Vaccine-based expansion of T cells is one approach to enhance the graft-versus-tumor effect of allogeneic bone marrow transplantation (BMT), but the complex immunobiology of the allogeneic environment on responses to tumor vaccines has not been well characterized. We hypothesized that subclinical graft-versus-host disease (GVHD) impairs immunity, but modulation of gamma interferon (IFN-γ) signaling could reverse this effect. Dendritic cell vaccines and donor lymphocyte infusions (DLIs) were incorporated into a minor histocompatibility antigen–mismatched, T cell–depleted, allogeneic BMT mouse model. Animals were then challenged with H-Y expressing tumors. CD4+ and CD8+ responses to H-Y were diminished in vaccinated allogeneic versus syngeneic BMT recipients with DLI doses below the threshold for clinical GVHD, especially in thymectomized hosts. IFN-γ receptor 1–deficient (IFN-γR1−/−) T cells cannot cause GVHD but also have diminished vaccine responses. Remarkably, IFN-γR1−/− bone marrow abrogates GVHD, allowing higher DLI doses to be tolerated, but improves vaccine responses and tumor protection. We conclude that tumor vaccines administered after allogeneic BMT can augment graft-versus-tumor if GVHD is avoided and that prevention of IFN-γ signaling on donor bone marrow is an effective approach to preventing GVHD while preserving immunocompetence.
Introduction
The allogeneic bone marrow transplantation (BMT) environment provides a milieu for a potent graft-versus-tumor (GVT) effect that contributes substantially to the cure of certain malignancies. Recognition of minor histocompatibility antigens (mHAs) by donor T cells contributes to antitumor responses.1,2 Elevated levels of inflammatory mediators generated by the BMT preparative regimen, such as gamma interferon (IFN-γ), have the potential to increase the ability to preferentially expand specific T-cell populations.3,4 Although IFN-γ is immune activating in many models, recent studies have also demonstrated immunosuppressive effects of this molecule as well.5,–7 In several murine models, IFN-γ levels are elevated early in graft-versus-host disease (GVHD), peaking before clinical symptoms of GVHD appear.8 IFN-γ levels are also elevated in T cells isolated from patients with GVHD.5 Thus, the relative contributions of IFN-γ to beneficial GVT effects versus immunosuppressive effects of GVHD appear contradictory
Despite the immunosuppressive environment associated with GVHD, patients with mild GVHD appear to have better survival than patients with no GVHD, but as GVHD worsens, there is a drop-off in survival, secondary to mortality from GVHD and the associated immunosuppression.9,–11 This paradigm suggests that one could allow GVHD to occur as long as it remains mild, accept the accompanying GVT benefit, and then treat once GVHD gets more severe. Unfortunately, the treatments for GVHD are globally immunosuppressive, which would also impact GVT. Another approach could be to minimize GVHD from the beginning, through T-cell depletion, and then rebuild the immunity in a systematic fashion, such as with vaccines to skew the T-cell repertoire toward the tumor. One problem is that it is not known to what extent the T cells that mediate GVT and GVHD are identical or overlapping.2,12 Because residual disease after BMT may be resistant to standard chemotherapy, strategies are needed to selectively orient the post-BMT immune environment toward GVT and away from GVHD.13 Immunizing donor T cells expanding in the recipient to antigens expressed on the tumor, but with limited expression on GVHD target tissues, could increase the number of GVT-causing T cells while avoiding generation of GVHD-causing T cells. We and others have previously shown that this approach is very effective in the autologous setting, resulting in marked “skewing” of the resultant T-cell repertoire toward specific antigens provided during the expansion process.14,,–17 Although tumor vaccines are beginning to demonstrate success in the autologous setting, there are only limited data on the use of vaccination after allogeneic BMT.12,13,18 In particular, it remains unknown whether the inflammatory environment associated with GVHD will serve to augment vaccine responses via an adjuvant effect or if the immunosuppressive effects of GVHD will lead to diminished vaccine responses.
Because BMT is characterized by prolonged host lymphopenia and a lack of adequate donor lymphocyte immunity, it is difficult to achieve significant responses to a vaccine.19,20 Furthermore, in humans, T-cell immune reconstitution is often compromised because of the inability of the thymus to regenerate effectively in adults21 and therapy-related thymic toxicity.22 In addition, GVHD can also adversely affect thymic function.22 Thus, providing a source of mature, potentially alloreactive T cells through donor lymphocyte infusions (DLIs), preferably well after the initial cytokine storm from the preparative regimen to minimize GVHD, is one approach to gradually replenish the lymphopenic environment after BMT. By combining the DLI with a vaccine against a tumor antigen, one could exploit the lymphopenic environment to help expand T cells mediating GVT. To maximize this approach, it will be important to understand the impact of the complex allogeneic BMT environment on vaccine-mediated T-cell expansion. We therefore hypothesized that the immunosuppressive effects of GVHD would impact the induction of antigen-specific immune responses by vaccination and that modulation of donor IFN-γ signaling could abrogate this immunosuppression.
Methods
Mice
C57BL/6 (H-2b) (B6), C3H.SW (H-2b), and C57BL/6 × C3H.SW (H-2b) (F1) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). These mice are major histocompatibility complex (MHC) antigen-matched and mHAg-mismatched at multiple antigens.23 B6 gamma interferon receptor 1 knockout mice (IFN-γR1−/−) were also obtained from The Jackson Laboratory and used as bone marrow and/or DLI donors where indicated. Mice were age-matched and used between 4 and 8 weeks of age. Where indicated, thymectomized mice underwent vacuum suction removal of the thymus according to standard protocol. The mice were housed in a specific pathogen–free facility throughout the study. The Animal Care and Use Committee at the National Institutes of Health approved all protocols.
T cell–depleted BMT
Bone marrow cells were flushed from the tibias and fibulas of B6 female mice using 10% complete mouse media (CMM; RPMI 1640 with 10% heat-inactivated fetal calf serum, 1% N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer, 1% nonessential amino acids, 1% sodium pyruvate, 1% penicillin/streptomycin, 1% l-glutamine, all Invitrogen, Carlsbad, CA; and 50 μM 2-mercaptoethanol, Sigma-Aldrich, St Louis, MO), passed through a 70-μm nylon filter, and erythrocyte-depleted using ACK lysing buffer (Lonza Walkersville, Walkersville, MD). T cells were depleted from donor bone marrow grafts using anti-CD4, anti-CD8, and anti-CD90 microbeads through magnetic cell sorting (Miltenyi Biotec, Auburn, CA). T cell–depleted marrow was resuspended in serum-free RPMI media for intravenous tail vein injection. Lethally irradiated (10 Gy) B6 (syngeneic) or F1 (allogeneic) mice were injected intravenously through the tail vein with 4 × 106 T cell–depleted bone marrow cells. Recipients were weighed twice every 7 days. Survival and clinical monitoring of GVHD, including observation for skin changes (ruffling or hair loss), diarrhea, and hunched posture, occurred daily. GVHD was further assessed by weight loss, diminished splenic B cells, and histologic grading of GVHD target organs. Subclinical GVHD was defined as a mouse with no apparent weight loss or clinical symptoms, but with decreased splenic B cells or with histologic changes consistent with GVHD. Moribund mice were killed.
DLIs and DC vaccines
Lymphocytes were generated from single-cell suspensions of inguinal, axillary, and cervical lymph nodes harvested from B6 female mice in CMM. Cells were washed, counted, and resuspended in serum-free RPMI media for intravenous injection through the tail vein at 14 and 28 days after BMT. Tolerized DLIs were generated from thymus-bearing F1 recipients transplanted with T cell–depleted B6 bone marrow. At day 42 after BMT, the lymph nodes of the F1 recipients reconstituted with B6 bone marrow were processed and then adoptively transferred into the experimental F1 recipient.
Dendritic cells (DCs) used for vaccines were prepared from male B6 bone marrow as previously described.24 DCs were activated with 4 μg/mL anti-CD40 on day 7 and collected within 24 hours of activation, resuspended in serum-free media, and injected intraperitoneally at 105 cells per recipient at the time of DLI.
MB49 tumor challenge
The MB49 tumor cell line (generously provided by Dr Edmund Lattime,Robert Wood Johnson Medical School, New Brunswick, NJ) is derived from a chemically induced urothelial carcinoma in a male B6 mouse and expresses the male-specific mHA H-Y.25 MB49 cells were maintained in culture at 37°C in 5% CO2 in CMM. Exponentially growing tumor cells were prepared as a single cell suspension in serum-free media and injected into the subcutaneous fat of the flank at a dose of 2 × 106 tumor cells on day 42 after BMT. Tumors were measured in 2 dimensions (length × width) 2 times a week by digital caliper, and approximate spherical volumes were calculated (L/2) × (W/2) × (L + W/4) × (4π/3) after each measurement. Mice were killed with CO2 when tumor diameters reached 2 cm, in accordance with animal protocols. If a mouse was found dead, the previously recorded tumor measurement was carried for the rest of the experiment.
ELISPOT
Enzyme-linked immunosorbent spot (ELISPOT) assay was performed on day 42 after BMT as previously described25 with splenocytes placed in a 96-well cellulose membrane plate precoated with anti-γ interferon (IFN-γ) antibody for 24 hours with H-Y peptide-pulsed female stimulators. All samples were run in triplicate, and the net number of peptide responding cells was determined by subtracting background from wells containing irrelevant peptide.
Histopathologic analysis of GVHD
Livers, small intestines, and skin from killed BMT recipients were fixed in 10% buffered neutral formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. A veterinary pathologist graded tissue sections in blinded fashion. A semiquantitative scale from 0 to 4 was used where histopathologic changes were identified as follows: 1 indicates minimal; 2, mild; 3, moderate; and 4, severe. Cumulative histopathology scores were calculated based on the sum of individual changes of 2 to 6 parameters in each organ: hepatocellular inflammation, vacuolation, angiectasis, necrosis, bile duct hyalinosis, and oval-cell hyperplasia in the liver; villous blunting, crypt-cell hyperplasia, crypt-cell apoptosis, GALT hyperplasia, and inflammation in the small intestine; goblet-cell depletion, gland dilation, sloughing of epithelial cells into the lumen, and crypt-cell apoptosis in the colon; and melanosis dermis and lymphocytic infiltrates in the skin. Images were visualized using an Olympus Vanox AHBS3 microscope with an Olympus SPlan Apo 20×/0.70 NA objective (Olympus, Woodbury, NY). A Diagnostic Instrument Spot RT color digital camera using Spot software, version 4.0.2, was used to acquire the images (Diagnostic Instruments, Sterling Heights, MI).
Statistical analysis
Statistical tests were performed using GraphPad Prism version 4.0c for Macintosh (GraphPad Software, San Diego, CA). The last tumor volume recorded for each mouse at the time of death was used in the calculations of average tumor volume at each time point for each group after death. A one-way analysis of variance was used to assess statistical differences between cumulative tumor volumes in selected pairs of groups. Kaplan-Meier survival curves were generated and analyzed using a log-rank test to compare the survival curves. Significant differences comparing 2 groups were determined by 2-tailed Mann-Whitney test. A P value less than .05 was considered significant.
Results
Mild alloreactivity inhibits quantitative T-cell responses to DC vaccination in both thymus-bearing and thymectomized recipients
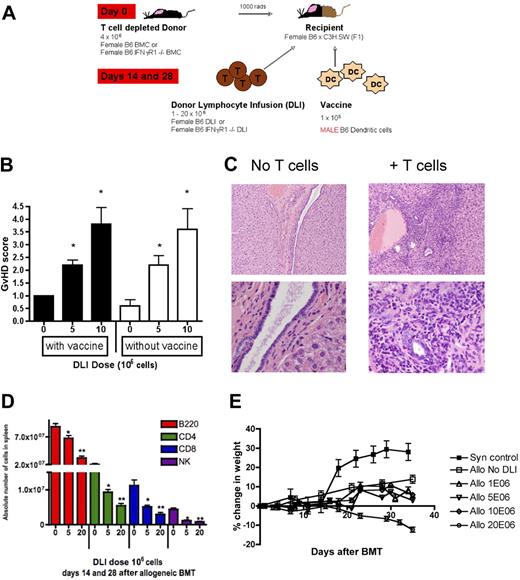
Our model was designed to study the influence of the allogeneic BMT environment on T-cell expansion mediated by DC vaccines administered with delayed administration of DLI (Figure 1A). GVHD in this BMT model was demonstrated histologically as lymphocytic infiltration of the liver at a DLI dose of 5 × 106 cells (moderate), with the severity increasing at a dose of 10 × 106 cells (high), and was not exacerbated by the DC vaccine (Figure 1B,C). Skin, small intestine, and colon were not affected (data not shown). GVHD was subclinical and sublethal at these doses of DLI. A significant reduction of B220+ cells in the spleen, a surrogate marker of GVHD in other murine models,8 as well as other lymphocyte subsets, was seen as GVHD increased in a DLI dose-dependent manner (Figure 1D). Although weight loss is a traditional symptom of GVHD in MHC mismatch models, the only DLI dose that induced appreciable weight loss in this model was 20 × 106 cells (Figure 1E). Thus, this model is a clinically relevant platform to test the impact of alloreactivity on vaccines.
GVHD after a B6→B6 × C3H.SW T cell–depleted BMT is both subclinical and sublethal, with hepatic inflammation evident before development of weight loss, and a DLI dose-dependent reduction in lymphocyte reconstitution. (A) In all experiments, T cell–depleted bone marrow was given to irradiated F1 recipients on day 0, followed by a delayed DLI with or without a male DC vaccine on days 14 and 28. (B) Histopathologic analysis of liver GVHD with a dose escalation of DLI. * P < .01, compared with no DLI group, 5 mice/group. (C) Only the liver showed significant lymphocytic infiltration on day 42, shown at both original magnifications ×10 (top panels) and ×40 (bottom panels) from recipients of allogeneic bone marrow alone and allogeneic BMT followed by DLI. (D) Spleens harvested on day 42 were analyzed by flow cytometry for enumeration of lymphocyte subsets. The percentage of the lymphocyte subset was multiplied by the splenocyte count to obtain an absolute number of cells. *P < .01; **P < .001. (E) The DLI dose-response of 0 to 20 × 106 lymph node cells was compared for GVHD-associated weight loss, 5 or 6 mice/group.
GVHD after a B6→B6 × C3H.SW T cell–depleted BMT is both subclinical and sublethal, with hepatic inflammation evident before development of weight loss, and a DLI dose-dependent reduction in lymphocyte reconstitution. (A) In all experiments, T cell–depleted bone marrow was given to irradiated F1 recipients on day 0, followed by a delayed DLI with or without a male DC vaccine on days 14 and 28. (B) Histopathologic analysis of liver GVHD with a dose escalation of DLI. * P < .01, compared with no DLI group, 5 mice/group. (C) Only the liver showed significant lymphocytic infiltration on day 42, shown at both original magnifications ×10 (top panels) and ×40 (bottom panels) from recipients of allogeneic bone marrow alone and allogeneic BMT followed by DLI. (D) Spleens harvested on day 42 were analyzed by flow cytometry for enumeration of lymphocyte subsets. The percentage of the lymphocyte subset was multiplied by the splenocyte count to obtain an absolute number of cells. *P < .01; **P < .001. (E) The DLI dose-response of 0 to 20 × 106 lymph node cells was compared for GVHD-associated weight loss, 5 or 6 mice/group.
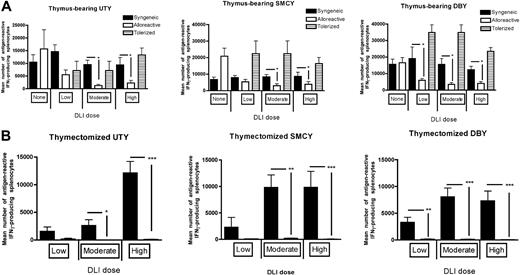
It is possible that either the inflammatory environment associated with GVHD will augment vaccine responses via an adjuvant effect or that the immunosuppressive effects of GVHD will predominate and lead to diminished vaccine responses. To address this question, we administered HY-expressing vaccines to mice with subclinical GVHD and then measured IFN-γ production of splenic T cells to the CD8+ dominant H-Y antigen, UTY, and CD8+ subdominant antigen SMCY, and the CD4+ dominant antigen, DBY. In thymus-bearing recipients, equivalent responses were observed to both class I antigens at the “low” DLI dose in all recipients, indicating that these recipients were below the threshold for GVHD. Vaccine responses were diminished in allogeneic recipients after both “moderate” and “high” DLI doses were given compared with identically treated syngeneic controls (Figure 2A). This was directly attributable to T cell–mediated alloreactivity because animals that received DLI-containing T cells tolerized to recipient alloantigens demonstrated robust vaccine responses. This observation was also not specific to B6 T cells because using C3H.SW-derived donor cells resulted in similar effects (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Vaccine responses after allogeneic BMT are significantly decreased to both class I and class II H-Y antigens in both thymus-bearing and thymectomized recipients but can be restored using T cells tolerized in a separate thymus-bearing host. None indicates no DLI was given; Low, a DLI dose of 106 cells; Moderate, 5 × 106 cells; and High, 10 or 20 × 106 cells, which produce equivalent results. All DLIs were given on days 14 and 28. (A) ELISPOT analysis of CD8+ and CD4+ T cells to the H-Y antigens was assessed in thymus-bearing recipients on day 42 after BMT, 7 to 11 mice/group, *P < .05. (B) ELISPOT analysis of CD8+ and CD4+ T cells to the H-Y antigens were assessed in thymectomized recipients on day 42 after BMT, 8 to 11 mice/group. *P < .05, **P < .01, ***P < .001.
Vaccine responses after allogeneic BMT are significantly decreased to both class I and class II H-Y antigens in both thymus-bearing and thymectomized recipients but can be restored using T cells tolerized in a separate thymus-bearing host. None indicates no DLI was given; Low, a DLI dose of 106 cells; Moderate, 5 × 106 cells; and High, 10 or 20 × 106 cells, which produce equivalent results. All DLIs were given on days 14 and 28. (A) ELISPOT analysis of CD8+ and CD4+ T cells to the H-Y antigens was assessed in thymus-bearing recipients on day 42 after BMT, 7 to 11 mice/group, *P < .05. (B) ELISPOT analysis of CD8+ and CD4+ T cells to the H-Y antigens were assessed in thymectomized recipients on day 42 after BMT, 8 to 11 mice/group. *P < .05, **P < .01, ***P < .001.
Although thymic function in mice after BMT recovers rapidly, adverse effects of age, therapy, and GVHD on human thymic function render most BMT recipients dependent primarily on thymic-independent pathways of immune reconstitution to generate antitumor immune responses during the first 6 to 12 months after BMT. For this reason, we included thymectomized recipients in these studies to ascertain whether sufficient DLI could be administered to accomplish immunocompetence as measured by vaccine responses. As we have previously shown in syngeneic BMT recipients,26 CD8+ and CD4+ HY responses could be induced but required substantial numbers of T cells. In allogeneic BMT recipients, however, little, if any, vaccine responses were observed at any DLI dose (Figure 2B) as the number of cells required for responses in syngeneic recipients exceeded the threshold for GVHD. We have previously shown that interleukin-7 (IL-7) can augment vaccine-mediated T-cell expansion from a limited T-cell dose in thymectomized mice.26 However, in allogeneic recipients, although T cells were expanded overall by IL-7, there was no improvement in vaccine-responding T cells (Figure S2), possibly because of exacerbation of GVHD.27 Similar results were observed with IL-15 (data not shown). Thus, in thymectomized recipients, where large doses of DLI are required for vaccine responses, the generation of GVHD presents a barrier to effective vaccination.
Vaccination of allogenic recipients with mild GVHD results in enhanced tumor protection
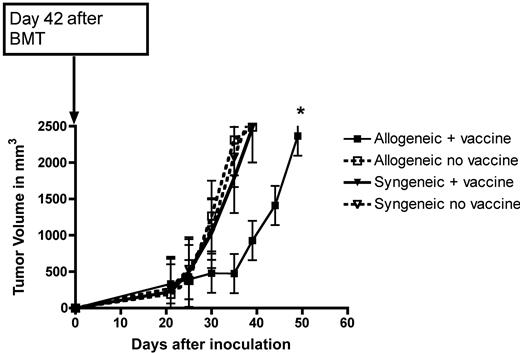
Although the loss of quantitative H-Y immune responses was dramatic and was observed with very mild GVHD, we next sought to determine whether the lowest DLI dose (5 × 106 cells) that caused this effect was functionally relevant and would translate into a loss of qualitative immunity after a tumor challenge. By challenging female recipients with a B6 HY-expressing tumor, MB49, syngeneic to the donor T cells, using a male vaccine to expand tumor-specific T cells allows assessment of the vaccine effect alone without contribution from alloantigens to GVT. Although we have previously demonstrated vaccine-induced protection in syngeneic recipients using a lower tumor inoculum,14 at this dose there was no protection for that group. Surprisingly, despite the decrease in quantitative immune responses noted with this DLI dose in allogeneic recipients (Figure 2A), there was improved tumor protection compared with syngeneic BMT recipients (Figure 3). Because this benefit occurred despite no contribution from alloantigens, these results suggest that there may be an enhanced vaccine-mediated antitumor effect in the allogeneic setting. We saw similar vaccine-mediated protection after allogeneic BMT even without a DLI; however, with DLI doses greater than 5 × 106 cells and more significant GVHD (albeit nonlethal), there was complete loss of a tumor-protective effect (data not shown).
Vaccinated, allogeneic BMT recipients have slower tumor growth. MB49 was placed on BMT recipients on day 42 and measured for growth in syngeneic and allogeneic BMT recipients who received 5 × 106 cells for their DLI on days 14 and 28 with or without a male DC vaccine, 5 or 6 mice/group. *P < .01.
Vaccinated, allogeneic BMT recipients have slower tumor growth. MB49 was placed on BMT recipients on day 42 and measured for growth in syngeneic and allogeneic BMT recipients who received 5 × 106 cells for their DLI on days 14 and 28 with or without a male DC vaccine, 5 or 6 mice/group. *P < .01.
IFN-γ signaling on both donor T cells and bone marrow contributes to GVHD, but absence of IFN-γ signaling on donor marrow improves quantitative vaccine responses to a tumor-associated antigen
Whereas the tumor challenge experiments suggest that the allogeneic environment may be beneficial for enhancing GVT effects when adequate numbers of tumor-specific T cells can be expanded from a thymically derived repertoire, the inability to expand tumor-specific T cells in thymus-deficient hosts prompted us to explore methods to overcome the remarkably immunosuppressive effect of GVHD. We hypothesized that a GVHD-associated inflammatory mediator was down-modulating immune responsiveness to the DC vaccine. Because the immunosuppressive effects of IFN-γ and the clear role for IFN-γ in GVHD pathophysiology have been well established,5 we sought to determine whether IFN-γ could be implicated in the loss of vaccine responses and tumor protection observed in this model.
To test this hypothesis, recipients underwent lethal irradiation and received either T cell–depleted, wild-type bone marrow followed by delayed administration of an allogeneic IFN-γR1−/− DLI, or T cell–depleted, allogeneic IFN-γR1−/− bone marrow with a high alloreactive DLI dose (20 × 106 cells) to induce weight loss. As shown in Figure 4A, IFN-γR1 signaling on the DLI was required for alloreactive T cells to cause GVHD-associated weight loss. Remarkably, loss of IFN-γR1 signaling on donor bone marrow–derived, non–T-cells abrogated GVHD, even in the presence of a DLI dose capable of inducing weight loss. If alloreactive T cells were given at the time of marrow infusion, GVHD was not abrogated (Figure S3A). In addition, using a 50:50 mixture of normal marrow with IFN-γR1−/− marrow did not abrogate GVHD (Figure S3B).
Absence of IFN-γ signaling on donor bone marrow abrogates GVHD but maintains vaccine responses. (A) Weights were recorded on mice that received either wild-type or IFN-γR1−/− allogeneic DLI at a dose of 20 × 106 cells given on days 14 and 28 after reconstitution with wild-type bone marrow. Other recipients received wild-type or IFN-γR1−/− allogeneic bone marrow followed by a normal, alloreactive DLI at a dose of 20 × 106 cells on days 14 and 28; 7 mice/group. (B) Spleens harvested on day 42 were analyzed by flow cytometry for enumeration of lymphocyte subsets. The percentage of the lymphocyte subset was multiplied by the splenocyte count to obtain an absolute number of cells. *P < .05; **P < .01. (C) ELISPOT analysis of CD8+ and CD4+ T cells to the H-Y antigens were performed on day 42 comparing 4 groups of thymus-bearing mice: mice who received allogeneic bone marrow (BM) without DLI, allogeneic BM with an alloreactive DLI, allogeneic BM with IFN-γR1−/− allogeneic DLI, and IFN-γR1−/− BM with an alloreactive DLI. All DLIs used 5 × 106 cells and were given on day 14 and 28, 8 mice/group. *P < .05, **P < .01, ***P < .001.
Absence of IFN-γ signaling on donor bone marrow abrogates GVHD but maintains vaccine responses. (A) Weights were recorded on mice that received either wild-type or IFN-γR1−/− allogeneic DLI at a dose of 20 × 106 cells given on days 14 and 28 after reconstitution with wild-type bone marrow. Other recipients received wild-type or IFN-γR1−/− allogeneic bone marrow followed by a normal, alloreactive DLI at a dose of 20 × 106 cells on days 14 and 28; 7 mice/group. (B) Spleens harvested on day 42 were analyzed by flow cytometry for enumeration of lymphocyte subsets. The percentage of the lymphocyte subset was multiplied by the splenocyte count to obtain an absolute number of cells. *P < .05; **P < .01. (C) ELISPOT analysis of CD8+ and CD4+ T cells to the H-Y antigens were performed on day 42 comparing 4 groups of thymus-bearing mice: mice who received allogeneic bone marrow (BM) without DLI, allogeneic BM with an alloreactive DLI, allogeneic BM with IFN-γR1−/− allogeneic DLI, and IFN-γR1−/− BM with an alloreactive DLI. All DLIs used 5 × 106 cells and were given on day 14 and 28, 8 mice/group. *P < .05, **P < .01, ***P < .001.
IFN-γR1−/− bone marrow enhanced host lymphocyte reconstitution to levels equivalent of syngeneic BMT controls (Figure 4B). Although loss of IFN-γR1 signaling on the DLI abrogates its ability to cause GVHD despite it being allogeneic (Figure 4A), it also abrogates the ability of the DLI to respond to a vaccine (Figure 4C). Surprisingly, IFN-γR1−/− bone marrow given with an alloreactive DLI abrogates GVHD (Figure 4A) but also improves vaccine responses (Figure 4C). Thus, it appears that the absence of IFN-γR1 signaling on donor T cells prevents their ability to cause GVT or GVHD responses, whereas absence of IFN-γR1 signaling on bone marrow preserves quantitative GVT responses while abrogating GVHD.
IFN-γR1−/− T cells do not show GVT activity, but IFN-γR1−/− bone marrow enhances GVT effects to syngeneic BMT controls
We next looked at the impact of IFN-γ modulation on functional responses to HY-expressing tumor to determine whether this approach will achieve the goal of controlling GVHD with preservation of vaccine-mediated tumor protection. We intentionally chose a tumor dose that would produce lethal tumors in all mice. To examine whether IFN-γR1−/− T cells from the DLI could protect against tumor, thymectomized recipients were chosen so that T cells generated from IFN-γR1+/+ marrow would not be present. As would be predicted, the loss of vaccine responses as measured by ELISPOT with a DLI deficient in IFN-γR1 signaling (Figure 4C) correlated with poor tumor protection in terms of growth and survival (Figure 5A), demonstrating that targeting IFN-γ signaling in vaccine-responding T cells is not an optimal approach. In contrast, using IFN-γR1−/− bone marrow resulted in enhanced vaccine-mediated tumor protection in terms of tumor growth and overall survival (Figure 5B), even with a normal alloreactive DLI dose that is sufficient to cause GVHD-induced loss of vaccine responses when given with wild-type bone marrow (Figure 2A).
Absence of IFN-γ signaling on donor marrow, but not the DLI, leads to enhanced tumor protection, even in the absence of the thymus. (A) All groups were thymectomized and received allogeneic bone marrow but received either an alloreactive or host-tolerized DLI of 20 × 106 cells on days 14 and 28 that could or could not signal through IFN-γ. All groups were then challenged on day 42 with MB49 tumor, 7 mice/group, *P < .05. Survival differences were not significant (P = .33). (B) Thymus-bearing allogeneic BMT recipients were infused with wild-type or IFN-γR1−/− allogeneic bone marrow and given a normal alloreactive DLI of 20 × 106 cells on days 14 and 28. All groups were then challenged on day 42 with MB49 tumor, 5 mice/group, *P < .05. (C) Thymectomized allogeneic BMT recipients were infused with wild-type or IFN-γR1−/− bone marrow and given a normal alloreactive DLI of 20 × 106 cells on days 14 and 28. All groups were then challenged on day 42 with MB49 tumor, 10 mice/group. *P < .05.
Absence of IFN-γ signaling on donor marrow, but not the DLI, leads to enhanced tumor protection, even in the absence of the thymus. (A) All groups were thymectomized and received allogeneic bone marrow but received either an alloreactive or host-tolerized DLI of 20 × 106 cells on days 14 and 28 that could or could not signal through IFN-γ. All groups were then challenged on day 42 with MB49 tumor, 7 mice/group, *P < .05. Survival differences were not significant (P = .33). (B) Thymus-bearing allogeneic BMT recipients were infused with wild-type or IFN-γR1−/− allogeneic bone marrow and given a normal alloreactive DLI of 20 × 106 cells on days 14 and 28. All groups were then challenged on day 42 with MB49 tumor, 5 mice/group, *P < .05. (C) Thymectomized allogeneic BMT recipients were infused with wild-type or IFN-γR1−/− bone marrow and given a normal alloreactive DLI of 20 × 106 cells on days 14 and 28. All groups were then challenged on day 42 with MB49 tumor, 10 mice/group. *P < .05.
Lastly, we examined responses to lethal tumor challenge in thymectomized recipients who had clinical GVHD after a high DLI dose to determine the extent of GVHD protection that could be mediated by IFN-γR1−/− bone marrow. Survival is poor in all thymectomized recipients because of the critical contribution of thymic-derived T cells toward GVT responses in this model (Figure 5C). Importantly, the use of IFN-γR1−/− bone marrow restored vaccine-mediated protection to that of syngeneic controls, as was observed in thymus-bearing recipients (Figure 5B). Thus, modulation of IFN-γR1 signaling on bone marrow–derived, non-T cells represents a potential strategy to overcome GVHD-associated inhibition of vaccine-mediated tumor protection.
Discussion
Allogeneic BMT is a potent form of immunotherapy against several high-risk malignancies, but strategies that separate the GVT effect without causing GVHD remain elusive. Immunizing patients with tumor antigens that have a relatively restricted tissue distribution in the milieu of an allogeneic environment could, in theory, tilt GVT effects over GVHD.28 Although prior studies have demonstrated that immunizing donor cells can augment GVT without exacerbating GVHD,12,13,18,29 only one model specifically induced GVHD to examine the impact on their vaccine response, but these mice had clinically overt GVHD.13 We chose to explore the role of subclinical GVHD, akin to humans with mild GVHD after fully MHC-matched, minor antigen–mismatched BMT, on vaccination. We demonstrate a substantial deleterious impact on quantitative and qualitative immunity with mild GVHD, leading to profound DLI dose-dependent lymphopenia, and without clinical signs of GVHD apparent in the mice. Importantly, this loss of vaccine responses was not absolutely inherent to the allogeneic environment and could be reversed through modulation of IFN-γ signaling on donor bone marrow without loss of the benefits of GVT.
Vaccine efficacy to a tumor-associated antigen (TAA) was assessed in 3 ways: absolute number of IFN-γ–producing T cells after challenge with H-Y class I and II antigens (via ELISPOT assays), rate of tumor growth after challenge with MB49, an H-Y–expressing tumor, and overall survival after MB49 challenge, allowing assessment of both quantitative and functional immunity. As expected, in the thymus-bearing recipients, the low DLI dose of 106 cells does allow a quantitative vaccine response against 2 CD8 epitopes, UTY and SMCY, but the moderate and high DLI doses do not (Figure 2A). However, in the thymectomized recipients, we could not identify a nontolerized DLI dose that was adequate for vaccine responses (Figure 2B), emphasizing the importance of identifying an approach to prevent GVHD yet retain the capacity to respond to vaccination. Amazingly, even very mild subclinical GVHD can negatively impact quantitative vaccine responses after allogeneic BMT, probably through the presence of inflammatory mediators30 and through lymphopenia.9,10 However, if the potential to cause GVHD is eliminated using host-tolerant T cells, vaccine responses are preserved (Figure 2A). This observation implies that there is nothing inherent to the allogeneic environment that is immunosuppressive outside of the process of GVHD, and one can maintain antigen-specific alloreactivity in the absence of GVHD. These data also complicate the current clinical paradigm that “mild GVHD” is beneficial,11 as we clearly show an impact on immunocompetence, in terms of vaccine responses and lymphocyte reconstitution. Indeed, these observations demonstrate the challenges of rebuilding the recipient immune system after allogeneic BMT using vaccines or other strategies.
It has been clearly demonstrated that thymic function is often limited after BMT. We demonstrate that thymectomized allogeneic BMT recipients are unable to generate vaccine responses because the DLI dose required for effective vaccination exceeds the threshold for GVHD. Although IL-7 has been previously shown to optimize quantitative immune responses in thymic-deficient hosts, this could not be achieved in allogeneic BMT recipients (Figure S2), probably through exacerbation of GVHD at lower DLI doses.27 Similar results were also observed with IL-15 (data not shown). These results indicate that effective vaccine responses can be generated in allogeneic transplant recipients but will require approaches to preserve thymic function or selective modulation of the DLI (either in vitro before infusion or in vivo after infusion) in the absence of thymic function.
Although there are decreased quantitative vaccine responses during subclinical GVHD, there is still a benefit of alloreactivity because vaccinated recipients of allogeneic BMT showed GVT effects in vivo at similar DLI doses. For example, vaccinated allogeneic BMT recipients who received a DLI dose of 5 × 106 cells had smaller tumors (Figure 3), despite lower quantitative responses (Figure 2A). Interestingly, there was no benefit in this model from using an allogeneic BMT without a vaccine, implying that the vaccine is necessary for tumor protection and that “nonspecific” mediators of GVT (ie, cytokines, Fas ligand) are relatively noncontributory, yet the allogeneic milieu is clearly providing a “nonspecific” advantage given that, in the context of lower quantitative responses, vaccinated allogeneic recipients have smaller tumors. One possible explanation for this discrepancy is that, although the allogeneic milieu decreases the number of antigen-reactive T cells, the cells that remain are more “potent” and able to eliminate tumor at a lower effector/target ratio or are better able to traffic to tumor.31 Alternatively, it is possible that other cell subsets that are not dependent on specifically reacting with the TAA for their cytotoxicity, such as natural killer cells, may also contribute to antitumor effects.32,–34 Studies are under way to explore the mechanism of this effect. Regardless, this finding supports the use of an allogeneic BMT platform to enhance tumor-specific immunotherapy.
Because of the critical role of IFN-γ in GVHD pathophysiology, we chose to explore the impact of eliminating IFN-γ signaling on the DLI and bone marrow as a means of preventing GVHD while potentially maintaining immune competence. Indeed, DLIs that cannot signal through IFN-γ cannot cause GVHD (Figure 4A), but such T cells are also unable to respond to vaccines or protect against a tumor (Figures 4C, 5A). This result was expected because IFN-γ signaling on T cells is a critical step in initiating an adaptive immune response35 and plays a role in antitumor activity.6 In contrast, bone marrow from IFN-γR1−/− mice also abrogated GVHD (Figure 4A) but at the same time enhanced immune reconstitution (Figure 4B), restoring the capacity to induce vaccine-directed immune responses to a TAA (Figure 4C), leading to delays in tumor growth and improved overall survival (Figure 5B,C) equivalent to syngeneic BMT controls. The abrogation of GVHD was not observed in the setting of a T cell–replete BMT (Figure S3A), probably because those T cells could be primed by IFN-γR1+/+ antigen-presenting cells (APCs) still present in the recipient, leading to GVHD.36 Using a 50:50 mixture of normal and IFN-γR1−/− marrow also did not abrogate GVHD, implying that the effects of IFN-γR1−/− marrow do not act through a dominant mechanism (Figure S3B). Thus, the benefit observed in the delayed T-cell add-back models suggests that the timing of the T-cell and bone marrow–derived, IFN-γR1−/− cell interaction is critical because sufficient time must pass to allow host APC turnover to occur.
These data demonstrate an important dichotomy between preventing IFN-γ signaling on donor T cells and bone marrow–derived non-T cells and the subsequent impact on alloreactivity. They also present an attractive target for selective modulation of alloreactivity with preserved immune competence to DC vaccines. In terms of translating these observations to the clinic, this would suggest that interfering with IFN-γ receptor signaling systemically through antibody approaches would probably decrease GVHD but worsen GVT because both bone marrow–derived populations and T cells from the DLI would be impacted. Identifying a specific subset from the marrow will be critical because one could potentially target this population ex vivo with shRNA or with a targeted inhibitor against IFN-γR1 (or a downstream molecule, such as JAK1/STAT1) and then adoptively transfer that subset with the bone marrow graft at the time of BMT. The advantage of using a targeted inhibitor is that it could be given to the recipient immediately after BMT for a defined period until the DLI, then stopped, minimizing a permanent impact on immunocompetence.
Other BMT models have examined the impact of using donor bone marrow that cannot produce IFN-γ,37,38 which accelerates GVHD, but using donor marrow deficient in IFN-γ receptor has only been explored in a few studies of GVHD.8,38,39 Although informative, these are not optimal models of clinical BMT practice, where MHC-matched, mHAg-mismatched BMT is preferred. All of these studies support our finding that absence of IFN-γ signaling on donor cells can abrogate GVHD. To our knowledge, this is the first report of enhancing GVT while abrogating GVHD using IFN-γR1−/− bone marrow. The marrow-derived cell responsible for mediating this effect was not a T cell (because T cell–depleted bone marrow was used), and we are currently attempting to identify the responsible cell. We hypothesize it will be an APC, given their clear role in GVHD.40 Indeed, IFN-γ plays a critical role in priming macrophages to secrete tumor necrosis factor-α, a major cytokine in the GVHD-associated cytokine storm, in response to lipopolysaccharide,6 and thus absence of IFN-γ signaling on donor APCs may prevent this initiating step of GVHD.
It will be important to understand the mechanism by which IFN-γ modulation of bone marrow can inhibit GVHD. Generation of proinflammatory soluble factors, such as IFN-γ, can induce both the maturation of mHA-expressing DCs and the up-regulation of target molecules on malignant cells, so introducing cells unable to signal through IFN-γ could provide more cytokine available for this purpose. Examination of donor and host cytokine production will be helpful in this regard. Studies will also need to be performed in mice with tumors at the time of BMT to mimic patients with minimal residual disease at the time of BMT. Lastly, identification of the bone marrow–derived “GVHD-inducing” cell that requires IFN-γ signaling will be critical.
In conclusion, our findings demonstrate that posttransplantation DC vaccines can effectively expand T cells and mediate antitumor responses. However, they also indicate that even mild GVHD should be avoided to prevent loss of vaccine responses. Importantly, the current paradigm of achieving mild GVHD to also mediate GVT effects may also prohibit vaccination approaches. To overcome this, it may be optimal to use a T cell–depleted platform that can effectively prevent GVHD. However, given the prolonged period of lymphopenia associated with this approach, it will be necessary to incorporate strategies to accelerate thymic recovery that in our model allowed for robust immune responses and perhaps an advantage over syngeneic platforms in terms of functional responses to tumor. We also show, for the first time, that selective targeting of IFN-γ on bone marrow–derived non-T cells creates a platform where large doses of unmanipulated, alloreactive T cells can be given to mediate tumor protection, even when thymic function is absent. Thus, if these caveats are taken into consideration, posttransplantation vaccination represents a useful strategy for enhancing GVT in patients who have received an allogeneic BMT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Richard Childs and Daniel Fowler for their critical review of the data and manuscript drafts.
The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
This work was supported by intramural research funds at the National Cancer Institute (C.L.M.), the Children's Cancer Foundation (T.J.F.), and in part with federal funds from the NCI, NIH, funded by NCI contract no. HHSN261200800001E (M.R.A.).
National Institutes of Health
Authorship
Contribution: C.M.C. designed and performed research, collected, analyzed, and interpreted data, performed statistical analysis, and drafted the manuscript; S.H. and M.M. performed research, collected, analyzed, and interpreted data, and performed statistical analysis; M.R.A. analyzed and interpreted histopathologic data; C.L.M. analyzed and interpreted data and revised the manuscript; and T.J.F. designed research, analyzed and interpreted data, performed statistical analysis, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christian M. Capitini, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, 10 Center Dr, Rm 1W-5832, Bethesda, MD 20892; e-mail: capitinic@mail.nih.gov.