Abstract
Vav proteins are guanine-nucleotide exchange factors implicated in leukocyte functions by relaying signals from immune response receptors and integrins to Rho-GTPases. We here provide first evidence for a role of Vav3 for β2-integrins–mediated macrophage functions during wound healing. Vav3−/− and Vav1−/−/Vav3−/− mice revealed significantly delayed healing of full-thickness excisional wounds. Furthermore, Vav3−/− bone marrow chimeras showed an identical healing defect, suggesting that Vav3 deficiency in leukocytes, but not in other cells, is causal for the impaired wound healing. Vav3 was required for the phagocytotic cup formation preceding macrophage phagocytosis of apoptotic neutrophils. Immunoprecipitation and confocal microscopy revealed Vav3 activation and colocalization with β2-integrins at the macrophage membrane upon adhesion to ICAM-1. Moreover, local injection of Vav3−/−or β2-integrin(CD18)−/− macrophages into wound margins failed to restore the healing defect of Vav3−/− mice, suggesting Vav3 to control the β2-integrin–dependent formation of a functional phagocytic synapse. Impaired phagocytosis of apoptotic neutrophils by Vav3−/− macrophages was causal for their reduced release of active transforming growth factor (TGF)-β1, for decreased myofibroblasts differentiation and myofibroblast-driven wound contraction. TGF-β1 deficiency in Vav3−/− macrophages was causally responsible for the healing defect, as local injection of either Vav3-competent macrophages or recombinant TGF-β1 into wounds of Vav3−/− mice fully rescued the delayed wound healing.
Introduction
Normal tissue repair follows a sequence of events involving clotting, inflammation, granulation tissue formation and re-epithelialization.1,2 Within few hours after injury, first polymorphonuclear neutrophils (PMN) and later macrophages (Mφ) invade the wound tissue to combat contaminating organisms by producing proteases and reactive oxygen species (ROS).
By phagocytosing apoptotic PMN before these undergo secondary necrosis, Mφ terminate the inflammatory phase and prevent spillage of toxic proteases and ROS that might amplify tissue injury.3 After engulfment of PMN, Mφ release transforming growth factor (TGF)–β1 at the wound site,3,4 which initiates myofibroblast differentiation, wound contraction, and neo-angiogenesis.4,5
A large number of leukocyte and endothelial adhesion molecules are required to coordinately regulate the influx and intimate cell-to-cell interactions of distinct leukocyte subpopulations at wound sites.4,6 The β2-integrins CD11a/CD18 (LFA-1), CD11b/CD18 (Mac-1), CD11c/CD18 (gp 150,95), and CD11d/CD18 are constitutively expressed on the surface of leukocytes; these heterodimers consist of a common β2 subunit (CD18) and a variable α subunit (CD11a, CD11b, CD11c, CD11d).7 β2-integrins are responsible for PMN recruitment4,8-12 and for the recruitment of Mφ subsets13 in different mouse models of inflammation. In addition, CD18 is crucial for the formation of the phagocytic synapse between Mφ and apoptotic PMN14 that initiates TGF-β1 release and is essential for myofibroblasts differentiation4 and vessel formation.5 The importance of β2-integrins is emphasized in leukocyte adhesion deficiency (LAD1) patients suffering from severe wound healing disturbances and bacterial infections due to decreased β2-integrin expression and/or function.15,16 Also CD18−/− mice share the essential characteristics of LAD1 patients.4,10 Thus, β2-integrins are critical mediators of PMN and Mφ functions in innate immunity and tissue repair. However, apart from a few downstream target proteins of β2-integrin signaling such as the nonreceptor tyrosine kinase Syk activated by adaptors bearing immunoreceptor tyrosine based activation motifs (ITAMs),17,18 little is known on the CD18 downstream signaling in phagocytes.
The guanine-nucleotide exchange factors Vav couple to diverse signaling receptors and, due to their exchange activity and adaptor domains, are ideally suited to transduce signals to RhoGTPases and the actin cytoskeleton.19-21 Targeted disruption of Vav1 results in severe impairment of T lymphocyte function in response to antigen receptor stimulation19,20 with defective TCR capping and actin patch formation.21,22 Various Vav double-knockout mice reveal distinctive B- and T-cell phenotypes,23-25 and in case of Vav triple knockouts, the function of B and T cells is severely compromised.25 Mainly in vitro evidence exists for FcγR, β1, β2 and β3-integrin–dependent roles for Vav proteins in T cells,26 platelets,27 neutrophils,28-30 and Mφ.31,32
To dissect carefully the in vivo role of Vav proteins for β2-integrin–dependent PMN and Mφ functions in cutaneous wound healing, we here used Vav1−/−, Vav3−/−, and Vav1−/−/Vav3−/− mice. We identify Vav3 as a critical downstream target in the CD18-dependent formation of the phagocytic synapse between Mφ and PMN required for tissue repair.
Methods
Mice
Vav1−/−, Vav2−/−, Vav3−/−, Vav1−/−/Vav3−/−, Vav2−/−/Vav3−/−, and WT littermates were previously described.24,33 Wound-healing experiments were performed with male cohorts between 8 and 12 weeks of age. All experiments were carried out in compliance with the German Law for Welfare of Laboratory Animals and were approved by the Institutional Review Board of the University of Ulm.
Generation of bone marrow chimeras
Bone marrow chimeras were generated by intravenous injection of whole bone marrow cells from WT or Vav3−/− mice on C57BL/6 (B6) background carrying the Ly5.2/CD45.2 allele, into lethally irradiated congenic recipients bearing the Ly5.1/CD45.1 allele on B6 background (The Jackson Laboratory, Bar Harbor, ME). Complete repopulation with either Vav3−/− or WT leukocyte compartment was confirmed by flow cytometry and immunoblotting (Figure S1A,B, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Wound-healing model
Wound-healing experiments were performed as described.4 Briefly, full-thickness (including the panniculus carnosus) excisional wounds were produced under anesthesia with 6-mm round knives (Stiefel, Offenbach, Germany) on both sides of the shaved backs of Vav1−/−, Vav2−/−, Vav3−/−, Vav1−/−/Vav3−/−, Vav2−/−/Vav3−/−, and WT mice, and of WT or Vav3−/− chimeras. Four wounds per mouse were induced. At indicated time points, each wound was digitally photographed, and wound areas were quantified using Adobe Photoshop software version 7.0.1 (Adobe Systems, San Jose, CA). Wound sizes at any time point were expressed as percentage of initial (day 0) wound area. For early time point analyses, wounds including adjacent margins were snap-frozen and stored at −80°C.
Immunofluorescence staining, Western blot analysis, and generation of murine bone marrow-derived Mφ and apoptotic PMN
Details of immunofluorescence staining, Western blot analysis, and generation of murine bone marrow-derived Mφ and apoptotic PMN are contained in Document S1.
In vitro adhesion and phagocytosis assays
WT or Vav3−/− mature Mφ were plated to adhere onto 24-well cell culture plates (Nunc/Thermo Fisher Scientific, Langenselbold, Germany) before addition of CMRA-labeled apoptotic PMN from indicated genotypes at Mφ:PMN ratio of 1:10. After 15 minutes of coculture for adhesion and 45 minutes for phagocytosis, nonadherent/ingested PMN were removed with cold phosphate-buffered saline (PBS). Adherent cells were collected by scraping and stained for the Mφ marker F4/80. CMRA+F4/80+ double positive complexes either of Mφ adherent to PMN or Mφ phagocytosing PMN were quantified by flow cytometry as previously described.4
Macrophage adhesion assay
Ninety-six-well cell culture plates (Nunc) were coated with 20 μg/mL fibronectin (BD Biosciences, Heidelberg, Germany), 20 μg/mL rmICAM-1 or with 20 μg/mL vitronectin (R&D Systems, Wiesbaden, Germany) overnight at 4°C. Bovine serum albumin (BSA; 0.1%)–coated wells served as controls for unspecific adhesion. BSA covered the plastic, reported to bind β2-integrins, and thus abolished β2-integrin–mediated adhesion. WT or Vav3−/− 5-chloromethylfluorescein diacetate (CMFDA)–labeled Mφ preactivated with 10 ng/mL TNFα (PeproTech, Hamburg, Germany) were seeded onto the coated plates at 5 × 104/well, shortly placed on ice to synchronize adhesion and incubated to adhere at 37°C for 15 minutes. Nonadherent cells were rigorously washed with PBS. Adhesion was assessed as percentage of fluorescence intensity related to total input macrophages (100%) measured at 485 nm with a Twinkle LB970 Fluorometer (Berthold Technologies, Bad Wildbad, Germany).
Oxidative burst
WT and Vav3−/− Mφ loaded with the ROS-sensitive fluorescence quenched substrate 5-(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (Invitrogen, Karlsruhe, Germany) were incubated with apoptotic PMN at 1:10 ratio for 180 minutes at 37°C. The increase in green fluorescence intensity reflecting oxidative burst was measured by fluorometry.
Immunoprecipitation
To study Vav3 activation, Mφ lysates were immunoprecipitated with anti-Vav3 pAb (Millipore, Billerica, MA), followed by Western blotting using anti-Phosphotyrosine mAb (4G10;, Millipore). A detailed description is included in Document S1.
Rescue and transfer experiments
Recombinant human (rh) TGF-β1 (R&D Systems) was subcutaneously injected at physiologic concentrations of 0.45 μg/wound into wound margins at days 1, 3, 5 and 7 after wounding as published.4 Mice treated with 0.9% saline served as mock controls. For transfer experiments, 106 WT, Vav3−/− or CD18−/− Mφ/wound were injected at 4 sites into the wound margins of WT or Vav3−/− mice. Control wounds were injected with PBS.
Cytokine enzyme-linked immunosorbent assay ELISA
Details of cytokine enzyme-linked immunosorbent assay (ELISA) appear in Document S1.
Binding of soluble ICAM-1
Bone marrow-derived WT, Vav3−/− and CD18−/− unstimulated (resting) or stimulated (with 100 nm fMLP, 100 nm LTB4; Sigma-Aldrich) or 50 ng/mL TNFα (eBioscience, Frankfurt, Germany) Mφ were incubated with 5 μg rmICAM-1 fused to the Fc region of human IgG (ICAM-1/Fc; R&D Systems) in 100 μL binding buffer (PBS, 2 mM MgCl2, 1 mM CaCl2, 1 mM D-Glucose and 0.1% BSA) at 37°C for 10 minutes with continuous agitation. Mφ incubated with human IgG served as controls. Mφ were immediately fixed on ice in 3.7% paraformaldehyde, washed in PBS and labeled with FITC-conjugated F(ab′) anti–human IgG to detect bound ICAM-1 on Mφ by flow cytometry. Bound ICAM-1/Fc was assessed as ratio between the mean fluorescence intensities of Mφ incubated with ICAM-1/Fc and Mφ incubated with human IgG for each stimulation.
Image acquisition and analysis
Wounds were photographed using a Sony Super SteadyShot DSC-H5 digital camera (Sony Germany, Berlin, Germany). Histology samples were visualized using a Zeiss Axiophot microscope with Plan-NEOFLUAR 20×/0.75 (∞/0.17) objective and images were captured using AxioCam MRc camera and AxioVision Version 4.7 imaging software (all Carl Zeiss, Jena, Germany). Confocal microscopy was performed using LSM 410/Axiovert 135M microscope (Zeiss) with Leica TCS SP% 63×/1.2 oil objective (Leica Microsystems, Wetzlar, Germany). Images were imported into and processed using Adobe Photoshop Version 7.0.1 software (Adobe Systems, San Jose, CA).
Statistical analysis
Quantitative data are presented as mean values plus or minus standard deviation (SD). Statistical significance was determined by the 2-tailed Student t test, or the Mann-Whitney U test in cases of a non-Gaussian distribution. A P value less than .05 was considered statistically significant.
Results
Vav3−/− mice display delayed wound healing with diminished numbers of Mφ and normal PMN recruitment at the wound site
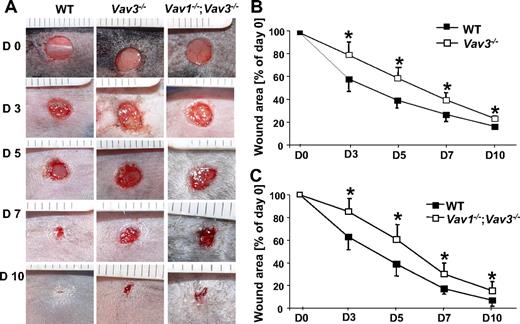
Full-thickness wounds were produced on Vav1−/−, Vav2−/−, Vav3−/− mice, intercrosses thereof, and WT controls. Wound sizes were monitored as described.4 Wound closure of Vav3−/− mice was significantly delayed between days 3 and 10 after wounding compared with WT mice (Figure 1A,B). No delay in wound closure was observed in Vav1−/− and Vav2−/− mice, whereas their intercrosses with Vav3−/− mice (Vav1−/−/Vav3−/− and Vav2−/−/Vav3−/−) did not aggravate the wound closure deficiency of Vav3−/− mice, suggesting that neither Vav1 nor Vav2 deficiency contributed to the delayed wound healing of Vav3−/− mice (Figure 1C and data not shown). Therefore, we concentrated on Vav3−/− and for control purposes in selected experiments also on Vav1−/−/Vav3−/− mice.
Wound closure of full-thickness wounds is delayed in Vav3−/− and Vav1−/−/Vav3−/− mice. (A) Representative macroscopic aspects of wounds from Vav3−/−, Vav1−/−/Vav3−/− and WT mice at different healing stages. (B,C) Statistical analysis of 24 wound areas quantified at days 0, 3, 5, 7 and 10 after wounding expressed as percentage of the initial (day 0) wound size for WT, Vav3−/− and Vav1−/−/Vav3−/− mice. Results given as mean ± SD (n = 6) reflect 1 of 3 independent experiments. *P < .05 by Mann-Whitney test.
Wound closure of full-thickness wounds is delayed in Vav3−/− and Vav1−/−/Vav3−/− mice. (A) Representative macroscopic aspects of wounds from Vav3−/−, Vav1−/−/Vav3−/− and WT mice at different healing stages. (B,C) Statistical analysis of 24 wound areas quantified at days 0, 3, 5, 7 and 10 after wounding expressed as percentage of the initial (day 0) wound size for WT, Vav3−/− and Vav1−/−/Vav3−/− mice. Results given as mean ± SD (n = 6) reflect 1 of 3 independent experiments. *P < .05 by Mann-Whitney test.
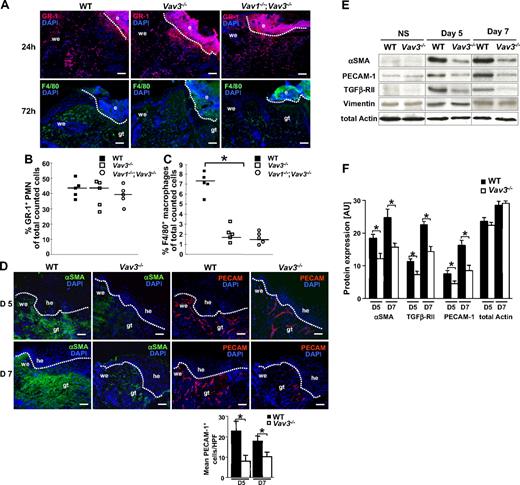
Immunostaining for GR-1 revealed virtually no differences in PMN numbers at wound sites of Vav3−/− and Vav1−/−/Vav3−/− compared with WT mice at 24 hours after wounding (Figure 2A). Also at earlier (1 hour, 6 hours) and later (72 hours) time points, no obvious differences in PMN recruitment were observed for the studied genotypes (data not shown). By contrast, immunostaining of 72-hour wounds revealed a significant reduction in recruitment of F4/80+ Mφ to wound sites of Vav3−/− and Vav1−/−/Vav3−/− compared with WT mice (Figure 2A). These data were confirmed by fluorescence-activated cell sorting (FACS) quantification of GR-1+ PMN enzymatically isolated from wound tissue 24 hours after wounding (Figure 2B) and of F4/80+ Mφ isolated 3 days after wounding (Figure 2C). The expression levels of apoptosis markers such as active caspase-3 and PARP-1 were identical in wounds of WT and Vav3−/− mice at different time points (data not shown), suggesting that enhanced apoptosis was not responsible for the reduced Mφ numbers at Vav3−/− wound sites.
