Adult hematopoietic stem cells (HSCs) are routinely used to reconstitute hematopoiesis after myeloablation; however, transplantation efficacy and multilineage reconstitution can be limited by inadequate HSC number, or poor homing, engraftment, or self-renewal. Here we report that mouse and human HSCs express prostaglandin E2 (PGE2) receptors, and that short-term ex vivo exposure of HSCs to PGE2 enhances their homing, survival, and proliferation, resulting in increased long-term repopulating cell (LTRC) and competitive repopulating unit (CRU) frequency. HSCs pulsed with PGE2 are more competitive, as determined by head-to-head comparison in a competitive transplantation model. Enhanced HSC frequency and competitive advantage is stable and maintained upon serial transplantation, with full multilineage reconstitution. PGE2 increases HSC CXCR4 mRNA and surface expression, enhances their migration to SDF-1 in vitro and homing to bone marrow in vivo, and stimulates HSC entry into and progression through cell cycle. In addition, PGE2 enhances HSC survival, associated with an increase in Survivin mRNA and protein expression and reduction in intracellular active caspase-3. Our results define novel mechanisms of action whereby PGE2 enhances HSC function and supports a strategy to use PGE2 to facilitate hematopoietic transplantation.
Introduction
Hematopoietic stem cell (HSC) transplantation with bone marrow, mobilized peripheral blood, or umbilical cord blood (UCB) is a proven therapy for malignant and nonmalignant hematologic diseases and metabolic disorders. Repopulation of hematopoiesis is a multistep process that can be adversely affected by the inability of HSCs to migrate/home to appropriate marrow niches or poor engrafting efficiency and self-renewal. Insight into the intrinsic and extrinsic mechanisms regulating these critical functions can lead to new strategies to improve HSC transplantation efficacy.
Prostaglandin E2 (PGE2) is the most abundant eicosanoid and a mediator of numerous physiological systems.1 We and others have demonstrated regulatory roles for PGE2 in hematopoiesis. PGE2 dose-dependently inhibits growth of human and colony-forming units granulocyte/macrophage (CFU-GM) in vitro2,3 and myelopoiesis in vivo4 but stimulates erythroid and multilineage progenitor cells.5,6 Short-term ex vivo treatment of marrow cells with PGE2 increases the proportion of mouse colony-forming units spleen (CFU-S)7 and human CFU-GM8 in cell cycle. In addition, PGE2 can stimulate production of cycling human CFU-GM from a population of quiescent cells, possibly stem cells, which is critically dependent on timing, duration of exposure, and concentration.9 Recently, it was shown that pulse exposure to PGE2 ex vivo increased HSC frequency of murine bone marrow cells and enhanced kidney marrow recovery in zebrafish.10 However, although it is clear that PGE2 can affect hematopoietic stem and progenitor cells, the mechanisms of action of PGE2 on stem cell function have yet to be determined.
In this report, we show that PGE2 acts directly on murine HSCs to enhance their frequency after transplantation and also provides a competitive advantage that is maintained during serial transplantation, with full multilineage reconstitution. Enhanced HSC engraftment induced by PGE2 results from increased homing of HSCs, mediated through up-regulation of the chemokine receptor CXCR4, implicated in HSC homing,11 and selective stimulation of primitive HSC survival and self-renewal associated with up-regulation of the inhibitor of apoptosis protein Survivin, required for HSC maintenance and entry into cell cycle.12,13 Our studies describe novel mechanisms for enhancement of HSC function by PGE2 and support a translational strategy to facilitate HSC transplantation.
Methods
Mice and human cord blood
C57Bl/6 (CD45.2) mice were purchased from Jackson Laboratories (Bar Harbor, ME). B6.SJL-PtrcAPep3B/BoyJ (BOYJ) (CD45.1), C57Bl/6 × BOYJ-F1 (CD45.1/CD45.2), and NOD.Cg-Prkdcscid IL2rgtm1Wjl/Sz (NS2) mice were bred in-house. Mice used in transplant studies received doxycycline feed for 30 days after transplantation. The Animal Care and Use Committee of IUSM approved all protocols. Human umbilical cord blood (UCB) was obtained from Wishard Hospital (Indianapolis, IN) with IUSM IRB approval.
Flow cytometry
All antibodies were purchased from BD Biosciences (BD, San Jose, CA) unless noted. For detection and sorting of KL and SKL cells, streptavidin conjugated with PE-Cy7 (to stain for biotinylated magnetic-activated cell separation [MACS] lineage antibodies; Miltenyi Biotec, Auburn, CA), c-kit-APC, Sca-1-PE or APC-Cy7, CD45.1-PE, CD45.2-FITC, and CD34-PE were used. For SLAM SKL, we used Sca-1-PE-Cy7, c-kit-FITC, CD150-APC (eBiosciences, San Diego, CA), CD48-biotin (eBiosciences), and streptavidin-PE. UCB CD34+ cells were detected using anti–human-CD34-APC. For multilineage analysis, APC-Cy7-Mac-1, PE-Cy7-B-220, and APC-CD3 were used. EP receptors were detected with rabbit anti-EP(1-4) antibodies (Cayman Chemicals, Ann Arbor, MI) and FITC-goat-anti–rabbit IgG (Southern Biotech, Birmingham, AL). CXCR4 expression was analyzed using streptavidin-PECy7, c-kit-APC, Sca-1-APC-Cy7, and CXCR4-PE. Apoptosis was measured with FITC–annexin-V or FITC-anti–active caspase-3. For Survivin and active caspase-3 detection, cells were permeabilized and fixed using the CytoFix/CytoPerm kit (BD) and stained with anti–active-caspase-3-FITC Flow Kit (BD) or Survivin-PE (R&D Systems, Minneapolis, MN). For cell-cycle analysis, cells were stained with Hoechst-33342 (Molecular Probes, Eugene, OR) and Pyronin-Y (Sigma-Aldrich, St Louis, MO) or FITC-BrdU Flow Kit (BD). Analyses were performed on an LSRII and sorting was performed on either a FACSAria or FACSVantage sorter (BD).
dmPGE2 pulse-exposure
16,16-Dimethyl prostaglandin E2 (dmPGE2) in methyl acetate (Cayman Chemicals) was evaporated on ice under N2, reconstituted in 100% ETOH at a final concentration of 0.1 M, and stored at −80°C. For pulse exposure, cells were incubated with dmPGE2 diluted in media, on ice, for 2 hours, with gentle vortexing every 30 minutes. After incubation, cells were washed twice in media before use. Vehicle-treated cells were processed in an identical manner, using the equivalent ETOH concentration.
Limiting dilution competitive and noncompetitive transplantation
Whole bone marrow (WBM) cells (CD45.2) were treated on ice for 2 hours with 1 μM dmPGE2 (Cayman Chemicals) or 1 × 10−3 % ETOH per 1 × 106 cells in PBS. After incubation, cells were washed twice and mixed with 2 × 105 congenic CD45.1 competitor marrow cells at various ratios and transplanted intravenously into lethally irradiated CD45.1 mice (1100-cGy split dose). Peripheral blood (PB) CD45.1 and CD45.2 cells were determined monthly by flow cytometry. For head-to-head competitive analysis, WBM from CD45.1 and CD45.2 mice was treated with vehicle or dmPGE2 and mixed with 2 × 105 competitor marrow cells from CD45.1/CD45.2 mice at various ratios and transplanted into lethally irradiated CD45.1/CD45.2 mice. The proportion of CD45.1, CD45.2, and CD45.1/CD45.2 cells in PB was determined monthly. HSC frequency was quantitated by Poisson statistics using L-CALC software (StemCell Technologies, Vancouver, BC) with less than 5% contribution to chimerism considered negative. Competitive repopulating units (CRUs) were calculated as described.14 For secondary transplantations, 2 × 106 WBM from CD45.1/CD45.2 mice that had previously undergone transplantation at a 1:1 ratio at 20 weeks after transplantation was injected into lethally irradiated CD45.1/CD45.2 mice in noncompetitive fashion.
Analysis of hematopoietic stem and progenitor cell homing
WBM from CD45.2 mice was labeled with 5-(and -6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes), washed, and treated with either 1 μM dmPGE2 or vehicle and 2 × 107 cells were transplanted into lethally irradiated CD45.2 mice. After 16 hours, femurs and tibias were flushed, Lin+ cells depleted using MACS microbeads, and Linneg cells stained for SKL and the total number of CFSE+ WBM, KL, and SKL cells was determined. For congenic homing studies, Linneg CD45.1 cells were treated with 1 μM dmPGE2, vehicle, or PBS and 2 × 106 cells transplanted into lethally irradiated CD45.2 mice. After 16 hours, CD45.1 SKL cells in recipient BM were quantitated. For competitive homing studies, Linneg cells from CD45.2 and CD45.1 mice were fluorescence-activated cell sorting (FACS) sorted and treated with dmPGE2 or vehicle, and 3 × 104 CD45.1 (vehicle or dmPGE2 treated) plus 3 × 104 CD45.2 (dmPGE2 or vehicle treated) SKL cells were transplanted into lethally irradiated CD45.1/CD45.2 mice. To evaluate the role of CXCR4 in homing, Linneg CD45.2 cells were treated with vehicle or 1 μM dmPGE2 plus 10 μM AMD3100 (AnorMed, Vancouver, BC), 2 × 106 treated cells injected into lethally irradiated CD45.1 mice, and homed SKL cells analyzed 16 hours after transplantation. Homing of human UCB cells was evaluated in NS2 mice. UCB mononuclear cells were isolated on Ficoll-Paque Plus (Amersham Biosciences, Piscataway, NJ) and treated with either dmPGE2 or vehicle, and 4 × 107 cells were transplanted into each of 5 sublethally irradiated (250 cGy) mice. Homed CD34+ cells were analyzed 16 hours after transplantation.
Expression of EP receptors, CXCR4, and Survivin
Linneg marrow cells were stained for SKL, SLAM, or CD34, and each of the 4 EP receptors, and surface receptor expression on KL, SKL, SLAM SKL, and CD34neg SKL cells was determined by FACS. For human EP receptors, UCB CD34+ cells were positively selected with MACS microbeads12 and stained for CD34 and CD38 and each of the EP receptors, and surface receptor expression was determined by FACS. To evaluate CXCR4, Survivin, and active caspase-3, Linneg cells or CD34+ UCB was treated on ice with either 1 μM dmPGE2 or vehicle control for 2 hours, washed, and then cultured in RPMI-1640/10% HI-FBS at 37°C for 24 hours, stained for SKL (murine cells) and CXCR4, Survivin, and/or active caspase-3 as described earlier, and analyzed by FACS.
Migration assays
Chemotaxis to SDF-1 was determined using a 2-chamber Costar Transwell (6.5-mm diameter, 5-μm pore; Cambridge, MA) as previously described.15 Briefly, dmPGE2- and vehicle-treated Linneg bone marrow cells were cultured in RPMI/10% HI-FBS overnight to allow for up-regulation of CXCR4, washed, resuspended at 2 × 106 cells/mL in RPMI/0.5% BSA (0.1 mL was added to the top chamber of the transwells, with or without rmSDF-1α [R&D Systems] in the bottom and/or top chamber), and incubated for 4 hours at 37°C. Cells completely migrating to the bottom chamber were enumerated by flow cytometry. Percentage migration was calculated by dividing total cells migrated to the lower well by the cell input multiplied by 100. SKL cell migration was determined by comparison of the proportion of SKL cells in input and migrated populations. For UCB migration, CD34+ cells were MACS selected as described and migration assays performed as described for mouse, using rhSDF-1α (R&D Systems).
Cell-cycle analysis
For in vitro cell-cycle analysis, Linneg cells were treated with either 1 μM dmPGE2 or vehicle and cultured in Stem Cell Pro Media (StemCell Technologies) with rmSCF (50 ng/mL) (R&D Systems), rhFlt-3, and rhTPO (100 ng/mL each) (Immunex, Seattle, WA). After 20 hours, cells were stained for SLAM SKL, fixed, permeabilized, and stained with Hoechst-33342 followed by Pyronin-Y. The proportion of SLAM SKL cells in G0, G1, S, and G2/M phase was determined by FACS quantitation of DNA and RNA. For in vivo cell-cycle analysis, CD45.2 mice were lethally irradiated and received a transplant of 5 × 106 dmPGE2- or vehicle-treated Linneg CD45.1 cells. Recipient mice received 1 mg/mL BrdU (Sigma-Aldrich) in drinking water and 1 mg/mouse BrdU intraperitoneally. After 16 hours, recipient marrow was isolated, lineage depleted, and stained for CD45.1, SKL, and BrdU, and the proportion of homed (CD45.1+) SKL cells that were BrdU+ was determined by FACS.
Apoptosis assays
Linneg cells were treated with 1 μM dmPGE2 or vehicle, and incubated in RPMI/2% HI-FCS at 37°C without growth factors. After 24 hours, the cells were stained for SLAM SKL and annexin-V or active caspase-3, and the proportion of apoptotic cells was determined by FACS. For dose-ranging studies, cells were cultured as described using a dose range of 0.1 nM to 1 μM dmPGE2 or vehicle control.
Quantitative-RT-PCR
Total RNA was obtained using the absolute RNA purification kit (Stratagene, La Jolla, CA). A constant amount of RNA was reverse transcribed with random primers (Promega, Madison, WI) and Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega) as described.12 DNase- and RNase-free water (Ambion, Austin, TX) was added to obtain a final concentration equivalent of 10 ng RNA/μL and 5 μL used for quantitative reverse-transcription–polymerase chain reaction (QRT-PCR). Primers for SYBR Green QRT-PCR were designed to produce an amplicon size of 75 to 150 bp. Sequences of primers are listed in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). QRT-PCR was performed using Platinum SYBR Green qPCR supermix UDG with ROX (Invitrogen, Carlsbad, CA) in an ABI-7000 (Applied Biosystems, Carlsbad, CA) or MxPro-3000 (Agilent, LaJolla, CA). Dissociation curves were determined on each analysis to confirm that only one product was obtained.
Statistical analysis
All pooled values are expressed as mean plus or minus SEM. Statistical differences were determined using the paired or unpaired 2-tailed t test function in Microsoft Excel (Redmond, WA) as appropriate.
Results
PGE2 increases LTR-HSC frequency and engraftment
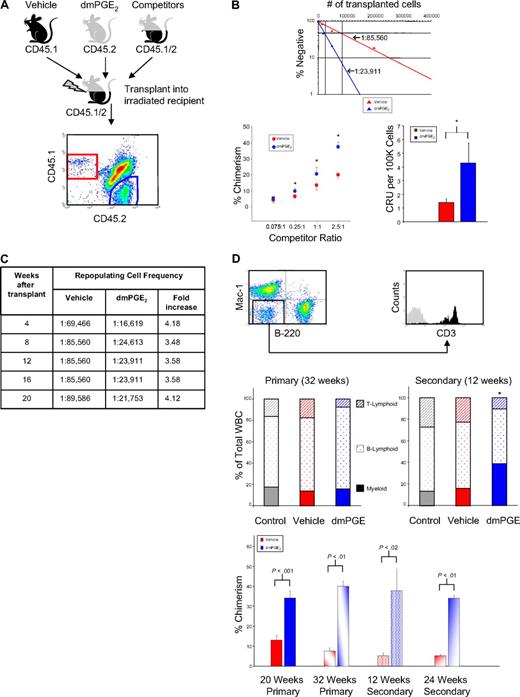
We previously showed that PGE2 stimulates proliferation, cycling, and differentiation of quiescent bone marrow cells into colony-forming cells, suggesting that PGE2 enhances HSC function.9 However, hematopoietic repopulation in myeloablated hosts is the only true measure of HSC function.14 A recent report by North et al10 showed that pulse exposure to 16,16-dimethyl PGE2 (dmPGE2) enhanced HSC frequency when transplanted into irradiated mice. We have confirmed enhancement of HSC frequency by PGE2. In addition, using a limiting-dilution, competitive head-to-head transplant model of CD45.2 and CD45.1 congenic grafts in CD45.1/CD45.2 hybrid mice that permits quantitative comparison of engraftment and competitiveness of HSCs from control and dmPGE2 treatment groups, as well as endogenous repopulation of host cells within the same animal, we now show that short-term dmPGE2 exposure produces stable long-term enhancement of HSC frequency and engraftment upon serial transplantation and that short-term exposure to dmPGE2 increases the number of CRUs and stably enhances HSC competitiveness (Figure 1A). At 12 weeks after transplantation, analysis of PB showed significantly increased chimerism of dmPGE2-treated cells compared with vehicle-treated cells, with approximately 4-fold increase in HSC frequency and CRUs, quantitative measures of long-term repopulating capacity (Figure 1B). Throughout 20 weeks after transplantation, an approximately 4-fold increase in HSC frequency was maintained, indicating that the effect of dmPGE2 pulse-exposure was stable (Figure 1C). At 32 weeks after transplantation, reconstitution was seen for peripheral B- and T-lymphoid and myeloid lineages, with no discernible differences in lineage contribution between untreated competitor cells, dmPGE2-treated cells, or vehicle-treated cells (Figure 1D).
PGE2 enhances hematopoietic stem cell engraftment. (A) Test bone marrow from CD45.1 or CD45.2 mice was treated with vehicle or dmPGE2, respectively. CD45.1/CD45.2 hybrid marrow cells were used as competitors. Limiting dilutions were transplanted into lethally irradiated (1100 cGy, split dose) CD45.1/CD45.2 hybrid mice and chimerism in PB was analyzed for 20 weeks. A representative flow plot detecting each cell population is shown. (B) Frequency analysis (top) for vehicle (red)– or dmPGE2 (blue)–pulsed cells, determined by Poisson statistics, at 12 weeks; P0 = 85 560 (vehicle) and P0 = 23 911 (dmPGE2 treated). Chimerism in PB and CRU analysis is shown at 12 weeks (mean ± SEM). Data represent 2 pooled experiments; n = 5 mice/group/experiment, each assayed individually. (*P < .05 compared with vehicle control.) (C) HSC frequency analysis in recipients of vehicle- or dmPGE2-treated bone marrow over 20 weeks. Fold change indicates increase in frequency of engraftment of dmPGE2-pulsed cells compared with vehicle. (D) Representative FACS plots of multilineage reconstitution (M indicates myeloid; B, B lymphoid; and T, T lymphoid). Multilineage analysis for primary transplantation (32 weeks) and a cohort of 4 mice that received transplants from mice that underwent primary transplantation at 20 weeks, with analysis 12 weeks later. For mice that underwent primary transplantation at 32 weeks, vehicle-treated cells were (mean ± SEM) 14.1% plus or minus 3.5% M, 70.8% plus or minus 1.1% B, and 17.8% plus or minus 1.4% T, versus dmPGE2-treated cells, which were 15.7% plus or minus 2.5% M, 76.9% plus or minus 3.4% B, and 7.5% plus or minus 1.2% T. For mice that underwent secondary transplantation at 12 weeks, vehicle-treated cells were 15.7% plus or minus 5.3% M, 60.3% plus or minus 4.8% B, and 22.1% plus or minus 3.6% T, versus dmPGE2-treated cells, which were 37.0% plus or minus 6.5% M, 52.3% plus or minus 5.4% B, and 9.0% plus or minus 1.4% T. (*P < .05 vs vehicle control.) Increased chimerism of dmPGE2-treated cells versus vehicle is shown for primary transplantation at 20 weeks (time of secondary transplantation) and in a subcohort at 32 weeks (time of 12-week analysis of secondary transplantation), for secondary transplantation at 12 weeks and 24 weeks. Data for 20-week primary transplantation were from 2 pooled experiments; n = 5 mice/group/experiment, each assayed individually. Data for secondary transplantations were from n = 5 mice/group, each assayed individually.
PGE2 enhances hematopoietic stem cell engraftment. (A) Test bone marrow from CD45.1 or CD45.2 mice was treated with vehicle or dmPGE2, respectively. CD45.1/CD45.2 hybrid marrow cells were used as competitors. Limiting dilutions were transplanted into lethally irradiated (1100 cGy, split dose) CD45.1/CD45.2 hybrid mice and chimerism in PB was analyzed for 20 weeks. A representative flow plot detecting each cell population is shown. (B) Frequency analysis (top) for vehicle (red)– or dmPGE2 (blue)–pulsed cells, determined by Poisson statistics, at 12 weeks; P0 = 85 560 (vehicle) and P0 = 23 911 (dmPGE2 treated). Chimerism in PB and CRU analysis is shown at 12 weeks (mean ± SEM). Data represent 2 pooled experiments; n = 5 mice/group/experiment, each assayed individually. (*P < .05 compared with vehicle control.) (C) HSC frequency analysis in recipients of vehicle- or dmPGE2-treated bone marrow over 20 weeks. Fold change indicates increase in frequency of engraftment of dmPGE2-pulsed cells compared with vehicle. (D) Representative FACS plots of multilineage reconstitution (M indicates myeloid; B, B lymphoid; and T, T lymphoid). Multilineage analysis for primary transplantation (32 weeks) and a cohort of 4 mice that received transplants from mice that underwent primary transplantation at 20 weeks, with analysis 12 weeks later. For mice that underwent primary transplantation at 32 weeks, vehicle-treated cells were (mean ± SEM) 14.1% plus or minus 3.5% M, 70.8% plus or minus 1.1% B, and 17.8% plus or minus 1.4% T, versus dmPGE2-treated cells, which were 15.7% plus or minus 2.5% M, 76.9% plus or minus 3.4% B, and 7.5% plus or minus 1.2% T. For mice that underwent secondary transplantation at 12 weeks, vehicle-treated cells were 15.7% plus or minus 5.3% M, 60.3% plus or minus 4.8% B, and 22.1% plus or minus 3.6% T, versus dmPGE2-treated cells, which were 37.0% plus or minus 6.5% M, 52.3% plus or minus 5.4% B, and 9.0% plus or minus 1.4% T. (*P < .05 vs vehicle control.) Increased chimerism of dmPGE2-treated cells versus vehicle is shown for primary transplantation at 20 weeks (time of secondary transplantation) and in a subcohort at 32 weeks (time of 12-week analysis of secondary transplantation), for secondary transplantation at 12 weeks and 24 weeks. Data for 20-week primary transplantation were from 2 pooled experiments; n = 5 mice/group/experiment, each assayed individually. Data for secondary transplantations were from n = 5 mice/group, each assayed individually.
Marrow was harvested from primary transplant recipient animals at 20 weeks after transplantation and transplanted into secondary recipients (Figure 1D) to validate expansion and self-renewal of LTRC previously exposed to dmPGE2 and vehicle. Analysis of PB 12 and 24 weeks after secondary transplantation showed multilineage reconstitution by cells from all mice that underwent transplantation, clearly demonstrating the self-renewal of primary transplanted LTRC. Unlike the primary transplantation, multilineage reconstitution by dmPGE2-treated cells showed an elevated myeloid lineage reconstitution. The increase in chimerism resulting from dmPGE2 exposure seen in primary donors was also observed in secondary transplantations without any additional treatments.
Murine and human hematopoietic stem and progenitor cells express PGE2 receptors
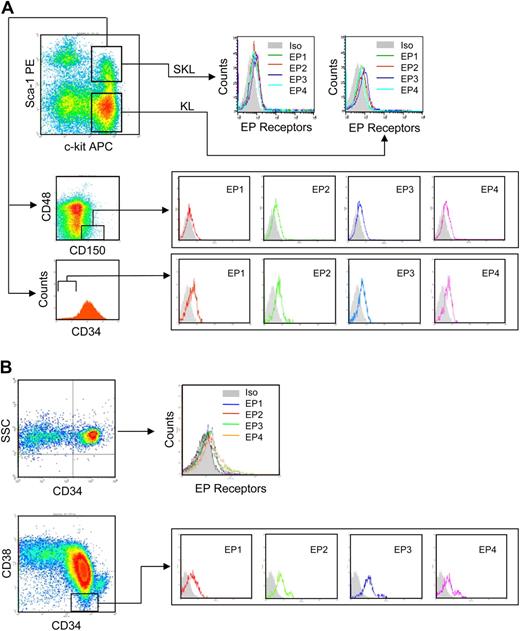
PGE2 interacts with 4 specific, highly conserved G-protein–coupled receptors: EP1-EP4,16 with EP receptor repertoire accounting for multiple, sometimes opposing responses attributed to PGE2.17 Although EP receptor expression has been observed in dendritic cells,18 monocytes,19 and early zebrafish hematopoietic tissue,20 EP receptor expression on hematopoietic stem and progenitor cell populations is not known. Analysis of EP receptors on c-kit+ Linneg (KL) cells, enriched for hematopoietic progenitor cells; Sca-1+ c-kit+ Linneg (SKL) cells, enriched for multipotent progenitor cells as well as HSCs; and SLAM (CD150+, CD48−) SKL and CD34− SKL cells, highly enriched for primitive repopulating HSCs21,22 showed that all 4 EP receptors are expressed on these hematopoietic cell populations (Figure 2A). Analogous to murine cells, all 4 receptors are expressed on human CD34+ UCB cells enriched for HSCs and CD34+, CD38− cells that contain the most primitive human HSCs (Figure 2B). Quantitative RT-PCR showed that mRNA for all 4 EP receptors is detected in the whole bone marrow cell population and in FACS-sorted KL, SKL, and primitive CD34− SKL cells (Figure 3A) and on common lymphoid progenitor cells (CLPs) (Linneg, c-kitlow, Sca-1low, IL7R+) and myeloid progenitor cells (CMPs) (Linneg, c-kit+, Sca-1−, CD34+) (not shown). Similarly, QRT-PCR analysis detected mRNA for all 4 EP receptors in purified CD34+ and CD34+, CD38− UCB cells (Figure 3B).
PGE2 receptors are expressed on murine and human HSPCs. (A) Representative FACS gating of Linneg murine bone marrow showing c-kit+ and Sca-1+ gates and SLAM (CD150+, CD48−) and CD34 gating of SKL cells. EP1-EP4 surface receptor expression on murine KL, SKL, SLAM SKL, and CD34neg SKL cells is shown. (B) Representative FACS gating of human CD34+ and CD34+, CD38− UCB cells. EP surface receptor expression on CD34+ and CD34+, CD38− cells is shown.
PGE2 receptors are expressed on murine and human HSPCs. (A) Representative FACS gating of Linneg murine bone marrow showing c-kit+ and Sca-1+ gates and SLAM (CD150+, CD48−) and CD34 gating of SKL cells. EP1-EP4 surface receptor expression on murine KL, SKL, SLAM SKL, and CD34neg SKL cells is shown. (B) Representative FACS gating of human CD34+ and CD34+, CD38− UCB cells. EP surface receptor expression on CD34+ and CD34+, CD38− cells is shown.
Amplification plots for mRNA for PGE2 receptors. (A) Primers designed to specifically detect murine EP1, EP2, EP3, or EP4 were used for QRT-PCR (with SYBR green) and plots with an activation step of 50°C for 2 minutes, denaturation at 95°C for 2 minutes, and amplification for 45 cycles at 95°C for 15 seconds, 50°C for 30 seconds, and 72°C for 30 seconds are shown. Plots corresponding to specific EP receptors are indicated in each amplification plot, where the legend key on the right shows the relative order of transcripts top to bottom. *Denotes the presence of at least 2 dissociation peaks indicating the presence of splice variants. (B) EP receptor amplification on human UCB CD34+ and CD34+ CD38− cells with the same QRT-PCR procedure as described for panel A.
Amplification plots for mRNA for PGE2 receptors. (A) Primers designed to specifically detect murine EP1, EP2, EP3, or EP4 were used for QRT-PCR (with SYBR green) and plots with an activation step of 50°C for 2 minutes, denaturation at 95°C for 2 minutes, and amplification for 45 cycles at 95°C for 15 seconds, 50°C for 30 seconds, and 72°C for 30 seconds are shown. Plots corresponding to specific EP receptors are indicated in each amplification plot, where the legend key on the right shows the relative order of transcripts top to bottom. *Denotes the presence of at least 2 dissociation peaks indicating the presence of splice variants. (B) EP receptor amplification on human UCB CD34+ and CD34+ CD38− cells with the same QRT-PCR procedure as described for panel A.
PGE2 increases HSC homing efficiency
Enhanced HSC engraftment by PGE2 could result from increased HSC number and/or cell-cycle status,23 effects on facilitating cells,24 or effects on HSC homing or proliferation in the host marrow.11 To evaluate the mechanism of action of PGE2 on HSC engraftment, we first used CFSE-labeled dmPGE2- or vehicle-treated WBM cells transplanted into lethally irradiated hosts to assess HSC homing. Total CFSE+ cells homing to bone marrow as well as the number of homed events within the KL and SKL cell populations were quantitated (Figure 4A). No difference in the percentage of CFSE+ cells homing to marrow was observed between dmPGE2- and vehicle-treated cells when total WBM cells were evaluated; however, significantly more SKL cells homed to the marrow compared with control. In a congenic model, where homed cells are detected based upon CD45 cell surface variants, a significantly greater percentage of dmPGE2-treated SKL cells homed to marrow (Figure 4B) compared with vehicle-treated or to nonmanipulated control cells. No difference in homing efficiency was seen between untreated and vehicle-treated cells.
PGE2 increases homing efficiency of HSPCs. (A) Test murine bone marrow cells were labeled with CFSE and treated with vehicle (red) or dmPGE2 (blue) and 2 × 107 labeled and treated WBM cells were transplanted into lethally irradiated mice. Sixteen hours later, bone marrow was analyzed by FACS for homed events. Data are expressed as mean plus or minus SEM; n = 6 mice/group, each assayed individually. (B) Test bone marrow cells from CD45.1 mice were treated with PBS, vehicle, or dmPGE2, and 2 × 107 treated WBM cells were transplanted into lethally irradiated CD45.2 mice. Sixteen hours later bone marrow was analyzed for homed SKL cells. The left panel shows representative data from 1 experiment; n = 3 mice/group, each assayed individually. The right panel shows the combined increase in homing efficiency of SKL cells after dmPGE2 treatment for 3 experiments (n = 6 mice/group per experiment, each assayed individually). (C) SKL cells from CD45.1 and CD45.2 mice were isolated by FACS sorting and treated with either dmPGE2 or vehicle. Five lethally irradiated CD45.1/CD45.2 hybrid mice received 3 × 104 vehicle-treated CD45.1-sorted SKL plus 3 × 104 dmPGE2-treated CD45.2 SKL cells (top panel). Five mice received a similar transplant with treatment groups switched between strains (bottom panel). Representative flow gating of marrow 16 hours after transplantation and combined data for the homing efficiency of dmPGE2- or vehicle-treated, sorted SKL cells (mean ± SEM; n = 10 mice, each assayed individually) are shown. (D) Low-density UCB mononuclear cells (LDMCs) were isolated and treated with either dmPGE2 or vehicle. Five sublethally irradiated NS2 mice received dmPGE2-treated LDMCs and 5 received vehicle-treated LDMCs. Bone marrow was analyzed 16 hours later and the number of CD34+ cells determined and homing efficiency calculated. Data are mean plus or minus SEM for n = 5 mice, each assayed individually.
PGE2 increases homing efficiency of HSPCs. (A) Test murine bone marrow cells were labeled with CFSE and treated with vehicle (red) or dmPGE2 (blue) and 2 × 107 labeled and treated WBM cells were transplanted into lethally irradiated mice. Sixteen hours later, bone marrow was analyzed by FACS for homed events. Data are expressed as mean plus or minus SEM; n = 6 mice/group, each assayed individually. (B) Test bone marrow cells from CD45.1 mice were treated with PBS, vehicle, or dmPGE2, and 2 × 107 treated WBM cells were transplanted into lethally irradiated CD45.2 mice. Sixteen hours later bone marrow was analyzed for homed SKL cells. The left panel shows representative data from 1 experiment; n = 3 mice/group, each assayed individually. The right panel shows the combined increase in homing efficiency of SKL cells after dmPGE2 treatment for 3 experiments (n = 6 mice/group per experiment, each assayed individually). (C) SKL cells from CD45.1 and CD45.2 mice were isolated by FACS sorting and treated with either dmPGE2 or vehicle. Five lethally irradiated CD45.1/CD45.2 hybrid mice received 3 × 104 vehicle-treated CD45.1-sorted SKL plus 3 × 104 dmPGE2-treated CD45.2 SKL cells (top panel). Five mice received a similar transplant with treatment groups switched between strains (bottom panel). Representative flow gating of marrow 16 hours after transplantation and combined data for the homing efficiency of dmPGE2- or vehicle-treated, sorted SKL cells (mean ± SEM; n = 10 mice, each assayed individually) are shown. (D) Low-density UCB mononuclear cells (LDMCs) were isolated and treated with either dmPGE2 or vehicle. Five sublethally irradiated NS2 mice received dmPGE2-treated LDMCs and 5 received vehicle-treated LDMCs. Bone marrow was analyzed 16 hours later and the number of CD34+ cells determined and homing efficiency calculated. Data are mean plus or minus SEM for n = 5 mice, each assayed individually.
To determine whether the enhancing effect of dmPGE2 on HSC homing was direct or indirect, we compared homing of highly purified SKL cells from both CD45.2 and CD45.1 mice in a head-to-head model. FACS-sorted SKL cells were treated with dmPGE2 or vehicle and transplanted into CD45.1/CD45.2 mice. An additional cohort received a transplant in which congenic strain and treatment groups were switched to test for strain bias. Similar to studies using WBM, dmPGE2 pulse-exposure of purified SKL cells increased their homing efficiency by 2-fold (Figure 4C), strongly suggesting a direct effect on HSCs. Although SKL cells are not a homogenous HSC population, they are highly enriched for LTRC.25
Immunodeficient mice offer the ability to evaluate human HSC function in an in vivo setting26 and are a validated model for human HSC homing.27 To verify that the enhancing effect of dmPGE2 on mouse HSC homing is also seen on human HSCs, UCB mononuclear cells were pulsed with dmPGE2 or vehicle and HSC homing was evaluated in sublethally irradiated NS2 mice (Figure 4D). Similar to mouse HSCs, dmPGE2 pulse-exposure significantly enhanced the homing efficiency of UCB CD34+ cells.
PGE2 increases hematopoietic stem and progenitor cell CXCR4 and chemotaxis to SDF-1α
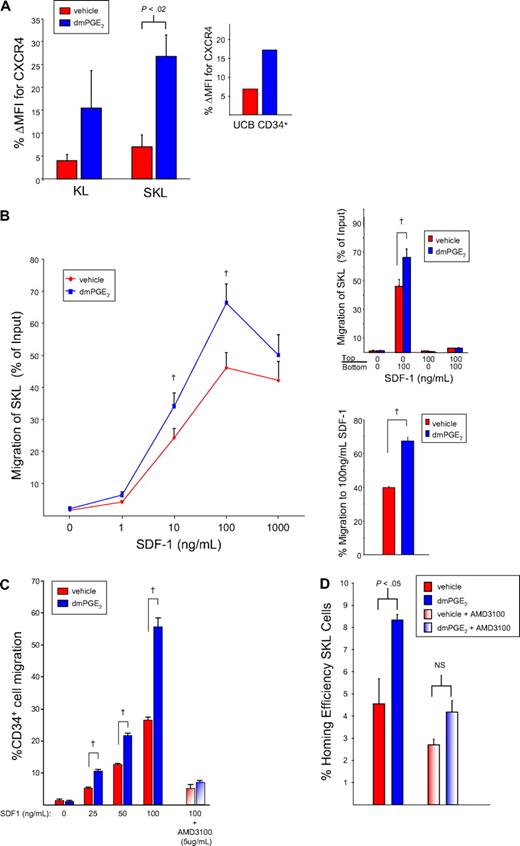
The stromal cell–derived factor-1α (SDF-1α)/CXCR4 axis is believed to play a major role in hematopoietic stem and progenitor cell (HSPC) trafficking and chemoattraction/homing to the bone marrow microenvironment.11 In addition, up-regulation of CXCR4 on human CD34+ cells28 and endothelial cells29 by PGE2 has been reported, and PGE2 can increase monocyte chemotaxis to SDF-1α.19 We therefore evaluated whether the mechanism of improved homing of dmPGE2-treated HSPCs resulted from up-regulation of SDF-1α/CXCR4 signaling. Pulse exposure to dmPGE2 increased CXCR4 expression on KL and SKL cells (Figure 5A). Similarly, dmPGE2 increased CXCR4 expression on CD34+ UCB cells. QRT-PCR demonstrated elevated CXCR4 mRNA levels in dmPGE2-treated SKL cells compared with vehicle (2.65-fold).
CXCR4 receptor expression is increased on murine and human HSPCs after dmPGE2 treatment. (A) CXCR4 expression (mean ± SEM; n = 3) on murine KL and SKL cells, and human CD34+ UCB cells 24 hours after treatment with dmPGE2. Data are expressed as percentage change in mean fluorescence intensity (MFI) of CXCR4 due to treatment with dmPGE2 or vehicle. (B) Freshly isolated Linneg cells were pulsed with dmPGE2 or vehicle for 2 hours, washed, and resuspended in media with 10% HI-FCS and cultured at 37°C for 16 hours. After incubation, cells were washed, resuspended in RPMI/0.5% BSA, and allowed to migrate to rmSDF-1α for 4 hours. Total cell migration was quantitated by flow cytometry. Data are the mean plus or minus SEM percentage migration for 3 experiments. (†P < .05 for dmPGE2-treated cells compared with cells treated with vehicle.) (Top inset) Percentage migration of gated SKL cells to positive (100 ng/mL SDF-1 in bottom chamber), negative (100 ng/mL SDF1 in upper chamber), or neutral (100 ng/mL SDF-1 in both upper and bottom chambers) gradients. Data are the mean ± SEM percentage migration for 3 experiments. (†P < .05 for dmPGE2-treated cells compared with cells treated with vehicle.) (Bottom inset) Percentage migration of sorted SKL cells to 100 ng/mL SDF-1. Data are the mean plus or minus SEM percentage migration for 3 experiments. (†P < .05 for dmPGE2-treated cells compared with cells treated with vehicle.) (C) Freshly isolated UCB CD34+ cells were pulsed with dmPGE2 or vehicle for 2 hours, washed, and resuspended in media with 10% HI-FCS and cultured at 37°C for 16 hours. After incubation, cells were washed and resuspended in RPMI/0.5% BSA, and migration to rhSDF-1 was quantitated by flow cytometry. To block the CXCR4 receptor, replicate cells were incubated with 5 μg/mL AMD3100 for 30 minutes prior to the migration assay. Data are the mean plus or minus SEM percentage migration for 3 experiments. (†P < .05 for dmPGE2-treated cells compared with cells treated with vehicle.) (D) Homing efficiency of vehicle- and dmPGE2-treated cells to bone marrow in the absence and presence of 10 μM AMD3100. Cells were incubated with AMD3100 for 30 minutes prior to the homing assay. Data are expressed as mean plus or minus SEM; n = 3 mice/group, each assayed individually.
CXCR4 receptor expression is increased on murine and human HSPCs after dmPGE2 treatment. (A) CXCR4 expression (mean ± SEM; n = 3) on murine KL and SKL cells, and human CD34+ UCB cells 24 hours after treatment with dmPGE2. Data are expressed as percentage change in mean fluorescence intensity (MFI) of CXCR4 due to treatment with dmPGE2 or vehicle. (B) Freshly isolated Linneg cells were pulsed with dmPGE2 or vehicle for 2 hours, washed, and resuspended in media with 10% HI-FCS and cultured at 37°C for 16 hours. After incubation, cells were washed, resuspended in RPMI/0.5% BSA, and allowed to migrate to rmSDF-1α for 4 hours. Total cell migration was quantitated by flow cytometry. Data are the mean plus or minus SEM percentage migration for 3 experiments. (†P < .05 for dmPGE2-treated cells compared with cells treated with vehicle.) (Top inset) Percentage migration of gated SKL cells to positive (100 ng/mL SDF-1 in bottom chamber), negative (100 ng/mL SDF1 in upper chamber), or neutral (100 ng/mL SDF-1 in both upper and bottom chambers) gradients. Data are the mean ± SEM percentage migration for 3 experiments. (†P < .05 for dmPGE2-treated cells compared with cells treated with vehicle.) (Bottom inset) Percentage migration of sorted SKL cells to 100 ng/mL SDF-1. Data are the mean plus or minus SEM percentage migration for 3 experiments. (†P < .05 for dmPGE2-treated cells compared with cells treated with vehicle.) (C) Freshly isolated UCB CD34+ cells were pulsed with dmPGE2 or vehicle for 2 hours, washed, and resuspended in media with 10% HI-FCS and cultured at 37°C for 16 hours. After incubation, cells were washed and resuspended in RPMI/0.5% BSA, and migration to rhSDF-1 was quantitated by flow cytometry. To block the CXCR4 receptor, replicate cells were incubated with 5 μg/mL AMD3100 for 30 minutes prior to the migration assay. Data are the mean plus or minus SEM percentage migration for 3 experiments. (†P < .05 for dmPGE2-treated cells compared with cells treated with vehicle.) (D) Homing efficiency of vehicle- and dmPGE2-treated cells to bone marrow in the absence and presence of 10 μM AMD3100. Cells were incubated with AMD3100 for 30 minutes prior to the homing assay. Data are expressed as mean plus or minus SEM; n = 3 mice/group, each assayed individually.
In vitro, HSPCs selectively migrate to a gradient of SDF-1α,30 a process that is believed to reflect their marrow-homing capacity. We evaluated the effect of dmPGE2 treatment on HSC chemotaxis to a gradient of SDF-1 in in vitro transwell migration assays to determine whether PGE2-mediated CXCR4 up-regulation enhanced chemotaxis. Both vehicle- and dmPGE2-treated SKL cells demonstrated significant migration to 1 to 1000 ng/mL SDF-1, however, chemotaxis was significantly higher in cells treated with dmPGE2 (Figure 5B). Analysis of positive and negative gradients indicated that the dmPGE2-enhancing effect on SKL cell chemotaxis did not result from a nonspecific increase in chemokinesis (Figure 5B top inset). Enhanced migration to SDF-1 by dmPGE2 was also observed using FACS-sorted SKL cells, suggesting a direct effect on HSCs (Figure 5B bottom inset). Chemotaxis of UCB CD34+ cells to SDF-1 was also significantly enhanced by pulse exposure to dmPGE2 and migration was blocked by the selective CXCR4 antagonist AMD3100,31 indicating a specific effect mediated through the CXCR4 receptor (Figure 5C).
To specifically determine whether up-regulated CXCR4 played a role in the enhanced homing observed after dmPGE2 treatment, cells were treated with AMD3100 prior to evaluation of in vivo homing. PGE2 pulse-exposure increased homing of SKL cells as described earlier, and incubation of vehicle or dmPGE2-pulsed cells with AMD3100 significantly reduced SKL cell homing (Figure 5D) and abrogated the improved homing efficiency of dmPGE2-pulsed cells. Pulse exposure to dmPGE2 enhanced SKL cell homing efficiency by 2.6- plus or minus 0.3-fold (P < .05), which was reduced to 1.3- plus or minus 0.2-fold (P = NS) in the presence of AMD3100. AMD3100 reduced overall homing by 42% plus or minus 5% (range, 31%-64%), consistent with previous reports.32,33
PGE2 decreases HSC apoptosis and increases Survivin
PGE2 treatment produces an approximately 4-fold increase in HSC and CRU frequency (Figure 1), but only an approximately 2-fold enhancement in homing (Figure 4), which suggests that other mechanisms are involved in enhanced engraftment. Apoptosis is an important regulatory process in normal and malignant hematopoiesis,34 and PGE2 has been implicated in antiapoptotic signaling.35,36 Moreover, activation of cAMP, a downstream signaling molecule of EP receptors, inhibits apoptosis in CD34+ cells.37 We hypothesized that dmPGE2 treatment affects survival and/or proliferation of HSCs, contributing to enhanced engraftment. Under conditions of reduced serum concentration, dmPGE2 pulse-exposure significantly reduced intracellular active caspase-3 in SLAM SKL cells (Figure 6A). Dose-ranging studies using annexin-V as an additional marker of apoptosis indicated that dmPGE2 inhibited apoptosis in a dose-dependent fashion, reaching approximately 65% inhibition at 1 μM (Figure 6A inset).
PGE2 decreases apoptosis, increases Survivin expression, and decreases active caspase-3 in SKL cells. (A) Linneg bone marrow cells were treated with dmPGE2 or vehicle and cultured in media supplemented with 2% FBS without growth factors for 24 hours to induce apoptosis. Cultured cells were stained for SKL and SLAM and PE-anti–active caspase-3 and/or FITC–annexin-V and the proportion of SKL or SLAM SKL cells undergoing apoptosis was determined by FACS. (Inset) dose-response analysis of the effects of dmPGE2 on SKL cell apoptosis. (B) Fold increase in intracellular Survivin levels (mean fluorescence intensity [MFI]) in control and dmPGE2-pulsed murine SKL and human CD34+ cells 24 hours after treatment; data are mean plus or minus SEM from 3 experiments; n = 3 mice/group, each assayed individually, or 3 separate cord blood samples. (C) Evaluation of intracellular Survivin and active caspase-3 levels (MFI) in SKL cells 24, 48, and 72 hours after treatment with dmPGE2. Data are expressed as mean plus or minus SEM for 3 experiments; n = 3-6 mice/group, each assayed individually (*P < .05).
PGE2 decreases apoptosis, increases Survivin expression, and decreases active caspase-3 in SKL cells. (A) Linneg bone marrow cells were treated with dmPGE2 or vehicle and cultured in media supplemented with 2% FBS without growth factors for 24 hours to induce apoptosis. Cultured cells were stained for SKL and SLAM and PE-anti–active caspase-3 and/or FITC–annexin-V and the proportion of SKL or SLAM SKL cells undergoing apoptosis was determined by FACS. (Inset) dose-response analysis of the effects of dmPGE2 on SKL cell apoptosis. (B) Fold increase in intracellular Survivin levels (mean fluorescence intensity [MFI]) in control and dmPGE2-pulsed murine SKL and human CD34+ cells 24 hours after treatment; data are mean plus or minus SEM from 3 experiments; n = 3 mice/group, each assayed individually, or 3 separate cord blood samples. (C) Evaluation of intracellular Survivin and active caspase-3 levels (MFI) in SKL cells 24, 48, and 72 hours after treatment with dmPGE2. Data are expressed as mean plus or minus SEM for 3 experiments; n = 3-6 mice/group, each assayed individually (*P < .05).
We previously showed that the inhibitor of apoptosis protein Survivin regulates apoptosis and proliferation of HSCs,12,38 and PGE2 has been reported to alter Survivin levels in cancer cells.39,40 We therefore evaluated if PGE2 affected Survivin expression in HSPCs. At 24 hours after dmPGE2 pulse, intracellular Survivin levels were significantly higher in murine SKL cells and human CD34+ UCB cells (1.7- and 2.4-fold, respectively) compared with control (Figure 6B). QRT-PCR analysis of treated SKL cells similarly indicated elevated Survivin mRNA compared with control (2.94-fold). In a kinetic analysis, decreased active caspase-3 coincident with an increase in Survivin was seen at 24, 48, and 72 hours after exposure of SKL cells to dmPGE2 compared with control (Figure 6C), consistent with the caspase-3 inhibiting activity of Survivin.41
PGE2 increases HSC proliferation
In previous reports, we showed that Survivin regulates HSC entry into and progression through cell cycle.12,13 Furthermore, β-catenin, implicated in HSC proliferation and self-renewal,42 lies downstream of EP receptor pathways.43 The ability of PGE2 to modulate these cell-cycle regulators suggests that an increase in HSC self-renewal and proliferation might contribute to the enhanced engraftment of dmPGE2-pulsed cells. To test this hypothesis, we analyzed the cell-cycle status of dmPGE2- or vehicle-pulsed SKL cells in vitro. Pulse exposure to dmPGE2 increased SKL cell cycling (Figure 7A), with 60% more SKL cells in G1+S/G2M phase of the cell cycle after dmPGE2 treatment compared with controls. To evaluate the effect of PGE2 exposure on primitive, quiescent HSCs, we performed additional in vitro studies using SLAM SKL cells. Similar to SKL cells, in vitro dmPGE2 pulse-exposure significantly increased the proportion of SLAM SKL cells in cell cycle (G1+ S/G2M) by 24% (Table 1). No significant effect on cell-cycle rate of KL or Linneg cells was seen (not shown), suggesting that dmPGE2 selectively increases HSC cycling state.
PGE2 increases the proliferation of SKL cells in vitro and in vivo. (A) Linneg cells were treated with either vehicle or 1 μM dmPGE2 for 2 hours, washed, and cultured in media with rmSCF, rhFlt3, and rhTpo. After 20 hours, cells were stained for SKL and Hoechst-33342 and Pyronin-Y. The proportion of SKL cells in cell cycle was quantitated by FACS. Representative flow plot showing cell-cycle distribution of gated SKL cells and combined data for fold increase in cell cycle for dmPGE2-treated cells compared with vehicle control from 3 experiments; mean plus or minus SEM; n = 9 mice, each assayed individually. (B) CD45.1 Linneg bone marrow cells were treated with dmPGE2 or vehicle and transplanted into lethally irradiated CD45.2 mice. Immediately after transplantation, BrdU was provided in drinking water and administered by intraperitoneal injection. Bone marrow was analyzed 16 hours later and the proportion of CD45.1+, SKL cells that was BrdU+ was analyzed by FACS analysis. Data are mean plus or minus SEM; n = 5 per mice/group, each assayed individually.
PGE2 increases the proliferation of SKL cells in vitro and in vivo. (A) Linneg cells were treated with either vehicle or 1 μM dmPGE2 for 2 hours, washed, and cultured in media with rmSCF, rhFlt3, and rhTpo. After 20 hours, cells were stained for SKL and Hoechst-33342 and Pyronin-Y. The proportion of SKL cells in cell cycle was quantitated by FACS. Representative flow plot showing cell-cycle distribution of gated SKL cells and combined data for fold increase in cell cycle for dmPGE2-treated cells compared with vehicle control from 3 experiments; mean plus or minus SEM; n = 9 mice, each assayed individually. (B) CD45.1 Linneg bone marrow cells were treated with dmPGE2 or vehicle and transplanted into lethally irradiated CD45.2 mice. Immediately after transplantation, BrdU was provided in drinking water and administered by intraperitoneal injection. Bone marrow was analyzed 16 hours later and the proportion of CD45.1+, SKL cells that was BrdU+ was analyzed by FACS analysis. Data are mean plus or minus SEM; n = 5 per mice/group, each assayed individually.
Effects of short-term in vitro exposure of SLAM SKL cells to dmPGE2 on cell cycle
| In vitro treatment . | SLAM SKL cells* . | |||
|---|---|---|---|---|
| G0 . | G1 . | S+G2M . | % cells in cycle† . | |
| Vehicle | 63.4 ± 2.5 | 2.6 ± 0.7 | 33.8 ± 2.1 | 36.4 ± 2.4 |
| 1 μM dmPGE2 | 54.8 ± 2.2‡ | 6.8 ± 1.9‡ | 38.4 ± 1.6‡ | 45.2 ± 2.2‡ |
| In vitro treatment . | SLAM SKL cells* . | |||
|---|---|---|---|---|
| G0 . | G1 . | S+G2M . | % cells in cycle† . | |
| Vehicle | 63.4 ± 2.5 | 2.6 ± 0.7 | 33.8 ± 2.1 | 36.4 ± 2.4 |
| 1 μM dmPGE2 | 54.8 ± 2.2‡ | 6.8 ± 1.9‡ | 38.4 ± 1.6‡ | 45.2 ± 2.2‡ |
Linneg cells, treated with either 1 μM dmPGE2 or vehicle for 2 hours and cultured in the presence of growth factors (50 ng/mL rmSCF, 100 ng/mL each of rhFlt-3 and rhTPO) for 20 hours, were stained with SLAM SKL, Hoechst-33342, and Pyronin-Y and the proportion of SLAM SKL cells in G0, G1, S, and G2/M phase of the cell cycle was determined by quantitation of DNA and RNA content by FACS. Data are mean plus or minus SEM for n = 9 mice, each assayed individually.
Percentage of cells in G1+S+G2M; combined data for n = 9 mice.
P < .05 compared with vehicle control.
To confirm the effect of dmPGE2 on enhancement of HSC cell cycle observed in vitro, bone marrow cells were pulsed with dmPGE2 and injected into congenic mice treated with BrdU after transplantation, and the proportion of donor BrdU+ SKL cells was determined 16 hours later (Figure 7B). An approximately 2-fold increase in the proportion of homed SKL cells in S+G2/M phase was observed for cells pulsed with dmPGE2 prior to transplantation, confirming that short-term exposure of HSCs to dmPGE2 stimulates HSCs to enter and progress through cell cycle in vivo.
Discussion
It is well documented that PGE2 participates in regulation of hematopoiesis, inhibiting myelopoiesis both in vitro2 and in vivo4 ; promoting erythroid and multilineage colony formation5,6 ; and enhancing proliferation of CFU-S7 and CFU-GM.8 In addition, PGE2 stimulates production of cycling hematopoietic progenitor cells (HPCs) from the quiescent bone marrow compartment,9 suggesting that PGE2 has biphasic effects on hematopoiesis. These studies implicated PGE2 in stem cell function, but did not directly evaluate HSCs. Moreover, one cannot rule out that inhibition of colony formation by PGE2 resulted from modulation of HSC commitment to self-renewal versus differentiation, thus reducing colony formation. Recently, ex vivo exposure of bone marrow cells to PGE2 was shown to facilitate murine hematopoietic cell engraftment,10 validating previous studies that PGE2 enhances HPC production and extending the role of PGE2 to stimulation of HSC function. However, the mechanism by which PGE2 produced this effect was not defined. We now demonstrate, for the first time, that PGE2 has direct and stable effects on long-term repopulating HSCs, as determined by serial transplantation, and facilitates HSC engraftment by increasing CXCR4, enhancing migration to SDF-1 and homing to bone marrow, up-regulating Survivin expression that blocks HSC apoptosis, and increasing the proportion of LTR-HSCs entering into and progressing through cell cycle.
Direct comparison in competitive transplant models showed that short-term exposure of HSCs to PGE2 produces an approximately 4-fold competitive advantage, consistent with published results.10 However, previous studies showed a maximal effect on HSC frequency at 12 weeks after transplantation with reduced HSC frequency at 24 weeks, suggesting a greater effect on short-term rather than long-term repopulating cells. Our studies show that PGE2-induced enhancement of HSC frequency was stable throughout a more than 20-week period and was maintained in secondary transplantations through 24 weeks, clearly indicating a sustained effect on LTRC. The reasons for this difference in repopulating stability are not clear, but may relate to more precise head-to-head quantitation of HSC competition in our model.
Full hematopoietic reconstitution was observed in recipients of serial transplantations using either control- or PGE2-treated cells, indicating no adverse impact of PGE2 on HSC self-renewal. In fact, a trend toward increased LTRC activity was seen, indicating that the enhancing effect of short-term PGE2 exposure on HSCs observed in primary transplantations was long-lasting, since no additional treatment was performed on cells or animals before secondary transplantations. Although it is commonly assumed that a single HSC compartment gives rise to all hematopoietic lineages, recent studies have demonstrated the presence of normal HSCs biased toward lymphoid or myeloid differentiation.44 In secondary transplantations, we observed a myeloid bias in mice that received a transplant of PGE2-treated HSCs, suggesting a possible selective effect of PGE2 on myeloid-biased HSCs. However, white blood cell counts in mice that underwent secondary transplantation have remained within normal ranges. Continued analysis of mice that have undergone transplantation and retransplantation studies will validate these findings and determine their significance, if any.
Although it was suggested that PGE2 does not affect HSC homing, earlier studies evaluated WBM10 and did not specifically assess HSPCs. When evaluating total transplanted cells, we also observed no difference in homing efficiency between control- and PGE2-treated cells; however, enhanced homing of SKL cells by PGE2 was clearly evident. Furthermore, enhanced homing efficiency of PGE2-treated, sorted SKL cells was observed, suggesting a direct effect on HSCs. PGE2 also enhanced homing of human CD34+ UCB in immunodeficient NS2 mice, strongly indicating translation of HSC enhancement to human stem cell products. Although more primitive populations of HSCs than defined by SKL can be identified (eg, CD34− SKL and SLAM SKL), the small number of homed events that can ultimately be detected using these markers precludes the ability to define effects of PGE2 on these extremely rare cells in vivo in individual mice as we performed. The fact that we see similar activities of PGE2 on LTRC and on SKL and SLAM SKL cells in several assays of HSC function without significant effects on the progenitor cell–enriched KL cell population indicates that the SKL cell fraction is a valid indicator of the effects of PGE2 on HSC homing.
PGE2 treatment increased SKL CXCR4 mRNA and surface expression, consistent with effects of PGE2 on CXCR4 in CD34+ cells.28 This increase in CXCR4 corresponds directly with a functional increase in chemotaxis to SDF-1, and chemotaxis was blocked using AMD3100. In addition, AMD3100 significantly reduced the enhancing effect of PGE2 on homing in vivo, suggesting that increased CXCR4 expression and chemoattraction to marrow SDF-1 are largely responsible for the enhanced homing effect, although additional effects on adhesion molecule expression or function cannot be excluded. We also found elevated mRNA and protein levels of Survivin, with concomitant reduced active caspase-3 in PGE2-treated SLAM SKL cells. It is likely that enhanced HSC survival, mediated through Survivin, also contributes to enhanced engraftment.
Pulse exposure to PGE2 increased the proportion of HSCs in cell cycle by approximately 2-fold, with increased frequency of HSCs, CRUs, and homed BrdU+ SKL cells and maintenance of enhanced HSC frequency in primary and secondary transplantations, suggesting that PGE2 pulse-exposure initiated at least a single round of HSC self-renewal. EP2 and EP4 receptor activation can result in phosphorylation of glycogen synthase kinase-3 (GSK-3) and increased β-catenin signaling,43 which is downstream of the Wnt pathway that has been implicated in HSPC survival and self-renewal.42,45 Signaling by PGE2 through EP4 can directly increase β-catenin, and synergistic cross-talk between COX-2 and Wnt pathways has been suggested.46 Further exploration of specific signaling pathways and EP receptors involved in mediating the effects of PGE2 may refine our understanding of the role of PGE2 on HSC function. Although it has been suggested that cycling cells have reduced marrow homing, which may be the result of triggered apoptosis,27 it is clear that PGE2-treated cells have both enhanced homing and enhanced migration, despite their enhanced cycling. This may be explained by the increase in CXCR4 migratory response overcoming deficits in the homing of cycling cells and/or increased homing occurring before an increase in cycling. In addition, PGE2 may protect homed cycling HSCs from apoptosis, thus allowing for simultaneous enhanced homing, survival, and proliferation in these cells.
We previously reported that Survivin is required for HSCs to enter and progress through cell cycle,13,38 and deletion in conditional knockout mice indicates Survivin is required for HSC maintenance.47,48 Survivin also facilitates HSPC cell cycle through p21WAF1/CDKN1,49 known to be involved in HSC function,50 and blocks caspase-3 activity,41 recently implicated in HSC self-renewal.51 Our findings that PGE2 up-regulates Survivin, which is consistent with previous reports in cancer40 and dendritic cells,39 and decreases intracellular levels of active caspase-3 in primitive HSCs suggest that the Survivin pathway may also be involved in the effects of PGE2 on self-renewal. It is interesting to note that transcription of both Survivin52 and CXCR453 is up-regulated by the transcription factor hypoxia-inducible factor-1α (HIF-1α), which can be stabilized by PGE2,54 potentially linking these PGE2 responsive pathways.
In summary, we have defined specific mechanisms of action and new pathways for enhancement and regulation of HSC function by PGE2. The 4-fold increase in HSC frequency and engraftment produced by exposure to PGE2 results from the cumulative effect of a 2-fold increase in HSC homing and a 2-fold increase in HSC cell-cycle activity. Although the precise signaling pathways remain to be determined, enhanced engraftment involves up-regulation of CXCR4 and Survivin, with subsequent increased chemotactic response to SDF-1 and reduced apoptosis. The ability to easily improve homing and survival/proliferation of HSCs by short-term PGE2 exposure is exciting from a clinical perspective, especially in transplantation settings where insufficient or low HSC numbers are found (eg, UCB and some mobilized peripheral blood stem cell products). Our limiting dilution transplant studies show that equivalent engraftment is achieved with one fourth the number of PGE2-treated cells compared with controls, supporting a use for PGE2 when HSC numbers are limiting. Homing and migration studies using UCB CD34+ cells clearly suggest potential translation to human hematopoietic grafts. Lastly, it will be interesting to determine whether enhanced engraftment/recovery can be achieved by administering PGE2 in vivo or if PGE2 used in vivo can further facilitate engraftment of HSCs exposed to PGE2 ex vivo. In COX2 knockout mice, recovery from 5-fluorouracil (5-FU) is delayed,55 suggesting that COX2 activation and subsequent PGE2 production may be critical for HSC expansion.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Giao Hangcoc for technical assistance and Hal Broxmeyer and Edward Srour for critically reading the paper. Flow cytometry was performed in the Flow Cytometry Resource Facility of the Indiana University Simon Cancer Center (NCI P30 CA082709).
These studies were supported by NIH grants HL069669 and HL079654 (L.M.P.) from the National Institutes of Health. J.H. is supported by NIH Training Grant DK07519.
National Institutes of Health
Authorship
Contribution: J.H. designed and performed research, collected and analyzed data, performed statistical analysis, and wrote the paper; P.S. designed and performed research and collected and analyzed data; J.S. participated in performance of research and collection and analysis of data; L.M.P. participated in designing research, analyzing data, and coordination and performance of the study, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Louis M. Pelus, Department Microbiology & Immunology, Indiana University School of Medicine, 950 W Walnut St, Indianapolis, IN 46202; e-mail: lpelus@iupui.edu.



![Figure 6. PGE2 decreases apoptosis, increases Survivin expression, and decreases active caspase-3 in SKL cells. (A) Linneg bone marrow cells were treated with dmPGE2 or vehicle and cultured in media supplemented with 2% FBS without growth factors for 24 hours to induce apoptosis. Cultured cells were stained for SKL and SLAM and PE-anti–active caspase-3 and/or FITC–annexin-V and the proportion of SKL or SLAM SKL cells undergoing apoptosis was determined by FACS. (Inset) dose-response analysis of the effects of dmPGE2 on SKL cell apoptosis. (B) Fold increase in intracellular Survivin levels (mean fluorescence intensity [MFI]) in control and dmPGE2-pulsed murine SKL and human CD34+ cells 24 hours after treatment; data are mean plus or minus SEM from 3 experiments; n = 3 mice/group, each assayed individually, or 3 separate cord blood samples. (C) Evaluation of intracellular Survivin and active caspase-3 levels (MFI) in SKL cells 24, 48, and 72 hours after treatment with dmPGE2. Data are expressed as mean plus or minus SEM for 3 experiments; n = 3-6 mice/group, each assayed individually (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/22/10.1182_blood-2009-01-201335/5/m_zh89990936440006.jpeg?Expires=1764960833&Signature=tFw57BhmhDRrPTJ8LlBazDzb5xeVcpzTNi~JKrdQFUDecxPTsa~5ZcaS6qmo8WBFNCFPo79XHROtDkHQUaNPc2l21E9Q61n1eXku2-AzYz1C~UiHbwk~dxmCDLmxJosO6msGmykdN9yU-kQTs18u9oQWsSdmohEwy9wFgegWR-hDFg8XF80UaTzBAVfSLirJpEukUkWVJ6-NtqqIUfSbKoUX0ZqQebaPYPkiHsnzOIiPNcFuLI4fczrTBLH7BlakwZAM6GduXbjgRYikhmdEqUSt3VwY8jpOMAhU-yPRm~zFoVtuUhu-pQ~R~epwjwgJnFHV6mIgXvW9yqMFe1f3gA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal