Abstract
Correction of murine models of β-thalassemia has been achieved through high-level globin lentiviral vector gene transfer into mouse hematopoietic stem cells (HSCs). However, transduction of human HSCs is less robust and may be inadequate to achieve therapeutic levels of genetically modified erythroid cells. We therefore developed a double gene lentiviral vector encoding both human γ-globin under the transcriptional control of erythroid regulatory elements and methylguanine methyltransferase (MGMT), driven by a constitutive cellular promoter. MGMT expression provides cellular resistance to alkylator drugs, which can be administered to kill residual untransduced, diseased HSCs, whereas transduced cells are protected. Mice transplanted with β-thalassemic HSCs transduced with a γ-globin/MGMT vector initially had subtherapeutic levels of red cells expressing γ-globin. To enrich γ-globin–expressing cells, transplanted mice were treated with the alkylator agent 1,3-bis-chloroethyl-1-nitrosourea. This resulted in significant increases in the number of γ-globin–expressing red cells and the amount of fetal hemoglobin, leading to resolution of anemia. Selection of transduced HSCs was also obtained when cells were drug-treated before transplantation. Mice that received these cells demonstrated reconstitution with therapeutic levels of γ-globin–expressing cells. These data suggest that MGMT-based drug selection holds promise as a modality to improve gene therapy for β-thalassemia.
Introduction
Hematopoietic stem cell (HSC)–targeted gene therapy is an attractive approach for several hematopoietic disorders caused by single gene defects.1 In the past few years, murine models of hemoglobin disorders, including β-thalassemia and sickle cell disease (SCD), have been successfully corrected using globin lentiviral vectors for targeting HSCs.2-7 The success of these preclinical studies occurred in the setting of highly efficient gene transfer into HSCs coupled with myeloablative conditioning of transplantation recipients to insure high-level engraftment of transduced cells. However, current gene transfer efficiencies into human HSCs may be inadequate to achieve successful gene therapy for patients with hemoglobin disorders because there is only a moderate selective advantage for corrected cells in late-stage erythropoiesis.
Despite improvements in HSC gene transfer protocols using retroviral vectors, levels of HSC-targeted gene transfer in nonhuman primate models and in human clinical marking protocols are significantly lower than those obtained in mice.8-10 Levels of genetically marked blood cells up to 5% to 10% using γ-retroviral vectors have been obtained in only a small number of human patients, and long-term marking at these levels has not yet been reported.8,9,11 Although lentiviral vectors appear to be promising for improving human stem cell gene transfer efficiency, it remains unknown whether they will provide higher levels of HSC gene transfer in the clinical context. In a long-term study of primates transplanted with lentiviral vector-transduced CD34+ cells, we observed stable gene transfer levels of 3% to 12% 4 years after transplantation.12 Levels of at least 15% and higher will probably be needed for significant clinical benefit for SCD and β-thalassemia. In addition, if submyeloablative conditioning is used to reduce the regimen-related toxicity to patients, there would be a further reduction of the proportion of engrafting, genetically corrected cells resulting from dilution with residual endogenous HSCs. One approach to selectively enrich genetically corrected autologous HSCs to therapeutic levels is to incorporate a drug-resistance gene into the therapeutic vector.13-15 After transplantation, treatment with a stem cell toxic drug could then be used to enrich the drug-resistant, genetically modified HSCs while eradicating the endogenous, diseased HSCs.
Methylguanine methyltransferase (MGMT) is an alkyltransferase that normally functions to repair cellular DNA damage at the O6 position of guanine.16,17 The cytotoxic effects of alkylating agents, such as temozolomide and 1,3-bis-chloroethyl-1-nitrosourea (BCNU), can be prevented if there is adequate expression of MGMT, which removes the O6 adduct from the modified DNA. Variant MGMT proteins with specific amino acid changes, such as P140K and G156A, retain significant activity while possessing the useful property of resistance to inactivation by O6-benzylguanine (BG).18 BG can be used to inactivate endogenous MGMT to enhance the specificity of alkylator-mediated cell death to cells not expressing the variant form.
Previously, we showed that MGMT-mediated in vivo selection of HSCs could ameliorate the anemia in a murine model of β-thalassemia through modulation of normal HSC chimerism.19 In that study, a minority population of normal HSCs that had been transduced with a γ-retroviral vector encoding only a variant MGMT drug-resistance protein could be enriched to therapeutic levels in β-thalassemic mice after treatment with the alkylating agent, temozolomide. This study, along with others using a human xenograft model and a canine transplantation model,20,21 provides evidence for the feasibility of modulating transduced normal cell chimerism in the context of allogeneic transplantation. Here, we use a model of gene-corrected autologous cells to demonstrate the successful development and use of a dual gene, lentiviral vector encoding both γ-globin and MGMT to correct murine β-thalassemia intermedia. Our data show that coexpression of MGMT allowed autologous, γ-globin vector-transduced β-thalassemic HSCs to be enriched to therapeutic levels through either pre- or posttransplantation selection.
Methods
Mice
Heterozygous β-thalassemic male mice were a gift from Dr T. Townes (University of Alabama, Birmingham, AL) and have been bred with HW80 (B6.C-TyrcH1b Hbbd/By; The Jackson Laboratory, Bar Harbor, ME) female mice for more than 5 years. C57Bl/6J mice (congenic with the HW80 background) were purchased from the Jackson Laboratory. Three- to 5-month-old β-thalassemic male and female mice were used as bone marrow (BM) donors. All experimentation with mice was performed under protocols approved by the Institutional Animal Care and Use Committee of St Jude Children's Research Hospital.
Plasmid constructions
To generate γ-globin lentiviral vectors that also contain a selectable MGMT drug-resistance gene, 2 different P140KMGMT cDNA expression cassettes (encoding human MGMT with a proline to lysine amino acid change at position 140, which confers resistance to MGMT pseudosubstrate inhibitor O6 benzylguanine), were separately placed in our γ-globin vector, mLARV5 (V5), in the opposite transcriptional direction from the γ-globin cassette (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). The γ-globin expression cassette consists of 3.1 kb of transcriptional regulatory elements from the β-globin locus control region, a 130-bp β-globin promoter, and the γ-globin coding sequences, as previously described.19,22 Additional details of plasmid construction are presented in the supplemental data, and all plasmid sequences and plasmid DNAs are available on request.
Preparation of virus
VSV-G pseudotyped lentiviral vector particles were prepared using a 4-plasmid system by transient transfection of 293T cells, as previously described23 and detailed in supplemental methods.
Transduction of β-thalassemic BM cells
BM cells obtained from 3- to 5-month-old β-thalassemic mice were subjected to lineage depletion by immunomagnetic separation using the Lineage Cell Depletion Kit (Miltenyi Biotec, Auburn, CA) and transduced essentially as previously described.7 Additional details are presented in the supplemental methods.
Drug treatment
O6-BG (Sigma-Aldrich, St Louis, MO) was dissolved in polyethylene glycol and diluted in prewarmed phosphate-buffered saline to a final concentration of 2.5 mg/mL BCNU (Bristol-Myers Squibb, Stamford, CT) was dissolved in ethanol and diluted in phosphate-buffered saline to a final concentration of 2 mg/mL. Ten or 12 weeks after transplantation, each mouse was injected intraperitoneally with O6-BG (15 mg/kg body weight), followed 1 hour later by an injection of BCNU (10 mg/kg). Drugs were administered every 4 to 6 weeks in a total of 3 courses.
Hematologic analysis
Hemoglobin (Hb) cellulose acetate gel electrophoresis and fluorescence-activated cell sorter (FACS) analysis of red cells for expression of human γ-globin were performed as previously described.22 Fractionation and quantitation of mouse Hb and HbF were performed by high performance liquid chromatography (HPLC) using a cation-exchange column (Ultra2 Variant Resolution Analyzer; Primus Diagnostics, Kansas City, MO).7 Hb peaks were quantitated with the software accompanying the HPLC apparatus.
Spleen colony-forming unit assay
A total of 50 000 to 80 000 BM cells from primary transplanted mice were injected into C57BL/6J mice irradiated with 950 cGy. Mice were killed 14 days later, and well-separated individual spleen colonies were obtained for genomic DNA preparation using the PureGene Kit (QIAGEN, Valencia, CA).
DNA analysis
The presence of vector and copy number was determined by Southern blot analysis using standard laboratory procedures. The details of this analysis are presented in supplemental methods.
Identification of vector insertion sites using linear amplification–mediated polymerase chain reaction
Host genomic-vector proviral junction sequences were detected using linear amplification–mediated polymerase chain reaction (LAM-PCR) as previously described.24 Details are presented in supplemental methods.
Statistical analysis
Student 2-tailed t tests were used to determine statistically significant differences between mean values of different datasets using Microsoft Excel and GraphPad Prism software (GraphPad, San Diego, CA), unless otherwise stated. Descriptive statistics (mean ± SEM) were determined using Microsoft Excel. Instances in which subset analysis was performed on mice determined to be responders to the in vivo selection treatment are indicated in the text and figure legends.
Results
Development of lentiviral vectors encoding both human γ-globin and MGMT
Figure S1A shows the design of 2 different γ-globin/MGMT SIN lentiviral vectors. One vector uses an internal murine stem cell virus (MSCV) enhancer/promoter to drive MGMT expression, whereas the second vector uses an internal cellular elongation factor 1 α (EF1α) promoter. Both vectors contain an identical γ-globin expression cassette in reverse orientation, driven by 3.1 kb of β-globin locus control region elements and a 130-bp β-globin promoter, as previously described.22 Both vectors could be generated to relatively high titers (0.7-1.0 × 106 transducing units per milliliter, unconcentrated) and were concentrated 550- to 625-fold with efficient recovery of viral particles (Figure S2). Importantly, the viral genome was transmitted without evidence of rearrangement, as demonstrated by Southern blot analysis using an enzyme that cuts twice in the vector genome to liberate a nearly full-length provirus (Figures S1A, S2).
Therapeutic levels of γ-globin–expressing red blood cells after in vivo selection of β-thalassemic hematopoietic cells
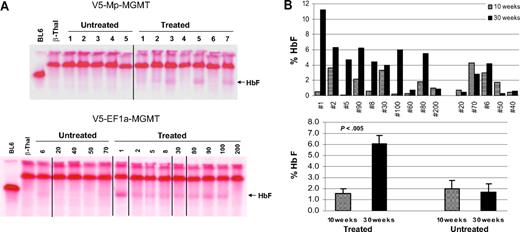
To test whether in vivo drug selection could be used to enrich a minority population of β-thalassemic red blood cells (RBCs) expressing human γ-globin, we transplanted wild-type mice with lineage-depleted β-thalassemic BM cells transduced with each of the γ-globin/MGMT dual-gene lentiviral vectors. Relatively low multiplicity of infection (MOI) was used to obtain animals with low levels of transduced HSCs, as one might expect in a human clinical trial (supplemental materials). The experimental design shown is in Figure S1B. Animals transplanted with mock-transduced cells served as controls. Mice transplanted with vector-transduced cells were allowed 10 to 12 weeks to achieve full hematopoietic engraftment. Baseline complete blood counts, FACS analysis for γ-globin–expressing RBCs, and analysis for HbF protein by HPLC were then performed. Animals transplanted with cells transduced with the 2 vectors were randomly assigned to receive either drug treatment with BCNU and O6-benzylguanine, which inactivates endogenous MGMT in HSCs, or a sham treatment with saline. We chose to use BCNU in these studies, rather than temozolomide, as we successfully used previously19 because a single injection of BCNU per treatment cycle was significantly better tolerated than 5 oral gavage doses per cycle using temozolomide resulting from much less animal manipulation. BCNU treatment was administered every 4 to 6 weeks for a total of 3 courses, after which time these parameters were again measured 1 month after the last drug treatment. All 17 treated mice survived the regimen of 3 courses of 15 mg/kg O6-benzylguanine and 10 mg/kg BCNU. Six of 7 mice in the V5-Mp-MGMT group had increases in γ-globin–expressing RBCs (henceforth termed “F cells”) from baseline values after drug treatment, whereas 8 of 10 mice in the V5-EF1-MGMT group also responded with increases (Figures S3, 1A). In contrast, only 1 of 11 mice in the control, untreated groups showed a modest, stochastic increase in F cells. On average, F cells in treated mice that responded in the V5-Mp-MGMT group increased from 11% to 47% (P < .001), whereas untreated mice showed a decline from 5% to 2% (Figure 1B). Similarly, starting from a higher baseline level, F cells in the responding V5-EF1-MGMT group displayed a robust increase after in vivo selection (24% increasing to 86% F cells, P < .001). Several mice had increases from less than 10% F cells up to greater than 80% F cells. Untreated, control V5-EF1-MGMT mice demonstrated an average decrease in F cells (Figure 1B).
In vivo selection results in significant increases in F cells in mice transplanted with γ-globin/MGMT lentiviral vector-transduced β-thalassemic BM cells. (A) The percentages of F cells in the peripheral blood of mice transplanted with the indicated γ-globin/MGMT vector-transduced β-thalassemic BM cells are shown before (10-12 weeks after transplantation) and 1 month after completing (30-32 weeks after transplantation) administration of 3 doses of BG/BCNU. F-cell percentages are also shown for control mice that received no drug treatment. (B) Percentages of F cells in control untreated mice and in treated responding mice before and after drug administration. Data represent the mean ± SEM. Statistically significant differences are indicated by the P values, as determined by the Student t test.
In vivo selection results in significant increases in F cells in mice transplanted with γ-globin/MGMT lentiviral vector-transduced β-thalassemic BM cells. (A) The percentages of F cells in the peripheral blood of mice transplanted with the indicated γ-globin/MGMT vector-transduced β-thalassemic BM cells are shown before (10-12 weeks after transplantation) and 1 month after completing (30-32 weeks after transplantation) administration of 3 doses of BG/BCNU. F-cell percentages are also shown for control mice that received no drug treatment. (B) Percentages of F cells in control untreated mice and in treated responding mice before and after drug administration. Data represent the mean ± SEM. Statistically significant differences are indicated by the P values, as determined by the Student t test.
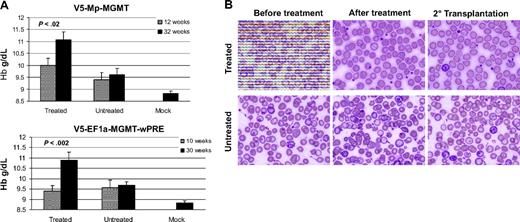
Commensurate with the increase in F cells, animals in both responding, drug-treated γ-globin/MGMT vector groups had increases in total HbF protein from baseline levels that were often undetectable, as determined by HPLC analysis (Figure S4). In contrast, HbF levels remained low or undetectable in the control groups (Figure 2). Importantly, in vivo selection was associated with significant increases in the Hb level with amelioration of anemia in the treated, responding groups of mice, relative to the control, untreated, and mock groups (Figure 3A). Interestingly, we observed that even the small amounts of HbF in the control, untreated groups caused a modest increase in Hb level relative to animals transplanted with mock-transduced cells, an effect also observed by others.25 Along with the improved Hb level, we observed resolution of β-thalassemic RBC morphologic abnormalities in the treated mice, whereas the RBC abnormalities persisted in untreated, control mice (Figure 3B).
In vivo selection results in significant increases in HbF in mice transplanted with γ-globin/MGMT lentiviral vector transduced β-thalassemic BM cells. (A) Cellulose acetate gel electrophoresis of RBC lysates from individual mice transplanted with V5-Mp-MGMT vector-transduced β-thalassemic cells (top panel) or V5-EF1-MGMT vector-transduced β-thalassemic cells (bottom panel) and either not treated or administered drug treatment as indicated in the Figure 1 legend. Vertical lines have been inserted to indicate a repositioned gel lane. (B) HbF levels as determined by quantitative HPLC are shown for individual mice transplanted with V5-EF1-MGMT–transduced β-thalassemic cells and left untreated or after drug selection, as indicated. Data represent the mean ± SEM. Statistically significant differences are indicated by the P value, as determined by the Student t test.
In vivo selection results in significant increases in HbF in mice transplanted with γ-globin/MGMT lentiviral vector transduced β-thalassemic BM cells. (A) Cellulose acetate gel electrophoresis of RBC lysates from individual mice transplanted with V5-Mp-MGMT vector-transduced β-thalassemic cells (top panel) or V5-EF1-MGMT vector-transduced β-thalassemic cells (bottom panel) and either not treated or administered drug treatment as indicated in the Figure 1 legend. Vertical lines have been inserted to indicate a repositioned gel lane. (B) HbF levels as determined by quantitative HPLC are shown for individual mice transplanted with V5-EF1-MGMT–transduced β-thalassemic cells and left untreated or after drug selection, as indicated. Data represent the mean ± SEM. Statistically significant differences are indicated by the P value, as determined by the Student t test.
Amelioration of the anemia and RBC morphologic abnormalities of β-thalassemia after in vivo selection of mice transplanted with γ-globin/MGMT lentiviral vector-transduced cells. (A) Hb levels in the indicated groups of control, untreated and drug-treated animals, both before and after drug administration. Values are also shown for mice transplanted with mock-transduced β-thalassemic cells at 32 weeks after transplantation. Data represent the mean plus or minus SEM. Statistically significant differences are indicated by the P values, as determined by the Student t test. (B) Wright-Giemsa–stained blood smears are shown for a representative treated and untreated animal transplanted with V5-EF1-MGMT-transduced β-thalassemic cells, as indicated. Photomicrographs were generated using an Olympus microscope (model BX60F-3; Tokyo, Japan) and an Olympus digital camera (model DP71), 100×/1.3 NA oil objective.
Amelioration of the anemia and RBC morphologic abnormalities of β-thalassemia after in vivo selection of mice transplanted with γ-globin/MGMT lentiviral vector-transduced cells. (A) Hb levels in the indicated groups of control, untreated and drug-treated animals, both before and after drug administration. Values are also shown for mice transplanted with mock-transduced β-thalassemic cells at 32 weeks after transplantation. Data represent the mean plus or minus SEM. Statistically significant differences are indicated by the P values, as determined by the Student t test. (B) Wright-Giemsa–stained blood smears are shown for a representative treated and untreated animal transplanted with V5-EF1-MGMT-transduced β-thalassemic cells, as indicated. Photomicrographs were generated using an Olympus microscope (model BX60F-3; Tokyo, Japan) and an Olympus digital camera (model DP71), 100×/1.3 NA oil objective.
Increased levels of HbF protein per expressing RBC after in vivo selection
We hypothesized that an additional benefit of drug selection might be enrichment of cells that express higher levels of γ-globin per cell. This could occur if cells containing transcriptionally permissive vector integration sites were enriched by drug selection. This, coupled with elimination of nontransduced cells as well, might result in a population of γ-globin–expressing cells with an overall mean higher level HbF per vector copy. To evaluate this possibility, we compared the amount of HbF protein per expressing RBC in each animal of the V5-EF1-MGMT vector group before and after completing the courses of drug treatment. Of the 8 V5-EF1-MGMTmice that demonstrated increases in the percentage of red cells expressing γ-globin, 4 showed an increase in the amount of HbF per γ-globin–positive cell, 3 showed no change in the amount of HbF per cell, and one animal showed a decrease in HbF per cell (Figure 4). In the mice in which HbF protein per expressing cell increased, we infer that the increase was not the result of an increase in copy number per cell, as secondary spleen colony-forming cells (CFU-S) assays performed with BM from both untreated and drug-treated mice showed clones containing only one or 2 vector copies per cell (see “In vivo drug selection enriches vector-transduced HSCs” and Figure S5).
In vivo selection results in an increase in the amount of HbF per expressing RBCs. HbF per positive cell (pg/cell) is shown for individual mice transplanted with V5-EF1-MGMT vector–transduced β-thalassemic BM cells both before (■) and after (▨) drug administration.
In vivo selection results in an increase in the amount of HbF per expressing RBCs. HbF per positive cell (pg/cell) is shown for individual mice transplanted with V5-EF1-MGMT vector–transduced β-thalassemic BM cells both before (■) and after (▨) drug administration.
In vivo drug selection enriches vector-transduced HSCs
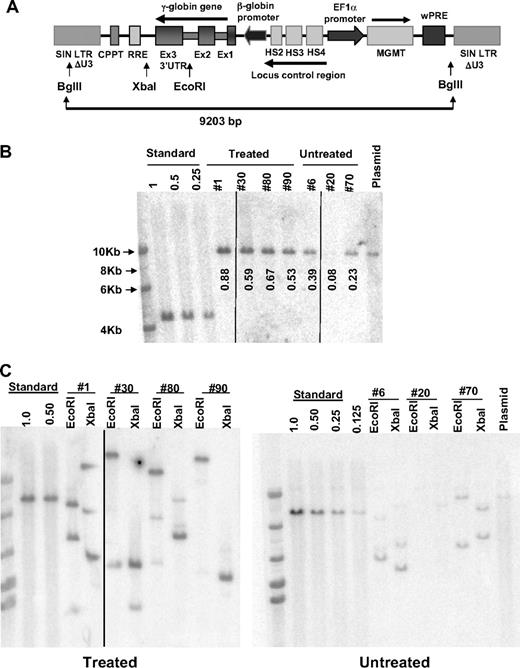
To estimate the fraction of vector-containing HSCs after the 3 courses of drug treatment at 31 weeks after transplantation, BM cells from 4 mice in the drug-treated, V5-EF1-MGMT group and from 3 mice in the untreated, control group were transplanted into new, lethally irradiated recipients for secondary CFU-S and for secondary long-term analysis (see below). An aliquot of these BM cells was also used to prepare genomic DNA. Two weeks later, CFU-S were obtained from the transplanted mice and DNA extracted for Southern blot analysis to determine the presence of and number of vector copies in each CFU-S clone. CFU-S are primitive cells contained in the multipotential progenitor population, which is directly fed by the HSC pool.26 When BM DNAs from both the primary treated and untreated animals were digested with BglII, which cuts twice in the integrated provirus, a single band of the expected size was observed on Southern blot analysis (Figure 5A,B). The intensity of the band, relative to vector copy standards, was consistent with most of the BM cells in the animals of the drug-treated group containing vector (average vector copy number [VCN] = 0.67). In comparison, all 3 mice from the untreated group had lower levels of vector-containing cells (average VCN = 0.23). Consistent with these data, 69% (25 of 39) of CFU-S derived from the drug-treated mice contained the V5-EF1-MGMT vector, whereas only 15% (5 of 33) of the CFU-S from the untreated, control group were vector positive (Figure S5). These data, along with that of the long-term secondary recipients described in “Sustained increases in F cells and Hb level in long-term secondary transplant recipients of BM from primary, drug-treated donor mice,” strongly support selection at the HSC level. Analysis of BM and CFU-S DNA using enzymes that cut only once in the provirus, which generates a unique vector-genome junction fragment for each vector insertion, showed the presence of a limited number of unique CFU-S clones, each containing only 1 or 2 vector copies, in both drug-treated and untreated control mice (Figures 5C, S5).
Increased levels of vector-transduced HSCs after in vivo selection of mice transplanted with γ-globin/MGMT lentiviral vector-transduced cells. (A) Schematic diagram of the γ-globin/MGMT lentiviral vector showing the pertinent restriction enzyme sites used in the Southern blot analyses. (B) Southern blot analysis of genomic DNA, cut with BglII, from the BM of individual mice (indicated by mouse number) transplanted with V5-EF1-MGMT–transduced β-thalassemic BM cells. DNA size ladder is shown in the leftmost lane, whereas the vector plasmid DNA as a positive control is shown in the last lane (plasmid). Numbers below each lane represent the vector copy number as determined by densitometry, relative to the “Standard,” which is DNA from a K562 clone that contains a single copy of an integrated GFP lentiviral vector (1.0). Dilutions of this DNA with naive K562 DNA to establish samples with vector copy numbers of 0.5 and 0.25. Vertical lines have been inserted to indicate a repositioned gel lane. (C) Southern blot analysis of genomic DNA, cut with EcoRI or XbaI as indicated, from the BM of individual mice (indicated by mouse number) transplanted with V5-EF1-MGMT-transduced β-thalassemic BM cells, as indicated. These enzymes cut once within the provirus; therefore, each band reflects a unique host genomic-vector junction fragment. Vertical lines have been inserted to indicate a repositioned gel lane.
Increased levels of vector-transduced HSCs after in vivo selection of mice transplanted with γ-globin/MGMT lentiviral vector-transduced cells. (A) Schematic diagram of the γ-globin/MGMT lentiviral vector showing the pertinent restriction enzyme sites used in the Southern blot analyses. (B) Southern blot analysis of genomic DNA, cut with BglII, from the BM of individual mice (indicated by mouse number) transplanted with V5-EF1-MGMT–transduced β-thalassemic BM cells. DNA size ladder is shown in the leftmost lane, whereas the vector plasmid DNA as a positive control is shown in the last lane (plasmid). Numbers below each lane represent the vector copy number as determined by densitometry, relative to the “Standard,” which is DNA from a K562 clone that contains a single copy of an integrated GFP lentiviral vector (1.0). Dilutions of this DNA with naive K562 DNA to establish samples with vector copy numbers of 0.5 and 0.25. Vertical lines have been inserted to indicate a repositioned gel lane. (C) Southern blot analysis of genomic DNA, cut with EcoRI or XbaI as indicated, from the BM of individual mice (indicated by mouse number) transplanted with V5-EF1-MGMT-transduced β-thalassemic BM cells, as indicated. These enzymes cut once within the provirus; therefore, each band reflects a unique host genomic-vector junction fragment. Vertical lines have been inserted to indicate a repositioned gel lane.
Vector insertion site analysis in CFU-S clones
Vector insertion sites were identified and analyzed from both the BM cells of the primary recipients and from secondary CFU-S clones. Vector insertions were mapped by LAM-PCR in 4 drug-treated and 2 untreated control V5-EF1-MGMT mice. Table S1 summarizes the vector insertion sites. We found no insertions into “worrisome” genes in either set of animals. None of the targeted genes appears in the Sanger Cancer Gene Census (http://www.sanger.ac.uk/genetics/CGP/Census), a database of known oncogenes. Interestingly, we identified the same identical vector insertion representing a unique HSC clone in 2 different treated mice, clones 30-B and 80-A (Table S1). This probably represents an HSC self-renewal division that occurred in vitro, with progeny HSCs subsequently engrafting in 2 different recipients that received grafts from the same pool of transduced cells. In this clone, the vector inserted into intron 5 of the Tial1 gene, which is an RNA-binding protein that may function as a nucleolysin in cytotoxic T lymphocytes. In addition, the Rgs10 and Bag3 genes, encoding a G-protein regulator and a Bcl-2 interacting death suppressor, respectively, reside 30 kb 5′ (former) and 76 kb 3′ (latter) from the vector insertion. It is unclear whether insertional gene dysregulation played a role in the outgrowth of this particular HSC clone in vitro or whether the presence of the clone in 2 animals is the outcome of a stochastic process. However, the fact that untreated mice did not show increases in transduced cells, as measured by both γ-globin expression and VCN, argues against clonal outgrowth as a mechanism for “in vivo selection.”
Sustained increases in F cells and Hb level in long-term secondary transplant recipients of BM from primary, drug-treated donor mice
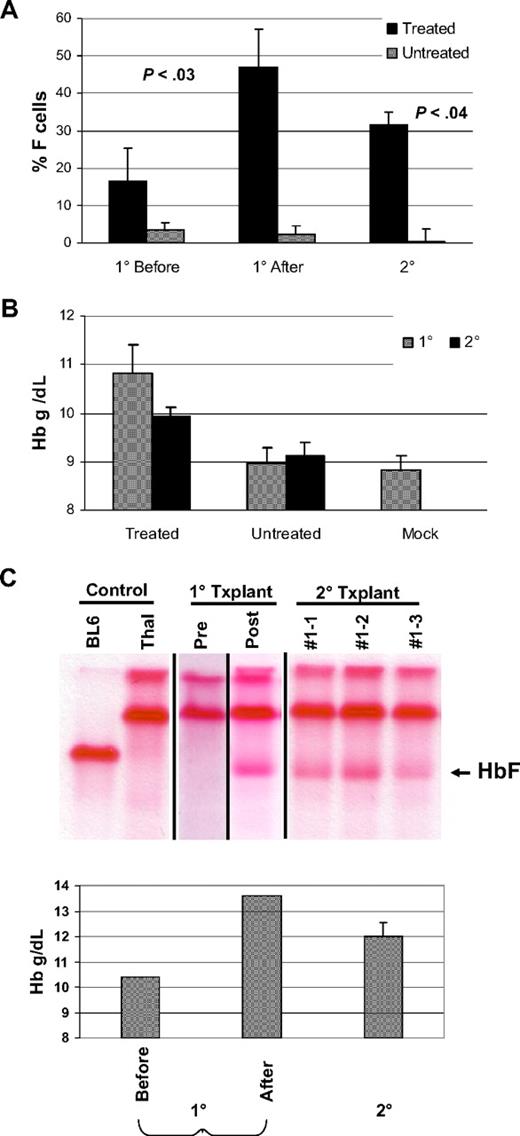
BM cells from drug-treated and control untreated mice were transplanted into secondary, wild-type recipients to further substantiate whether transduced, long-term repopulating hematopoietic cells had been selected by the drug treatment in the primary mice. Secondary recipient animals received no drug treatment and were analyzed 12 to 14 weeks after transplantation. F cells and HbF remained increased, relative to the low levels observed in secondary mice transplanted with BM from the untreated, control animals (Figure 6A,C). Similarly, Hb levels remained improved compared with control and mock-transplanted mice (Figure 6B,C). These data, along with the secondary CFU-S data in “In vivo drug selection enriches vector-transduced HSCs” strongly support that selection of transduced HSCs occurred as a consequence of drug treatment. This is consistent with the our previous data and that of others indicating that HSCs can be selected with the MGMT system.17,19,27-29
Secondary transplantation demonstrates sustained, increased levels of F cells, HbF, and Hb. (A) The percentages of F cells in control, untreated, and treated V5-MSCV-MGMT animal groups (as indicated), both at baseline (1° Before) and after (1° After) drug treatment are shown. Also shown are the values in secondary transplantation recipients (2°) of BM from control, untreated, and drug-treated animals. The levels of F cells in the mice after treatment and in secondary recipients derived from them were statistically different from the baseline values before treatment as determined by the Student t test. P values are shown for each, compared with the baseline value. (B) The Hb levels in 1° and 2° control (untreated) and treated animal groups (as indicated) are shown. Also shown is the mean Hb level in animals transplanted with mock-transduced cells. Data represent the mean plus or minus SEM. (C) Top panel: cellulose acetate gel electrophoresis of RBC lysates from a drug-treated, V5-EF1-MGMT animal before (Pre) and after (Post) BG/BCNU administration and from 2° transplantation recipients derived from this primary mouse. The samples of the first 2 control lanes and the 2° transplantation lanes were run on one gel as indicated by the vertical lines. Pre and post samples in the 1° transplantation lanes were run on different gels at different times because samples could not be preserved intact for 20 weeks. Bottom panel: Hb levels of the primary animal before (Pre) and after (Post) drug treatment and of 2° transplantation recipients derived from the primary animal.
Secondary transplantation demonstrates sustained, increased levels of F cells, HbF, and Hb. (A) The percentages of F cells in control, untreated, and treated V5-MSCV-MGMT animal groups (as indicated), both at baseline (1° Before) and after (1° After) drug treatment are shown. Also shown are the values in secondary transplantation recipients (2°) of BM from control, untreated, and drug-treated animals. The levels of F cells in the mice after treatment and in secondary recipients derived from them were statistically different from the baseline values before treatment as determined by the Student t test. P values are shown for each, compared with the baseline value. (B) The Hb levels in 1° and 2° control (untreated) and treated animal groups (as indicated) are shown. Also shown is the mean Hb level in animals transplanted with mock-transduced cells. Data represent the mean plus or minus SEM. (C) Top panel: cellulose acetate gel electrophoresis of RBC lysates from a drug-treated, V5-EF1-MGMT animal before (Pre) and after (Post) BG/BCNU administration and from 2° transplantation recipients derived from this primary mouse. The samples of the first 2 control lanes and the 2° transplantation lanes were run on one gel as indicated by the vertical lines. Pre and post samples in the 1° transplantation lanes were run on different gels at different times because samples could not be preserved intact for 20 weeks. Bottom panel: Hb levels of the primary animal before (Pre) and after (Post) drug treatment and of 2° transplantation recipients derived from the primary animal.
Pretransplantation in vitro selection of HSCs transduced with the V5-EF1-MGMT vector facilitates engraftment of therapeutic levels of γ-globin–expressing cells
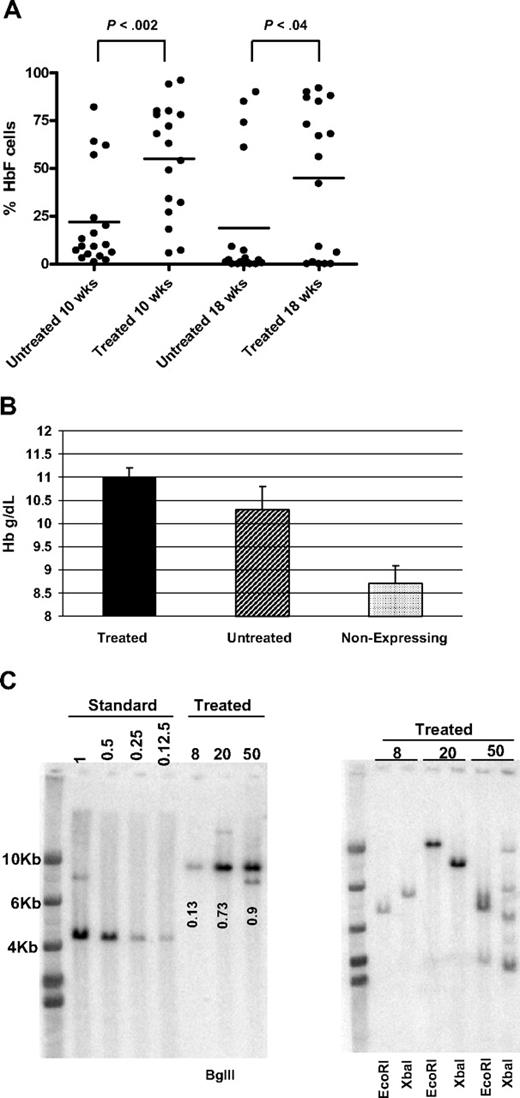
Because our data showed that HSCs could be selected with the V5-EF1-MGMT vector in vivo, we next tested whether selection of transplantable, transduced HSCs was possible during ex vivo culture before transplantation. If feasible, this might allow posttransplantation drug administration to be reduced or eliminated. Lineage-negative β-thalassemic BM cells were transduced with the V5-EF1-MGMT vector and either transplanted into lethally irradiated mice (n = 18) or maintained in culture for an additional 36 hours to allow for MGMT transgene expression. Cells were then treated with BG/BCNU in vitro followed by transplantation (n = 17). Both cohorts of mice were analyzed at 10 and 18 weeks after transplantation. Animals that received transplantations of cells pretreated with BG/BCNU had a significantly higher mean level of γ-globin–expressing cells, compared with the mice that received transduced, untreated cells at both time points (Figure 7A). Only 4 of 18 mice (22%) that received untreated cells had 40% or greater F cells, a level that could be therapeutic,30 whereas 10 of 17 mice (59%) achieved this level in the group of mice that received treated cells. When we evaluated the Hb level in animals that demonstrated the presence of vector-expressing cells, both groups showed an improvement in Hb level compared with mice that lacked γ-globin–expressing cells, with the group receiving pretreated cells demonstrating the greatest improvement (Figure 7B). Analysis of BM DNA from 3 of the treated mice demonstrated an average VCN of 0.59, with a range of 0.13 to 0.9. DNA restricted with an enzyme that cuts once in the vector genome demonstrated that a single clone was selected in 2 animals, whereas a third animal had bands consistent with several clones (Figure 7C). Analysis of secondary CFU-S from the mice with the higher BM VCNs showed that all 24 CFU-S clones studied were vector positive (data not shown). These data show that transduced HSCs can be enriched in culture before transplantation using the MGMT system, at least in a model where adequate numbers of HSCs remain after selection.
In vitro selection of transduced cells before transplantation results in increased engraftment of γ-globin–expressing cells and improved Hb levels. (A) The percentage of HbF-positive cells at 10 and 18 weeks after transplantation, as assessed by FACS, is shown for mice receiving transplantations of either untreated (n = 18) or drug-treated (n = 17) lineage-negative BM cells. P values of Student t test are shown. (B) The mean Hb levels of mice receiving transplantations of untreated or drug-treated BM cells are shown. As a control, the mean Hb level of all mice from both groups that had no γ-globin–expressing cells is shown. One-way analysis of variance with Bonferroni multiple comparison showed that Hb levels of treated and untreated groups significantly differed from the Hb level of mice that lacked γ-globin expression (P < .001 and P < .05, respectively). The difference between treated and untreated groups did not reach statistical significance. Data are expressed as mean ± SEM. (C) Vector copy number determination in animals receiving in vitro selected cells. Left panel: Southern blot analysis using BglII-digested BM DNA from the indicated animals. Left lanes indicate the copy number standards, as described in Figure 5 legend. Numbers indicate the VCN, as determined by densitometry, relative to the copy number standards. In addition to the hybridizing band of correct size, animal 50 also shows a second band that is smaller and probably represents vector containing a small deletion. Right panel: Southern blot analysis of BM DNA from the indicated animals, digested with the enzymes as labeled.
In vitro selection of transduced cells before transplantation results in increased engraftment of γ-globin–expressing cells and improved Hb levels. (A) The percentage of HbF-positive cells at 10 and 18 weeks after transplantation, as assessed by FACS, is shown for mice receiving transplantations of either untreated (n = 18) or drug-treated (n = 17) lineage-negative BM cells. P values of Student t test are shown. (B) The mean Hb levels of mice receiving transplantations of untreated or drug-treated BM cells are shown. As a control, the mean Hb level of all mice from both groups that had no γ-globin–expressing cells is shown. One-way analysis of variance with Bonferroni multiple comparison showed that Hb levels of treated and untreated groups significantly differed from the Hb level of mice that lacked γ-globin expression (P < .001 and P < .05, respectively). The difference between treated and untreated groups did not reach statistical significance. Data are expressed as mean ± SEM. (C) Vector copy number determination in animals receiving in vitro selected cells. Left panel: Southern blot analysis using BglII-digested BM DNA from the indicated animals. Left lanes indicate the copy number standards, as described in Figure 5 legend. Numbers indicate the VCN, as determined by densitometry, relative to the copy number standards. In addition to the hybridizing band of correct size, animal 50 also shows a second band that is smaller and probably represents vector containing a small deletion. Right panel: Southern blot analysis of BM DNA from the indicated animals, digested with the enzymes as labeled.
Discussion
Successful gene therapy for patients with β-thalassemia will require high-level, erythroid-specific globin transgene expression in a substantial number of stem cell–derived erythroid progeny. Based on experimental and clinical evidence, a significant clinical benefit would probably require an HSC gene transfer level of 15% to 30%, depending on the β-thalassemia genotype (β+ or β0, respectively), which dictates the degree of β-globin protein deficiency and disease severity.31-34 Here, we show that amelioration of a murine model of severe β-thalassemia intermedia can be achieved through in vivo drug selection using a dual γ-globin/MGMT vector. Using a relatively low MOI transduction protocol designed to achieve low, nontherapeutic levels of globin vector–transduced cells that might occur in a human clinical trial, we showed that therapeutic levels of transduced cells could be achieved in 14 of 17 mice after drug treatment to eliminate nontransduced and nonexpressing cells. Control cohorts of mice transplanted with the same pool of transduced cells but that did not receive drug treatment showed no increase in transduced cells. Others have observed successful in vivo selection using nontherapeutic, single-gene γ-retroviral or foamy viral marking vectors and low MOI for HSC transduction to result in initial low levels of transduced cells.35,36 Chang et al, using a lentiviral vector encoding both human factor IX and MGMT driven by the cellular PGK promoter, showed that in vivo selection could be used to increase factor IX levels from very low levels to those that would be curative.37 However, in this study, the VCN reached 2.3 after therapeutic selection, consistent with some clones having multiple vector copies. No experiments were done to evaluate the VCN in transduced CFU-S clones. In contrast to these data, selection with our vector, which used an EF1α-MGMT cassette, resulted in therapeutic selection with a VCN of less than one. Analysis of CFU-S clones documented that VCN in transduced clones was one or 2 copies. Because the EF1α promoter is more transcriptionally active than the PGK promoter in both primitive mouse and human hematopoietic cells,38,39 this may account for the difference in the VCN after selection in the 2 studies. More copies of vector containing the PKG-MGMT cassette may have been needed to provide drug resistance than in the case of our vector using the EF1a-MGMT cassette. Recent data from Milsom et al also suggest that the EF1a promoter is an optimal promoter to avoid issues of toxicity that can be associated with overexpressed MGMT, which can occur when strong transcriptional regulatory elements, such as strong spleen focus-forming viral enhancer/promoter, are used.40 All of these data together suggest that the EF1α promoter could be a better choice to drive MGMT selection in HSCs, so to minimize selection of higher copy clones as well as HSC toxicity.
We obtained significant improvement of the Hb level with an average VCN of 0.7 after in vivo selection. This compares favorably to the globin VCNs obtained by other groups and ourselves using single-gene globin vectors, which led to resolution of the anemia in the same or similar β-thalassemia models (May et al, 0.82 ; Imren et al, 3.05 ; Hanawa et al, 1.322 ; and Miccio et al, 0.830 ). Because such high levels of gene transfer with a single-gene vector are not realistic in humans, our data demonstrate the feasibility of ultimately achieving a therapeutic proportion of transduced HSCs using in vivo selection. In several instances, vector-expressing cells increased from levels approximately 10% or less to levels in excess of 80%, with up to 50-fold increases observed after drug treatment. Much higher overall levels of F cells were observed after completion of drug treatment in the V5-EF1-MGMT group compared with the V5-Mp-MGMT group (86% vs 47%); however, the starting baseline levels in the former were also higher (24% vs 11%; Figure 1B). Others have also observed that more efficient in vivo selection occurs when there is an initial threshold number of vector-expressing cells.35 Thus, it is difficult to conclude whether the EF1α-MGMT-wPRE–containing vector is superior to the MSCV-MGMT-based vector. However, given recent genotoxicity studies from our and other groups, the cellular EF1α promoter offers an enhanced safety profile.39,41
After in vivo selection, we observed approximately a 2-fold higher level of Hb F protein per expressing cell in 4 of 8 mice in the V5-EF1-MGMT transplanted group, whereas 3 remained stable and one animal showed a decrease. The results in the former suggest that MGMT and γ-globin expression were linked, despite being driven by different promoters, which are active in different compartments and developmental stages of hematopoiesis. The increase in HbF per expressing cell was probably not attributable to an increase in vector copy per cell as DNA analysis showed that the selected clones contributing to hematopoiesis after drug selection harbored only one or 2 vector copies (Figure S5). Our observation is consistent with previous in vitro work, which demonstrated that MGMT expression per cell increased 8-fold after 2 rounds of drug treatment in the absence of selecting high copy number clones.42 However, this is the first in vivo evidence that, in some cases, selecting for cells with a more chemoresistant phenotype (ie, higher MGMT expression) can lead to increased expression of a linked gene. We think that this is probably the result of elimination by drug treatment of cells containing vector insertions with poor expression because of an unfavorable genomic location, with some contribution also from the elimination of nontransduced cells.
In this study, the dual gene vector expressed 9.9% (± 1.4%) HbF per vector copy, whereas previously we observed 17% HbF per vector copy with the single-gene mLARV5 vector, containing the same γ-globin cassette. However, the method of HbF measurement in that study was different and not as precise as HPLC, thus making the 2 studies difficult to directly compare. In contrast, using Hb output per VCN, which is a common method of expressing vector potency,6,43 we found a 3.3 g/dL per vector copy improvement in the Hb level in the treated mice, relative to mock control group. This value compares favorably with that we previously observed using our single-gene vector in this model (Hanawa et al,22 2 g/dL per vector copy) and also to those observed by others using β-globin lentiviral vectors and similar mouse models (May et al, 3.8 g/dL2 ; Imren et al, 1.5 g/dL5 ; Rivella et al, 2.3 g/dL43 ). Although this level of HbF per cell was adequate to significantly improve this model of severe β-thalassemia intermedia, a higher level of γ-globin transgene expression may be required to improve β-thalassemia major. Promoter interactions in bi-promoter vectors are well documented in the literature, and recently one group has shown that placement of an insulator between 2 nearby expression cassettes in a lentiviral vector can reduce negative interactions.44 We are currently testing whether inclusion of an insulator between the 2 expression cassettes improves globin output per vector copy.
There are several implications of our data with respect to the potential safety of this approach. We did not find that vivo selection selected for high-copy-number clones. If clones with higher copy numbers were selected for, the risk of insertional mutagenesis may be increased because the likelihood of such appears to increase with copy number.45 Similarly, Zielske et al, using the K562 cell line, also found that strong BG/BCNU selective pressure did not result in the preferential survival of clones with increased copy numbers.42 In other work using the same β-thalassemia mouse model, we recently found that our single-gene globin vector, although capable in some instances of altering expression of gene neighboring the insertion site, did not result in clonal dominance or altered hematopoiesis.25 Thus, coupling the γ-globin expression cassette with the MGMT system would not seem to necessarily increase the insertional genotoxicity risk. However, ultimate assessment of the safety and efficacy of the MGMT selection system will require testing in a nonhuman primate model before clinical translation in humans.
We also observed that selection of HSCs was as successful using an EF1α-driven MGMT-wPRE cassette as it was using an MSCV-MGMT cassette. Because the EF1α promoter appears to have a lower propensity to disturb the normal transcriptional pattern of genes near its integration site,39,41 we think that this validation of its activity in the primitive cells of mice provides a strong foundation and rationale to evaluate its HSC activity in a large animal model. In this study, we also observed a limited number of clones contributing to hematopoiesis in both the treated and untreated groups. These results agree with those of others in an reemerging consensus that, in these mouse models of HSC gene transfer, oligoclonality is a common finding and not necessarily indicative of insertional mutagenesis.30 Indeed, an early study demonstrated monoclonality or oligoclonality as the norm in mouse gene transfer studies.46
Using a different approach, we found that we could enrich γ-globin/MGMT vector-transduced HSCs before transplantation by briefly exposing the cells to BG/BCNU in vitro. Mice transplanted with such drug-treated cells showed increased levels of F cells, compared with animals that received transduced but untreated cells. Pretreating the transduced cell graft further improved the resolution of the anemia, relative to that of animals transplanted with untreated transduced or mock transduced cells. Pretransplantation selection resulted in levels of γ-globin–expressing cells in some animals that were similar to those obtained in experiments in which animals received 3 courses of posttransplantation drug administration. Thus, this approach could reduce the toxicity of the selection process by reducing or eliminating the need for posttransplantation drug selection. Although this approach was successful in mice, which can be reconstituted with as few as one HSC, further work is needed to determine whether this strategy can be successful in large animals because it is probable that significant stem cell depletion occurs in the graft after drug treatment. An attractive option would be to couple in vitro selection with in vitro HSC expansion to provide an adequate transplantation graft. Future experiments will focus on evaluating new cytokines47,48 or recombinant growth-promoting molecules49 for the ability to expand selected HSCs in vitro before transplantation.
In conclusion, these studies demonstrate the proof of principle that a globin vector containing an MGMT cassette can be produced with clinically relevant titers and used to increase a small population of globin-expressing cells to therapeutic levels in a model of β-thalassemia. Further work will be needed in a large animal transplantation model to evaluate the feasibility of this strategy for eventual use in patients with hemoglobin disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Brian Sorrentino and Arthur W. Nienhuis for critical reading of the manuscript and useful discussion, the laboratory of Dr Dennis Jay for performing the HPLC analysis of red cell lysates, the Flow Cytometry Core Facility at St Jude Children's Research Hospital (SJCRH), and the staff of the Animal Resource Center of SJCRH for animal care and experimental analysis.
This work was supported in part by the National Heart, Lung, and Blood Institute (NHLBI) Comprehensive Sickle Cell Center (grant U54 HL070590), NHLBI Program Project (PO1HL053749), NHLBI Basic and Translational Research Program for Sickle Cell Disease (grant U54 HL070590-06), SJCRH Cancer Center Support (Core; grant CA-21765), and the American Lebanese Associated Charities.
National Institutes of Health
Authorship
Contribution: H.Z. designed research, performed research, collected, analyzed, and interpreted data, and helped write the manuscript; T.I.P., M.N., and P.W.H. performed research; P.M. performed bioinformatics analysis; D.A.P. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Derek A. Persons, St Jude Children's Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105; e-mail: derek.persons@stjude.org.