Impaired recruitment of Mφ, but not of PMN, and reduced numbers of myofibroblasts and blood vessels at the wound sites of Vav3−/− mice. (A) GR-1+ PMN (red; top panel) and F4/80+ Mφ (green; bottom panel) recruitment to wound sites assessed by immunostaining of cryosections from Vav3−/−, Vav1−/−/Vav3−/− and WT mice. Quantification of GR-1+ PMN recruitment at 24 hours after wounding (B) and F4/80+ Mφ recruitment at 72 hours after wounding (C) by FACS analysis of wound cells isolated by enzymatic disruption from wound tissue. Results given as scatter plots. Bars indicate the median (n = 5). *P < .05 by Student t test. (D) Granulation tissue formation in 5 and 7 days old WT and Vav3−/− wounds assessed by immunostaining with myofibroblasts-specific αSMA (green) and endothelial cell–specific PECAM-1 (red). Cell nuclei are counterstained with DAPI (blue). Original magnification ×20, scale bar indicates 200μm; e, eschar, he, hyperproliferative epidermis; we, wound edge, gt, granulation tissue. Quantification of PECAM-1+ cells in 10 high-power fields (HPFs) of 5- and 7-day-old wounds of 4 Vav3−/− and WT mice. Data are given as mean ± SD numbers of PECAM-1+ cells counted per HPF. *P < .05 by Student t test. (E) Western blot analysis of snap-frozen nonwounded normal skin (NS) and of wound tissue at days 5 and 7 equilibrated to total actin and vimentin levels to measure expression of αSMA, TGFβ-RII and PECAM-1. αSMA, TGFβ-RII, PECAM-1, actin, and vimentin are expressed at identical levels in WT and Vav3−/− nonwounded skin, excluding that these molecules are globally down-regulated in Vav3−/− mice. (F) Semiquantitative balance analysis of immunoblots performed by densitometry of digitized Western blots. Data are given as mean ± SD. *P < .05 by Student t test.
Impaired recruitment of Mφ, but not of PMN, and reduced numbers of myofibroblasts and blood vessels at the wound sites of Vav3−/− mice. (A) GR-1+ PMN (red; top panel) and F4/80+ Mφ (green; bottom panel) recruitment to wound sites assessed by immunostaining of cryosections from Vav3−/−, Vav1−/−/Vav3−/− and WT mice. Quantification of GR-1+ PMN recruitment at 24 hours after wounding (B) and F4/80+ Mφ recruitment at 72 hours after wounding (C) by FACS analysis of wound cells isolated by enzymatic disruption from wound tissue. Results given as scatter plots. Bars indicate the median (n = 5). *P < .05 by Student t test. (D) Granulation tissue formation in 5 and 7 days old WT and Vav3−/− wounds assessed by immunostaining with myofibroblasts-specific αSMA (green) and endothelial cell–specific PECAM-1 (red). Cell nuclei are counterstained with DAPI (blue). Original magnification ×20, scale bar indicates 200μm; e, eschar, he, hyperproliferative epidermis; we, wound edge, gt, granulation tissue. Quantification of PECAM-1+ cells in 10 high-power fields (HPFs) of 5- and 7-day-old wounds of 4 Vav3−/− and WT mice. Data are given as mean ± SD numbers of PECAM-1+ cells counted per HPF. *P < .05 by Student t test. (E) Western blot analysis of snap-frozen nonwounded normal skin (NS) and of wound tissue at days 5 and 7 equilibrated to total actin and vimentin levels to measure expression of αSMA, TGFβ-RII and PECAM-1. αSMA, TGFβ-RII, PECAM-1, actin, and vimentin are expressed at identical levels in WT and Vav3−/− nonwounded skin, excluding that these molecules are globally down-regulated in Vav3−/− mice. (F) Semiquantitative balance analysis of immunoblots performed by densitometry of digitized Western blots. Data are given as mean ± SD. *P < .05 by Student t test.
Wounds of Vav3−/− mice reveal reduced numbers of myofibroblasts and blood vessels
Differences in wound size between WT and Vav3−/− mice were most significant between days 3 and 7 after wounding. While at day 3 the early inflammatory phase prevails, we have focused on days 5 and 7, when mainly myofibroblast-dependent wound contraction34 and neo-angiogenesis occur, and studied the expression of myofibroblasts and endothelial cell markers in granulation tissues by histology and Western blotting. In 5- and 7-day-old wounds, the myofibroblast-specific α smooth muscle actin (αSMA) was abundantly present at wound edges and below the hyperproliferative epidermis of WT wounds. In contrast, only faint αSMA staining was detected in the correspondent areas of 5- and 7-day-old Vav3−/− wounds (Figure 2D). Densitometric assessment of Western blots revealed lower expression of αSMA and another myofibroblast marker, TGFβ-RII, in wounds of Vav3−/− mice compared with WT mice (Figure 2E,F). Vimentin served to equilibrate loading for similar numbers of fibroblastic cells. These results suggested that lower numbers of myofibroblasts resided in wound beds of Vav3−/− mice compared with WT mice.
Apart from myofibroblast-driven wound contraction, neo-angiogenesis plays an important role in tissue repair. Notably, immunostaining for platelet/endothelial cell adhesion molecule 1 (PECAM-1) revealed high numbers of vessels in 5 and 7 days WT wounds, whereas significantly fewer PECAM-1+ vessels were found in Vav3−/− wounds (Figure 2D). This was confirmed by Western blotting using wound lysates of Vav3−/− and WT mice (Figure 2E,F), suggesting that neo-angiogenesis is reduced and delayed in Vav3−/− mice. αSMA, TGFβ-RII, and PECAM-1 in nonwounded normal skin were expressed at identical low levels in the studied Vav3−/− and WT mice, excluding the possibility that these molecules are globally down-regulated in Vav3−/− mice.
Local injection of TGF-β1 rescues myofibroblast-driven wound contraction and neo-angiogenesis, but not defective Mφ recruitment in Vav3−/− mice
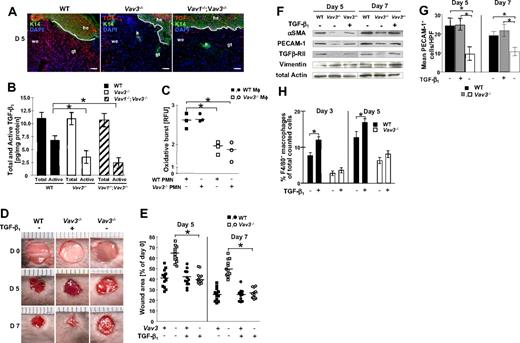
TGF-β1 is the pivotal growth factor promoting myofibroblast differentiation35 and neo-angiogenesis during wound healing.5 Rough assessment of TGF-β1 expression by immunohistology revealed in all genotypes (WT, Vav3−/−, Vav1−/−/Vav3−/−) a strong TGF-β1 staining in the epidermis and hair follicles, identified by epidermis-specific K14 staining (Figure 3A). There was, however, clearly reduced staining in the dermal wound margins and granulation tissue at days 5 and 7 after wounding of Vav3−/− and Vav1−/−/Vav3−/− mice compared with WT mice (Figure 3A and data not shown). To substantiate these findings, total and active TGF-β1 concentrations were quantified by specific ELISA. Although no significant differences in total TGF-β1 concentrations were found between WT, Vav3−/− and Vav1−/−/Vav3−/− wounds, active TGF-β1 concentrations were significantly reduced in 5-day-old wound lysates from Vav3−/− and Vav1−/−/Vav3−/− mice (Figure 3B). Moreover, upon phagocytosis of apoptotic PMN, Vav3−/− Mφ revealed a significantly decreased oxidative burst compared with WT Mφ (Figure 3C). As ROS have been shown to convert latent TGF-β1 to active TGF-β1,36 the reduced ROS formation in Vav3−/− Mφ may contribute to the reduced activation of TGF-β1 in Vav3−/− and Vav1−/−/Vav3−/− wound lysates compared with WT wounds. These results provide circumstantial evidence that reduced active TGF-β1 at wound sites accounts for decreased numbers of αSMA+ myofibroblasts, reduced wound contraction and diminished neovascularization of the restoration tissue in Vav3−/− mice.
Reduced release of active TGF-β1 in the wound margins is causal for the wound healing defect of Vav3−/− and Vav1−/−/Vav3−/− mice. (A) Immunostaining of 5-day-old wounds from Vav3−/−, Vav1−/−/Vav3−/− and WT mice showing TGF-β1 (red) localization throughout the K14+ epidermis (green) and the wound tissue. Blue staining indicates nuclear staining with DAPI. Original magnification ×20, scale bar indicates 200μm; he, hyperproliferative epidermis; we, wound edge, gt, granulation tissue, h, hair follicle. (B) Quantitative evaluation of total and active TGF-β1 release from 5 days old Vav3−/−, Vav1−/−/Vav3−/− and WT wound lysates by specific ELISA. Results representative of 2 independent experiments are expressed as mean ± SD (n = 4). *P < .05 by Student t test. (C) Oxidative burst of WT and Vav3−/− Mφ upon phagocytosis of apoptotic WT or Vav3−/− PMN measured at 3 hours and expressed as the increase in fluorescence intensity of oxidized carboxy H2DCFDA. Data representative for at least 2 different experiments is given in RFU (relative fluorescence units) as scatter plot. Each symbol represents triplicate measurements, Mφ derived from 3 different mice of each genotype (n = 3). Bars indicate medians. *P < .05 by Student t test. (D) Representative macroscopic pictures of wounds derived from WT and Vav3−/− mice at days 5 and 7 after wounding and repetitive injection with a physiologic concentration of rhTGF-β1 (TGF-β1+) or of NaCl (TGF-β1−). (E) Statistical analysis of 16 wound areas expressed as percentage of the initial (day 0) wound size. Results presented as scatter plots. Bars indicate medians of each cohort (n = 4). *P < .05 by Mann-Whitney test. (F) Western blot analysis of snap-frozen wound tissue equilibrated to total actin and vimentin levels to assess expression levels of αSMA, TGFβ-RII and PECAM-1 in wound margins of different genotypes at different time points after injection of either rhTGF-β1 (TGF-β1+) or of NaCl (TGF-β1-). (G) Quantification of PECAM-1+ cells in 10 high power fields (HPF) of 5- and 7-day-old wounds of WT, Vav3−/− and TGF-β1–treated Vav3−/− mice. Data are given as mean ± SD numbers of PECAM-1+ cells counted per HPF. *P < .05 by Student t test. (H) Recruitment of F4/80+ Mφ assessed by FACS analysis of cells isolated by enzymatic disruption of 3- and 5-day-old wounds. Results given as scatter plots. Bars indicate the median (n = 4). *P < .05 by Student t test.
Reduced release of active TGF-β1 in the wound margins is causal for the wound healing defect of Vav3−/− and Vav1−/−/Vav3−/− mice. (A) Immunostaining of 5-day-old wounds from Vav3−/−, Vav1−/−/Vav3−/− and WT mice showing TGF-β1 (red) localization throughout the K14+ epidermis (green) and the wound tissue. Blue staining indicates nuclear staining with DAPI. Original magnification ×20, scale bar indicates 200μm; he, hyperproliferative epidermis; we, wound edge, gt, granulation tissue, h, hair follicle. (B) Quantitative evaluation of total and active TGF-β1 release from 5 days old Vav3−/−, Vav1−/−/Vav3−/− and WT wound lysates by specific ELISA. Results representative of 2 independent experiments are expressed as mean ± SD (n = 4). *P < .05 by Student t test. (C) Oxidative burst of WT and Vav3−/− Mφ upon phagocytosis of apoptotic WT or Vav3−/− PMN measured at 3 hours and expressed as the increase in fluorescence intensity of oxidized carboxy H2DCFDA. Data representative for at least 2 different experiments is given in RFU (relative fluorescence units) as scatter plot. Each symbol represents triplicate measurements, Mφ derived from 3 different mice of each genotype (n = 3). Bars indicate medians. *P < .05 by Student t test. (D) Representative macroscopic pictures of wounds derived from WT and Vav3−/− mice at days 5 and 7 after wounding and repetitive injection with a physiologic concentration of rhTGF-β1 (TGF-β1+) or of NaCl (TGF-β1−). (E) Statistical analysis of 16 wound areas expressed as percentage of the initial (day 0) wound size. Results presented as scatter plots. Bars indicate medians of each cohort (n = 4). *P < .05 by Mann-Whitney test. (F) Western blot analysis of snap-frozen wound tissue equilibrated to total actin and vimentin levels to assess expression levels of αSMA, TGFβ-RII and PECAM-1 in wound margins of different genotypes at different time points after injection of either rhTGF-β1 (TGF-β1+) or of NaCl (TGF-β1-). (G) Quantification of PECAM-1+ cells in 10 high power fields (HPF) of 5- and 7-day-old wounds of WT, Vav3−/− and TGF-β1–treated Vav3−/− mice. Data are given as mean ± SD numbers of PECAM-1+ cells counted per HPF. *P < .05 by Student t test. (H) Recruitment of F4/80+ Mφ assessed by FACS analysis of cells isolated by enzymatic disruption of 3- and 5-day-old wounds. Results given as scatter plots. Bars indicate the median (n = 4). *P < .05 by Student t test.
To provide evidence for a causal role of TGF-β1 deficiency in the impaired wound closure, we injected rhTGF-β1 (or NaCl as control) subcutaneously around the wounds as previously described.4 Gross assessment (Figure 3D) and digital measurement (Figure 3E) of wound sizes during healing showed that local injection of rhTGF-β1 fully rescued impaired wound healing of Vav3−/− mice. Notably, in contrast to wounds of mock-treated Vav3−/− mice with reduced αSMA, TGFβ-RII, and PECAM-1 expression, Vav3−/− wounds injected with rhTGF-β1 showed increased myofibroblast αSMA, TGFβ-RII, and vascular PECAM-1 levels in wound lysates (Figure 3F) and increased numbers of PECAM-1+ cells in wound tissue sections (Figure 3G), comparable with wounds from WT controls. Thus, in absence of Vav3, injection of rhTGF-β1 during healing was sufficient to fully restore myofibroblast-driven wound contraction and neo-angiogenesis, unequivocally supporting a causal role for the reduced release of active TGF-β1 in the delayed wound healing of Vav3−/− mice.
As TGF-β1 has been reported to modulate Mφ chemotaxis,37 we investigated whether TGF-β1 injection enhanced Mφ recruitment in Vav3−/− wounds. In fact, exogenous TGF-β1 increased the Mφ influx only in WT, but not in Vav3−/− mice (Figure 3H), very much suggesting that TGF-β1–induced Mφ recruitment at wound sites distinctly depends on Vav3 and does not activate alternate emigration pathways.
Defective release of active TGF-β1 is due to impaired adhesion with defective phagocytosis of apoptotic PMN by Mφ in Vav3−/− mice
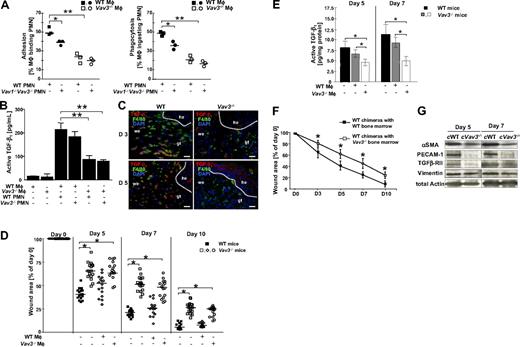
After cleaning the wound from invading pathogens, PMN undergo apoptosis and are removed by Mφ by adhesion-dependent phagocytosis, which stimulates Mφ to release large amounts of TGF-β1.3,4 Mφ activation to release active TGF-β1 (Figure S2B) preferentially depends on β2-integrin–mediated adhesion to and phagocytosis of apoptotic PMN (Figure S2A), as also shown with β2-integrin(CD18)−/− cocultures of Mφ and PMN.4 To analyze the adhesion-mediated phagocytosis of apoptotic PMN by Mφ, apoptotic Vav3−/−, Vav1−/−/Vav3−/− and WT PMN were cocultured with Vav3−/− or WT Mφ in all possible combinations. Mφ-PMN conjugates were quantified using microscopy and flow cytometry after 15 minutes of coculture to assess PMN adhesion to Mφ and after 45 minutes to assess Mφ phagocytosing PMN. Adhesion of Vav3−/− Mφ to apoptotic WT PMN was severely impaired, and this defect was not further reduced by coculture of Vav3−/− Mφ with Vav3−/− PMN (Figure 4A and data not shown). Instead, when coculturing WT Mφ with Vav1−/−/Vav3−/− PMN, a minor, though significant, decrease in adhesion was observed. These data suggest that Vav3 deficiency in Mφ is predominantly responsible for the observed adhesion defect, whereas in PMN a double Vav1/Vav3 deficiency is required to affect adhesion to some extent. In fact, previous reports confirm that Vav1 and Vav3 play redundant roles in PMN adhesion and spreading, functions that are only impaired by double deficiency.28
Defective release of active TGF-β1 is due to impaired adhesion resulting in defective phagocytosis of apoptotic PMN by macrophages in Vav3−/− mice. (A) In vitro adhesion and phagocytosis of WT and Vav3−/− Mφ cocultured with CMRA-labeled Vav1−/−/Vav3−/− or WT apoptotic PMN for 15 minutes (adhesion; left panel) or 45 minutes (phagocytosis; right panel) assessed by flow cytometry as CMRA+F4/80+ conjugates. Results are expressed as percentages of PMN-binding Mφ of the total Mφ count (PMN-binding Mφ × 100/total number of Mφ). Each symbol indicates the median of a triplicate analysis. *P < .05; **P < .005. (B) Active TGF-β1 concentrations measured by ELISA in supernatants of unstimulated WT and Vav3−/− Mφ and in cocultures between Mφ and PMN of the indicated genotypes. Data given as mean ± SD (n = 4). **P < .005. (C) Active TGF-β1–producing Mφ (yellow) identified by immunostaining of cryosections from 3- and 5-day-old Vav3−/− and WT wounds for TGF-β1 (red) and F4/80 (green). Cell nuclei are counterstained with DAPI (blue; original magnification ×20, scale bar indicates 150 μm; he, hyperproliferative epidermis; we, wound edge; and gt, granulation tissue). (D) Statistical analysis of 16 wounds derived from WT mice and from Vav3−/− mice after wounding and injection of viable WT Mφ (WT Mφ+) or of Vav3−/− Mφ (Vav3−/− Mφ+). Wound areas are expressed as percentages of the initial (day 0) wound size. Results represented as scatter plots. Bars indicate medians of each cohort (n = 4). *P < .05 by Mann-Whitney test. (E) Quantitative evaluation of active TGF-β1 measured by specific ELISA in lysates of 5- and 7-day-old wounds of WT mice and Vav3−/− mice after wounding and injection of viable WT Mφ (WT Mφ+) or Vav3−/− Mφ (Vav3−/− Mφ+) into wound margins. Results representative of 2 independent experiments are expressed as mean plus or minus SD (n = 4). *P < .05 by Student t test. (F) Lethally irradiated WT mice reconstituted with bone marrow from Vav3−/− mice reveal a significant delay in wound healing. Statistical analysis of 16 wounds per studied time point derived from lethally irradiated WT mice reconstituted with bone marrow from either WT (■) or Vav3−/− (□) mice were subjected to image analysis. Wound areas are expressed as percentages of the initial (day 0) wound size. Results given as mean ± SD (n = 4) reflect 1 of 2 independent experiments. *P < .05 by Mann-Whitney test. (G) Western blot analysis of expression levels of αSMA, TGFβ-RII and PECAM-1 equilibrated to total actin and vimentin levels in wound margins of WT and Vav3−/− bone marrow chimeric mice at days 5 and 7 after wounding (cWT, WT chimera with WT bone marrow; cVav3−/−, WT chimera with Vav3−/− bone marrow).
Defective release of active TGF-β1 is due to impaired adhesion resulting in defective phagocytosis of apoptotic PMN by macrophages in Vav3−/− mice. (A) In vitro adhesion and phagocytosis of WT and Vav3−/− Mφ cocultured with CMRA-labeled Vav1−/−/Vav3−/− or WT apoptotic PMN for 15 minutes (adhesion; left panel) or 45 minutes (phagocytosis; right panel) assessed by flow cytometry as CMRA+F4/80+ conjugates. Results are expressed as percentages of PMN-binding Mφ of the total Mφ count (PMN-binding Mφ × 100/total number of Mφ). Each symbol indicates the median of a triplicate analysis. *P < .05; **P < .005. (B) Active TGF-β1 concentrations measured by ELISA in supernatants of unstimulated WT and Vav3−/− Mφ and in cocultures between Mφ and PMN of the indicated genotypes. Data given as mean ± SD (n = 4). **P < .005. (C) Active TGF-β1–producing Mφ (yellow) identified by immunostaining of cryosections from 3- and 5-day-old Vav3−/− and WT wounds for TGF-β1 (red) and F4/80 (green). Cell nuclei are counterstained with DAPI (blue; original magnification ×20, scale bar indicates 150 μm; he, hyperproliferative epidermis; we, wound edge; and gt, granulation tissue). (D) Statistical analysis of 16 wounds derived from WT mice and from Vav3−/− mice after wounding and injection of viable WT Mφ (WT Mφ+) or of Vav3−/− Mφ (Vav3−/− Mφ+). Wound areas are expressed as percentages of the initial (day 0) wound size. Results represented as scatter plots. Bars indicate medians of each cohort (n = 4). *P < .05 by Mann-Whitney test. (E) Quantitative evaluation of active TGF-β1 measured by specific ELISA in lysates of 5- and 7-day-old wounds of WT mice and Vav3−/− mice after wounding and injection of viable WT Mφ (WT Mφ+) or Vav3−/− Mφ (Vav3−/− Mφ+) into wound margins. Results representative of 2 independent experiments are expressed as mean plus or minus SD (n = 4). *P < .05 by Student t test. (F) Lethally irradiated WT mice reconstituted with bone marrow from Vav3−/− mice reveal a significant delay in wound healing. Statistical analysis of 16 wounds per studied time point derived from lethally irradiated WT mice reconstituted with bone marrow from either WT (■) or Vav3−/− (□) mice were subjected to image analysis. Wound areas are expressed as percentages of the initial (day 0) wound size. Results given as mean ± SD (n = 4) reflect 1 of 2 independent experiments. *P < .05 by Mann-Whitney test. (G) Western blot analysis of expression levels of αSMA, TGFβ-RII and PECAM-1 equilibrated to total actin and vimentin levels in wound margins of WT and Vav3−/− bone marrow chimeric mice at days 5 and 7 after wounding (cWT, WT chimera with WT bone marrow; cVav3−/−, WT chimera with Vav3−/− bone marrow).
Firm adhesion of target cells to phagocytes markedly contributes to phagocytic efficacy.4,14,38 Indeed, we observed a defective phagocytosis of apoptotic WT or Vav3−/− PMN by Vav3−/− Mφ, at least partly caused by the impaired physical attachment of apoptotic PMN to Mφ (Figure 4A). Similar to the defective adhesion, coculture of Vav1−/−/Vav3−/− PMN with WT Mφ moderately reduced PMN engulfment. Vav3−/− and WT Mφ engulfed identical amounts of latex beads (data not shown), suggesting that the phagocytic machinery itself is functional, and that Vav3 deficiency specifically affected phagocytosis of apoptotic PMN by Vav3−/− Mφ.
Next, we cocultured Mφ with apoptotic PMN for 24 hours to assess the supernatants for concentrations of active TGF-β1 by ELISA (Figure 4B). WT or Vav3−/− Mφ cultivated in the absence of PMN, as well as cultures of viable PMN with either genotype of Mφ rendered negligible amounts of TGF-β1, whereas high concentrations of active TGF-β1 of approximately 215 pg/mL were found in supernatants of WT Mφ/PMN cocultures. Active TGF-β1 decreased significantly to below 100 pg/mL after coculture of Vav3−/− Mφ with either WT or Vav3−/− PMN, very much suggesting that Vav3 deficiency in Mφ predominantly contributes to defective adhesion-dependent phagocytosis of apoptotic PMN by Mφ, with significantly decreased active TGF-β1.
The finding that TGF-β1 concentrations particularly depend on Vav3 expression in Mφ was confirmed by immunostaining in vivo, with lower numbers of F4/80+ TGF-β1-producing Mφ present at Vav3−/− wound edges at days 3 and 5 after wounding, by contrast with high numbers of F4/80+ TGF-β1–producing Mφ at WT wound sites (Figure 4C).
To differentiate whether reduced Mφ recruitment, or rather their impaired phagocytic function with reduced release of active TGF-β1 were causal for the impaired wound healing in Vav3 deficiency, we next injected WT or Vav3−/− Mφ subcutaneously directly in the wound margins of Vav3−/− and WT mice at day 2 after wounding, thus circumventing the Vav3−/− Mφ transmigration defect. Before injection, viability and proper functions of Mφ were confirmed by Trypan blue exclusion, appropriate phagocytosis of apoptotic PMN and robust interleukin-12 (IL-12) release upon maximal stimulation (data not shown). Notably, injection of WT Vav3-competent Mφ in wound margins of Vav3−/− mice significantly increased the level of active TGF-β1 at days 5 and 7 after wounding (Figure 4E), accelerated wound closure at day 5 and fully rescued the wound healing of Vav3−/− mice by day 10. By contrast, injection of Vav3−/− Mφ in Vav3−/− wound margins failed to increase active TGF-β1 levels and to rescue impaired wound healing (Figure 4D,E), clearly indicating the requirement for Vav3 in Mφ for phagocytic uptake of PMN and subsequent release of active TGF-β1, eventually promoting proper wound healing.
To further confirm the exclusive requirement for Vav3 in leukocytes, but not in other cells for tissue repair, we next studied wound healing of lethally irradiated WT mice reconstituted with WT or Vav3−/− bone marrow. Notably, Vav3-competent mice reconstituted with Vav3−/− bone marrow displayed a delay in wound healing similar to that observed for Vav3−/− mice (Figure 4F). Moreover, compared with WT control chimeras, wounds from Vav3−/− chimeras showed reduced myofibroblast differentiation and impaired angiogenesis as assessed by αSMA, TGFβ-RII, and PECAM-1 expression in tissue lysates (Figure 4G), confirming that Vav3 deficiency exclusively in leukocytes is causal for the impaired wound healing.
Collectively, wild-type levels of Vav3 in Mφ are essential for proper wound healing and, if absent, result in defective phagocytosis of apoptotic PMN by Mφ with reduced oxidative burst and reduced release of active TGF-β1, impaired myofibroblasts-driven wound closure, and neo-angiogenesis. The delayed wound healing phenotype of Vav3−/− mice closely resembles that observed in LAD1 patients and CD18−/− mice,4 and neither Vav3−/− nor CD18−/− Mφ injected into wound margins of Vav3−/− mice could rescue the wound healing defect (Figures 4D, 5E). This may indicate that CD18 deficiency and Vav3 deficiency result in identical defects in Mφ and, thus, suggest that CD18 and Vav3 are within the same signaling pathway controlling engulfment of PMN by Mφ and active TGF-β1 release. Therefore, we set out to further investigate whether Vav3 may qualify as a downstream target in the β2-integrin (CD18)–dependent signaling in Mφ.
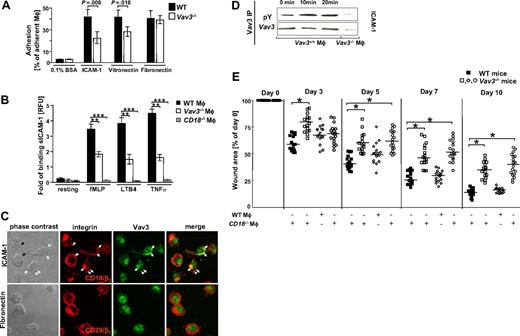
Vav3 is a downstream target of β2-integrin–dependent macrophage adhesion. (A) Adherence of TNFα-stimulated, CMFDA-loaded WT and Vav3−/− Mφ plated onto β1-integrin (fibronectin)–, β2-integrin (ICAM-1)–, or β3-integrin (vitronectin)–specific ligands or on 0.1% BSA as control is expressed as percentage of adherent Mφ related to input cells measured after 15 minutes of incubation. Representative results for 3 independent experiments are shown as mean ± SD (n = 3). P assessed by Student t test. (B) Binding of soluble ICAM-1/Fc-FITC by unstimulated (resting) or fMLP-, LTB4- and TNFα-stimulated WT, Vav3−/− and CD18−/− Mφ. Results are given in RFU, representing the ratio between the mean green fluorescence intensities of Mφ incubated with ICAM-1/Fc and Mφ incubated with human IgG for each stimulation. Bars indicate means ± SD of triplicate measurements and are representative for 3 different experiments. **P < .005; ***P < .001 by Student t test. (C) Confocal microscopy of WT Mφ plated for 15 minutes on ICAM-1 (top panel) revealed β2 integrins (CD18) (red) clustering at focal adhesion sites (open and filled arrows). Vav3 (green) colocalized with CD18 in more than 60% of the plated Mφ within the focal adhesion contacts (filled arrows). Colocalization is indicated by the yellow staining. No colocalization occurred between Vav3 and CD29 upon plating Mφ onto fibronectin (bottom panel). (D) Vav3 phosphorylation detected by Vav3 immunoprecipitation and subsequent blotting against anti-phosphotyrosine antibody (pY) induced 10 minutes or 20 minutes after plating Mφ on ICAM-1–coated plates. Total Vav3 levels served as loading controls, Vav3−/− Mφ served as technical controls. Data are representative of at least 3 independent experiments. (E) Injection of β2-integrin (CD18)−/− Vav3 competent Mφ into wound margins of Vav3−/− mice does not rescue the wound healing defect. WT or CD18−/− bone marrow–derived Mφ were injected at 4 sites into wound margins of Vav3−/− mice. Statistical analysis of 16 wounds of each studied time point derived from WT mice or from Vav3−/− mice after wounding and injection of viable WT Mφ (WT Mφ) or CD18−/− Mφ (CD18−/− Mφ+). Wound areas are expressed as percentages of the initial (day 0) wound size. Results represented as scatter plots. Bars indicate medians of each cohort (n = 4). *P < .05 by Mann-Whitney test.
Vav3 is a downstream target of β2-integrin–dependent macrophage adhesion. (A) Adherence of TNFα-stimulated, CMFDA-loaded WT and Vav3−/− Mφ plated onto β1-integrin (fibronectin)–, β2-integrin (ICAM-1)–, or β3-integrin (vitronectin)–specific ligands or on 0.1% BSA as control is expressed as percentage of adherent Mφ related to input cells measured after 15 minutes of incubation. Representative results for 3 independent experiments are shown as mean ± SD (n = 3). P assessed by Student t test. (B) Binding of soluble ICAM-1/Fc-FITC by unstimulated (resting) or fMLP-, LTB4- and TNFα-stimulated WT, Vav3−/− and CD18−/− Mφ. Results are given in RFU, representing the ratio between the mean green fluorescence intensities of Mφ incubated with ICAM-1/Fc and Mφ incubated with human IgG for each stimulation. Bars indicate means ± SD of triplicate measurements and are representative for 3 different experiments. **P < .005; ***P < .001 by Student t test. (C) Confocal microscopy of WT Mφ plated for 15 minutes on ICAM-1 (top panel) revealed β2 integrins (CD18) (red) clustering at focal adhesion sites (open and filled arrows). Vav3 (green) colocalized with CD18 in more than 60% of the plated Mφ within the focal adhesion contacts (filled arrows). Colocalization is indicated by the yellow staining. No colocalization occurred between Vav3 and CD29 upon plating Mφ onto fibronectin (bottom panel). (D) Vav3 phosphorylation detected by Vav3 immunoprecipitation and subsequent blotting against anti-phosphotyrosine antibody (pY) induced 10 minutes or 20 minutes after plating Mφ on ICAM-1–coated plates. Total Vav3 levels served as loading controls, Vav3−/− Mφ served as technical controls. Data are representative of at least 3 independent experiments. (E) Injection of β2-integrin (CD18)−/− Vav3 competent Mφ into wound margins of Vav3−/− mice does not rescue the wound healing defect. WT or CD18−/− bone marrow–derived Mφ were injected at 4 sites into wound margins of Vav3−/− mice. Statistical analysis of 16 wounds of each studied time point derived from WT mice or from Vav3−/− mice after wounding and injection of viable WT Mφ (WT Mφ) or CD18−/− Mφ (CD18−/− Mφ+). Wound areas are expressed as percentages of the initial (day 0) wound size. Results represented as scatter plots. Bars indicate medians of each cohort (n = 4). *P < .05 by Mann-Whitney test.
Vav3 is a downstream target of β2-integrin–dependent Mφ adhesion
As the physical attachment of Mφ to apoptotic PMN depends on β2-integrins, we studied the adhesive property of WT and Vav3−/− Mφ onto the β2-integrin ligand intercellular adhesion molecule 1 (ICAM-1), which mediates β2-integrin–dependent formation of the phagocytic synapse4 (Figure S2A). Integrin-mediated Mφ activation was achieved by TNFα stimulation before plating on defined ligands. Comparable CD18 expression levels were confirmed for WT and Vav3−/− Mφ and PMN before and after TNFα stimulation as assessed by flow cytometry (Figure S2D and data not shown). Notably, Vav3−/− Mφ revealed a significantly reduced adhesion to ICAM-1 compared with WT Mφ. This Vav3-dependent β2-integrin–mediated adhesion to ICAM-1 was specific, as adhesion of both WT and Vav3−/− Mφ to BSA was insignificant. Compared with the β2-integrin–dependent reduced adhesion to ICAM-1, a minor, but significant decrease in adhesion was detected when Vav3−/− Mφ were plated onto the β3-integrin ligand vitronectin, indicating that Vav3, at least in part, controls β3-integrin–dependent Mφ adhesion. This finding is in line with previous reports indicating Vav3 to control αvβ3–dependent polarization and spreading of osteoclasts.32 By contrast, Vav3 deficiency did not affect β1-integrin–mediated binding to fibronectin, suggesting that Vav3 is preferentially responsible for β2-integrin–mediated Mφ adhesion and, to a lesser extent, contributes to β3-integrin–mediated Mφ adhesion (Figure 5A).
Moreover, these findings suggested a role for Vav3 in β2-integrin–dependent strengthening of the synapse between Mφ and PMN, which depends on ICAM-1 binding. To investigate the contribution of Vav3 to β2-integrin activation in more detail, we assessed β2-integrin affinity of WT, Vav3−/− and CD18−/− Mφ by their capacity to bind soluble ICAM-1 (sICAM-1). Activated WT Mφ bound sICAM-1 up to 4 times more efficiently compared with unstimulated or to CD18−/− Mφ. Remarkably, in the absence of Vav3 the capacity of Mφ to bind sICAM-1 was diminished to 50% when stimulated with fMLP and to 30% when activated by TNFα (Figure 5B). These results strongly suggest that Vav3 controls integrin affinity upon activation and thus regulates the extent of outside-in signaling required for firm adhesion, phagocytosis and oxidative burst.
To further characterize the role of Vav3 as a downstream target of β2-integrin–dependent signaling in Mφ, we analyzed the cellular localization of Vav3 and CD18 during Mφ adhesion on ICAM-1 using confocal microscopy. CD18 (Figure 5C second top panel, red) redistributed and clustered within focal adhesion contacts (Figure 5C second top panel, open arrows) at the cell membrane when Mφ were plated onto the β2-integrin specific ligand ICAM-1, which is also present in the phagocytic synapse (Figure 5C). In more than 60% of the Mφ, Vav3 (Figure 5C third top panel, green) colocalized with CD18 at focal adhesion sites as revealed by the yellow staining indicated with filled arrows (Figure 5C fourth top panel). In contrast, no stringent colocalization of CD29 (β1-integrins) with Vav3 was observed when Mφ were plated onto fibronectin (Figure 5C bottom panels), suggesting that Vav3 is not involved in the β1-integrin–dependent adhesion, but in the β2-integrin–dependent adhesion to ICAM-1 and, thus, in the phagocytic cup formation.
Activation of Vav proteins requires phosphorylation of specific tyrosine residues as a prerequisite to activate downstream targets. So far, integrin signaling via Vav3 has rarely been addressed in Mφ.31,32 To study whether Vav3 is activated in response to β2-integrin engagement, the phosphorylation status of Vav3 after Mφ binding to ICAM-1 was assessed. Before adhesion, Mφ were treated with TNFα to enhance integrin adhesive activity. No phosphorylation occurred upon TNFα treatment when Mφ were plated on BSA-coated dishes, excluding any direct effect of TNFα on the phosphorylation status of Vav3 (data not shown). Induction of Vav3 phosphorylation was observed at 10 minutes after plating Mφ onto ICAM-1, whereas the level of total Vav3 remained unchanged (Figure 5D).
Additional evidence for the β2-integrin–dependent Vav3 activation was provided by the observation that CD18hypomorphic Mφ (with only 15% of CD18 WT expression levels39 ) revealed significantly reduced Vav3 phosphorylation upon adherence on ICAM-1, whereas CD18−/− Mφ attached poorly and could not be harvested in sufficient quantities (unpublished data).
These data, together with the finding that, similar to injection of Vav3−/− Mφ, injection of CD18−/− Mφ in wound margins does not restore the impaired wound healing of Vav3−/− mice (Figure 5E), suggest that Vav3 in Mφ constitutes a key molecule in the β2-integrin–dependent formation of a functional phagocytic synapse essentially required for proper wound healing.
Discussion
We here found Vav3 proteins to be specifically required for proper healing of cutaneous full-thickness wounds, whereas their absence results in delayed angiogenesis and impaired wound closure. This phenotype closely resembles nonhealing wounds in LAD1 patients with functional mutations in the CD18 gene16,40 and CD18−/− mice.4 The impaired wound closure in Vav3−/− mice is essentially due to a β2-integrin–dependent deficient formation of the phagocytic synapse between Vav3−/− Mφ and apoptotic PMN. The defective engulfment of PMN leads to reduced release of active TGF-β1 from Mφ, which in turn is responsible for defective myofibroblast-driven wound contraction and reduced angiogenesis. Injection of either recombinant TGF-β1 or Vav3 competent Mφ into wound margins of Vav3−/− mice fully rescued the defective wound healing.
We here identified a previously unrecognized role for Vav3 in Mφ, linking to a central role for Vav3 in β2-integrin downstream signaling in Mφ, which, if defective, may contribute to disturbances in tissue repair and host defense in general. In particular, between days 5 and 7 after wounding, the phase of granulation tissue contraction and neo-angiogenesis, Vav3−/− wounds were significantly larger compared with WT wounds. Reduced αSMA and TGFβ-RII expression in Vav3−/− granulation tissue suggest that, similar to CD18 deficiency,4 impaired wound closure in Vav3−/− mice results from reduced myofibroblasts differentiation. Myofibroblasts differentiation is characterized by the de novo expression of αSMA, conferring wound contraction in vivo41 and in vitro.42,43 TGF-β1 is the major growth factor inducing αSMA+myofibroblast differentiation44 through specific binding to TGFβ-RII.45 TGF-β1 amplifies its response via an autocrine mechanism with up-regulation of TGFβ-RII and enhanced release of active TGF-β1 from myofibroblasts and inflammatory cells. Therefore, decreased TGF-β1 release from Mφ in the initial phases of wound healing will result in substantially amplified effects on myofibroblasts differentiation and angiogenesis5,44 driving tissue repair. We here demonstrate that reduced active TGF-β1 in granulation tissue of Vav3−/− mice is the major pathogenic event in impaired wound healing, since wound contraction is fully rescued after subcutaneous injection of rhTGF-β1. TGF-β1 release from Mφ is essentially induced upon phagocytosis of apoptotic PMN at wound sites.3,4 As both PMN and Mφ are mandatory for the formation of the phagocytic synapse with sufficient TGF-β1 release, we studied their emigration patterns.
Mφ recruitment at wound sites was reduced in Vav3 deficiency; however, the recruitment defect was similar in Vav3−/− and Vav1−/−; Vav3−/− mice, suggesting that Vav3 alone is critical for the Mφ extravasation to wound sites. This is in line with previous data reporting Vav3 to control cytoskeleton organization, polarization and spreading of Mφ32 or MCS-F-induced Mφ chemotaxis,46 all critical functions for Mφ emigration. Irrespective of the underlying mechanism, defective Mφ recruitment was not causal for the impaired wound healing in Vav3−/− mice, as circumventing this defect by injection of Vav3−/− Mφ directly into the wound margin failed to rescue the healing disturbance. However, differences in the recruitment of Mφ and PMN between Vav3−/− and the β2-integrin(CD18)−/− mice may explain the earlier onset of the wound healing delay in Vav3−/− mice compared with CD18−/− mice (see also “Discussion” section of online data supplement).
In this report, we define the defective formation of the phagocytic synapse between Vav3−/− Mφ and apoptotic PMN as the major cause for the reduced release of active TGF-β1 and the resulting wound healing deficiency. In fact, injection of Vav3−/− Mφ directly into Vav3−/− wound margins failed to restore the impaired wound closure. In contrast, injection of WT Mφ fully restored the wound defect and the diminished TGF-β1 in Vav3−/− mice at levels comparable with WT mice, clearly demonstrating that Vav3 deficiency in Mφ with impaired adhesion-dependent phagocytosis of apoptotic PMN is causal for the wound healing defect in Vav3−/− mice.
Phagocytosis of apoptotic PMN by Mφ and the concomitant release of active TGF-β1 were significantly reduced in absence of Vav3 in Mφ, but not in PMN. Even Vav1/Vav3 double deficiency in PMN cocultured with WT Mφ reduced the phagocytic cup formation only moderately, but to much lower extent compared with Vav3−/− Mφ cocultured with WT PMN. Because adhesion of target cells to phagocytes before engulfment contributes to the efficacy of cell uptake,4,14,38 the impaired attachment between PMN and Vav3−/− Mφ may account for the disturbed phagocytosis. Thus, Vav3 is essentially required in Mφ for efficient cell-cell contacts and thus promotes engulfment of apoptotic PMN by Mφ. Vav3 deficiency leads to the inefficient Mφ-PMN attachment and impaired PMN uptake, resulting in reduced release of active TGF-β1 from Mφ. Possibly, the diminished oxidative burst in Vav3−/− Mφ upon phagocytosis of apoptotic PMN contributes to the reduced TGF-β1 activation and consequences thereof. In fact, activation of TGF-β1 has been reported to be mediated by ROS,36 and VaV guanin nucleotide exchange factors link integrin-mediated signaling with the assembly of the nicotinamid-adenin dinucleotide phosphate oxidase complex responsible for the production of ROS in Mφ.47
The almost identical phenotypes with delayed wound closure, defective phagocytic synapse formation and reduced release of active TGF-β1 in CD18−/− and Vav3−/− mice very much suggest that β2-integrins may preferentially control these key steps via the guanine exchange factor Vav3 in Mφ. Several lines of evidence support this view.
First, β2-integrins are mandatory for the attachment-mediated phagocytosis of PMN by Mφ and for the phagocytic synapse formation. For this, binding of Mac-1 (CD11b/CD18) on Mφ to its counterreceptor ICAM-1 on PMN and vice versa are essential.4 In case Vav3 constituted a downstream target of CD18, we predicted that Vav3−/− Mφ would display reduced adhesion to ICAM-1. And exactly that is what we found. By contrast, Vav3−/− Mφ adhesion onto fibronectin, a β1-integrin–dependent ligand, was not altered, strongly suggesting a critical role for Vav3 in β2-integrin–dependent Mφ adhesion. Even though we found a decrease in adhesion of Vav3−/− Mφ to the β3-integrin ligand vitronectin, suggesting that Vav3 plays a role in β3-integrin signaling. Vitronectin is an extracellular matrix protein that does not occur at the synapse between Mφ and apoptotic PMN, and thus may not be relevant in the phagocytic cup formation studied here. In fact, β3-integrins/CD36 complexes interact with unknown moieties on PMN via a thrombospondin bridge.48
In support of Vav3 preferentially relaying β2-integrin–mediated signals, Vav3 distinctly controlled the Mφ capacity to bind β2-integrin–specific ligands upon activation, as Vav3−/− Mφ presented a severely reduced capacity to bind sICAM-1 compared with WT Mφ. In contrast to this finding, a previous report on phagocytosis of complement-opsonized red blood cells by Vav3−/− and Vav1−/−Vav3−/− Mφ indicated that Vav3 alone has no effect on β2-integrin–mediated phagocytosis and that even the Vav1/Vav3 double deficiency does not impair β2-integrin–mediated adhesion to Mφ.31 However, this study investigated rapid phagocytosis of C3-biopsonized particles by Mφ previously stimulated with PMA, which is known to directly activate the protein kinase C required for complement-mediated phagocytosis.49 This may have completely compensated for the absence of upstream Vav proteins during adhesion and may have partially compensated for the lack of Vav3 during β2-integrin–dependent phagocytosis. We here studied binding of sICAM-1 to Mφ stimulated with fMLP, LTB4, or TNFα, which initiate a high-affinity ICAM-1–binding state for β2-integrins.50 Our experimental approach using a physiologic ligand (ICAM-1) and macrophage stimuli occurring in a variety of inflammatory conditions closely reflects the adhesion-dependent phagocytosis of apoptotic PMN during wound healing, and these conditions may additionally require Vav3 signaling.
Second, binding of WT Mφ to ICAM-1, the major ligand for Mac-1 in the phagocytic synapse resulted in the phosphorylation of Vav3, essentially required for Vav3 exchange activity and activation of Rho-GTPases. Vav3 phosphorylation in response to macrophage adhesion to ICAM-1 was reduced in macrophages with reduced CD18 expression, further strengthening the role of Vav3 as a potential downstream target of β2-integrin–dependent signaling.
Third, colocalization of CD18 and Vav3 specifically occurred in Mφ upon adhesion to ICAM-1 but not to fibronectin.
Fourth, apart from the almost exact recapitulation of the wound healing phenotype of CD18−/− mice with deficient phagocytic cup formation, reduced release of active TGF-β1 and impaired myofibroblast-driven wound contraction, Vav3−/− mice exhibit spontaneous ulcerations, as earlier reported for CD18−/− mice.10
Finally and most importantly, injection of either Vav3−/− or CD18−/− Mφ into wound margins failed to rescue the wound healing defect of Vav3−/− mice. These data unequivocally support a critical role for Vav3 as a downstream target in β2-integrin–dependent signaling. However, the β2-integrins–Vav3 axis may not exclusively mediate phagocytic synapse formation, engulfment of apoptotic PMN, and release of active TGF-β1 from Mφ, but other receptors may play a role.51 At least, inhibition of β3-integrins resulted in a significant, though—compared with blocking the CD18 or ICAM-1 function—markedly lesser decrease in adhesion-dependent phagocytosis of apoptotic PMN and a milder impairment of active TGF-β1 release by Mφ in vitro. These data suggest β2-integrins to preferentially, but not exclusively control these processes. Even though β3-integrins most likely contribute to the phagocytosis of apoptotic PMN and Mφ in vitro, their in vivo role is unclear. In contrast to a severe wound healing defect in β2-integrin(CD18)−/− mice and LAD1 patients15 due to a decrease in active TGF-β1,4 β3-integrin−/− mice were reported to present accelerated wound healing and enhanced TGF-β1 release and signaling.52
Our data show that Vav3 cell-specifically relays β2-integrin signaling, thus fine-tuning distinct cellular functions. Although Vav3 is not required for β2-integrin–dependent PMN functions, it is distinctly required for Mφ recruitment and their capacity to build the phagocytic synapse efficiently. There has been a recent focus on targeting signaling molecules as an approach to modulate and rebalance inflammation in vivo.53 Targeting Vav3 in Mφ by agonists or antagonists may specifically modulate Mφ recruitment and phagocytosis of apoptotic PMN with subsequent modulation of TGF-β1 release, while preserving PMN recruitment, a strategy that would minimize overall immune suppression. Alternatively, as enhanced TGF-β1 release from activated Mφ is pathogenic in a variety of fibrotic conditions54 including hypertrophic scar and keloid formation, targeting of Vav3 may be particularly beneficial for these difficult-to-treat conditions.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Heidi Hainzl, Henriette Peter, and Carmen Hauser for excellent technical assistance.
K.S.-K., A.S., K.L.R., A.G., C.S., and K.D.F. are supported by Deutsche Forschungsgemeinschaft (DFG) grants SFB497-C7 (K.S.-K., A.S.), KFO142 (K.S.-K.), SFB497-C11 (K.L.R., A.G.), DFG SU195/3-1 (C.S.), and SFB497-C6 (K.D.F.).
Authorship
Contribution: A.S. and T.P. designed and performed research, analyzed data, and wrote the manuscript; J.S., T.O., H.W., F.M., M.W., C.S., and B.W. designed research and analyzed data; A.G., K.L.R., X.R.B., and K.D.F. provided research tools, designed research and analyzed data; and K.S.-K. designed research and analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karin Scharffetter-Kochanek, MD, Department of Dermatology and Allergic Diseases, University of Ulm, Maienweg 12, 89081 Ulm, Germany; e-mail: karin.scharffetter-kochanek@uniklinik-ulm.de.
References
Author notes
*A.S. and T.P. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal