Abstract
To understand the molecular basis underlying 15(S)-hydroxyeicosatetraenoic acid (15(S)-HETE)–induced angiogenesis, we have studied the role of the Janus kinase-signal transducer and activator of transcription (Jak-STAT) signaling. The 15(S)-HETE stimulated tyrosine phosphorylation of Jak2 in a time-dependent manner in human retinal microvascular endothelial cells (HRMVECs). Inhibition of Jak2 activation via adenovirus-mediated expression of its dominant-negative mutant attenuated 15(S)-HETE–induced HRMVEC migration and tube formation and Matrigel plug angiogenesis. Similarly, 15(S)-HETE activated tyrosine phosphorylation of STAT-5B in a time-dependent manner. Dominant-negative mutant-mediated interference of STAT-5B activation suppressed 15(S)-HETE–induced HRMVEC migration and tube formation and Matrigel plug angiogenesis. The 15(S)-HETE induced interleukin-8 (IL-8) expression in Jak2-STAT-5B–dependent manner in HRMVECs. In addition, neutralizing anti–IL-8 antibodies reduced 15(S)-HETE–induced HRMVEC migration and tube formation and Matrigel plug angiogenesis. Cloning and Transfac analysis of IL-8 promoter revealed the presence of 1 putative STAT-binding sequence at −476 nt, and electrophoretic mobility shift assay and chromatin immunoprecipitation analysis showed the binding of STAT-5B to this site in response to 15(S)-HETE. Mutational analysis showed that STAT binding site is essential for 15(S)-HETE–induced IL-8 promoter activity. Together, these observations suggest that 15(S)-HETE–induced angiogenesis requires Jak2-STAT-5B–dependent expression of IL-8.
Introduction
The 15-lipoxygenase 1 (15-LOX1) converts arachidonic acid (AA) to 15(S)-hydroxyeicosatetraenoic acid (15(S)-HETE) to a major level and to 12(S)-HETE to a small extent.1,2 On the other hand, 15-lipoxygenease 2 (15-LOX2) metabolizes AA exclusively to 15(S)-HETE.2,3 Both 15-LOX1 and 15-LOX2 are expressed in humans, although in a tissue-specific manner.4 The 15-LOX1 mRNA and protein were found to be colocalized with the epitopes of oxidized low-density lipoprotein in atherosclerotic plaques.5 Similarly, atherosclerotic arteries upon incubation ex vivo with AA converted it mainly to 15-HETE.6 Knockout mice for 12/15-LOX, a murine ortholog of 15-LOX1, exhibited decreased atherosclerotic lesions in response to high-fat diet and reduced neointima formation in response to injury.7-9 Conversely, overexpression of 15-LOX1 in vascular endothelium enhanced atherosclerosis in low-density lipoprotein receptor-deficient mice,10 findings that suggest a role for 15-LOX1-15(S)-HETE/12/15-LOX-12(S)-HETE axis in vascular diseases. In regard to their role in carcinogenesis, opposite actions have been observed for 15-LOX1 and 15-LOX2 in influencing prostate cancer, the former being procarcinogenic.11-14 However, in these carcinogenic effects, although 12(S)-HETE mimicked 15-LOX1, 15(S)-HETE exhibited effects similar to 15-LOX2.15-17 Specifically, whereas 15-LOX1-12(S)-HETE appear to exert procarcinogenic effects, 15-LOX2-15(S)-HETE exhibited proapoptotic actions in prostate carcinomas. In addition, 15-LOX1 induced apoptosis in colorectal carcinomas.18,19 In regard to their role in other diseases, particularly retinopathy, 15(S)-HETE levels have been found to be elevated in epiretinal membranes of proliferative vitreoretinal and proliferative diabetic retinopathy patients.20 Furthermore, hypoxia increased the expression of 15-LOX1 and production of 15(S)-HETE in pulmonary arteries, causing vasoconstriction.21 Hypoxia also increased the production of 15(S)-HETE in human retinal microvascular endothelial cells, causing angiogenesis.22-24 In addition, we showed that 15(S)-HETE induces angiogenesis, and this phenomenon requires phosphatidylinositol 3-kinase-Akt–dependent fibroblast growth factor-2 expression and signal transducer and activator of transcription (STAT)-3–mediated vascular endothelial growth factor induction.25,26 Thus, these differential effects of 15(S)-HETE in various tissues may be due to the result of its different dosages used in specific studies or different actions.
The Janus kinase (Jak)-STAT signaling pathway plays a crucial role in the regulation of cell proliferation, migration, and apoptosis.27,28 Whereas STAT-3 and 5 appear to be directed toward mediating cell proliferation, differentiation, or migration, STAT-1 has been shown to be involved in apoptosis.29-37 In regard to the mechanism of their activation, both the Jak and Src family of tyrosine kinases have been reported to phosphorylate STATs.38,39 Furthermore, mutations in Jak2 were found to account for the deficiency of endothelial progenitor cells in the reparation of neovascularization,40 the observations that highlight the importance of the Jak-STAT signaling in the regulation of angiogenesis. To understand the molecular basis underlying the potency of 15(S)-HETE in influencing pathologic angiogenesis, and thereby its possible involvement in various diseases, including atherosclerosis, cancer, and retinopathy, in this study we have examined the role of the Jak-STAT signaling. We found that 15(S)-HETE–induced angiogenesis requires Jak2-STAT-5B–mediated expression of interleukin (IL)-8.
Methods
Reagents
The 15(S)-HETE was bought from Cayman Chemical (Ann Arbor, MI). Growth factor-reduced Matrigel (catalog no. 354250) was obtained from BD Biosciences (Bedford, MA). Phosphospecific anti-Jak2 antibodies (catalog no. 3771) and anti–STAT-5 antibodies (catalog no. 9351) were bought from Cell Signaling Technology (Beverly, MA). Anti-Jak2 antibodies (catalog no. 06-255) were obtained from Upstate Biotechnology (Lake Placid, NY). Anti–STAT-5 antibodies (SC-836), anti–STAT-5B antibodies (SC-1656), and anti–β-tubulin antibodies (SC-9104) and normal mouse serum (SC-2025) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Neutralizing anti–IL-8 antibodies (catalog no. MAB208) were bought from R&D Systems (Minneapolis, MN). Anti-CD31 antibodies (catalog no. 550274) were purchased from BD Pharmingen (Palo Alto, CA). Human IL-8 enzyme-linked immunosorbent assay (ELISA) kit (catalog no. EH2IL8) was obtained from Pierce (Rockford, IL). T4 polynucleotide kinase was procured from New England Biolabs (Ipswich, MA). [γ-32P]adenosine triphosphate (ATP; 3000 Ci/mmol) was bought from GE Healthcare Biosciences (Piscataway, NJ). All the primers were made by IDT (Coralville, IA).
Adenoviral vectors
To construct adenoviral vector for dominant-negative (dn)Jak2, kinase-inactive Jak2K882E cDNA was released from pRK5-dnJak2 plasmid by digestion with BamHI and EcoRV and cloned into the same sites of entry vector pENTR3C. The pAd-dnJak2 was generated by specific recombination of pENTR3C-dnJak2 with pAdCMV5DEST (Invitrogen, Carlsbad, CA). The plasmid pAd-dnJak2 was linearized by digestion with PacI and transfected into HEK293A cells to amplify adenovirus (Ad)-dnJak2. Construction of Ad-GFP (green fluorescent protein) and Ad-dnSTAT-5B was described previously.35 Adenovirus was purified with cesium chloride centrifugation, and the titer was determined by plaque assay, as described previously.41
Cell culture
Human retinal microvascular endothelial cells (HRMVECs) were bought from Cell Systems (Kirkland, WA) and grown in medium 131 containing microvascular growth supplements, 10 μg/mL gentamycin, and 0.25 μg/mL amphotericin B. Cultures were maintained at 37°C in a humidified 95% air and 5% CO2 atmosphere. HRMVECs were growth arrested by incubating in medium 131 for 24 hours and used to perform the experiments, unless otherwise indicated. All animal studies reported in this manuscript were approved by the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center.
Cell migration
Cell migration was performed using modified Boyden chamber method, as described by Nagata et al.42 The cell-culture inserts containing membranes 10 mm in diameter and 8.0 μm pore size (Nalgene Nunc International, Rochester, NY) were placed in a 24-well tissue culture plate (Costar, Corning, Corning, NY). The lower surface of the porous membrane was coated with 70% Matrigel at 4°C overnight and then blocked with 0.1% heat-inactivated bovine serum albumin (BSA) at 37°C for 1 hour. HRMVECs were quiesced for 24 hours in medium 131, trypsinized, and neutralized with trypsin-neutralizing solution (TNS). Cells were seeded into the upper chamber at 1 × 105 cells/well. Vehicle or 15(S)-HETE was added to the lower chamber at the indicated concentration. Both the upper and lower chambers contained medium 131. When the effect of dnJak2 and dnSTAT-5B mutants was tested on 15(S)-HETE–induced HRMVEC migration, cells were infected first with Ad-GFP, Ad-dnJak-2, or Ad-dnSTAT-5B at 80 multiplicity of infection (MOI) and quiesced before they were subjected to migration assay. In the case of testing the effect of neutralizing anti–IL-8 antibodies on 15(S)-HETE–induced HRMVEC migration, cells were incubated with antibodies (3 μg/mL) for 30 minutes at 37°C and washed with medium 131. Cells were then seeded into each well and, wherever appropriate, the antibodies were added to both the upper and lower chambers before the addition of 15(S)-HETE. After 6 hours of incubation at 37°C, nonmigrated cells were removed from the upper side of the membrane with cotton swabs and the cells on the lower surface of the membrane were fixed in methanol for 15 minutes. The membrane was then stained with DAPI (4,6 diamidino-2-phenylindole) in vectashield mounting medium (Vector Laboratories, Burlingame, CA) and observed under Nikon diaphot fluorescence microscope with photometrics CH250 charge-coupled device (CCD) camera (Nikon, Garden City, NY). Cells were counted in 5 randomly selected squares per well and presented as number of migrated cells per field.
Tube formation
Tube formation assay was performed, as described by Nagata et al.42 Twenty-four–well culture plates (Costar, Corning) were coated with growth factor–reduced Matrigel (BD Biosciences) in a total volume of 280 μL/well and allowed to solidify for 30 minutes at 37°C. HRMVECs were trypsinized, neutralized with TNS, and resuspended at 5 × 105 cells/mL, and 200 μL of this cell suspension was added into each well. Vehicle or 15(S)-HETE, at the indicated concentration, was added to the appropriate well, and the cells were incubated at 37°C for 6 hours. When the effect of dnJak2 and dnSTAT-5B mutants was tested on 15(S)-HETE–induced HRMVEC tube formation, cells were infected first with Ad-GFP, Ad-dnJak2, or Ad-dnSTAT-5B at 80 MOI and quiesced before they were subjected to tube formation. In the case of testing the effect of neutralizing anti–IL-8 antibodies on 15(S)-HETE–induced HRMVEC tube formation, cells were incubated with antibodies (3 μg/mL) for 30 minutes at 37°C and washed with medium 131. Cells were then seeded into each well and, wherever appropriate, the antibodies were added to the well before the addition of 15(S)-HETE. Tube formation was observed under an inverted microscope (model, Eclipse TS100; Nikon, Tokyo, Japan). Images were captured with a CCD color camera (model, KP-D20AU; Hitachi, Tokyo, Japan) attached to the microscope, and tube length was measured using the National Institutes of Health (NIH, Bethesda, MD) ImageJ software (http://rsb.info.nih.gov/ij).
Western blotting
After appropriate treatments and rinsing with cold phosphate-buffered saline (PBS), HRMVECs were lysed in 500 μL of lysis buffer (PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 100 μg/mL phenylmethylsulfonyl fluoride (PMSF), 100 μg/mL aprotinin, 1 μg/mL leupeptin, and 1 mM sodium orthovanadate) and scraped into 1.5-mL Eppendorf tubes. After standing on ice for 20 minutes, the cell lysates were cleared by centrifugation at 12 000 rpm for 20 minutes at 4°C. Cell lysates containing equal amount of protein were resolved by electrophoresis on 0.1% SDS and 10% polyacrylamide gels. The proteins were transferred electrophoretically to a nitrocellulose membrane (Hybond; Amersham Biosciences, Piscataway, NJ). After blocking in 10 mM Tris-HCl buffer, pH 8.0, containing 150 mM sodium chloride, 0.1% Tween 20, and 5% (wt/vol) nonfat dry milk, the membrane was treated with appropriate primary antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibodies. The antigen-antibody complexes were detected using chemiluminescence reagent kit (Amersham Biosciences). The band intensities were quantified using NIH ImageJ version 1.31.
RT-PCR
After appropriate treatments, total cellular RNA was isolated from HRMVECs using TRIzol reagent, as per the manufacturer's guidelines (Invitrogen). Reverse transcription was carried out with Superscript III First-Strand Synthesis System for reverse transcription-polymerase chain reaction (RT-PCR) based on supplier's protocol (Invitrogen). The cDNA was then used as template for PCR using specific primers for human IL-8 (accession no. NM_000584) (forward, 5′-TGTGCCTTGGTTTCTCCTTTAT-3′; reverse, 5′-GGGTACCCAATTGTTTGTTTGT-3′) and human β-actin (accession no. NM_001101) (forward, 5′-AGCCATGTACGTTGCTAT-3′; reverse, 5′-GATGTCCACGTCACACTTCA-3′). The amplification was carried out on Gene Amp PCR System 2400 (Applied Biosystems, Foster City, CA), using the following amplification conditions for the above-mentioned genes, as follows: for IL-8, at 95°C for 5 minutes, followed by 35 cycles at 95°C for 45 seconds, 50°C for 45 seconds, and 72°C for 55 seconds, with final extension at 72°C for 7 minutes; for β-actin, at 95°C for 5 minutes, followed by 35 cycles at 95°C for 45 seconds, 55°C for 45 seconds, and 72°C for 50 seconds, with final extension at 72°C for 7 minutes. The amplified RT-PCR products were separated on 1.2% (wt/vol) agarose or 8% native polyacrylamide gels and stained with ethidium bromide; pictures were captured using AlphaEase Digital Imaging System (Alpha Innotech, San Leandro, CA); and the band intensities were quantified using NIH ImageJ.
Cloning of human IL-8 promoter-luciferase constructs
From HRMVEC genomic DNA, IL-8 promoter fragment starting from −1481 nucleotides (nt) upstream to +25 nt downstream of transcription start site was generated by polymerase chain reaction using forward primer, 5′-GGTACCGAATTCAGTAACCCAGGCA-3′ and reverse primer, 5′-CTCGAGCTCTGAAAGTTTGTGCCTTATG-3′. Site-directed mutagenesis in STAT binding site located at −476 nt in IL-8 promoter, TTCCTAGAA to TTAAACTAA, was introduced by PCR overlap extension method. The mutation was centered within the overlap of 2 primary amplification products that were generated using the following primers: forward, 5′-GGTACCGAATTCAGTAACCCAGGCA-3′; reverse, 5′-TTAGTTTAACTGTTACTGCTCTTAGG-3′ (expected PCR product 1012 bp) and forward, 5′-GTAACAGTTAAACTAAACTCTCTAAAATGCTTAG-3′; reverse, 5′-CTCGAGCTCTGAAAGTTTGTGCCTTATG-3′ (expected PCR product 522 bp), respectively. The 2 fragments were then linked by overlap extension PCR using 50 fmole of each amplicon and nested outside primers (forward, 5′-GGTACCGAATTCAGTAACCCAGGCA-3′ and reverse, 5′-CTCGAGCTCTGAAAGTTTGTGCCTTATG-3′). The PCR-generated promoter sequences were directly cloned into pGEMT vector (Promega, Madison, WI). The cloned sequences were released by restriction digestion with KpnI and XhoI and subcloned into pGL3 basic vector (Promega) at the same sites. The underlined regions are KpnI and XhoI sites in the forward and reverse primers, respectively. Mutated sequences are shown in boldfaces. Sequence of the cloned promoter fragments was confirmed by DNA sequencing.
Luciferase reporter-gene assay
HRMVECs were transfected with IL-8 promoter-luciferase constructs or pGL3 basic vector using Lipofectamine 2000 reagent. After growth synchronization in growth factor-free medium for 12 hours, cells were treated with and without 15(S)-HETE (0.1 μM) for 6 hours. Cells were then washed once with ice-cold PBS and lysed with 200 μL of lysis buffer. The cell extracts were collected into microcentrifuge tubes and centrifuged for 2 minutes at 12 000g at 4°C. The supernatants were assayed for luciferase activity using Luciferase Assay System (Promega) and a single tube luminometer (TD20/20; Turner Designs, Sunnyvale, CA) and expressed as relative luciferase units.
Electrophoretic mobility shift assay
After appropriate treatments, nuclear extracts were prepared from HRMVECs, as described previously.26 The protein content of the nuclear extracts was determined using a Micro BCA protein assay reagent kit (Pierce). Protein-DNA complexes were formed by incubating 10 μg of nuclear protein in a total volume of 25 μL consisting of 15 mM HEPES, pH 7.9, 3 mM Tris-HCl, pH 7.9, 60 mM KCl, 1 mM EDTA (ethylenediaminetetraacetic acid), 1 mM PMSF, 1 mM dithiothreitol, 2.5 μg/mL BSA, 1 μg/mL poly(dI-dC), 15% glycerol, and 100 000 cpm of [32P]-labeled oligonucleotide probe for 30 minutes on ice. The protein-DNA complexes were resolved by electrophoresis on a 4% polyacrylamide gel using 1× Tris-glycine-EDTA buffer (25 mM Tris-HCl, pH 8.5, 200 mM glycine, 0.1 mM EDTA). Double-stranded oligonucleotides (5′-GCAGTAACAGTTCCTAGAAACTCTCTA-3′, 3′-CGTCATTGTCAAGGATCTTTGAGAGAT-5′) encompassing a STAT-binding sequence from −476 to −484 region of human IL-8 promoter (accession no. M28130) were used as [32P]-labeled probes to measure STAT-DNA–binding activity. Double-stranded oligonucleotides were labeled with [γ-32P]ATP using the T4 polynucleotide kinase kit following the supplier's protocol.
ChIP
Chromatin immunoprecipitation (ChIP) assay was performed on HRMVECs by using ChIP assay kit following supplier's protocol (Upstate Biotechnology). STAT-5B-DNA complexes were immunoprecipitated using anti–STAT-5B antibody. Preimmune rabbit serum was used as a negative control. The immunoprecipitated DNA was uncross-linked, subjected to proteinase K digestion, and purified using QIAquick columns (QIAGEN, Valencia, CA). The purified DNA was used as a template for PCR amplification using primers (forward, 5′-GATCCCCCACATTACTCAGA-3′; reverse, 5′-GGTGAAGATAAGCCAGCCAAT-3′) flanking the putative STAT binding site located at −476 to −484 nt in human IL-8 promoter region (accession no. M28130). The primers designed for RT-PCR are approximately 2.9 kb away from the STAT binding site and were used as negative controls for ChIP-PCR. The PCR products were resolved on 1.2% agarose or 8% native polyacrylamide gels and stained with ethidium bromide, and the band intensities were quantified using NIH ImageJ.
Matrigel plug angiogenesis
Matrigel plug assay was performed essentially as described by Medhora et al.43 C57BL/6 mice (8 weeks old) were lightly anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally) and were injected subcutaneously with 0.5 mL of Matrigel that was premixed with vehicle or 50 μM 15(S)-HETE along the abdominal midline. In the later studies, 15(S)-HETE was used at lower doses (5 μM), and it was found that it is as effective as at higher doses in stimulating Matrigel plug angiogenesis. The injection was made rapidly with a B-D 30G1/2 needle to ensure the entire content was delivered as a single plug. Wherever the effect of Ad-GFP (5 × 109 plaque-forming units (pfu)/mL), Ad-dnJak-2 (5 × 109 pfu/mL), Ad-dnSTAT-5B (5 × 109 pfu/mL), preimmune serum (3 mg/mL), or neutralizing anti–IL-8 antibodies (3 mg/mL) tested on 15(S)-HETE-induced angiogenesis, they were added to the Matrigel before injecting into mice. The mice were allowed to recover, and 7 days later, unless otherwise stated, the animals were killed by inhalation of CO2 and the Matrigel plugs were harvested from underneath the skin. The plugs were homogenized in 1 mL of deionized H2O on ice and cleared by centrifugation at 10 000 rpm for 6 minutes at 4°C. The supernatant was collected and used in duplicate to measure hemoglobin content with Drabkin reagent along with hemoglobin standard essentially according to the manufacturer's protocol (Sigma-Aldrich, St. Louis, MO). The absorbance was read at 540 nm in an ELISA plate reader (Spectra Max 190; Molecular Devices, Sunnyvale, CA). These experiments were repeated at least 3 times with 6 mice for each group, and the values are expressed as g/dL hemoglobin/mg plug.
Immunohistochemistry
After retrieving the Matrigel plugs from mice, they were snap frozen in OCT compound. Cryosections (5 μm) were made using Leica Kryostat machine (model CM3050S) and stained with anti-CD31 antibody (1/500 dilution; BD Pharmingen), followed by sequential incubation with biotinylated anti–rat IgG (1/300 dilution), avidin-biotin peroxidase, and diaminobenzidine substrate (Vector Laboratories). The sections were counterstained with hematoxylin and examined under a light microscope (model, Eclipse 50i; Nikon) in 6 random fields (×400 magnification) from each group. Images were captured using Nikon Digital Sight DS-L1 system.
IL-8 ELISA
IL-8 released into the culture medium was measured using an ELISA kit following the manufacturer's instructions (Pierce).
Statistics
All the experiments were repeated 3 times, and data are presented as mean plus or minus SD. The treatment effects were analyzed by Student t test, and P values less than .05 were considered to be statistically significant. In the case of ChIP analysis, electrophoretic mobility shift assay (EMSA), and Western blotting, one representative set of data is shown.
Results
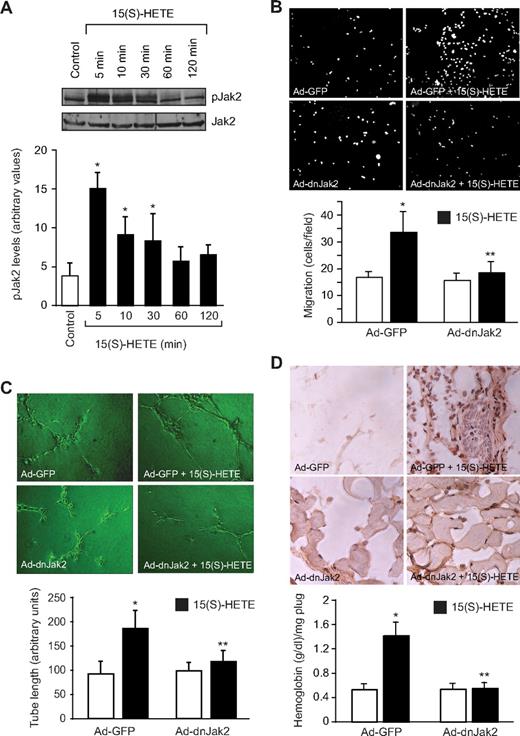
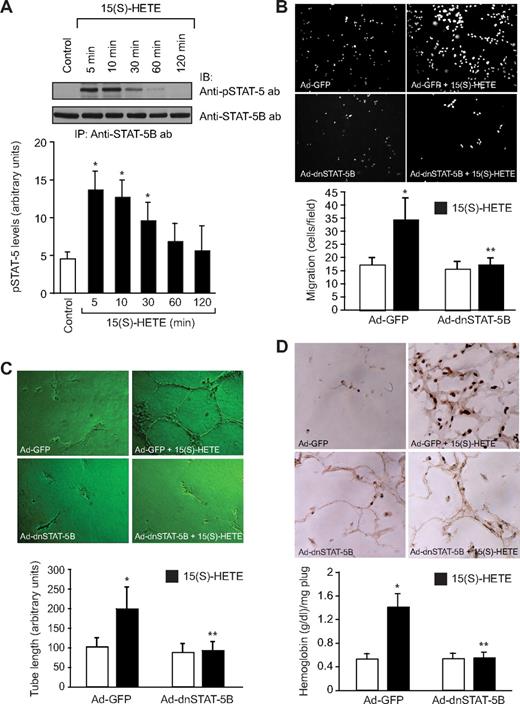
To understand the mechanisms of 15(S)-HETE–induced angiogenesis, we have studied the role of Jak2. The 15(S)-HETE stimulated tyrosine phosphorylation of Jak2 in a time-dependent manner in HRMVECs with a 4-fold increase at 5 minutes compared with control (Figure 1A). Next, we examined the role of Jak2 in 15(S)-HETE–induced HRMVEC migration and tube formation. As determined by modified Boyden chamber method, 15(S)-HETE induced HRMVEC migration by 2-fold compared with control (Figure 1B). Similarly, 15(S)-HETE induced HRMVEC tube formation by 2-fold compared with control (Figure 1C). Adenovirus-mediated expression of dnJak2 inhibited 15(S)-HETE–induced HRMVEC migration and tube formation substantially (Figure 1B,C). The 15(S)-HETE also induced Matrigel plug angiogenesis, as measured by platelet endothelial cell adhesion molecule (PECAM) expression with anti-CD31 antibody immunostaining and hemoglobin levels (Figure 1D). In addition, adenovirus-mediated expression of dnJak2 completely reduced 15(S)-HETE–induced PECAM-positive cells and hemoglobin levels in the Matrigel plugs (Figure 1D). These results suggest that Jak2 plays a role in 15(S)-HETE–induced angiogenesis. A large body of data indicates that Jaks phosphorylate and activate STATs.38 To test the capacity of 15(S)-HETE in the activation of STATs, we have studied its effects on tyrosine phosphorylation of STAT-5B. An equal amount of protein from control and various time periods of 15(S)-HETE–treated HRMVECs was immunoprecipitated with anti–STAT-5B antibodies, and the resulting immunocomplexes were analyzed by Western blotting using phosphospecific STAT-5 antibodies. The 15(S)-HETE induced STAT-5B phosphorylation in a time-dependent manner with a 3-fold increase at 5 minutes compared with control (Figure 2A). Using a dominant-negative approach, we next tested the role of STAT-5B in 15(S)-HETE–induced HRMVEC migration and tube formation. Adenovirus-mediated expression of dnSTAT-5B suppressed 15(S)-HETE–induced HRMVEC migration and tube formation (Figure 2B,C). DnSTAT-5B mutant also inhibited 15(S)-HETE–induced Matrigel plug angiogenesis (Figure 2D). These findings indicate an involvement for STAT-5B in 15(S)-HETE–induced angiogenesis.
Jak2 mediates 15(S)-HETE–induced HRMVEC migration and tube formation in vitro and Matrigel plug angiogenesis in vivo. (A) Quiescent HRMVECs were treated with and without 15(S)-HETE (0.1 μM) for the indicated time periods, and cell extracts were prepared and analyzed by Western blotting for pJak2 using its phosphospecific antibodies. The blot was reprobed with anti-Jak2 antibodies for normalization. (B,C) HRMVECs were transduced with Ad-GFP or Ad-dnJak2 at 80 MOI, quiesced, and subjected to 15(S)-HETE–induced migration (B) or tube formation (C). (D) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5 μM 15(S)-HETE in combination with Ad-GFP or Ad-dnJak2 (5 × 109 pfu/mL). One week later, the animals were killed and the Matrigel plugs were harvested from underneath the skin, and cryosections were either made and immunostained for CD31 (PECAM) using anti-CD31 antibodies or analyzed for hemoglobin content using Drabkin reagent. The bar graphs in panels A through D represent mean ± SD values of 3 independent experiments or 6 plugs from 6 animals. *P < .01 versus control or Ad-GFP; **P < .01 versus AD-GFP + 15(S)-HETE.
Jak2 mediates 15(S)-HETE–induced HRMVEC migration and tube formation in vitro and Matrigel plug angiogenesis in vivo. (A) Quiescent HRMVECs were treated with and without 15(S)-HETE (0.1 μM) for the indicated time periods, and cell extracts were prepared and analyzed by Western blotting for pJak2 using its phosphospecific antibodies. The blot was reprobed with anti-Jak2 antibodies for normalization. (B,C) HRMVECs were transduced with Ad-GFP or Ad-dnJak2 at 80 MOI, quiesced, and subjected to 15(S)-HETE–induced migration (B) or tube formation (C). (D) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5 μM 15(S)-HETE in combination with Ad-GFP or Ad-dnJak2 (5 × 109 pfu/mL). One week later, the animals were killed and the Matrigel plugs were harvested from underneath the skin, and cryosections were either made and immunostained for CD31 (PECAM) using anti-CD31 antibodies or analyzed for hemoglobin content using Drabkin reagent. The bar graphs in panels A through D represent mean ± SD values of 3 independent experiments or 6 plugs from 6 animals. *P < .01 versus control or Ad-GFP; **P < .01 versus AD-GFP + 15(S)-HETE.
STAT-5B mediates 15(S)-HETE–induced HRMVEC migration and tube formation in vitro and Matrigel plug angiogenesis in vivo. (A) Quiescent HRMVECs were treated with and without 15(S)-HETE (0.1 μM) for the indicated time periods, and cell extracts were prepared. An equal amount of protein from control and 15(S)-HETE–treated HRMVECs was immunoprecipitated with anti–STAT-5B antibodies, and the resulting immunocomplexes were analyzed by Western blotting using phosphospecific anti–STAT-5 antibodies. The blot was reprobed with anti–STAT-5B antibodies for normalization. (B,C) HRMVECs were transduced with Ad-GFP or Ad-dnSTAT-5B at 80 MOI, quiesced, and subjected to 15(S)-HETE–induced migration (B) or tube formation (C). (D) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5 μM 15(S)-HETE in combination with Ad-GFP or Ad-dnSTAT-5B (5 × 109 pfu/mL). One week later, the animals were killed and the Matrigel plugs were harvested from underneath the skin, and cryosections were either made and immunostained for CD31 using anti-CD31 antibodies or analyzed for hemoglobin content using Drabkin reagent. The bar graphs in panels A through D represent the mean ± SD values of 3 independent experiments or 6 plugs from 6 animals. *P < .01 versus control or Ad-GFP; **P < .01 versus Ad-GFP + 15(S)-HETE.
STAT-5B mediates 15(S)-HETE–induced HRMVEC migration and tube formation in vitro and Matrigel plug angiogenesis in vivo. (A) Quiescent HRMVECs were treated with and without 15(S)-HETE (0.1 μM) for the indicated time periods, and cell extracts were prepared. An equal amount of protein from control and 15(S)-HETE–treated HRMVECs was immunoprecipitated with anti–STAT-5B antibodies, and the resulting immunocomplexes were analyzed by Western blotting using phosphospecific anti–STAT-5 antibodies. The blot was reprobed with anti–STAT-5B antibodies for normalization. (B,C) HRMVECs were transduced with Ad-GFP or Ad-dnSTAT-5B at 80 MOI, quiesced, and subjected to 15(S)-HETE–induced migration (B) or tube formation (C). (D) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5 μM 15(S)-HETE in combination with Ad-GFP or Ad-dnSTAT-5B (5 × 109 pfu/mL). One week later, the animals were killed and the Matrigel plugs were harvested from underneath the skin, and cryosections were either made and immunostained for CD31 using anti-CD31 antibodies or analyzed for hemoglobin content using Drabkin reagent. The bar graphs in panels A through D represent the mean ± SD values of 3 independent experiments or 6 plugs from 6 animals. *P < .01 versus control or Ad-GFP; **P < .01 versus Ad-GFP + 15(S)-HETE.
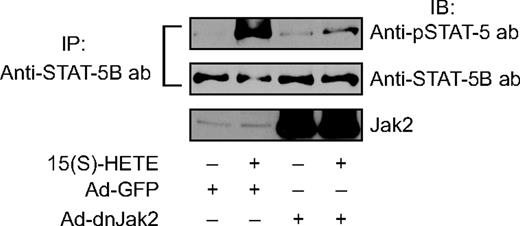
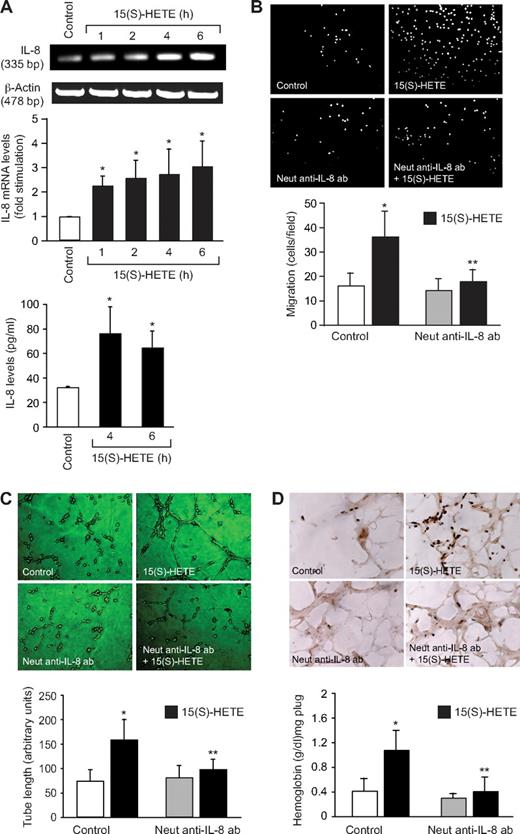
To find the mechanism by which 15(S)-HETE activates STAT-5B, we examined the role of Jak2. Interference with Jak2 activation via adenovirus-mediated expression of its dominant-negative mutant completely suppressed 15(S)-HETE–induced STAT-5B tyrosine phosphorylation (Figure 3). This observation suggests that 15(S)-HETE activates STAT-5B via Jak2. It was demonstrated that oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (Ox-PAPC) and 1-palmitoyl-2-epoxyisoprostane-sn-glycero-3-phosphorylcholine induce IL-8 expression via Jak-STAT signaling in endothelial cells.44 In addition, IL-8 has been reported to stimulate angiogenesis.45,46 Therefore, to find the downstream target gene(s) of STAT-5B in mediating 15(S)-HETE–induced angiogenesis, we first studied the time course effect of 15(S)-HETE on IL-8 expression. An equal amount of RNA from control and various time periods of 15(S)-HETE–treated HRMVECs was tested for IL-8 mRNA levels by RT-PCR. The 15(S)-HETE induced the expression of IL-8 mRNA in a time-dependent manner with a near-maximum effect at 4 hours (Figure 4A). To confirm the effect of 15(S)-HETE on IL-8 expression, we also measured its release into the medium by ELISA. A time-dependent release of IL-8 was observed in response to 15(S)-HETE with a maximum effect at 4 hours (Figure 4A). To find whether IL-8 plays a role in 15(S)-HETE–induced angiogenesis, we tested the effect of its neutralizing antibodies. As shown in Figure 4B,C, neutralizing IL-8 antibodies inhibited 15(S)-HETE–induced HRMVEC migration and tube formation. Furthermore, neutralizing IL-8 antibodies also reduced 15(S)-HETE–induced Matrigel plug angiogenesis (Figure 4D).
Blockade of Jak2 activation inhibits 15(S)-HETE–stimulated STAT-5B phosphorylation. HRMVECs were transduced with Ad-GFP or Ad-dnJak2 at 80 MOI, quiesced, and treated with and without 15(S)-HETE (0.1 μM) for the indicated time periods, and cell extracts were prepared and analyzed by Western blotting for tyrosine phosphorylation of STAT-5B, as described in Figure 2A legend (top panel). The blot was reprobed with anti–STAT-5B antibodies for normalization (middle panel). The same samples were analyzed by Western blotting using anti-Jak2 antibodies to show the overexpression of dnJak2 (bottom panel).
Blockade of Jak2 activation inhibits 15(S)-HETE–stimulated STAT-5B phosphorylation. HRMVECs were transduced with Ad-GFP or Ad-dnJak2 at 80 MOI, quiesced, and treated with and without 15(S)-HETE (0.1 μM) for the indicated time periods, and cell extracts were prepared and analyzed by Western blotting for tyrosine phosphorylation of STAT-5B, as described in Figure 2A legend (top panel). The blot was reprobed with anti–STAT-5B antibodies for normalization (middle panel). The same samples were analyzed by Western blotting using anti-Jak2 antibodies to show the overexpression of dnJak2 (bottom panel).
IL-8 mediates 15(S)-HETE–induced angiogenesis. (A) Quiescent HRMVECs were treated with and without 15(S)-HETE (0.1 μM) for the indicated time periods, and either RNA was isolated and analyzed for IL-8 mRNA levels by RT-PCR, or medium was collected and analyzed for IL-8 release by ELISA. (B,C) Quiescent HRMVECs were treated with and without neutralizing anti–IL-8 antibodies (3 μg/mL) for 30 minutes at 37°C, followed by washing with medium 131. The cells were then subjected to 0.1 μM 15(S)-HETE–induced migration (B) or tube formation (C) in the presence and absence of 3 μg/mL neutralizing anti–IL-8 antibodies. (D) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5 μM 15(S)-HETE with and without 3 μg/mL neutralizing anti–IL-8 antibodies. One week later, the animals were killed and the Matrigel plugs were harvested from underneath the skin, and cryosections were either made and immunostained for CD31 using anti-CD31 antibodies or analyzed for hemoglobin content using Drabkin reagent. Wherever appropriate, preimmune serum was added to the control groups. The bar graphs in panels A through D represent the mean ± SD values of 3 independent experiments or 6 plugs from 6 animals. *P < .01 versus control; **P < .01 versus 15(S)-HETE.
IL-8 mediates 15(S)-HETE–induced angiogenesis. (A) Quiescent HRMVECs were treated with and without 15(S)-HETE (0.1 μM) for the indicated time periods, and either RNA was isolated and analyzed for IL-8 mRNA levels by RT-PCR, or medium was collected and analyzed for IL-8 release by ELISA. (B,C) Quiescent HRMVECs were treated with and without neutralizing anti–IL-8 antibodies (3 μg/mL) for 30 minutes at 37°C, followed by washing with medium 131. The cells were then subjected to 0.1 μM 15(S)-HETE–induced migration (B) or tube formation (C) in the presence and absence of 3 μg/mL neutralizing anti–IL-8 antibodies. (D) C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5 μM 15(S)-HETE with and without 3 μg/mL neutralizing anti–IL-8 antibodies. One week later, the animals were killed and the Matrigel plugs were harvested from underneath the skin, and cryosections were either made and immunostained for CD31 using anti-CD31 antibodies or analyzed for hemoglobin content using Drabkin reagent. Wherever appropriate, preimmune serum was added to the control groups. The bar graphs in panels A through D represent the mean ± SD values of 3 independent experiments or 6 plugs from 6 animals. *P < .01 versus control; **P < .01 versus 15(S)-HETE.
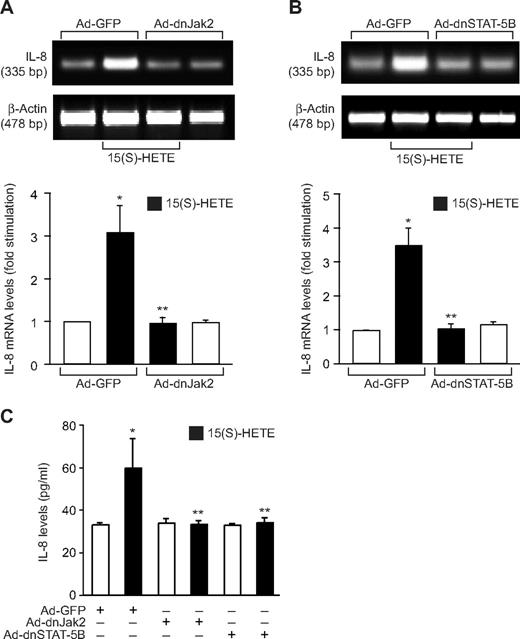
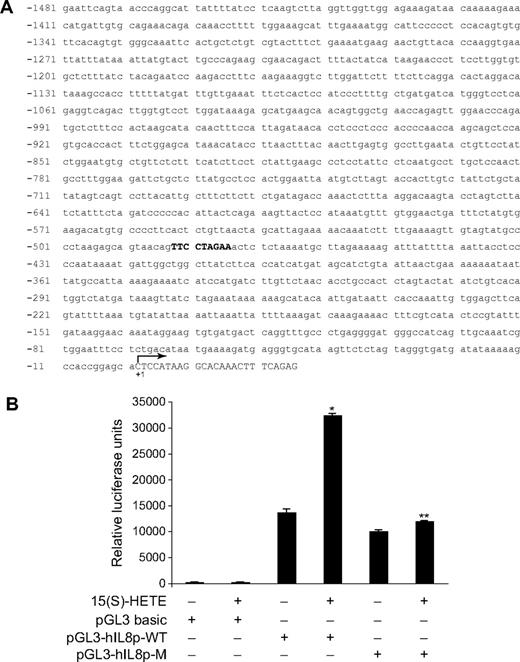
Next, we tested the role of Jak2-STAT-5B signaling in 15(S)-HETE–induced IL-8 expression. Adenovirus-mediated expression of either dnJak-2 or dnSTAT-5B completely inhibited 15(S)-HETE–induced IL-8 expression at both mRNA and protein levels (Figure 5). To understand the mechanisms by which 15(S)-HETE induces IL-8 expression, we cloned a 1.5-kb IL-8 promoter. Transfac analysis of the cloned IL-8 promoter showed a STAT binding site spanning from −476 to −484 nt (Figure 6A). To obtain support for the role of STATs in 15(S)-HETE–induced IL-8 expression, we subcloned the 1.5-kb IL-8 promoter that contains the STAT binding site into pGL3 basic vector and tested its capacity in modulating luciferase reporter-gene induction. The 15(S)-HETE induced IL-8 promoter-dependent luciferase reporter-gene (pGL3-hIL8p-WT) activity by at least 2- to 3-fold compared with control (Figure 6B). Furthermore, mutational disruption of STAT binding site (from TTCCTAGAA to TTAAACTAA) almost completely abolished the effect of 15(S)-HETE on induction of luciferase reporter-gene (pGL3-hIL8p-M) activity (Figure 6B). To gain additional support for the role of Jak2-STAT-5B signaling in 15(S)-HETE–induced IL-8 expression, we performed EMSA. An equal amount of nuclear protein from control and various time periods of 15(S)-HETE–treated HRMVECs was analyzed for STAT-DNA binding using a [32P]-labeled oligonucleotide probe that was designed based on STAT-binding sequence in the IL-8 promoter. The 15(S)-HETE induced STAT-DNA–binding activity in a time-dependent manner (Figure 7A). Interference with activation of Jak2 or STAT-5B via forced expression of their respective dominant-negative mutants blocked 15(S)-HETE–induced STAT-DNA–binding activity (Figure 7B). To test the binding of STAT-5B to IL-8 promoter region in vivo, we performed ChIP assay. ChIP analysis indicated that STAT-5B binds to IL-8 promoter in a time-dependent manner with maximum effect at 2 hours (Figure 7C). In addition, inhibition of either Jak2 or STAT-5B activation by adenovirus-mediated expression of their dominant-negative mutants attenuated STAT-5B binding to IL-8 promoter (Figure 7D). These findings reveal that 15(S)-HETE induces IL-8 expression via activation of Jak2-STAT-5B signaling in HRMVECs to influence the angiogenic events of these cells. The 15(S)-HETE metabolizes to lipoxins (LXs).47 To find whether 15(S)-HETE–induced angiogenesis requires its conversion to LXs, we studied the effect of LXA4 on angiogenesis. LXA4 had no significant effect on angiogenesis as measured by Matrigel plug assay (Figure 8).
15(S)-HETE–induced IL-8 expression requires activation of Jak2-STAT-5B signaling in HRMVECs. (A,B) HRMVECs were transduced with Ad-GFP, Ad-dnJak2, or Ad-dnSTAT-5B with 80 MOI, quiesced, and treated with and without 0.1 μM 15(S)-HETE for 4 hours, and RNA was isolated and analyzed for IL-8 mRNA levels by RT-PCR. (C) All the conditions were the same as in panels A and B, except that medium was collected and tested for IL-8 release by ELISA. The bar graphs in panels A through C represent the mean ± SD values of 3 independent experiments. *P < .01 versus Ad-GFP; **P < .01 versus Ad-GFP + 15(S)-HETE.
15(S)-HETE–induced IL-8 expression requires activation of Jak2-STAT-5B signaling in HRMVECs. (A,B) HRMVECs were transduced with Ad-GFP, Ad-dnJak2, or Ad-dnSTAT-5B with 80 MOI, quiesced, and treated with and without 0.1 μM 15(S)-HETE for 4 hours, and RNA was isolated and analyzed for IL-8 mRNA levels by RT-PCR. (C) All the conditions were the same as in panels A and B, except that medium was collected and tested for IL-8 release by ELISA. The bar graphs in panels A through C represent the mean ± SD values of 3 independent experiments. *P < .01 versus Ad-GFP; **P < .01 versus Ad-GFP + 15(S)-HETE.
15(S)-HETE–induced IL-8 promoter-driven luciferase reporter-gene expression requires STAT binding site. (A) Sequence of a 1.5-kb cloned human IL-8 promoter region showing the STAT binding site. (B) HRMVECs were transfected with either empty vector or IL-8 promoter-luciferase constructs, quiesced, and treated with and without 0.1 μM 15(S)-HETE for 6 hours, and cell extracts were prepared and analyzed for luciferase activity. *P < .01 versus pGL3-hIL8p-WT control; **P < .01 versus pGL3-hIL8p-WT + 15(S)-HETE. pGL3-hIL8p-WT, wild-type promoter construct; pGL3-hIL8p-M, mutant promoter construct. Sequence in bold represents STAT binding site; +1 indicates transcription start site.
15(S)-HETE–induced IL-8 promoter-driven luciferase reporter-gene expression requires STAT binding site. (A) Sequence of a 1.5-kb cloned human IL-8 promoter region showing the STAT binding site. (B) HRMVECs were transfected with either empty vector or IL-8 promoter-luciferase constructs, quiesced, and treated with and without 0.1 μM 15(S)-HETE for 6 hours, and cell extracts were prepared and analyzed for luciferase activity. *P < .01 versus pGL3-hIL8p-WT control; **P < .01 versus pGL3-hIL8p-WT + 15(S)-HETE. pGL3-hIL8p-WT, wild-type promoter construct; pGL3-hIL8p-M, mutant promoter construct. Sequence in bold represents STAT binding site; +1 indicates transcription start site.
STAT-5B binds to IL-8 promoter in response to 15(S)-HETE in Jak2-dependent manner. (A,C) Quiescent HRMVECs were treated with and without 0.1 μM 15(S)-HETE for the indicated time periods, and either nuclear extracts were prepared and analyzed by EMSA for STAT-DNA–binding activity using [32P]-labeled STAT-binding sequence of IL-8 promoter as a probe in vitro (A) or processed for ChIP analysis of STAT-5B binding to IL-8 promoter in vivo (C). (B,D) HRMVECs that were transduced with Ad-GFP, Ad-dnJak2, or Ad-dnSTAT-5B with 80 MOI and quiesced were treated with and without 0.1 μM 15(S)-HETE for 2 hours, and either nuclear extracts were prepared and analyzed by EMSA for STAT-DNA–binding activity (B), as described in (A), or processed for ChIP analysis of STAT-5B binding to IL-8 promoter in vivo (D).
STAT-5B binds to IL-8 promoter in response to 15(S)-HETE in Jak2-dependent manner. (A,C) Quiescent HRMVECs were treated with and without 0.1 μM 15(S)-HETE for the indicated time periods, and either nuclear extracts were prepared and analyzed by EMSA for STAT-DNA–binding activity using [32P]-labeled STAT-binding sequence of IL-8 promoter as a probe in vitro (A) or processed for ChIP analysis of STAT-5B binding to IL-8 promoter in vivo (C). (B,D) HRMVECs that were transduced with Ad-GFP, Ad-dnJak2, or Ad-dnSTAT-5B with 80 MOI and quiesced were treated with and without 0.1 μM 15(S)-HETE for 2 hours, and either nuclear extracts were prepared and analyzed by EMSA for STAT-DNA–binding activity (B), as described in (A), or processed for ChIP analysis of STAT-5B binding to IL-8 promoter in vivo (D).
LXA4 fails to induce angiogenesis. C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5 μM 15(S)-HETE or 5 μM LXA4. One week later, the animals were killed and the Matrigel plugs were harvested from underneath the skin, and cryosections were either made and immunostained for CD31 using anti-CD31 antibodies or analyzed for hemoglobin content using Drabkin reagent. The values in the bar graph are the mean plus or minus SD of 6 plugs from 6 animals. *P < .01 versus vehicle control.
LXA4 fails to induce angiogenesis. C57BL/6 mice were injected subcutaneously with 0.5 mL of Matrigel premixed with vehicle or 5 μM 15(S)-HETE or 5 μM LXA4. One week later, the animals were killed and the Matrigel plugs were harvested from underneath the skin, and cryosections were either made and immunostained for CD31 using anti-CD31 antibodies or analyzed for hemoglobin content using Drabkin reagent. The values in the bar graph are the mean plus or minus SD of 6 plugs from 6 animals. *P < .01 versus vehicle control.
Discussion
The important findings of the present study are as follows: (1) 15(S)-HETE stimulated tyrosine phosphorylation of Jak2 and STAT-5B in a time-dependent manner in HRMVECs. (2) 15(S)-HETE–induced tyrosine phosphorylation of STAT-5B requires Jak2 activation. (3) 15(S)-HETE induced IL-8 expression in HRMVECs via activation of Jak2-STAT-5B signaling. (4) 15(S)-HETE–induced migration and tube formation of HRMVECs and Matrigel plug angiogenesis require Jak2-STAT-5B–dependent expression of IL-8. In recent years, some studies showed that the lack of the capacity of endothelial progenitor cells to develop neovascularization is due to defects in Jak2.40 In addition, mutations that lead to constitutive activation of Jak2 are associated with enhanced angiogenesis in myeloproliferative diseases.48,49 These discoveries clearly indicate that Jak2 plays an important role in the maintenance of endothelial progenitor cell plasticity in forming new blood vessels, and thereby in the regulation of both developmental and pathologic angiogenesis. These observations together with the capacity of 15(S)-HETE to activate Jak2 in microvascular endothelial cells suggest that Jak2 may be one of the crucial signaling molecules in 15(S)-HETE–induced angiogenesis. Recently, we have shown that 15(S)-HETE activates Src in human dermal microvascular endothelial cells.39 Because both Jak2 and Src are activated by 15(S)-HETE and these 2 nonreceptor tyrosine kinases can phosphorylate and activate STATs, it is possible that in addition to Jak2, Src may also be involved in the mediation of 15(S)-HETE–induced STAT-5B phosphorylation in HRMVECs. In fact, a similar mechanism has been shown in the activation of STAT-3 by an oxidized lipid molecule, Ox-PAPC.44 Because blockade of Jak2 and STAT-5B attenuated 15(S)-HETE–induced HRMVEC migration and tube formation and Matrigel plug angiogenesis and dnJak2 suppressed 15(S)-HETE–stimulated STAT-5B phosphorylation, it is likely that Jak2-mediated STAT-5B activation is required for 15(S)-HETE–induced angiogenesis.
Upon incubation ex vivo, the capacity of atherosclerotic arteries was found to be significantly higher in the conversion of AA to 15(S)-HETE compared with normal arteries.6 In addition, many studies have reported a correlation between intraplaque angiogenesis and atherosclerotic plaque progression.50,51 Based on these observations, it can be predicted that 15(S)-HETE with its ability to induce angiogenesis may influence the development of atherosclerotic lesions. The levels of 15(S)-HETE have also been reported to be elevated in the epiretinal membranes of PVR (proliferative vitrioretinopathy) and PDR (proliferative diabetic retinopathy) patients.20 Similarly, hypoxia has been shown to produce higher levels of 15(S)-HETE compared with normoxia.21 Because hypoxia is one of the important factors that trigger angiogenesis and it stimulates the production of 15(S)-HETE, it is possible that hypoxia-induced angiogenesis may be mediated at least to some extent by 15(S)-HETE.
The most interesting observation of the present study is the ability of 15(S)-HETE to induce the expression of IL-8, a chemokine whose actions have been linked to the regulation of angiogenesis.45,46 Angiogenic growth factors such as vascular endothelial growth factor (VEGF) and oxidized lipid molecules such as Ox-PAPC have been reported to induce the expression of IL-8.44,52 Because neutralizing anti–IL-8 antibodies substantially attenuated 15(S)-HETE–induced HRMVEC migration and tube formation and Matrigel plug angiogenesis, IL-8 may be an important mediator of 15(S)-HETE–induced angiogenesis. Because angiogenesis is a multievent phenomenon, it can be speculated that IL-8 is involved in the sprouting, elongation, or maturation/stabilization of microvessels. The analysis of the cloned 1.5-kb IL-8 promoter for transcription factor binding sites revealed the presence of 1 putative STAT-binding sequence at −476 nt to −484 nt. In addition, this promoter was found to be able to mediate luciferase reporter-gene activity by 15(S)-HETE, and this induction was completely abolished by disruption of STAT binding site. Furthermore, using this sequence as a labeled probe, increased binding of nuclear proteins to this STAT binding site was observed in response to 15(S)-HETE. In a similar manner, in response to 15(S)-HETE, STAT-5B was bound to IL-8 promoter, and this response was dependent on the activation of Jak2. Based on these observations, it may be concluded that 15(S)-HETE–induced angiogenesis requires Jak-2-STAT-5B–dependent induction of expression of IL-8. Previously, we have shown that 15(S)-HETE induces fibroblast growth factor-2 and VEGF in human dermal microvascular endothelial cells.25,26 The capacity of 15(S)-HETE in the induction of expression of various angiogenic factors including IL-8 suggests a potential role for these factors in different stages of angiogenesis. For example, angiopoietin-2 has been reported to play a role in vessel maturation and stabilization.53 In this aspect, it will be interesting to study whether 15(S)-HETE also induces the expression of angiopoietins in HRMVECs.
Previously, it was demonstrated that a stable analog of aspirin-triggered LXs, namely, 15-epi-16-(para-fluro)-phenoxy LXA4 inhibits VEGF-induced angiogenesis.54 Consistent with these observations, in the present study, we found that LXA4, a metabolite of 15(S)-HETE, also had no effect on angiogenesis. This result suggests that 15(S)-HETE–induced angiogenesis does not require its conversion to LXA4. In view of the findings of the present and previous studies,24-26 one can speculate that 15(S)-HETE via its influence on angiogenesis may be involved in the development of several inflammatory diseases, such as atherosclerosis, cancer, and retinal neovascularization.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant (EY014856) from the National Eye Institute/National Institutes of Health.
National Institutes of Health
Authorship
Contribution: S.Y.C. performed Western blotting, RT-PCR, ELISA, migration, tube formation, and Matrigel plug angiogenesis assays; D.W. performed Matrigel plug angiogenesis assays and immunohistochemistry; M.K. performed EMSA and ChIP assay; V.K.-S. performed cloning and luciferase assays; Q.Z. performed Western blot analysis; K.R.C. performed EMSA; and G.N.R. designed the experiments, interpreted the results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Gadiparthi N. Rao, Department of Physiology, University of Tennessee Health Science Center, 894 Union Ave, Memphis, TN 38163; e-mail: grao@physio1.utmem.edu.

![Figure 7. STAT-5B binds to IL-8 promoter in response to 15(S)-HETE in Jak2-dependent manner. (A,C) Quiescent HRMVECs were treated with and without 0.1 μM 15(S)-HETE for the indicated time periods, and either nuclear extracts were prepared and analyzed by EMSA for STAT-DNA–binding activity using [32P]-labeled STAT-binding sequence of IL-8 promoter as a probe in vitro (A) or processed for ChIP analysis of STAT-5B binding to IL-8 promoter in vivo (C). (B,D) HRMVECs that were transduced with Ad-GFP, Ad-dnJak2, or Ad-dnSTAT-5B with 80 MOI and quiesced were treated with and without 0.1 μM 15(S)-HETE for 2 hours, and either nuclear extracts were prepared and analyzed by EMSA for STAT-DNA–binding activity (B), as described in (A), or processed for ChIP analysis of STAT-5B binding to IL-8 promoter in vivo (D).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/23/10.1182_blood-2008-10-183210/4/m_zh89990936950007.jpeg?Expires=1767721604&Signature=fj3posfplRE0F73a~2Ip3jV0EzoJdiudAYv4eAYrsmzyeQb6hiDYmbAIAb3xBtKE4tASNyr4kIbWUBa24jHrCU9JB3zXa~GlT-G4ksCwxYviJy3Gm9hAuCie6jKmvelCTwaqc-ANgq7HH34eafbunJTrSMDLpo3oGLq9N09eThwsO3v9KYHCLq1D-2TpskcK2cdnuS~djKvt4QaDto9tpUi~trr2QB4VALdCxM~z5ckNBLJrbjt7lf8ACgNyhdLY6PFKcEuna-ojhQB~CuwdaG6XqRw2DEWTSktfXyMELDdtDr7rGEjS6DQJu4nQROUStqvJ3aSyJt~dXdpBFbaQpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal