comparable process remains unknown.
We recently used intravital microscopy to demonstrate the ability of serum from MPO-immunized MPO−/− mice to induce glomerular leukocyte adhesion in LPS-pretreated mice, although adhesion mechanisms were not investigated in this study.5 The β2-integrins have been implicated as being of key importance in neutrophil adhesion induced in response to anti-MPO.12,13 However, highly activated neutrophils can use alternative adhesion molecules, specifically the α4-integrin. Strong stimuli can cause neutrophils to express the α4-integrin at functional levels, and this molecule can contribute to neutrophil–endothelial cell interactions in vivo, including in models of vasculitis.16-19 The role of the α4-integrin in anti-MPO–induced adhesion has not been addressed.
Studies of ANCA-positive patients show an association between bacterial infection and disease activity, suggesting that infection facilitates the pathogenesis of ANCA-associated glomerular injury.20,21 It is hypothesized that infection-related stimuli, such as LPS, and inflammatory mediators activate neutrophils leading to externalization of MPO, allowing for binding of anti-MPO and subsequent neutrophil activation, a concept supported by in vitro studies.7 Most studies that have demonstrated anti-MPO–induced adhesion in vivo have used inflammatory mediators such as cytokines and chemokines to facilitate anti-MPO–induced adhesion. However, there is little in vivo evidence of MPO externalization in response to inflammatory mediators, and under some circumstances, anti-MPO has been shown to promote adhesion in otherwise noninflamed animals.9,13,22 Therefore the aims of the present study were to use in vivo imaging to investigate the mechanisms whereby anti-MPO induces glomerular leukocyte adhesion, both in the presence and absence of an infection-related stimulus (ie, LPS) and to examine the effect of LPS stimulation in promoting binding of anti-MPO to neutrophils. The findings indicate that even in nonstimulated mice, anti-MPO binds to a substantial proportion of circulating neutrophils, and induces their adhesion in glomerular capillaries.
Methods
Animals
Male C57BL/6 wild-type mice (6-12 weeks, weighing 20-30 g) were bred in-house at Monash University. MPO-deficient (MPO−/−) mice on a C57BL/6 background were generously provided by Prof A. J. Lusis (University of California Los Angeles [UCLA]).23 All experimental procedures were approved by the Monash University Animal Ethics Committee.
Antibodies and reagents
Monoclonal antibodies (mAbs) against murine adhesion molecules used in vivo were from BD Biosciences (San Diego, CA), and raised in rats unless otherwise stated. mAbs used were as follows: 2E6, a hamster mAb against CD18/β2-integrin (2 mg/kg; ATCC); M17/4, a mAb against LFA-1 (170 μg/mouse); 5C6, a mAb against Mac-1 (100 μg/mouse; ATCC); M1/70, a mAb against Mac-1; RB6-8C5, a mAb against Gr-1; RB40.34, a mAb against P-selectin (20 μg/mouse); PS/2 (2 mg/kg; ATCC) and R1-2 (75 μg per mouse), both mAbs against the α4-integrin; 6C7.1, a mAb against VCAM-1 (180 μg per mouse); and 2.4G2 (100 μg/mouse), a mAb against murine FcγRII. In addition, rabbit antimouse thrombocyte serum (Accurate Chemical) was used for platelet depletion.15 LPS from Escherichia coli 0127:B8 (1.5 × 106 endotoxin units [EU]/mg) was purchased from Sigma-Aldrich (St Louis, MO).
Generation of anti-MPO antibodies
Anti-MPO antibodies were generated by immunizing MPO−/− mice against a peptide derived from amino acids 400 to 450 of the heavy chain of murine MPO (blocking peptide for antimurine MPO antibody, MPO heavy chain [L-20]: catalog no. sc-16129 P; Santa Cruz Biotechnology). MPO−/− mice were injected subcutaneously with 5 μg MPO peptide in Freund complete adjuvant (FCA; Sigma-Aldrich), and boosted via injection of the same mixture 7 days later. On day 14, mice were bled, and serum was prepared. Serum IgG was purified using a protein G column (GE Healthcare) by Biologic DuoFlow chromatography (Bio-Rad Australia). Antibody preparations were prepared by pooling serum from groups of 8 to 12 immunized MPO−/− mice. As control, antibodies against ovalbumin (anti-OVA) were generated and purified in an identical fashion. Purified antibodies were tested for LPS content using the E-toxate assay (Sigma-Aldrich). Antibodies were tested for MPO specificity via enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates were coated with recombinant mouse MPO (produced via baculovirus transfection of Sf21 insect cells, and purified via a batch/gravity-flow column method, as described24 ) at 5 μg/mL, and incubated at 4°C overnight.24 Plates were washed with 0.1% Tween/PBS and then blocked with 2% casein. Mouse serum diluted 1:100 in PBS was added to plates and incubated at 37°C for 1 hour. Plates were then washed and treated with horseradish peroxidase–conjugated sheep anti–mouse IgG (1/2000). After washing, color was developed using tetramethylbenzidine (Sigma-Aldrich), and the absorbance read at 450 nm.
Unilateral ureteric ligation and renal intravital microscopy
To prepare the kidney for intravital microscopy, mice underwent unilateral ureteric ligation as previously described.15 Twelve weeks were allowed for renal hydronephrosis. At the end of this period, mice were anesthetized using ketamine hydrochloride and xylazine, and the jugular vein was cannulated for the administration of further anesthetic and reagents. Animals were maintained at 37°C on a heating pad. The hydronephrotic kidney was exteriorized through a lateral incision, drained of urine using a 30-G needle and extended over a clear viewing platform using 4/0 silk. The kidney was superfused with bicarbonate-buffered saline (pH 7.4; 37°C) and covered with a coverslip. The renal microvasculature was observed with an intravital microscope (Axioplan 2 Imaging; Carl Zeiss) with a water-immersion objective (20 ×/0.50 numeric aperture). To visualize leukocytes, mice were injected with 50 μL of 0.05% rhodamine 6G (Sigma-Aldrich) and the tissue was examined via epifluorescence at 520 to 560 nm, using a 590-nm emission filter. Images were visualized using a video camera (IR-1000; Dage-MTI, Michigan City, IN) and recorded for playback analysis using a digital video recorder. Three to 4 randomly chosen glomeruli were recorded at intervals throughout the experiment and analyzed for leukocyte adhesion. A leukocyte was defined as adherent if it remained stationary within the glomerulus for at least 30 seconds.15
Experimental protocol
In the LPS-treated groups, mice received 0.1 μg LPS (150 EU) intraperitoneally. Subsequently either 1 or 4 hours later, mice were prepared for intravital microscopy. An initial recording of glomerular leukocyte parameters was made, immediately after which either anti-MPO or anti-OVA antibodies (50 μg) were administered. Subsequent recordings were made 15, 30, and 60 minutes after antibody administration. In mice that did not receive LPS, the kidney was prepared for intravital microscopy, and the recordings were performed and antibodies administered as for the LPS groups.
In experiments investigating adhesion molecule function, Abs against various adhesion molecules were administered intravenously 5 minutes prior to the initial glomerular recording. To examine the role of platelets, platelet depletion was achieved via injection of rabbit antimouse thrombocyte serum (15 μL, intraperitoneally) 4 hours prior to administration of anti-MPO.15 We have previously found that this antibody reduces circulating platelet counts by more than 97%.15
Preparation of the cremaster muscle for intravital microscopy
The cremaster muscle was prepared for intravital microscopy and leukocyte rolling and adhesion assessed as previously described.25
Assessment of splenic and lung MPO activity
MPO activity in spleen and lung was determined using a previously published technique.26 One unit (U) of MPO activity is defined as a change in A460 of 1.0 after 2 minutes, and results are expressed as unit per gram of tissue (U/g).
Immunohistochemical analysis of glomerular leukocyte recruitment
Glomerular neutrophil and monocyte accumulation was determined via immunohistochemistry, as previously described.15 In brief, cryostat sections, prepared from tissues fixed in periodate/lysine/paraformaldehyde, were stained with anti–Gr-1 (RB6-8C5, 1 μg/mL; BD Biosciences) or anti-CD68 (FA-11, 20 μg/mL; BD Biosciences), and neutrophil (anti–Gr-1) or monocyte (anti-CD68) accumulation was quantified in 40 to 50 glomeruli per mouse. Data were expressed as cells/glomerular cross section (cells/gcs).
Flow cytometric analysis of leukocyte anti-MPO binding, integrin expression, and VCAM-1 binding function
To assess the ability of intravenously administered anti-MPO to bind to circulating neutrophils and monocytes, anti-MPO or anti-OVA (50 μg), conjugated to Alexa 647 (Invitrogen Australia), was administered intravenously to anesthetized mice. Blood was collected 5 or 60 minutes later, and the erythrocytes were lysed in NH4Cl. To identify neutrophils within the leukocyte pellet, the cells were stained with PE-conjugated anti–Gr-1 and FITC-conjugated M1/70 (both 1/50), then examined using a MoFlo flow cytometer (Beckman Coulter). Neutrophils were identified via characteristic high forward and side scatter (“granulocyte” gate) and high staining for anti–Gr-1 (clone RB6-8C5) and M1/70 (BD Biosciences), and then assessed for binding of Alexa 647–conjugated anti-MPO. Monocytes were identified as cells positive for both CD115 (clone AFS98, 1/100; eBioscience) and F4/80 (1/50, BD Biosciences), as previously described.27 To limit leukocyte sequestration, additional mice were pretreated with an anti–β2-integrin mAb (2E6, 2 mg/kg, intravenously) 5 minutes prior to administration of either anti-MPO or anti-OVA.
To assess the effect of anti-MPO on neutrophil α4-integrin expression, neutrophils in whole blood or spleen were examined. Erythrocytes in whole blood or dissociated spleen samples were lysed via NH4Cl, and leukocytes pelleted via centrifugation. Leukocytes were treated with either anti-MPO or anti-OVA (50 μg/mL, 5-30 minutes, 37°C) then stained with anti–Gr-1-PE, M1/70-Alexa 647, and anti–α4-integrin (PS/2) conjugated to Alexa 488. α4-integrin expression was assessed via flow cytometry on neutrophils identified on the basis of high staining for anti–Gr-1 and M1/70. VCAM-1 binding by blood or splenic neutrophils was assessed following treatment of mice with anti-MPO or anti-OVA (50 or 200 μg intravenously, 5-30 minutes).28 Isolated splenocytes or leukocytes were treated with chimeric murine VCAM-1/human Fc (R&D Systems; 0.4 μg/mL in Tyrode buffer, 30 minutes, 37°C). Following washing, VCAM-1/Fc binding was assessed via flow cytometry using FITC-conjugated sheep anti–human IgG, in combination with anti–Gr-1-PE/M1/70-Alexa 647 to allow identification of neutrophils.
Statistics
Data are presented as mean plus or minus SEM. For comparisons involving 2 groups, either 2-way analysis of variance (ANOVA) or Student unpaired t tests were used. For comparisons involving 3 groups, either 2-way ANOVA, followed by posthoc tests, or Student t tests, followed by Bonferroni multiple comparisons test, were used.
Results
β2-Integrin use in glomerular adhesion induced by anti-MPO in LPS-stimulated mice
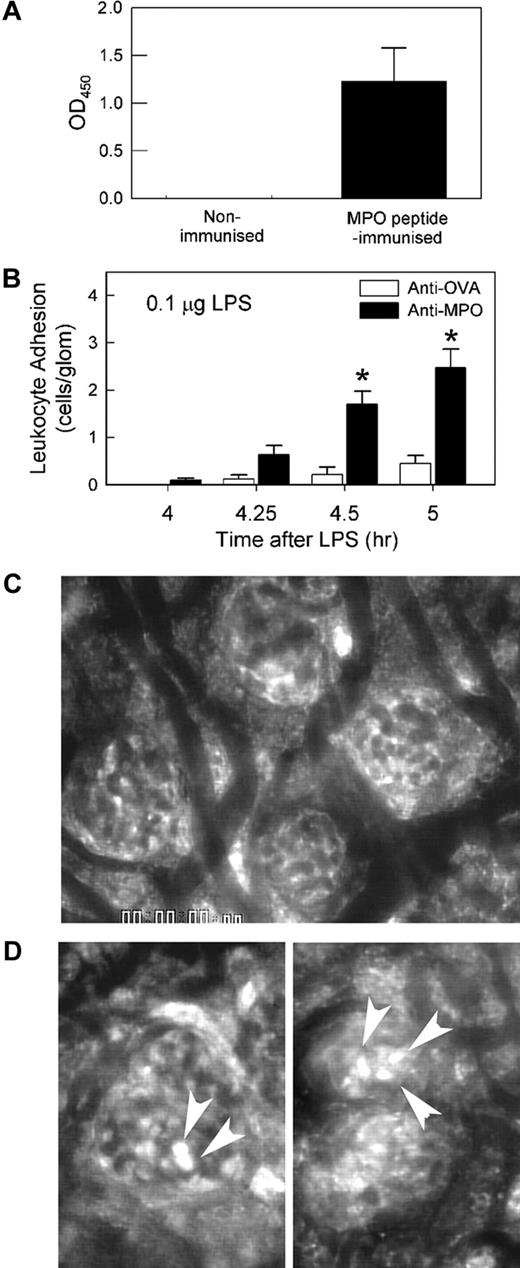
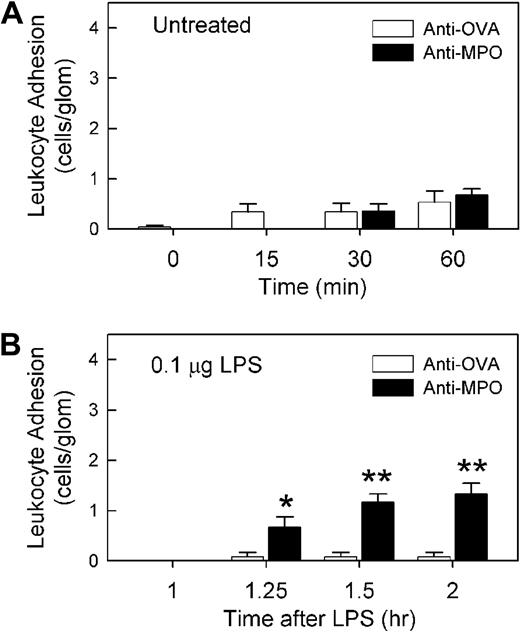
ELISA analysis of serum from mice immunized with MPO peptide demonstrated strong reactivity against recombinant murine MPO, indicating that the antibodies raised using this approach recognized the intact MPO protein (Figure 1A). We have previously observed that serum from MPO-immunized mice induced leukocyte recruitment to glomeruli, but only in mice pretreated with LPS.5 The aim of the initial series of experiments was to determine whether comparable responses were induced in response to IgG purified from serum of MPO-immunized mice (anti-MPO). In mice pretreated with 0.1 μg LPS, minimal glomerular adhesion was observed after 4 hours (Figure 1B). A similar result was observed after treatment with 1 μg/1500 EU LPS (data not shown). These findings demonstrate that 0.1 to 1 μg LPS is insufficient to induce leukocyte adhesion in the glomerulus. However, in these mice, administration of 50 μg IgG from MPO-immunized mice induced a significant increase in leukocyte adhesion, a response not seen in mice treated with IgG from OVA-immunized mice (Figure 1B,D). This response was MPO dependent in that administration of anti-MPO to LPS-pretreated MPO−/− mice induced a minimal response (data not shown). In mice that had not received LPS pretreatment, glomerular leukocyte adhesion following administration of 50 μg anti-MPO was minimal, and not different from that seen in anti-OVA–treated mice (Figure 2A). To assess the amount of time required for the effect of LPS to take place, mice were treated with anti-MPO only 60 minutes after LPS pretreatment (Figure 2B). In these animals, anti-MPO also induced a significant increase in leukocyte adhesion, although this response was not as large as that seen 4 hours after LPS pretreatment.
Anti-MPO recognizes MPO and induces glomerular leukocyte adhesion in LPS-pretreated mice. (A) Serum from MPO−/− mice immunized with MPO peptide was assessed via ELISA for its ability to recognize recombinant murine MPO. Serum from a nonimmunized mouse is shown as comparison. OD450 data are shown as mean ± SEM of samples from n = 6 immunized mice, diluted 1:100 and measured in duplicate. (B) Glomerular leukocyte adhesion was assessed via intravital microscopy in LPS (0.1 μg, 4 hours)–pretreated mice that received either anti-MPO or anti-OVA. Following a basal reading of adhesion 4 hours after LPS, mice received either anti-MPO (50 μg, intravenously, n = 14) or anti-OVA (50 μg, intravenously, n = 8), and adhesion was reassessed 15, 30, and 60 minutes later. (*P < .001 vs anti-OVA via 2-way ANOVA.) (C-D) Representative intravital microscopy images of glomeruli before (C) and 60 minutes after (D) administration of anti-MPO. Arrowheads denote adherent leukocytes stained via rhodamine 6G. Images were acquired with a Zeiss Axioplan 2 Imaging microscope equipped with a 20×/WI 0.5 NA objective lens.
Anti-MPO recognizes MPO and induces glomerular leukocyte adhesion in LPS-pretreated mice. (A) Serum from MPO−/− mice immunized with MPO peptide was assessed via ELISA for its ability to recognize recombinant murine MPO. Serum from a nonimmunized mouse is shown as comparison. OD450 data are shown as mean ± SEM of samples from n = 6 immunized mice, diluted 1:100 and measured in duplicate. (B) Glomerular leukocyte adhesion was assessed via intravital microscopy in LPS (0.1 μg, 4 hours)–pretreated mice that received either anti-MPO or anti-OVA. Following a basal reading of adhesion 4 hours after LPS, mice received either anti-MPO (50 μg, intravenously, n = 14) or anti-OVA (50 μg, intravenously, n = 8), and adhesion was reassessed 15, 30, and 60 minutes later. (*P < .001 vs anti-OVA via 2-way ANOVA.) (C-D) Representative intravital microscopy images of glomeruli before (C) and 60 minutes after (D) administration of anti-MPO. Arrowheads denote adherent leukocytes stained via rhodamine 6G. Images were acquired with a Zeiss Axioplan 2 Imaging microscope equipped with a 20×/WI 0.5 NA objective lens.
Sixty-minute LPS treatment is sufficient to promote anti-MPO–induced glomerular leukocyte adhesion. (A) Effect of anti-MPO or anti-OVA in otherwise untreated mice. An initial reading of glomerular adhesion was made; then mice received either anti-MPO or anti-OVA (n = 6-7/gp, 50 μg, intravenously), and adhesion was reassessed 15, 30, and 60 minutes later. (B) Assessment of the ability of anti-MPO to induce a glomerular adhesion response 60 minutes after LPS (0.1 μg) administration. Mice were treated in an identical fashion to Figure 1, except anti-MPO or anti-OVA (n = 6/gp, 50 μg, intravenously) was administered 1 hour after LPS. (*P < .05; **P < .01 vs anti-OVA at the corresponding time points.)
Sixty-minute LPS treatment is sufficient to promote anti-MPO–induced glomerular leukocyte adhesion. (A) Effect of anti-MPO or anti-OVA in otherwise untreated mice. An initial reading of glomerular adhesion was made; then mice received either anti-MPO or anti-OVA (n = 6-7/gp, 50 μg, intravenously), and adhesion was reassessed 15, 30, and 60 minutes later. (B) Assessment of the ability of anti-MPO to induce a glomerular adhesion response 60 minutes after LPS (0.1 μg) administration. Mice were treated in an identical fashion to Figure 1, except anti-MPO or anti-OVA (n = 6/gp, 50 μg, intravenously) was administered 1 hour after LPS. (*P < .05; **P < .01 vs anti-OVA at the corresponding time points.)
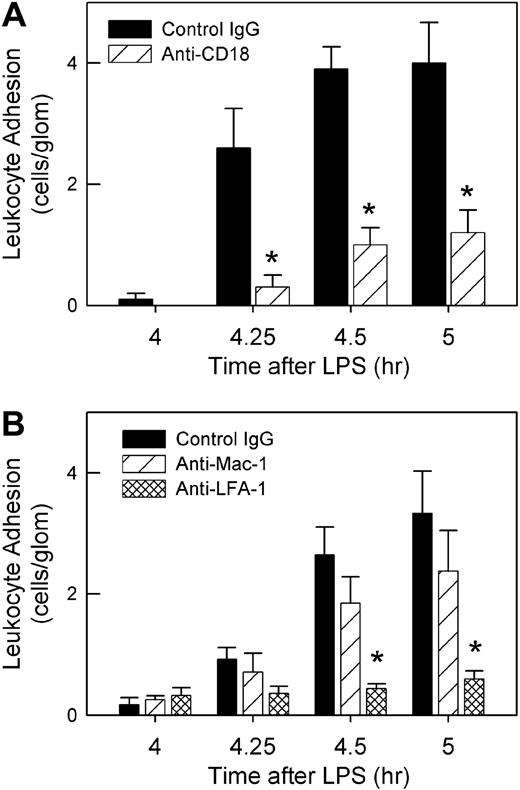
We next assessed the role of the β2-integrins in anti-MPO–induced adhesion. Pretreatment with the anti–β2–integrin mAb 2E6 inhibited anti-MPO–induced adhesion significantly (Figure 3A). Additional experiments showed that inhibition of LFA-1 (αLβ2) but not Mac-1 (αMβ2) prevented the increase in adhesion, indicating that LFA-1 was the major contributor to the β2-integrin–mediated glomerular adhesion (Figure 3B).
Roles of β2-integrins in anti-MPO–induced glomerular leukocyte adhesion in LPS-pretreated mice. (A) Mice underwent treatment with LPS (0.1 μg, 4 hour) and received either anti–β2-integrin (2E6, 2 mg/kg) or control hamster IgG immediately prior to anti-MPO (50 μg, intravenously) (n = 5/gp). (*P < .001 vs control IgG at the corresponding time points.) (B) Using a similar approach, mice were treated with mAbs against either LFA-1 (M17/4, 170 μg, n = 6) or Mac-1 (5C6, 100 μg, n = 6) to identify which β2-integrin was mediating anti-MPO–induced adhesion. Data were compared with mice treated with isotype control mAb (n = 6). (*P < .001 vs isotype control IgG at same time point, via 2-way ANOVA.)
Roles of β2-integrins in anti-MPO–induced glomerular leukocyte adhesion in LPS-pretreated mice. (A) Mice underwent treatment with LPS (0.1 μg, 4 hour) and received either anti–β2-integrin (2E6, 2 mg/kg) or control hamster IgG immediately prior to anti-MPO (50 μg, intravenously) (n = 5/gp). (*P < .001 vs control IgG at the corresponding time points.) (B) Using a similar approach, mice were treated with mAbs against either LFA-1 (M17/4, 170 μg, n = 6) or Mac-1 (5C6, 100 μg, n = 6) to identify which β2-integrin was mediating anti-MPO–induced adhesion. Data were compared with mice treated with isotype control mAb (n = 6). (*P < .001 vs isotype control IgG at same time point, via 2-way ANOVA.)
Anti-MPO antibody binds to circulating neutrophils in unstimulated mice
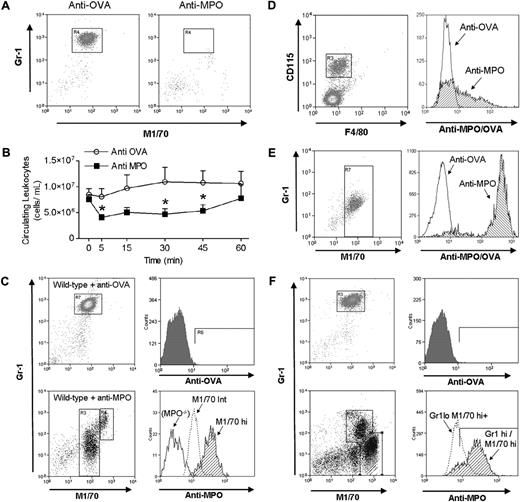
The glomerular adhesion in LPS-treated mice seen in response to anti-MPO is believed to stem from LPS-induced externalization of MPO by neutrophils, although this has not been documented in vivo. Therefore we used fluorochrome-conjugated anti-MPO to assess neutrophil anti-MPO binding. In initial experiments involving intravenous administration of anti-MPO, we found that very few neutrophils were present in the blood 5 minutes after anti-MPO administration (Figure 4A). This was supported by examination of circulating leukocyte counts, which were reduced significantly in anti-MPO–treated mice within 5 minutes, and examination of blood cytospins, which revealed an almost complete absence of granulocytes (Table 1). We hypothesized that neutrophils were undergoing sequestration within organs such as the spleen and lung. This hypothesis was tested by measuring tissue MPO activity as an indirect readout of neutrophil accumulation. Splenic MPO activity doubled within 5 minutes of anti-MPO administration, and was significantly elevated above that seen in anti-OVA–treated mice. In contrast, lung MPO levels did not differ between the groups (Table 1). To determine whether neutrophils remained sequestered in the spleen, we examined circulating leukocyte counts at multiple time points in mice treated with either anti-MPO or anti-OVA (Figure 4B). In anti-MPO–treated mice, circulating leukocytes were significantly reduced at 5, 30, and 45 minutes, but by 60 minutes were no longer significantly different from those in anti-OVA–treated mice. In addition, at 60 minutes, splenic MPO levels no longer differed between the groups (Table 1), indicating that the sequestration of neutrophils in the spleen was temporary.
Flow cytometric analysis of anti-MPO binding by circulating leukocytes from naive mice or mice pretreated with LPS. Mice were treated with fluorochrome-conjugated anti-MPO or anti-OVA and whole blood was removed either 5 or 60 minutes later. Neutrophils in blood samples were identified by initially gating on cells of high forward scatter/side scatter characteristics, and examining staining for Gr-1 and M1/70. (A) Neutrophils in mice treated with anti-OVA or anti-MPO alone, showing the rapid removal of neutrophils from the circulation in response to anti-MPO, but not anti-OVA (representative of n = 3). (B) Time course of circulating leukocyte counts of mice that received either anti-OVA or anti-MPO (50 μg, n = 6/gp). (*P < .05 vs anti-OVA at designated time points.) Following treatment with anti–β2-integrin mAb (2E6; C,D,F), sufficient leukocytes were retained in the circulation to allow assessment of anti-MPO binding. (C-F) Gates shown in the left-hand panels define the leukocytes analyzed for binding of anti-MPO/OVA in the right-hand panels. (C) Binding of anti-OVA or anti-MPO by circulating neutrophils in otherwise untreated wild-type C57BL/6 mice. In mice treated with anti-OVA (n = 8), neutrophils stain homogeneously for M1/70, although minimal binding of anti-OVA is observed. In contrast, in wild-type mice treated with anti-MPO (n = 8), 2 neutrophil populations are apparent: cells that stain moderately (M1/70int) or strongly (M1/70hi) for M1/70. Approximately 50% of M1/70int cells stain positively with anti-MPO, whereas the majority of M1/70hi cells stain positively for anti-MPO. Also shown is the anti-MPO staining pattern of neutrophils from MPO−/− mice treated with anti-MPO (n = 4), showing negligible binding. (D) Monocyte binding of anti-MPO. In the blood of mice treated as in panel C, monocytes were identified as cells positive for both CD115 and F4/80 (n = 5). These cells were not found in the high forward scatter/side scatter gate that contained the neutrophils. Approximately 40% of circulating monocytes stained positively for anti-MPO. Monocyte binding of anti-OVA (n = 4) is shown on the same trace. (E) Binding of anti-MPO by circulating neutrophils 60 minutes after anti-MPO administration (in the absence of β2-integrin blockade, n = 3). At this point, Gr1+/M170int cells were again present in the circulation, and the majority of cells stained strongly with anti-MPO. Also shown is neutrophil binding of anti-OVA at the corresponding time point. (F) Binding of anti-OVA or anti-MPO by circulating neutrophils in LPS (0.1 μg, 4 hours)–pretreated mice (n = 6). In mice treated with anti-OVA, neutrophil M1/70 staining is unaltered from that seen in the absence of LPS and minimal binding of anti-OVA is observed. However, in mice treated with LPS and anti-MPO, a wide spread of M1/70 staining is apparent within the granulocyte gate. The 2 major populations are as follows: Gr-1hi/M1/70hi cells, of which the majority stain strongly with anti-MPO; and cells of lower Gr-1 staining, but greater M1/70 staining (Gr-1lo/M1/70hi+), of which 70% are stained by anti-MPO.
Flow cytometric analysis of anti-MPO binding by circulating leukocytes from naive mice or mice pretreated with LPS. Mice were treated with fluorochrome-conjugated anti-MPO or anti-OVA and whole blood was removed either 5 or 60 minutes later. Neutrophils in blood samples were identified by initially gating on cells of high forward scatter/side scatter characteristics, and examining staining for Gr-1 and M1/70. (A) Neutrophils in mice treated with anti-OVA or anti-MPO alone, showing the rapid removal of neutrophils from the circulation in response to anti-MPO, but not anti-OVA (representative of n = 3). (B) Time course of circulating leukocyte counts of mice that received either anti-OVA or anti-MPO (50 μg, n = 6/gp). (*P < .05 vs anti-OVA at designated time points.) Following treatment with anti–β2-integrin mAb (2E6; C,D,F), sufficient leukocytes were retained in the circulation to allow assessment of anti-MPO binding. (C-F) Gates shown in the left-hand panels define the leukocytes analyzed for binding of anti-MPO/OVA in the right-hand panels. (C) Binding of anti-OVA or anti-MPO by circulating neutrophils in otherwise untreated wild-type C57BL/6 mice. In mice treated with anti-OVA (n = 8), neutrophils stain homogeneously for M1/70, although minimal binding of anti-OVA is observed. In contrast, in wild-type mice treated with anti-MPO (n = 8), 2 neutrophil populations are apparent: cells that stain moderately (M1/70int) or strongly (M1/70hi) for M1/70. Approximately 50% of M1/70int cells stain positively with anti-MPO, whereas the majority of M1/70hi cells stain positively for anti-MPO. Also shown is the anti-MPO staining pattern of neutrophils from MPO−/− mice treated with anti-MPO (n = 4), showing negligible binding. (D) Monocyte binding of anti-MPO. In the blood of mice treated as in panel C, monocytes were identified as cells positive for both CD115 and F4/80 (n = 5). These cells were not found in the high forward scatter/side scatter gate that contained the neutrophils. Approximately 40% of circulating monocytes stained positively for anti-MPO. Monocyte binding of anti-OVA (n = 4) is shown on the same trace. (E) Binding of anti-MPO by circulating neutrophils 60 minutes after anti-MPO administration (in the absence of β2-integrin blockade, n = 3). At this point, Gr1+/M170int cells were again present in the circulation, and the majority of cells stained strongly with anti-MPO. Also shown is neutrophil binding of anti-OVA at the corresponding time point. (F) Binding of anti-OVA or anti-MPO by circulating neutrophils in LPS (0.1 μg, 4 hours)–pretreated mice (n = 6). In mice treated with anti-OVA, neutrophil M1/70 staining is unaltered from that seen in the absence of LPS and minimal binding of anti-OVA is observed. However, in mice treated with LPS and anti-MPO, a wide spread of M1/70 staining is apparent within the granulocyte gate. The 2 major populations are as follows: Gr-1hi/M1/70hi cells, of which the majority stain strongly with anti-MPO; and cells of lower Gr-1 staining, but greater M1/70 staining (Gr-1lo/M1/70hi+), of which 70% are stained by anti-MPO.
Effect of 50 μg anti-MPO versus anti-OVA on circulating leukocyte counts, percentage of circulating neutrophils, and MPO levels in spleen and lung
| . | Anti-OVA . | Anti-MPO . |
|---|---|---|
| Circulating leukocyte count, cells/×10−6/mL, n = 5/gp | 5.6 ± 0.7 | 1.7 ± 0.5* |
| % of neutrophils in cytospun blood samples, n = 5/gp | 16.8 ± 1.8 % | 0.8 ± 0.5%* |
| Splenic MPO activity 5 min after anti-MPO, U/g, n = 7/gp | 24.8 ± 8.9 | 53.5 ± 9.9† |
| Lung MPO activity 5 min after anti-MPO, U/g, n = 7/gp | 2.5 ± 0.6 | 4.0 ± 1.1 (NS) |
| Splenic MPO activity 60 min after anti-MPO, U/g, n = 7/gp | 19.3 ± 3.2 | 19.4 ± 3.0 (NS) |
| Lung MPO activity 60 min after anti-MPO, U/g, n = 7/gp | 10.9 ± 2.1 | 8.6 ± 1.9 (NS) |
| . | Anti-OVA . | Anti-MPO . |
|---|---|---|
| Circulating leukocyte count, cells/×10−6/mL, n = 5/gp | 5.6 ± 0.7 | 1.7 ± 0.5* |
| % of neutrophils in cytospun blood samples, n = 5/gp | 16.8 ± 1.8 % | 0.8 ± 0.5%* |
| Splenic MPO activity 5 min after anti-MPO, U/g, n = 7/gp | 24.8 ± 8.9 | 53.5 ± 9.9† |
| Lung MPO activity 5 min after anti-MPO, U/g, n = 7/gp | 2.5 ± 0.6 | 4.0 ± 1.1 (NS) |
| Splenic MPO activity 60 min after anti-MPO, U/g, n = 7/gp | 19.3 ± 3.2 | 19.4 ± 3.0 (NS) |
| Lung MPO activity 60 min after anti-MPO, U/g, n = 7/gp | 10.9 ± 2.1 | 8.6 ± 1.9 (NS) |
Samples were removed 5 minutes after intravenous administration of 50 μg of either anti-MPO or anti-OVA, except for the 60-minute samples for splenic and lung MPO activity, which were removed 60 minutes after antibody administration.
NS indicates not significant relative to anti-OVA.
P < .001 vs anti-OVA.
P < .05 vs anti-OVA.
A previous study has reported that anti-MPO induces a reduction in circulating leukocyte counts in cytokine-treated mice, and that this was preventable via β2-integrin inhibition.13 We therefore performed additional experiments in which mice were pretreated with an anti–β2-integrin mAb. In mice that received both anti-MPO and anti–β2-integrin, neutrophils remained in the circulation 5 minutes after anti-MPO administration (Figure 4C), allowing analysis of anti-MPO binding. In mice treated with anti-OVA and anti–β2-integrin, neutrophils stained homogeneously for M1/70, although minimal binding of anti-OVA was observed. In contrast, in mice treated with anti-MPO alone, 2 populations of neutrophils were observed (Figure 4C; Table 2). The majority of neutrophils (∼ 84%) displayed an intermediate level of M1/70 binding (M1/70int), whereas a smaller population displayed a marked increase in M1/70 binding (M1/70hi), indicative of increased Mac-1 expression. Of the M1/70int population, 32% stained positively for anti-MPO. Moreover, 95% of the M1/70hi population, which presumably represented more activated neutrophils, bound anti-MPO, and in these cells, the mean fluorescence intensity (MFI) of MPO staining was elevated above that in the M1/70int population (Table 2). Anti-MPO treatment also resulted in a reduction in Gr-1 expression by many cells in the granulocyte gate (Figure 4C,F). The antibody preparations used in these experiments tested negative for endotoxin, indicating that these responses were not due to LPS contamination.
Mac-1 expression and binding of either anti-MPO or anti-OVA by circulating neutrophils in untreated wild-type and MPO−/− mice
| Population . | Proportion of total neutrophils . | M1/70 MFI . | % +ve for anti-OVA or anti-MPO . | Anti-MPO MFI . |
|---|---|---|---|---|
| Wild-type + anti-OVA, n = 8 | ||||
| Gr-1hi/M1/70int | All | 81 ± 8 | 0.3% ± 0.1% (anti-OVA+ve) | — |
| Wild-type + anti-MPO, n = 8 | ||||
| Gr-1hi/M1/70int | 84% ± 18% | 78 ± 17 | 32% ± 5% (anti-MPO+ve) | 9.5 ± 1.3 |
| Gr-1lo/M1/70hi | 16% ± 20% | 326 ± 48 | 95% ± 4% (anti-MPO+ve) | 31.9 ± 1.8 |
| MPO−/− + anti-MPO, n = 4 | ||||
| Gr-1int/M1/70int | 97% ± 3% | 64 ± 14 | 2% ± 1%, (anti-MPO+ve) | 4 ± 0.5 |
| Population . | Proportion of total neutrophils . | M1/70 MFI . | % +ve for anti-OVA or anti-MPO . | Anti-MPO MFI . |
|---|---|---|---|---|
| Wild-type + anti-OVA, n = 8 | ||||
| Gr-1hi/M1/70int | All | 81 ± 8 | 0.3% ± 0.1% (anti-OVA+ve) | — |
| Wild-type + anti-MPO, n = 8 | ||||
| Gr-1hi/M1/70int | 84% ± 18% | 78 ± 17 | 32% ± 5% (anti-MPO+ve) | 9.5 ± 1.3 |
| Gr-1lo/M1/70hi | 16% ± 20% | 326 ± 48 | 95% ± 4% (anti-MPO+ve) | 31.9 ± 1.8 |
| MPO−/− + anti-MPO, n = 4 | ||||
| Gr-1int/M1/70int | 97% ± 3% | 64 ± 14 | 2% ± 1%, (anti-MPO+ve) | 4 ± 0.5 |
Data are shown as mean ± SEM.
— indicates not applicable.
To determine whether anti-MPO bound to neutrophils via a nonspecific, Fc-mediated mechanism, neutrophil anti-MPO binding was assessed in mice pretreated with the Fc receptor–blocking antibody 2.4G2. In these experiments, the percentage of total neutrophils positive for anti-MPO was not significantly altered in the presence of 2.4G2 (anti-MPO alone: 44.1% ± 8.3%, n = 4 vs anti-MPO + 2.4G2: 30 ± 3.8, n = 4, NS), although the MFI of anti-MPO staining was significantly reduced (anti-MPO alone: 96.5 ± 24.8, n = 4 vs anti-MPO + 2.4G2: 30.4 ± 5.7, n = 4, P = .02). In addition, neutrophils from MPO−/− mice treated with anti-MPO displayed minimal antibody binding, and no Mac-1 up-regulation (Figure 4C; Table 2). These data indicate that neutrophil binding of anti-MPO was specific to MPO, but raise the possibility that Fc receptors have a role in retaining anti-MPO on the neutrophil surface. We also examined the binding of anti-MPO to circulating monocytes. Approximately 40% of monocytes were positive for anti-MPO (Figure 4D; Table 3), although at a lower MFI than for the M1/70hi neutrophil population (Table 2). Finally, in mice examined 60 minutes after anti-MPO administration (in the absence of β2-integrin blockade), neutrophils had returned to the circulation and were predominantly strongly positive for anti-MPO (Figure 4E).
Binding of either anti-MPO or anti-OVA by circulating monocytes in untreated wild-type mice
| . | % +ve for anti-OVA or anti-MPO . | Anti-MPO MFI . |
|---|---|---|
| Anti-OVA, n = 4 | 1.9% ± 0.5% (anti-OVA+ve) | — |
| Anti-MPO, n = 5 | 44% ± 5% (anti-MPO+ve) | 9.4 ± 1.7 |
| . | % +ve for anti-OVA or anti-MPO . | Anti-MPO MFI . |
|---|---|---|
| Anti-OVA, n = 4 | 1.9% ± 0.5% (anti-OVA+ve) | — |
| Anti-MPO, n = 5 | 44% ± 5% (anti-MPO+ve) | 9.4 ± 1.7 |
Data are shown as mean ± SEM.
— indicates not applicable.
To assess the effect of LPS on neutrophil MPO externalization, mice underwent LPS pretreatment (0.1 μg, 4 hours) prior to administration of anti–β2-integrin mAb and either anti-MPO or anti-OVA. In LPS-pretreated mice that received anti-OVA, there was minimal antibody binding, and the MFI for M1/70 staining (72 ± 12) was comparable with that in neutrophils from unstimulated wild-type mice treated with anti-OVA (77 ± 5) and unstimulated MPO−/− treated with anti-MPO (64 ± 14; Figure 4F; Table 4). This indicated that the LPS treatment alone was insufficient to induce Mac-1 up-regulation on circulating neutrophils. In contrast, in mice treated with anti-MPO, the neutrophil populations were markedly altered (Figure 4F). A wide range of M1/70 binding was observed, although 2 major populations were evident: a Gr-1hi/M1/70hi population, of which 91% bound anti-MPO; and a lesser population that expressed M1/70 at extremely high levels (MFI = 1252 ± 414) while displaying reduced Gr-1 (Gr-1lo/M1/70hi+). Approximately 70% of these cells were positive for anti-MPO. In addition, both of these populations showed substantially elevated MFI of MPO staining (Table 4) relative to cells from mice not treated with LPS.
Mac-1 expression and binding of either anti-MPO or anti-OVA by circulating neutrophils in wild-type mice pretreated with LPS (0.1 μg, 4 hours)
| Population . | Proportion of total neutrophils . | M1/70 MFI . | % +ve for anti-OVA or anti-MPO . | Anti-MPO MFI . |
|---|---|---|---|---|
| LPS + anti-OVA, n = 6 | ||||
| Gr-1hi/M1/70int | All | 72 ± 12 | 1% ± 1% (anti-OVA+ve) | — |
| LPS + anti-MPO, n = 6 | ||||
| Gr-1hi/M1/70hi | 70% ± 14% | 449 ± 74 | 91% ± 6% (anti-MPO+ve) | 81 ± 33 |
| Gr-1lo/M/1/70hi+ | 30% ± 14% | 1252 ± 414 | 70% ± 17% (anti-MPO+ve) | 90 ± 39 |
| Population . | Proportion of total neutrophils . | M1/70 MFI . | % +ve for anti-OVA or anti-MPO . | Anti-MPO MFI . |
|---|---|---|---|---|
| LPS + anti-OVA, n = 6 | ||||
| Gr-1hi/M1/70int | All | 72 ± 12 | 1% ± 1% (anti-OVA+ve) | — |
| LPS + anti-MPO, n = 6 | ||||
| Gr-1hi/M1/70hi | 70% ± 14% | 449 ± 74 | 91% ± 6% (anti-MPO+ve) | 81 ± 33 |
| Gr-1lo/M/1/70hi+ | 30% ± 14% | 1252 ± 414 | 70% ± 17% (anti-MPO+ve) | 90 ± 39 |
Data are shown as mean ± SEM.
— indicates not applicable.
High-dose anti-MPO antibody induces glomerular neutrophil adhesion in unstimulated mice
Given the capacity of anti-MPO to bind to circulating neutrophils in untreated mice, we next assessed the ability of higher concentrations of anti-MPO to induce glomerular leukocyte localization in the absence of LPS pretreatment. Mice treated with 200 to 450 μg anti-MPO displayed significant increases in glomerular leukocyte adhesion within 30 minutes (data not shown). Figure 5 shows the increase in glomerular leukocyte adhesion induced by 200 μg anti-MPO, a response not seen in mice treated with a comparable amount of anti-OVA. Immunohistochemical analysis revealed that glomeruli in these kidneys contained significantly more Gr-1+ cells, relative to those in anti-OVA–treated mice (anti-OVA: 0.25 ± 0.02 cells/gcs, n = 11 vs anti-MPO: 0.60 ± 0.03 cells/gcs, n = 5, P < .001). In contrast, few CD68+ monocytes (< 0.15 cells/gcs) were detected in glomeruli of anti-MPO–treated mice, suggesting that anti-MPO binding induces glomerular adhesion of neutrophils but not monocytes.
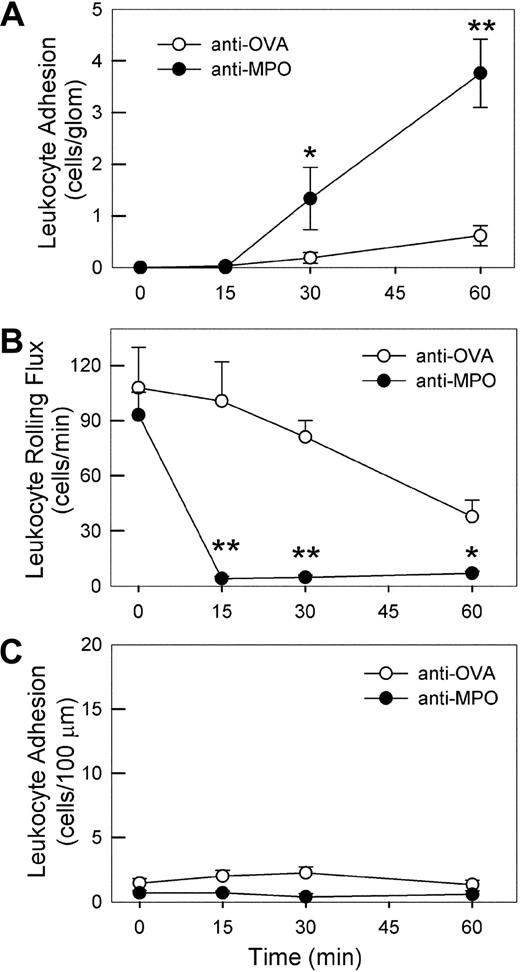
Effect of high-dose anti-MPO on leukocyte–endothelial cell interactions in glomerular capillaries and cremasteric postcapillary venules. (A) Intravital microscopic assessment of glomerular leukocyte adhesion in mice treated with high-dose (200 μg, intravenously) anti-MPO (n = 8) versus anti-OVA (n = 10). A basal assessment of glomerular adhesion was performed, anti-MPO or anti-OVA was administered, then recordings of glomerular adhesion were performed 15, 30, and 60 minutes later. (B,C) Leukocyte rolling flux (B) and adhesion (C) in cremasteric postcapillary venules of mice treated with high-dose anti-MPO (n = 5) or anti-OVA (n = 4). Following assessment of basal leukocyte rolling and adhesion, mice received 200 μg anti-MPO or anti-OVA intravenously, and rolling and adhesion were reassessed over the subsequent 60 minutes. (*P < .05; **P < .01 vs the anti-OVA group at the corresponding time points.)
Effect of high-dose anti-MPO on leukocyte–endothelial cell interactions in glomerular capillaries and cremasteric postcapillary venules. (A) Intravital microscopic assessment of glomerular leukocyte adhesion in mice treated with high-dose (200 μg, intravenously) anti-MPO (n = 8) versus anti-OVA (n = 10). A basal assessment of glomerular adhesion was performed, anti-MPO or anti-OVA was administered, then recordings of glomerular adhesion were performed 15, 30, and 60 minutes later. (B,C) Leukocyte rolling flux (B) and adhesion (C) in cremasteric postcapillary venules of mice treated with high-dose anti-MPO (n = 5) or anti-OVA (n = 4). Following assessment of basal leukocyte rolling and adhesion, mice received 200 μg anti-MPO or anti-OVA intravenously, and rolling and adhesion were reassessed over the subsequent 60 minutes. (*P < .05; **P < .01 vs the anti-OVA group at the corresponding time points.)
To assess whether this adhesion response also occurred in postcapillary venules, we examined leukocyte rolling and adhesion in cremasteric postcapillary venules of mice treated with 200 μg of either anti-OVA or anti-MPO. Anti-OVA administration caused no overt changes in leukocyte rolling and adhesion (Figure 5B,C). Anti-MPO also did not induce leukocyte adhesion (Figure 5C), but caused a marked reduction in leukocyte rolling flux within 15 minutes (Figure 5B). Analysis of neutrophils from anti-MPO–treated mice showed no alteration in expression of either PSGL-1 or L-selectin (data not shown), suggesting that this response was not due to loss of adhesion molecules associated with rolling. These findings show that the adhesion response induced by high-dose anti-MPO in glomeruli does not occur universally throughout the microvasculature.
Anti-MPO antibody–induced glomerular neutrophil adhesion requires α4-integrin
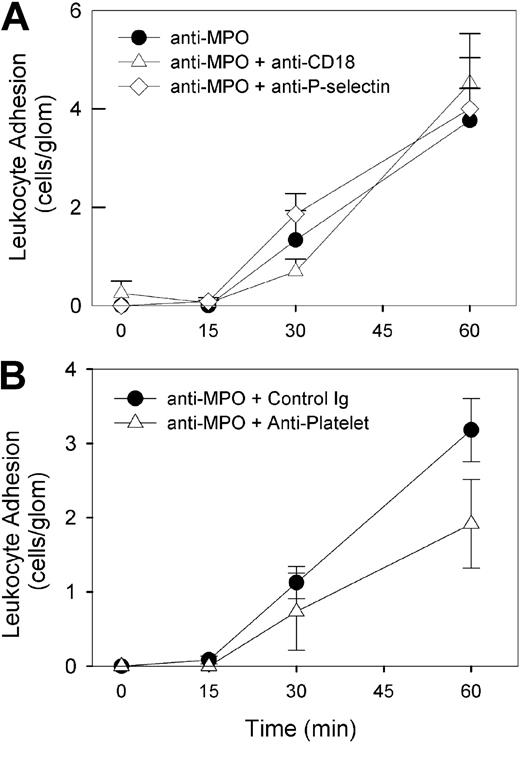
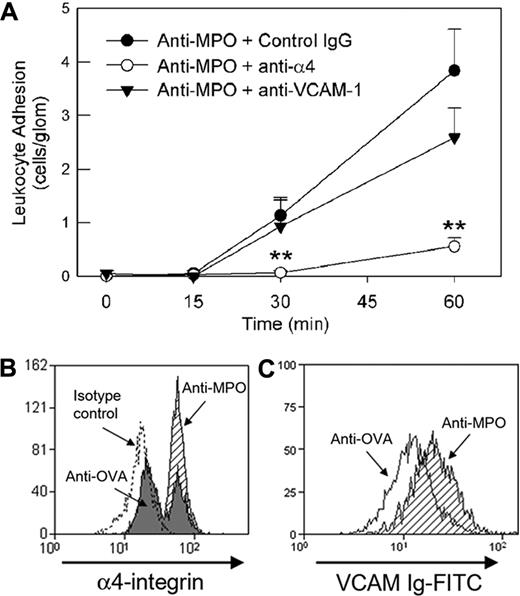
In contrast to the results in LPS-pretreated mice, blockade of the β2-integrin did not reduce leukocyte adhesion induced by high-dose anti-MPO (Figure 6). Both P-selectin and circulating platelets have been shown to contribute to glomerular neutrophil recruitment induced by antibodies against the glomerular basement membrane.15 However, neither platelet depletion nor P-selectin blockade caused a significant reduction in glomerular adhesion (Figure 6). In contrast, inhibition of the α4-integrin in mice treated with anti-MPO resulted in a significant reduction in glomerular adhesion (Figure 7A). This response was seen with 2 different anti–α4-integrin mAbs (PS/2 [Figure 7A] and R1/2 [data not shown]) and confirmed by immunohistochemical analysis of kidneys from these mice (anti-MPO + isotype control: 0.49 ± 0.02 cells/gcs, n = 6 vs anti-MPO + anti-α4: 0.22 ± 0.03 cells/gcs, n = 6, P < .001). However, blockade of the α4-integrin ligand VCAM-1 did not reduce anti-MPO–induced glomerular adhesion (Figure 7A).
Roles of β2-integrins, P-selectin, and platelets in anti-MPO–induced glomerular leukocyte adhesion in mice treated with high-dose anti-MPO alone. Mice underwent treatment with high-dose (200 μg) anti-MPO in combination with various inhibitory antibodies administered prior to anti-MPO. (A) Effect of mAbs against either β2-integrins/CD18 (n = 6) or P-selectin (n = 5) on glomerular leukocyte adhesion induced by high-dose anti-MPO. Also shown are results from mice treated with anti-MPO alone. (B) Effect of platelet depletion on glomerular leukocyte adhesion induced by high-dose anti-MPO. Mice received antiplatelet serum 4 hours prior to undergoing treatment with anti-MPO (n = 6). These experiments were compared with results from mice pretreated with nonspecific rabbit IgG (n = 6).
Roles of β2-integrins, P-selectin, and platelets in anti-MPO–induced glomerular leukocyte adhesion in mice treated with high-dose anti-MPO alone. Mice underwent treatment with high-dose (200 μg) anti-MPO in combination with various inhibitory antibodies administered prior to anti-MPO. (A) Effect of mAbs against either β2-integrins/CD18 (n = 6) or P-selectin (n = 5) on glomerular leukocyte adhesion induced by high-dose anti-MPO. Also shown are results from mice treated with anti-MPO alone. (B) Effect of platelet depletion on glomerular leukocyte adhesion induced by high-dose anti-MPO. Mice received antiplatelet serum 4 hours prior to undergoing treatment with anti-MPO (n = 6). These experiments were compared with results from mice pretreated with nonspecific rabbit IgG (n = 6).
Role of α4-integrin in mediating glomerular neutrophil adhesion in response to high-dose anti-MPO. (A) Effect of anti–α4-integrin (PS/2, 2 mg/kg, n = 9) or anti–VCAM-1 (6C7.1, 180 μg, n = 6) versus an isotype control mAb (n = 6) on glomerular leukocyte adhesion induced by high-dose anti-MPO (200 μg), as assessed by intravital microscopy. Anti–adhesion molecule mAbs were administered prior to administration of anti-MPO. (**P < .05 vs isotype control mAb.) (B) Up-regulation of neutrophil α4-integrin expression in response to anti-MPO in vitro. Following erythrocyte lysis, leukocytes isolated from whole blood were treated with anti-MPO (shaded) or anti-OVA (filled; 50 μg/mL, 30 minutes), and α4-integrin expression was assessed via flow cytometry. Data from cells treated with the appropriate isotype control antibody for the anti–α4-integrin antibody are also shown (dotted line). Neutrophils were identified by high expression of Gr-1 and Mac-1 (M1/70). Similar procedures were performed to assess resting splenic neutrophils (not shown). The data shown are representative of 3 to 4 individual experiments using both blood and splenic neutrophils. (C) Alteration in α4-integrin affinity on splenic neutrophils, as assessed by binding of soluble VCAM-1, in response to in vivo administration of anti-MPO (200 μg intravenously, 5-30 minutes, shaded). Data are also shown for cells from mice treated with anti-OVA (open). The data shown are representative of 4 individual experiments using splenic neutrophils. Similar anti-MPO–induced affinity changes were observed when treating splenic neutrophils from naive mice with anti-MPO in vitro.
Role of α4-integrin in mediating glomerular neutrophil adhesion in response to high-dose anti-MPO. (A) Effect of anti–α4-integrin (PS/2, 2 mg/kg, n = 9) or anti–VCAM-1 (6C7.1, 180 μg, n = 6) versus an isotype control mAb (n = 6) on glomerular leukocyte adhesion induced by high-dose anti-MPO (200 μg), as assessed by intravital microscopy. Anti–adhesion molecule mAbs were administered prior to administration of anti-MPO. (**P < .05 vs isotype control mAb.) (B) Up-regulation of neutrophil α4-integrin expression in response to anti-MPO in vitro. Following erythrocyte lysis, leukocytes isolated from whole blood were treated with anti-MPO (shaded) or anti-OVA (filled; 50 μg/mL, 30 minutes), and α4-integrin expression was assessed via flow cytometry. Data from cells treated with the appropriate isotype control antibody for the anti–α4-integrin antibody are also shown (dotted line). Neutrophils were identified by high expression of Gr-1 and Mac-1 (M1/70). Similar procedures were performed to assess resting splenic neutrophils (not shown). The data shown are representative of 3 to 4 individual experiments using both blood and splenic neutrophils. (C) Alteration in α4-integrin affinity on splenic neutrophils, as assessed by binding of soluble VCAM-1, in response to in vivo administration of anti-MPO (200 μg intravenously, 5-30 minutes, shaded). Data are also shown for cells from mice treated with anti-OVA (open). The data shown are representative of 4 individual experiments using splenic neutrophils. Similar anti-MPO–induced affinity changes were observed when treating splenic neutrophils from naive mice with anti-MPO in vitro.
We next assessed the possibility that high-dose anti-MPO altered neutrophil α4-integrin expression. Peripheral blood neutrophils showed a biphasic distribution of α4-integrin expression, both in the absence of treatment (data not shown) and in mice treated with anti-OVA (50 μg/mL). However, neutrophils exposed to anti-MPO all expressed α4-integrin at high levels (Figure 7B). The functional state of the α4-integrin was also assessed using a VCAM-1 binding assay. Treatment with anti-MPO induced a 50% increase in VCAM-1 binding relative to cells treated with anti-OVA (VCAM-Ig MFI: anti-MPO, 28 ± 2 vs anti-OVA, 19 ± 3, n = 4, P < .05; Figure 7C).
Discussion
Studies examining postcapillary venules have demonstrated the proadhesive capabilities of ANCA IgG in vivo.9,13 However, the nature of this response in the glomerular microvasculature is less clear. Here we show that anti-MPO can induce glomerular leukocyte adhesion, both at low doses in mice undergoing an infection-related inflammatory response and at higher doses in otherwise untreated mice. In the LPS-dependent model, adhesion was dependent on LFA-1, whereas at the higher dose the α4-integrin became the dominant leukocyte integrin used for adhesion. Moreover, we found that anti-MPO administered intravenously rapidly bound to circulating neutrophils, and monocytes to a lesser extent, in otherwise untreated mice, and induced increased expression and affinity of the α4-integrin on neutrophils. Together these data illustrate that the molecular basis of anti-MPO–induced glomerular leukocyte adhesion can vary according to the amount of anti-MPO present and the preexisting inflammatory state.
The major focus of these studies was to determine the mechanisms of glomerular leukocyte adhesion induced by anti-MPO, using in vivo imaging to directly visualize glomeruli. This approach allowed us to demonstrate unequivocally that the process of anti-MPO–induced glomerular leukocyte adhesion was not simply a process of physical trapping of neutrophils in response to an anti-MPO–induced reduction in deformability.14 In contrast, even under conditions sufficiently potent to result in increased α4-integrin expression and affinity on neutrophils, glomerular leukocyte adhesion remained adhesion-molecule dependent. The reason for different functional roles of the β2- and α4-integrins at the different doses of anti-MPO is not clear. Recent studies have demonstrated the complexity of integrin function in neutrophil recruitment, with different family members mediating distinct functions such as arrest, intravascular crawling, and transmigration.29,30 Further detailed studies will be required to examine these aspects of integrin function in this model.
The hypothesis describing the basis of disease development in anti-MPO–associated vasculitis indicates that ANCA binding to neutrophils is made possible when neutrophils are primed by an additional stimulus, such as that provided by systemic infection. This is believed to result in externalization of MPO, making it accessible for ANCA binding.7 Despite this, addition of anti-MPO ANCA to unprimed neutrophils has been shown to promote adhesion, suggesting that there is sufficient surface expression of MPO on resting neutrophils to allow for anti-MPO binding.10-12 The findings in the present study extend these observations by demonstrating that circulating anti-MPO can bind to nonprimed neutrophils, causing rapid alterations in leukocyte adhesion molecule expression and adhesive behavior. This suggests that the presence of high concentration ANCA may be sufficient to induce leukocyte adhesion in the glomerulus, thereby initiating ANCA-associated glomerulonephritis. This contention is consistent with the clinical observation that many ANCA-associated glomerulonephritis patients have no evidence of existing or antecedent infection.31
However, it is also clear from these data that LPS enables lower concentrations of anti-MPO to be capable of inducing a leukocyte adhesion response. In the present study, at the lower dose of anti-MPO used, anti-MPO did not induce significant glomerular leukocyte adhesion when administered to naive mice. However, in mice treated with 0.1 μg LPS, a substantial increase in glomerular adhesion occurred in response to anti-MPO. Furthermore, assessment of neutrophil Mac-1 expression showed that, whereas LPS alone did not alter surface Mac-1 expression, administration of anti-MPO to LPS-pretreated mice resulted in a dramatic up-regulation of Mac-1 on circulating neutrophils. This indicates the existence of a synergistic interaction between anti-MPO and LPS in terms of neutrophil activation and glomerular adhesion and supports the finding that ANCA-associated inflammation is exacerbated in the presence of a stimulus such as bacterial endotoxin.6 This interaction may contribute to the clinical association of bacterial infection and disease relapse in ANCA-associated glomerulonephritis.20,21
Human neutrophils typically express the α4-integrin at minimal levels. However strong stimuli both in vitro and in vivo have been found to induce its up-regulation and allow it to serve a functional role in neutrophil adhesion.16,19,32 Murine neutrophils have also been found to express the α4-integrin, and use it to undergo recruitment under inflammatory conditions.17,33 In the present study, exposure to anti-MPO increased neutrophil expression of the α4-integrin, and in mice treated with high-dose anti-MPO alone, neutrophil recruitment to glomerular capillaries was α4-integrin dependent. These findings indicate that anti-MPO has the capacity to act as a highly potent neutrophil-activating stimulus, with the potential to markedly alter neutrophil integrin function. The ligand via which the α4-integrin mediated adhesion in response to 200 μg anti-MPO was not VCAM-1, suggesting the involvement of alternative α4-integrin ligands. One possibility is fibronectin, an α4-integrin ligand expressed in normal glomeruli.34,35 This possibility will be the subject of further investigations. However, studies in a model of vasculitis-associated leukocyte recruitment showed that inhibition of both VCAM-1 and fibronectin did not inhibit α4-integrin–dependent interactions, suggesting the existence of an additional, unidentified α4-integrin ligand.36
Our observation of substantial neutrophil binding of anti-MPO in resting mice is in contrast to studies using isolated human neutrophils, which demonstrate minimal surface expression of MPO in resting cells.7,37 However, few studies have successfully examined binding of anti-MPO to neutrophils in the circulation. Hoshino et al examined in vivo accumulation of quantum dot–conjugated anti-MPO in mice undergoing a model of vasculitis.22 They found substantial tissue deposition of anti-MPO, although much of the anti-MPO was not cell associated, presumably representing MPO released as a result of neutrophil degranulation. MPO is also present in the serum of mice and humans, with increased levels of serum MPO associated with inflammatory conditions.6,38 Furthermore, MPO has been reported to bind to nonactivated neutrophils via a Mac-1–dependent pathway.39 This raises the intriguing possibility that MPO in the serum may be available for binding to the surface of circulating neutrophils, and thereby accessible for ANCA binding.
Given that this project used the hydronephrotic kidney to allow visualization of the glomerular microvasculature, the possibility that the process of hydronephrosis altered the physiology of the glomerular microvasculature, and subsequently, the mechanisms of leukocyte adhesion, must be considered. This issue was addressed in our previous study, in which we compared various inflammatory parameters in nonhydronephrotic and hydronephrotic kidneys from the same mice.15 Parameters such as adhesion molecule up-regulation and neutrophil recruitment were similar in both types of kidneys. Similarly in the present study, anti-MPO–induced glomerular neutrophil recruitment, as assessed histologically, was similar in hydronephrotic and nonhydronephrotic kidneys. Together these findings indicate that the process of inflammation, particularly delivery of leukocytes to glomeruli, is not markedly altered in hydronephrotic kidneys.
An unexpected finding was the apparent link between MPO and Gr-1, as demonstrated by the reduction in anti–Gr-1 staining in neutrophils exposed to anti-MPO. Gr-1 is a leukocyte differentiation marker recognized by the RB6-8C5 clone. This antibody recognizes an epitope on Ly-6G and Ly-6C. Little is known about the function of these molecules, although Ly-6C has been proposed to have a role in integrin-mediated adhesion of CD8+ T cells, and cross-linking of Ly-6G on neutrophils has been shown to induce up-regulation of CD11b and F-actin polymerization.40,41 However, the present findings indicate that Gr-1 can be rapidly down-regulated in response to activation induced by ligation of surface MPO by anti-MPO. More detailed analysis is required to understand the functional significance of these observations.
ANCA-induced adhesion of neutrophils in the microvasculature of target organs is believed to be a key step in the pathogenesis of ANCA-associated vasculitis.42 This step may be a prerequisite for deposition of ANCA antigens in vulnerable vascular beds, where they can damage tissues directly, or be recognized by the adaptive immune system, promulgating a further local immune response.5 The present findings show that anti-MPO can induce leukocyte adhesion in glomerular capillaries via diverse adhesive pathways. Furthermore, they demonstrate that glomerular leukocyte adhesion induced by these antibodies remains adhesion-molecule dependent, and is not a simple physical trapping of highly activated leukocytes. This raises the possibility than antiadhesion molecule antibodies, such as those in use for psoriasis (anti–LFA-1/efalizumab)43 or multiple sclerosis (anti–α4-integrin/natalizumab),44 may have potential as therapeutic agents in ANCA-associated vasculitis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Jake Lusis for generous provision of MPO−/− mice.
This work was supported by a program grant (no. 334067) from the National Health & Medical Research Council (NHMRC) of Australia, and a project grant from the Genzyme Renal Innovations Program. M.J.H. is an NHMRC Senior Research Fellow.
Authorship
Contribution: M.P.K., R.Y.Q.K., C.L., C.W., W.G.J., J.D.O., L.D.A., and P.H. performed research and analyzed data; D.B. designed and performed research and analyzed data; A.R.K. designed research, interpreted data, and wrote the paper; and M.J.H. designed and performed research, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael J. Hickey, Centre for Inflammatory Diseases, Monash University, Department of Medicine, Monash Medical Centre, 246 Clayton Rd, Clayton, Victoria, 3168, Australia; e-mail: michael.hickey@med.monash.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal