To the editor:
We read with interest the new consensus guidelines for the diagnosis of chronic lymphocytic leukemia (CLL).1 The new revisions have clarified the role of molecular prognostic markers, improved the classification of response, and defined the role of minimal residual disease monitoring, and we support these changes.
We are concerned, however, with the substantial changes in the CLL diagnostic criteria that have been suggested without clear supportive evidence. In particular, the new guidelines propose changing the diagnostic criteria for CLL from an absolute lymphocyte count (ALC) more than or equal to 5.0 × 109/L2 to a B-cell count more than or equal to 5.0 × 109/L. Although we recognize this change was made to align with previously proposed monoclonal B-cell lymphocytosis (MBL) criteria,3 it will significantly change the diagnostic criteria for CLL, MBL,3 and small lymphocytic lymphoma (SLL).
This proposed change has several shortcomings.
(1) Lack of established clinical relevance. Although the shift from an ALC to a B cell-based criterion may seem trivial, this modification will reclassify approximately 40% of Rai stage 0 patients from CLL to MBL.4 Such a change will dramatically alter the incidence rate and confound monitoring the epidemiology of CLL. The published data suggest that a cutoff of 5.0 × 109/L B cells does not have prognostic value, as there is no difference in time to treatment when current Rai stage 0 patients are reclassified.4 Indeed, recent data suggest that a numeric threshold for predicting overall survival in CLL and MBL patients may be as high as 11.0 × 109/L B cells.5 We hope that data like these can be used to support future classification changes.
With respect to epidemiologic studies, the change will also increase the number of patients classified as SLL, since those with lymphadenopathy and an ALC of 5.0 × 109/L or more, but a B-cell count less than 5.0 × 109/L (previously Rai stage I CLL), no longer fulfill the criteria to be classified as CLL.
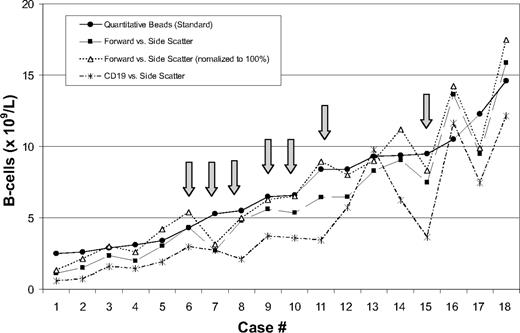
(2) Absence of a standardized way to measure B-cell counts. Flow cytometric immunophenotyping for leukemia/lymphoma analysis is not a quantitative test, and no standardized approach for determining B-cell counts in CLL has been proffered.5,6 The determination of a B-cell count will vary based on the methodology used and the gating strategy used (Figure 1). Having a standard clinical laboratory approach for making quantitative measurements is critical if B-cell counts are to be included in any new diagnostic criteria. In addition, moving the monitoring of disease from an ALC via a complete blood count (CBC), an easily accessible and economically prudent assay, to a B-cell count using a flow cytometry–based procedure will also have unintended consequences affecting cost and access.
Flow cytometric lymphocyte gating methodology influences B-cell counts. B-cell counts were calculated from peripheral blood using flow cytometric immunophenotyping in 18 patients with a clonal B-cell population of CLL phenotype, using 3 methods of lymphocyte gating and comparing with a quantitative bead-based methodology as the standard. All patients had an ALC greater than 5.0 × 109/L (range, 5.2-19.6 × 109/L). Variation in B-cell count was observed depending on the gating method used. Arrows indicate 7 of 18 patients had B-cell counts greater than 5.0 × 109/L using one method, but less than 5.0 × 109/L using one of the other methodologies.
Flow cytometric lymphocyte gating methodology influences B-cell counts. B-cell counts were calculated from peripheral blood using flow cytometric immunophenotyping in 18 patients with a clonal B-cell population of CLL phenotype, using 3 methods of lymphocyte gating and comparing with a quantitative bead-based methodology as the standard. All patients had an ALC greater than 5.0 × 109/L (range, 5.2-19.6 × 109/L). Variation in B-cell count was observed depending on the gating method used. Arrows indicate 7 of 18 patients had B-cell counts greater than 5.0 × 109/L using one method, but less than 5.0 × 109/L using one of the other methodologies.
(3) Artificial inflation of the risk of progression among MBL patients. The proposed change will decrease the number of patients diagnosed with CLL, and will dramatically increase the number of patients labeled as having MBL and SLL. In the seminal paper by Rawstron et al,3 the clonal B-cell count among MBL patients identified through population screening ranged from 0.003 to 1.458 × 109/L (median = 0.013 × 109/L). The MBL label is now being expanded, and many of these new MBL patients will be identified after they undergo evaluation for a lymphocytosis (> 3.0 × 109/L) discovered in clinical practice, rather than via population screening as in the original observations of MBL.3 The risk of progression to requiring chemotherapy treatment among such clinically identified MBL cases is 1% to 2% per year,7-10 profoundly different from that of patients with MBL diagnosed via population screening.9 Given the divergent progression risks, it may be confusing to group patients with a minuscule clone identified by population screening together with those that were previously labeled as CLL into a single category. Indeed this reclassification will likely worsen the prognosis for both Rai 0 CLL and MBL patients because the lowest risk patients from the previous Rai 0 CLL group will be moved to the MBL group and become their highest risk patients. Those patients remaining in the new Rai 0 CLL category will have higher ALCs and likely a greater risk of progression.
We commend the IWCLL for making much needed improvements to the guidelines, but we believe changing the diagnostic criteria for CLL to 5.0 × 109/L B cells requires further study as the current recommendation will likely create more questions than answers. The real challenge is not to focus on numeric cutoffs, but to devise a classification system that recognizes a common clonal B-cell immunophenotype and reflects today's genetic and biologic knowledge in a way that will best benefit our patients.
Authorship
Contribution: All authors conceived the letter and approved the final draft of the letter. C.A.H. wrote the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Curtis A. Hanson, MD, Division of Hematopathology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: cahanson@mayo.edu.