We evaluated 26 901 patients who underwent allogeneic hematopoietic cell transplantation (HCT) at 271 centers worldwide to define patterns of posttransplantation lymphoproliferative disorders (PTLDs). PTLDs developed in 127 recipients, with 105 (83%) cases occurring within 1 year after transplantation. In multivariate analyses, we confirmed that PTLD risks were strongly associated (P < .001) with T-cell depletion of the donor marrow, antithymocyte globulin (ATG) use, and unrelated or HLA-mismatched grafts (URD/HLA mismatch). Significant associations were also confirmed for acute and chronic graft-versus-host disease. The increased risk associated with URD/HLA-mismatched donors (RR = 3.8) was limited to patients with T-cell depletion or ATG use (P = .004). New findings were elevated risks for age 50 years or older at transplantation (RR = 5.1; P < .001) and second transplantation (RR = 3.5; P < .001). Lower risks were found for T-cell depletion methods that remove both T and B cells (alemtuzumab and elutriation, RR = 3.1; P = .025) compared with other methods (RR = 9.4; P = .005 for difference). The cumulative incidence of PTLDs was low (0.2%) among 21 686 patients with no major risk factors, but increased to 1.1%, 3.6%, and 8.1% with 1, 2, and more than 3 major risk factors, respectively. Our findings identify subgroups of patients who underwent allogeneic HCT at elevated risk of PTLDs for whom prospective monitoring of Epstein-Barr virus activation and early treatment intervention may be particularly beneficial.
Introduction
Currently, more than 15 000 patients undergo an allogeneic hematopoietic cell transplantation (HCT) annually throughout the world, with the number of long-term survivors increasing rapidly.1 Given the introduction of better therapies in parallel with advancements in supportive care in the past decades, there has been a steady improvement in survival after allogeneic HCT and growing interest in quantifying the late effects of transplantation
Single-center investigations, generally based on small numbers, have consistently reported that survivors of allogeneic HCT are at increased risk of developing posttransplantation lymphoproliferative disorders (PTLDs), which present clinically as aggressive and frequently fatal lymphomas.2,,,,,–8 To quantify the risk and define risk factors for PTLDs, we previously conducted a large international study including 18 014 patients who underwent allogeneic HCT at centers worldwide, including 78 patients who developed PTLDs.4 Risk of PTLDs was associated with unrelated or human leukocyte antigen (HLA)–mismatched related donor transplantations, T-cell depletion of donor marrow, and use of antithymocyte globulin (ATG) or anti-CD3 monoclonal antibody for prophylaxis or treatment of acute graft-versus-host disease (GVHD). Methods of T-cell depletion that selectively targeted T cells, or T and natural killer (NK) cells, were associated with markedly higher risks of PTLDs compared with methods that removed both T and B cells (such as anti-CD52 alemtuzumab [CAMPATH-1] monoclonal antibody or elutriation), although numbers of patients in the latter category were small. Currently, there is only limited information available on the long-term risk of developing PTLDs, and risk factors for late-onset PTLDs are largely unknown. In our previous study,4 we found chronic GVHD to be associated with increased risk of late-onset PTLD, indicating that risk factors associated with altered immunity and T-cell regulatory mechanisms might be predictors of both early- and late-onset PTLDs.
We have expanded and updated our previously assembled unique multi-institutional cohort to evaluate risk factors for PTLDs among 26 901 patients who underwent allogeneic HCT, making this the largest study to date to assess risk factors for early- and late-onset PTLDs after transplantation. The aims of this study were to further our understanding of the effects of age and time since transplantation on PTLD risk, to quantify the PTLD risk associated with various factors (such as immunosuppressive treatment), to evaluate differences in risks of polymorphic and monomorphic PTLDs, and to identify subgroups of patients at especially high risk. Given this foundation, investigators planning future allogeneic HCT strategies can minimize the risk of PTLDs and promote risk-adapted strategies for enhanced surveillance and treatment.
Methods
Patients and study centers
We evaluated 26 901 patients who underwent allogeneic HCT using bone marrow as a source of stem cells between 1964 and 1994 at 271 transplant centers reporting to the Center for International Blood and Marrow Transplant Research (CIBMTR; follow-up through 1995), or between 1969 and 1996 at the Fred Hutchinson Cancer Research Center (FHCRC, Seattle, WA; follow-up through 1996). The current analysis excluded patients with an inherent susceptibility to cancer, that is, those who underwent transplantation for either Fanconi anemia (n = 328, 1 PTLD) or primary immunodeficiency diseases (n = 532, 20 PTLDs). We also excluded patients with a primary disease of non-Hodgkin lymphoma (NHL; n = 1092) or Hodgkin lymphoma (n = 228, 1 PTLD); and patients receiving syngeneic transplants (n = 634). Finally, we excluded a small group of FHCRC patients (n = 22) who received treatment with a unique murine anti–T-cell monoclonal antibody (64.1) for acute GVHD and who were subsequently reported to have a high incidence of PTLDs (3 cases).2,4,9 The current study extends the follow up and expands the cohort of our earlier investigation,4 which included 18 014 transplant recipients (CIBMTR 1964-1990 [follow-up through 1991], FHCRC 1969-1992). In addition, other publications have included data from selected transplant teams participating in this study. Second cancer studies, which include PTLD, at the CIBMTR and FHCRC were conducted with a waiver of informed consent at their respective institutions and at the National Cancer Institute (NCI, Bethesda, MD).
Of the 127 PTLDs identified, 116 cases (91.3%) were confirmed by centralized histopathologic review of archived tissue or slides or by review of pathology/clinical reports. Eleven cases (8.6%) were based on transplant center report only. The WHO criteria10 were used to classify the 53 PTLD cases identified during the current expanded study (P.M.B.), whereas the 74 PTLD cases from our earlier investigation4 were classified using well-established criteria from Knowles et al11 (E.S.J., D.W.K., P.M.B.). Epstein-Barr virus (EBV)–related sequences were detected in 64 of 68 evaluable PTLD cases using in situ hybridization (EBER1) and LMP methods. No immunoglobulin gene rearrangement studies were performed.
The study was based on anonymized data and was classified as exempt by Institutional Review Boards.
Statistical analyses
Person-years at risk were calculated from the date of transplantation to the date of death, last known follow up, diagnosis of new malignancy (including PTLD), or study end, whichever occurred first. The pattern of PTLD occurrence by posttransplantation intervals was initially evaluated by calculating PTLD incidence rates, defined as the number of PTLD cases divided by the number of person-years in each interval. Although PTLDs are recognized to be disorders distinct from NHL,10 we also compared the observed numbers of PTLD cases (O) to the expected number of NHL cases (E) and calculated the observed-to-expected ratio (O/E) of NHL. Age-, sex-, calendar year–, and region-specific incidence rates for NHL from selected registries in the United States, England and Wales, continental Europe, and Asia12,–14 were applied to the appropriate person-years at risk to compute the expected number of NHL cases. The exact Poisson distribution was used to calculate 95% confidence intervals (CIs).15 The cumulative incidence was calculated as the percentage of patients developing a PTLD by a specified time after the initial transplantation, while taking into account competing risks of death among patients who did not develop a second malignancy.16
Multivariate analyses using Poisson regression methods for grouped survival data17 were conducted to compute estimates of the relative risk (RR) of PTLDs associated with various risk factors. Patient-related variables evaluated included time since transplantation, age and calendar year of transplantation, sex, primary disease, cohort (CIBMTR, Seattle, WA), and geographic region. Transplant-related variables included conditioning regimen, occurrence and method of T-cell depletion, prophylaxis or treatment for acute GVHD with antithymocyte globulin (ATG), degree of HLA match and donor relationship, occurrence of acute GVHD (grades II-IV), occurrence of chronic GVHD (moderate or severe disease in the CIBMTR cohort and clinically extensive disease in the FHCRC cohort), and occurrence of second allogeneic HCT. Occurrence of acute GVHD, chronic GVHD, and second transplantation, and treatment of acute GVHD with ATG were entered as time-dependent covariates. ATG was used only in vivo (no in vitro manipulations). Time since transplantation was stratified in 13 intervals (with cut points of 1, 2, 3, 4, 5, 6, 9, 12, 18, 30, 60, and 120 months); age at transplantation in 5-year intervals; year of transplantation in 3 intervals (with cut points of 1987 and 1991); and primary disease in 5 categories (acute lymphoblastic leukemia [ALL], acute myeloid leukemia [AML], chronic myeloid leukemia [CML], severe aplastic anemia, all other diseases). All models were stratified on time since transplantation using the categories noted earlier in this paragraph. Repeat analyses with additional stratification on primary disease yielded very similar results. All treatment and transplant-related variables identified as significant risk factors in our previous analyses4 were included in initial models. First-order interactions were tested for all models. Log-linear continuous variables were used to evaluate interactions of transplant- and patient-related variables with time since transplantation. Hypothesis tests and confidence intervals were based on likelihood ratio tests; all P values are 2-sided and are defined as statistically significant if P value is less than .05.
A nested case-control study was conducted to quantify the association of immunosuppressive therapy for acute and chronic GVHD (types of drugs, duration of use) with the development of late-onset PTLD. Transplant centers were selected for participation on the basis of completeness of patient follow up and willingness to collect supplemental detailed posttransplantation data from medical records. A standardized abstract form was used to collect data on GVHD occurrence and treatment up to the time of diagnosis for 35 PTLD cases developing 6 months or more after transplantation or the corresponding matched time interval for control patients. We attempted to match at least 3 controls using the following criteria: cohort (CIBMTR, FHCRC), primary disease, age at transplantation (within 3 years), sex, survival time at least as long as the interval from transplantation to PTLD diagnosis, race (US cases only: white, African American, other), and geographic region of the CIBMTR transplant team. In addition, controls were matched to PTLD cases on T-cell depletion of donor marrow and degree of HLA match. Estimates of the risk for PTLDs associated with specific risk factors were calculated by comparing the exposure histories of the PTLD cases with those of their matched controls within the matched time window of interest using multivariate conditional logistic regression methods.18
Results
Table 1 describes patient and transplant characteristics for the 26 901 patients included in the analysis by calendar year of allogeneic HCT. The percentage of the cohort undergoing transplantation at age 30 years or older increased from 30% during 1968 to 1986 to more than 50% during 1991 to 1996. The majority of patients (74%) underwent transplantation for leukemia, and more than 70% received a transplant from an HLA-identical sibling donor. However, patients who underwent transplantation in the period 1991 to 1996 were more likely to have a primary disease of CML or to have received cells from unrelated donors. Total body irradiation (TBI) was part of the conditioning regimen in 80% of the patients who underwent transplantation in 1968 to 1986, but in only 57% of patients who underwent transplantation in 1991 to 1996. In 15% of the patients donor cells were T-cell depleted, with proportions remaining relatively constant over the 3 time periods.
Characteristics of patients undergoing allogeneic HCT
| . | Year of allogeneic HCT . | |||||||
|---|---|---|---|---|---|---|---|---|
| 1968-1986 . | 1987-1990 . | 1991-1996 . | 1968-1996 . | |||||
| Patients . | % . | Patients . | % . | Patients . | % . | Patients . | % . | |
| Total cohort | 8719 | 100 | 8946 | 100 | 9236 | 100 | 26 901 | 100 |
| CIBMTR | 6721 | 77.1 | 7777 | 86.9 | 7645 | 82.8 | 22 143 | 82.3 |
| FHCRC | 1998 | 22.9 | 1169 | 13.1 | 1591 | 17.2 | 4758 | 17.7 |
| Sex | ||||||||
| Male | 5086 | 58.3 | 5296 | 59.2 | 5496 | 59.5 | 15 878 | 59.0 |
| Female | 3633 | 41.7 | 3650 | 40.8 | 3740 | 40.5 | 11 023 | 41.0 |
| Age at transplantation, y | ||||||||
| Younger than 10 | 1403 | 16.1 | 1262 | 14.1 | 1243 | 13.5 | 3908 | 14.5 |
| 10-19 | 2336 | 26.8 | 1628 | 18.2 | 1461 | 15.8 | 5425 | 20.2 |
| 20-29 | 2400 | 27.5 | 2137 | 23.9 | 1748 | 18.9 | 6285 | 23.4 |
| 30-39 | 1853 | 21.3 | 2218 | 24.8 | 2222 | 24.1 | 6293 | 23.4 |
| 40-49 | 656 | 7.5 | 1472 | 16.5 | 1917 | 20.8 | 4045 | 15.0 |
| 50 y or older | 71 | 0.8 | 229 | 2.6 | 645 | 7.0 | 945 | 3.5 |
| Median age (range) at transplantation, y | 22.5 (0.2-67) | NA | 27.5 (0.1-62) | NA | 30.8 (0.2-68) | NA | 26.6 (0.1-68) | NA |
| Geographic region of transplant team | ||||||||
| United States | 3979 | 45.6 | 3418 | 38.2 | 4077 | 44.1 | 11 474 | 42.7 |
| Canada | 130 | 1.5 | 369 | 4.1 | 529 | 5.7 | 1028 | 3.8 |
| Europe | 3870 | 44.4 | 3845 | 43.0 | 3070 | 33.2 | 10 785 | 40.1 |
| Other | 740 | 8.5 | 1314 | 14.7 | 1560 | 16.9 | 3614 | 13.4 |
| Primary disease* | ||||||||
| Acute lymphocytic leukemia | 2226 | 25.5 | 1861 | 20.8 | 1704 | 18.5 | 5791 | 21.5 |
| Acute nonlymphocytic leukemia | 2615 | 30.0 | 2387 | 26.7 | 2366 | 25.6 | 7368 | 27.4 |
| Chronic myelogenous leukemia | 1860 | 21.3 | 2716 | 30.4 | 2882 | 31.2 | 7458 | 27.7 |
| Severe aplastic anemia | 1398 | 16.0 | 734 | 8.2 | 656 | 7.1 | 2788 | 10.4 |
| Other | 620 | 7.1 | 1248 | 14.0 | 1628 | 17.6 | 3496 | 13.0 |
| Donor recipient relationship and histocompatibility | ||||||||
| HLA-identical sibling | 7495 | 86.0 | 7060 | 78.9 | 6318 | 68.4 | 20 873 | 77.6 |
| 1 HLA antigen–mismatched related donor | 615 | 7.1 | 700 | 7.8 | 444 | 4.8 | 1759 | 6.5 |
| 2+ HLA antigen–mismatched related donor | 465 | 5.3 | 330 | 3.7 | 274 | 3.0 | 1069 | 4.0 |
| Unrelated donor | 83 | 1.0 | 819 | 9.2 | 2103 | 22.8 | 3005 | 11.2 |
| Other, uncertain | 61 | 0.7 | 37 | 0.4 | 97 | 1.1 | 195 | 0.7 |
| Transplantation conditioning regimen | ||||||||
| TBI + Cy ± other drugs | 6245 | 71.6 | 4876 | 54.5 | 4578 | 49.6 | 15 699 | 58.4 |
| TBI + other drugs (no Cy) | 738 | 8.5 | 771 | 8.6 | 683 | 7.4 | 2192 | 8.2 |
| LFI ± Cy ± other drugs | 339 | 3.9 | 319 | 3.6 | 145 | 1.6 | 803 | 3.0 |
| Busulfan + Cy ± other drugs | 440 | 5.1 | 2482 | 27.7 | 3355 | 36.3 | 6277 | 23.3 |
| Cy ± other drugs | 903 | 10.4 | 422 | 4.7 | 372 | 4.0 | 1697 | 6.3 |
| Other | 54 | 0.6 | 76 | 0.9 | 103 | 1.1 | 233 | 0.9 |
| T-cell depletion of marrow | ||||||||
| No T-cell depletion | 7425 | 85.2 | 7741 | 86.5 | 8189 | 88.7 | 23 355 | 86.8 |
| Anti-T or anti-T + NK MoAb | 684 | 7.8 | 568 | 6.4 | 532 | 5.8 | 1784 | 6.6 |
| Sheep red blood cell (SRBC) rosetting | 73 | 0.8 | 91 | 1.0 | 61 | 0.7 | 225 | 0.8 |
| Lectins (including SRBC/MoAb) | 62 | 0.7 | 74 | 0.8 | 87 | 0.9 | 223 | 0.8 |
| Alemtuzumab (CAMPATH) MoAb | 319 | 3.7 | 286 | 3.2 | 79 | 0.9 | 684 | 2.5 |
| Elutriation/density gradient centrifugation | 100 | 1.2 | 152 | 1.7 | 260 | 2.8 | 512 | 1.9 |
| Unclassified/other | 56 | 0.6 | 34 | 0.4 | 28 | 0.3 | 118 | 0.4 |
| Acute GVHD† | ||||||||
| None, grade I | 5238 | 60.1 | 5691 | 63.6 | 5397 | 58.4 | 16 326 | 60.7 |
| Grades II-IV | 3481 | 39.9 | 3255 | 36.4 | 3839 | 41.6 | 10 575 | 39.3 |
| Chronic GVHD† | ||||||||
| None, mild | 7251 | 83.2 | 7327 | 81.9 | 7552 | 81.8 | 22 130 | 82.3 |
| Moderate, severe | 1468 | 16.8 | 1619 | 18.1 | 1684 | 18.2 | 4771 | 17.7 |
| ATG | ||||||||
| None | 7755 | 88.9 | 8384 | 93.7 | 8493 | 92.0 | 24 632 | 91.6 |
| GVHD prophylaxis only | 307 | 3.5 | 150 | 1.7 | 221 | 2.4 | 678 | 2.5 |
| Acute GVHD treatment only | 612 | 7.0 | 382 | 4.3 | 488 | 5.3 | 1482 | 5.5 |
| Both prophylaxis and acute GVHD treatment | 45 | 0.5 | 30 | 0.3 | 34 | 0.4 | 109 | 0.4 |
| Second allogeneic HCT† | ||||||||
| No | 8123 | 93.2 | 8464 | 94.6 | 8843 | 95.7 | 25 430 | 94.5 |
| Yes | 596 | 6.8 | 482 | 5.4 | 393 | 4.3 | 1471 | 5.5 |
| . | Year of allogeneic HCT . | |||||||
|---|---|---|---|---|---|---|---|---|
| 1968-1986 . | 1987-1990 . | 1991-1996 . | 1968-1996 . | |||||
| Patients . | % . | Patients . | % . | Patients . | % . | Patients . | % . | |
| Total cohort | 8719 | 100 | 8946 | 100 | 9236 | 100 | 26 901 | 100 |
| CIBMTR | 6721 | 77.1 | 7777 | 86.9 | 7645 | 82.8 | 22 143 | 82.3 |
| FHCRC | 1998 | 22.9 | 1169 | 13.1 | 1591 | 17.2 | 4758 | 17.7 |
| Sex | ||||||||
| Male | 5086 | 58.3 | 5296 | 59.2 | 5496 | 59.5 | 15 878 | 59.0 |
| Female | 3633 | 41.7 | 3650 | 40.8 | 3740 | 40.5 | 11 023 | 41.0 |
| Age at transplantation, y | ||||||||
| Younger than 10 | 1403 | 16.1 | 1262 | 14.1 | 1243 | 13.5 | 3908 | 14.5 |
| 10-19 | 2336 | 26.8 | 1628 | 18.2 | 1461 | 15.8 | 5425 | 20.2 |
| 20-29 | 2400 | 27.5 | 2137 | 23.9 | 1748 | 18.9 | 6285 | 23.4 |
| 30-39 | 1853 | 21.3 | 2218 | 24.8 | 2222 | 24.1 | 6293 | 23.4 |
| 40-49 | 656 | 7.5 | 1472 | 16.5 | 1917 | 20.8 | 4045 | 15.0 |
| 50 y or older | 71 | 0.8 | 229 | 2.6 | 645 | 7.0 | 945 | 3.5 |
| Median age (range) at transplantation, y | 22.5 (0.2-67) | NA | 27.5 (0.1-62) | NA | 30.8 (0.2-68) | NA | 26.6 (0.1-68) | NA |
| Geographic region of transplant team | ||||||||
| United States | 3979 | 45.6 | 3418 | 38.2 | 4077 | 44.1 | 11 474 | 42.7 |
| Canada | 130 | 1.5 | 369 | 4.1 | 529 | 5.7 | 1028 | 3.8 |
| Europe | 3870 | 44.4 | 3845 | 43.0 | 3070 | 33.2 | 10 785 | 40.1 |
| Other | 740 | 8.5 | 1314 | 14.7 | 1560 | 16.9 | 3614 | 13.4 |
| Primary disease* | ||||||||
| Acute lymphocytic leukemia | 2226 | 25.5 | 1861 | 20.8 | 1704 | 18.5 | 5791 | 21.5 |
| Acute nonlymphocytic leukemia | 2615 | 30.0 | 2387 | 26.7 | 2366 | 25.6 | 7368 | 27.4 |
| Chronic myelogenous leukemia | 1860 | 21.3 | 2716 | 30.4 | 2882 | 31.2 | 7458 | 27.7 |
| Severe aplastic anemia | 1398 | 16.0 | 734 | 8.2 | 656 | 7.1 | 2788 | 10.4 |
| Other | 620 | 7.1 | 1248 | 14.0 | 1628 | 17.6 | 3496 | 13.0 |
| Donor recipient relationship and histocompatibility | ||||||||
| HLA-identical sibling | 7495 | 86.0 | 7060 | 78.9 | 6318 | 68.4 | 20 873 | 77.6 |
| 1 HLA antigen–mismatched related donor | 615 | 7.1 | 700 | 7.8 | 444 | 4.8 | 1759 | 6.5 |
| 2+ HLA antigen–mismatched related donor | 465 | 5.3 | 330 | 3.7 | 274 | 3.0 | 1069 | 4.0 |
| Unrelated donor | 83 | 1.0 | 819 | 9.2 | 2103 | 22.8 | 3005 | 11.2 |
| Other, uncertain | 61 | 0.7 | 37 | 0.4 | 97 | 1.1 | 195 | 0.7 |
| Transplantation conditioning regimen | ||||||||
| TBI + Cy ± other drugs | 6245 | 71.6 | 4876 | 54.5 | 4578 | 49.6 | 15 699 | 58.4 |
| TBI + other drugs (no Cy) | 738 | 8.5 | 771 | 8.6 | 683 | 7.4 | 2192 | 8.2 |
| LFI ± Cy ± other drugs | 339 | 3.9 | 319 | 3.6 | 145 | 1.6 | 803 | 3.0 |
| Busulfan + Cy ± other drugs | 440 | 5.1 | 2482 | 27.7 | 3355 | 36.3 | 6277 | 23.3 |
| Cy ± other drugs | 903 | 10.4 | 422 | 4.7 | 372 | 4.0 | 1697 | 6.3 |
| Other | 54 | 0.6 | 76 | 0.9 | 103 | 1.1 | 233 | 0.9 |
| T-cell depletion of marrow | ||||||||
| No T-cell depletion | 7425 | 85.2 | 7741 | 86.5 | 8189 | 88.7 | 23 355 | 86.8 |
| Anti-T or anti-T + NK MoAb | 684 | 7.8 | 568 | 6.4 | 532 | 5.8 | 1784 | 6.6 |
| Sheep red blood cell (SRBC) rosetting | 73 | 0.8 | 91 | 1.0 | 61 | 0.7 | 225 | 0.8 |
| Lectins (including SRBC/MoAb) | 62 | 0.7 | 74 | 0.8 | 87 | 0.9 | 223 | 0.8 |
| Alemtuzumab (CAMPATH) MoAb | 319 | 3.7 | 286 | 3.2 | 79 | 0.9 | 684 | 2.5 |
| Elutriation/density gradient centrifugation | 100 | 1.2 | 152 | 1.7 | 260 | 2.8 | 512 | 1.9 |
| Unclassified/other | 56 | 0.6 | 34 | 0.4 | 28 | 0.3 | 118 | 0.4 |
| Acute GVHD† | ||||||||
| None, grade I | 5238 | 60.1 | 5691 | 63.6 | 5397 | 58.4 | 16 326 | 60.7 |
| Grades II-IV | 3481 | 39.9 | 3255 | 36.4 | 3839 | 41.6 | 10 575 | 39.3 |
| Chronic GVHD† | ||||||||
| None, mild | 7251 | 83.2 | 7327 | 81.9 | 7552 | 81.8 | 22 130 | 82.3 |
| Moderate, severe | 1468 | 16.8 | 1619 | 18.1 | 1684 | 18.2 | 4771 | 17.7 |
| ATG | ||||||||
| None | 7755 | 88.9 | 8384 | 93.7 | 8493 | 92.0 | 24 632 | 91.6 |
| GVHD prophylaxis only | 307 | 3.5 | 150 | 1.7 | 221 | 2.4 | 678 | 2.5 |
| Acute GVHD treatment only | 612 | 7.0 | 382 | 4.3 | 488 | 5.3 | 1482 | 5.5 |
| Both prophylaxis and acute GVHD treatment | 45 | 0.5 | 30 | 0.3 | 34 | 0.4 | 109 | 0.4 |
| Second allogeneic HCT† | ||||||||
| No | 8123 | 93.2 | 8464 | 94.6 | 8843 | 95.7 | 25 430 | 94.5 |
| Yes | 596 | 6.8 | 482 | 5.4 | 393 | 4.3 | 1471 | 5.5 |
The distribution under each subheading (in bold) is based on all subjects regardless of their status with respect to other variables in the table. Thus, the numbers under each subheading add to the totals at the top of the columns and the percentages add to 100%. Percentages do not always add to exactly 100% because of rounding.
CIBMTR indicates Center for International Blood and Marrow Research; FHCRC, Fred Hutchinson Cancer Research Center; TBI, total body irradiation; Cy, cyclophosphamide; LFI, limited field irradiation; GVHD, graft-versus-host disease; NK, natural killer; MoAb, monoclonal antibody; and ATG, antithymocyte globulin.
Primary diseases excluded from the analysis were non-Hodgkin lymphomas (n = 1092), Hodgkin lymphoma (n = 228), Fanconi anemia (n = 328), and immune-deficiency diseases (n = 532). Other primary diseases included other malignancies, myelodysplastic syndromes or myeloproliferative disorders, and other smaller groups of primarily nonmalignant diseases, including inherited disorders of metabolism, and hemoglobinopathies.
Number of patients with acute GVHD, chronic GVHD, or second transplantation that occurred before study exit date (date of last follow-up or date of PTLD diagnosis). Second allogeneic transplantation among patients with a first allogeneic transplantation.
Subtypes of PTLDs
Overall 127 transplant recipients developed PTLD, including 74 cases that were included in our previous investigation.4 Incidence rates for PTLDs peaked at 2 to 3 months after transplantation, and then declined sharply with increasing time since transplantation (Table 2). Of 127 PTLDs, 71 (56%) were polymorphic, 28 (22%) monomorphic, and 28 (22%) unclassified; rates for both polymorphic and monotypes of PTLDs declined over time. There were no polymorphic PTLDs diagnosed more than 18 months after transplantation, but monomorphic PTLDs continued to occur as long as 10+ years after transplantation. EBV-related sequences were detected in 62 of 66 evaluable cases.
Incidence rate and number of cases (in parentheses) of posttransplantation lymphoproliferative disorders by subtype and by latency
| Latency, mo since transplantation . | No. of patients . | Person-years at risk . | PTLD incidence rate per 104 person-years (no. of cases) . | |||
|---|---|---|---|---|---|---|
| All PTLDs . | Polymorphic PTLDs . | Monomorphic PTLDs . | Other and unspecified PTLDs . | |||
| 0 to less than 1 | 26 901 | 2168 | 0 (0) | 0 (0) | 0 (0) | 0 |
| 1 to less than 2 | 24 749 | 1959 | 86.8 (17) | 71.5 (14) | 5.1 (1) | 10.2 (2) |
| 2 to less than 3 | 22 226 | 1751 | 177.0 (31) | 125.6 (22) | 22.8 (4) | 28.6 (5) |
| 3 to less than 4 | 19 989 | 1608 | 111.9 (18) | 68.4 (11) | 12.4 (2) | 31.1 (5) |
| 4 to less than 5 | 18 698 | 1515 | 72.6 (11) | 72.6 (11) | 0 (0) | 0 (0) |
| 5 to less than 6 | 17 684 | 1438 | 62.6 (9) | 34.8 (5) | 13.9 (2) | 14 (2) |
| 6- | 16 876 | 3990 | 35.1 (14) | 12.5 (5) | 17.5 (7) | 5.0 (2) |
| 9- | 15 204 | 3668 | 13.6 (5) | 5.5 (2) | 2.7 (1) | 5.5 (2) |
| 12- | 14 224 | 6638 | 15.1 (10) | 1.5 (1) | 4.5 (3) | 9.0 (6) |
| 18- | 12 457 | 11 232 | 1.8 (2) | 0 (0) | 0.9 (1) | 0.9 (1) |
| 30- | 10 159 | 20 182 | 2.0 (4) | 0 (0) | 1.0 (2) | 1.0 (2) |
| 60- | 6233 | 18 404 | 2.7 (5) | 0 (0) | 2.2 (4) | 0.5 (1) |
| 120+ | 1846 | 5483 | 1.8 (1) | 0 (0) | 1.8 (1) | 0 (0) |
| Total years | 80 035 | (127) | (71) | (28) | (28) | |
| Latency, mo since transplantation . | No. of patients . | Person-years at risk . | PTLD incidence rate per 104 person-years (no. of cases) . | |||
|---|---|---|---|---|---|---|
| All PTLDs . | Polymorphic PTLDs . | Monomorphic PTLDs . | Other and unspecified PTLDs . | |||
| 0 to less than 1 | 26 901 | 2168 | 0 (0) | 0 (0) | 0 (0) | 0 |
| 1 to less than 2 | 24 749 | 1959 | 86.8 (17) | 71.5 (14) | 5.1 (1) | 10.2 (2) |
| 2 to less than 3 | 22 226 | 1751 | 177.0 (31) | 125.6 (22) | 22.8 (4) | 28.6 (5) |
| 3 to less than 4 | 19 989 | 1608 | 111.9 (18) | 68.4 (11) | 12.4 (2) | 31.1 (5) |
| 4 to less than 5 | 18 698 | 1515 | 72.6 (11) | 72.6 (11) | 0 (0) | 0 (0) |
| 5 to less than 6 | 17 684 | 1438 | 62.6 (9) | 34.8 (5) | 13.9 (2) | 14 (2) |
| 6- | 16 876 | 3990 | 35.1 (14) | 12.5 (5) | 17.5 (7) | 5.0 (2) |
| 9- | 15 204 | 3668 | 13.6 (5) | 5.5 (2) | 2.7 (1) | 5.5 (2) |
| 12- | 14 224 | 6638 | 15.1 (10) | 1.5 (1) | 4.5 (3) | 9.0 (6) |
| 18- | 12 457 | 11 232 | 1.8 (2) | 0 (0) | 0.9 (1) | 0.9 (1) |
| 30- | 10 159 | 20 182 | 2.0 (4) | 0 (0) | 1.0 (2) | 1.0 (2) |
| 60- | 6233 | 18 404 | 2.7 (5) | 0 (0) | 2.2 (4) | 0.5 (1) |
| 120+ | 1846 | 5483 | 1.8 (1) | 0 (0) | 1.8 (1) | 0 (0) |
| Total years | 80 035 | (127) | (71) | (28) | (28) | |
PTLD indicates posttransplantation lymphoproliferative disorder.
Of the 53 PTLD newly identified cases in the more recent follow-up period, we were able to classify 43 according to the current WHO criteria.10 Among these, we were able to further evaluate the clonality for 14 of 26 polymorphic and 7 of 9 monomorphic B-cell PTLDs. For the 14 polymorphic cases, we found 7 polyclonal and 7 monoclonal PTLDs. All of the 7 monomorphic cases had diffuse large B-cell PTLD.
Comparison with the general population
To place the incidence of PTLDs in context, a similar age-, sex-, and country-adjusted population would be expected to develop 4.3 cases of NHL. Thus, the observed to expected ratio (O/E) was 29.7 (95% CI = 24.7-35.2). The O/E ratio at 5 or more years after transplantation was substantially lower, but remained significantly elevated (O = 6, E = 1.43, O/E = 4.2; 95% CI = 1.5-9.1).
Risk factors for PTLD
Overall analyses.
Table 3 summarizes results of multivariate modeling that evaluated risk factors for PTLD. We confirmed strong associations of T-cell depletion of the donor bone marrow, ATG use, degree of HLA match (P < .001), and more modest associations of acute and chronic GVHD with increasing risk of PTLDs (P = .005 and P = .012, respectively). After adjusting for established risk factors, we also identified receipt of a second transplant and age at transplantation of 50 years or older as newly recognized risk factors for PTLDs (P < .001). Evaluation of individual T-cell depletion methods (Table 3) indicated that alemtuzumab monoclonal antibodies and elutriation/density gradient centrifugation (hereafter referred to as broad lymphocyte depletion methods) were associated with significantly increased risks of PTLDs (RR = 3.1), although lower than risks seen with other T-cell depletion methods (hereafter referred to as selective T-cell depletion methods; RR = 9.4, P value for difference = .005). Nine of the 10 cases in which T-cell depletion involved lectins also had anti–T-cell monoclonal antibody depletion (n = 4) or sheep red blood cell rosetting (n = 5) performed. Furthermore, 8 of the 10 cases in the lectin group had received ATG as part of the conditioning regimen (RR = 29.1, 95% CI = 2.3-82). The final model included 2 categories of lymphocyte depletion: selective T-cell depletion and broad lymphocyte depletion methods. Prophylaxis or treatment for acute GVHD with ATG was another strong risk factor for PTLDs (RR = 3.8). ATG prophylaxis (RR = 3.6) and ATG therapy (RR = 4.4) both independently increased PTLD risks, and the combined effects appeared to be greater than either given separately.
Relative risk (RR) of PTLDs by method of T-cell depletion of the bone marrow, ATG therapy, HLA and GVHD status, second transplantation, and age at transplantation
| Variable . | Patients . | PTLDs . | RR (95% CI) . |
|---|---|---|---|
| Part I: Main model* | |||
| T-cell depletion methods | |||
| Broad lymphocyte depletion (see Part II) | 1196 | 6 | 3.1 (1.2-6.7) |
| Selective T-cell depletion (see Part II) | 2350 | 68 | 9.4 (6.0-14.7) |
| ATG use (preventive or acute GVHD treatment†) | 2269 | 39 | 3.8 (2.5-5.8) |
| 2+ HLA antigen–mismatched related or unrelated donor, no ATG, no selective T-cell depletion | 2699 | 4 | 0.9 (0.3-2.2) |
| 2+ HLA antigen–mismatched related or unrelated donor, ATG and/or selective T-cell depletion | 1375 | 55 | 3.8 (2.4-6.1) |
| Acute GVHD grade II-IV† | 10 575 | 62 | 1.7 (1.2-2.5) |
| Chronic GVHD moderate/severe or clinical extensive† | 4771 | 22 | 2.0 (1.1-3.2) |
| Second transplantation† | 1471 | 11 | 3.5 (1.7-6.3) |
| Age 50 y or older at transplantation | 945 | 14 | 5.1 (2.8-8.7) |
| Part II: T-cell depletion methods‡ | |||
| No T-cell depletion | 23 355 | 53 | 1.0 |
| Broad lymphocyte depletion | |||
| Alemtuzumab (CAMPATH) MoAb | 684 | 3 | 3.1 (0.7-8.4) |
| Elutriation/density gradient centrifugation | 512 | 3 | 3.2 (0.8-8.8) |
| Selective T-cell depletion | |||
| Anti-T or anti-T+NK MoAb | 1784 | 49 | 8.4 (5.1-13) |
| SRBC rosetting | 225 | 7 | 14.6 (5.9-31) |
| Lectins with/without SRBC or anti-T MoAb§ | 223 | 10 | 15.8 (7.2-32) |
| Unclassified/unknown method | 118 | 2 | 6.0 (0.96-20) |
| Variable . | Patients . | PTLDs . | RR (95% CI) . |
|---|---|---|---|
| Part I: Main model* | |||
| T-cell depletion methods | |||
| Broad lymphocyte depletion (see Part II) | 1196 | 6 | 3.1 (1.2-6.7) |
| Selective T-cell depletion (see Part II) | 2350 | 68 | 9.4 (6.0-14.7) |
| ATG use (preventive or acute GVHD treatment†) | 2269 | 39 | 3.8 (2.5-5.8) |
| 2+ HLA antigen–mismatched related or unrelated donor, no ATG, no selective T-cell depletion | 2699 | 4 | 0.9 (0.3-2.2) |
| 2+ HLA antigen–mismatched related or unrelated donor, ATG and/or selective T-cell depletion | 1375 | 55 | 3.8 (2.4-6.1) |
| Acute GVHD grade II-IV† | 10 575 | 62 | 1.7 (1.2-2.5) |
| Chronic GVHD moderate/severe or clinical extensive† | 4771 | 22 | 2.0 (1.1-3.2) |
| Second transplantation† | 1471 | 11 | 3.5 (1.7-6.3) |
| Age 50 y or older at transplantation | 945 | 14 | 5.1 (2.8-8.7) |
| Part II: T-cell depletion methods‡ | |||
| No T-cell depletion | 23 355 | 53 | 1.0 |
| Broad lymphocyte depletion | |||
| Alemtuzumab (CAMPATH) MoAb | 684 | 3 | 3.1 (0.7-8.4) |
| Elutriation/density gradient centrifugation | 512 | 3 | 3.2 (0.8-8.8) |
| Selective T-cell depletion | |||
| Anti-T or anti-T+NK MoAb | 1784 | 49 | 8.4 (5.1-13) |
| SRBC rosetting | 225 | 7 | 14.6 (5.9-31) |
| Lectins with/without SRBC or anti-T MoAb§ | 223 | 10 | 15.8 (7.2-32) |
| Unclassified/unknown method | 118 | 2 | 6.0 (0.96-20) |
RR indicates relative risk; PTLD, posttransplantation lymphoproliferative disorder; CI, confidence interval; chronic GVHD, mild or moderate graft-versus-host disease for CIBMTR or clinical extensive FHCRC; NK, natural killer; MoAb, monoclonal antibodies; ATG, antithymocyte globulin; and SRBC, sheep red blood cell rosetting methods including SRBC plus density gradient centrifugation.
Part I: Poisson regression analysis stratified by time since transplantation in 13 categories (Table 2).
The following variables are time dependent: ATG, acute GVHD, chronic GVHD, and second transplantation. Second allogeneic transplantation among patients with a first allogeneic transplantation.
Part II model includes all variables in part I model with additional variables for T-cell depletion methods.
Group includes methods that remove both B and T cells (lectin agglutinations) and methods that selectively target T cells.
Risk modification of HLA mismatch by other factors.
In initial analyses, patients who received a transplant from unrelated donors or from related donors with 2 or more HLA antigen mismatches (URD/HLA mismatch) had significantly higher risks for PTLDs than those with marrow from HLA-identical or only 1 HLA antigen–mismatched siblings. However, more detailed analyses indicated that the risk associated with HLA matching was modified significantly by the use of T-cell depletion, ATG, or both (P value interaction = .004; Table 3). Among patients with URD/HLA-mismatched donors, those who did not receive either selective T-cell–depleted marrow or ATG had no increase risk in the absence of other risk factors (RR = 0.9; 4 PTLD cases, all unrelated donors). However, having an URD/HLA-mismatched donor greatly enhanced the already high risk associated with T-cell depletion, ATG therapy, or both, 3.8-fold. Significantly increased risks (over and above the elevated risks for T-cell depletion and ATG use) were observed for both patients with 2 or more HLA antigen–mismatched related donors (RR = 3.1; 95% CI = 1.7-5.7) and patients with unrelated donors (RR = 4.2; 95% CI = 2.6-6.9), but not for patients with only 1 antigen HLA–mismatched donors (RR = 1.8; 95% CI = 0.7-4.0) compared with HLA-identical siblings.
Acute and chronic graft-versus-host disease, second transplantation.
Both acute and chronic GVHD significantly increased the risk of developing PTLDs (RR = 1.7 and 2.0, respectively). For both acute and chronic GVHD, the risk increased with increasing severity of GVHD, although the trend was not statistically significant. Based on 11 cases, undergoing a second allogeneic transplantation also increased the risk (RR = 3.5). In 2 patients, both the first and second transplantation involved T-cell depletion. In 2 cases (including 1 with T-cell depletion), ATG was given for the second transplantation but not for the first transplantation. Thus, T-cell depletion or ATG administration may have increased the PTLD risk for 3 of these patients.
Age at transplantation.
Patients who were 50 years or older at HCT had a 5-fold increased risk of PTLDs compared with younger patients (RR = 5.1, 14 PTLD cases, Table 3). A trend test for increasing risk with increasing age was also significant (P = .005), but neither continuous age adjustment nor categoric age variables in decades(< 10, 10-19, 20-29, 30-39, 40-49 years) significantly improved the fit of the model shown in Table 3 (P > .35). RRs for older transplant recipients were significantly elevated independent of their status with respect to selective T-cell depletion, ATG therapy, and receiving a transplant from URD/HLA-mismatched donors (Table 4). The excess risk associated with older age at HCT was high even among patients without GVHD (RR = 8.1; 95% CI = 3.5-16). Among patients 50 years or older at allogeneic HCT, 5 of the 6 PTLD cases who did not have selective T-cell depletion or ATG use had at least 1 additional PTLD risk factor (moderate to severe acute or chronic GVHD, or both; T-cell depletion using alemtuzumab or elutriation; or a second allogeneic transplantation).
Relative risk of PTLDs by age at transplantation and combination of major risk factors
| Model . | Age younger than 50 y . | Age 50 y or older . | Age 50 y or older versus younger than 50 y . | ||||
|---|---|---|---|---|---|---|---|
| Patients . | PTLD . | RR (95% CI) . | Patients . | PTLD . | RR (95% CI) . | RR (95% CI) . | |
| No. of major risk factors*† | |||||||
| No risk factors | 21 686 | 29 | 1.0 (referent group) | 799 | 6 | 6.7 (2.5-15) | 6.7 (2.5-15) |
| 1 risk factor | 2871 | 29 | 9.3 (5.5-16) | 113 | 4 | 46 (14-118) | 5.0 (1.5-13) |
| 2 or more risk factors | 1399 | 55 | 45 (29-75) | 33 | 4 | 237 (68-637) | 5.1 (1.5-13) |
| GVHD status‡ | |||||||
| No GVHD | 13 938 | 53 | 1.0 (referent group) | 424 | 8 | 8.1 (3.5-16) | 8.1 (3.5-16) |
| Acute or chronic GVHD | 12 018 | 60 | 2.1 (1.4-3.1) | 521 | 6 | 7.0 (2.7-15) | 3.3 (1.3-7.2) |
| Model . | Age younger than 50 y . | Age 50 y or older . | Age 50 y or older versus younger than 50 y . | ||||
|---|---|---|---|---|---|---|---|
| Patients . | PTLD . | RR (95% CI) . | Patients . | PTLD . | RR (95% CI) . | RR (95% CI) . | |
| No. of major risk factors*† | |||||||
| No risk factors | 21 686 | 29 | 1.0 (referent group) | 799 | 6 | 6.7 (2.5-15) | 6.7 (2.5-15) |
| 1 risk factor | 2871 | 29 | 9.3 (5.5-16) | 113 | 4 | 46 (14-118) | 5.0 (1.5-13) |
| 2 or more risk factors | 1399 | 55 | 45 (29-75) | 33 | 4 | 237 (68-637) | 5.1 (1.5-13) |
| GVHD status‡ | |||||||
| No GVHD | 13 938 | 53 | 1.0 (referent group) | 424 | 8 | 8.1 (3.5-16) | 8.1 (3.5-16) |
| Acute or chronic GVHD | 12 018 | 60 | 2.1 (1.4-3.1) | 521 | 6 | 7.0 (2.7-15) | 3.3 (1.3-7.2) |
RR indicates relative risk; PTLD, posttransplantation lymphoproliferative disorder; CI, confidence interval; and ATG, antithymocyte globulin.
Major PTLD risk factors are defined as follows: (1) T-cell depletion using selective T-cell depletion methods (see definitions in Table 3), (2) use of ATG therapy to prevent or treat acute graft-versus-host disease, (3) 2+ HLA antigen–mismatched sibling/relative, or unrelated donor, accompanied by selective T-cell depletion or ATG therapy.
Adjusted for acute and chronic GVHD, T-cell depletion using broad lymphocyte depletion methods, and second transplantation.
Adjusted for major risk factors noted above, T-cell depletion using broad lymphocyte depletion methods, and second transplantation.
Radiation and other factors.
In contrast to our previous study in a smaller numbers of patients,4 use of irradiation in the conditioning regimen did not increase PTLD risk (RR = 1.2; 95% CI = 0.7-2.0, 109 PTLD cases). Other widely used conditioning regimes including cyclophosphamide or busulfan also did not significantly increase the risk. Neither primary disease, donor age, nor sex was significantly associated with PTLD risk when added to the model shown in Table 3. Receiving a transplant from female (rather than male) donors decreased the risk for males who underwent HCT (RR = 0.45; 95% CI = 0.3-0.7, 22 PTLD cases), but not for female recipients (RR = 1.06; 95% CI = 0.6-1.9, 24 PTLD cases).
Time since transplantation.
Among 1-year survivors, risk of PTLDs remained elevated for most variables, but only for chronic GVHD (RR = 3.0; P = .02) and selective T-cell depletion (RR = 4.2; P = .06) did the increased risk approach statistical significance. The RR associated with having URD/HLA-mismatched donors among patients who received ATG or selective T-cell depletion declined sharply with time since transplantation (Ptrend < .001, Table 5), with no PTLDs occurring in this group later than 1 year after HCT. The risk after T-cell depletion and ATG administration appeared to decrease beyond 1 year. However, the test for trend over time since transplantation was not significant (Ptrend > .40).
Risk of PTLDs by time since transplantation
| Variable . | Time since transplantation, mo* . | |||||||
|---|---|---|---|---|---|---|---|---|
| Less than 3 mo . | 3 to less than 6 mo . | 6 to less than 12 mo . | More than 12 mo . | |||||
| No. PTLDs . | RR (95% CI) . | No. PTLDs . | RR (95% CI) . | No. PTLDs . | RR (95% CI) . | No. PTLDs . | RR (95% CI) . | |
| T-cell depletion methods | ||||||||
| Broad lymphocyte depletion† | 1 | 1.9 (0.1-9.3) | 0 | 0.0 (0.0-3.4) | 3 | 14.0 (2.8-59) | 2 | 3.4 (0.5-12) |
| Selective T-cell depletion† | 30 | 10.3 (5.1-21) | 24 | 12.3 (5.4-28) | 11 | 15.4 (4.2-58) | 3 | 4.2 (0.96-13) |
| ATG (preventative or acute GVHD treatment) | 23 | 5.4 (2.8-10) | 10 | 3.8 (1.6-8.2) | 4 | 3.2 (0.8-10) | 2 | 1.8 (0.3-6.9) |
| 2+ HLA antigen–mismatched related or unrelated donor | ||||||||
| No ATG, no selective T-cell depletion†‡ | 1 | 1.0 (0.1-5.1) | 1 | 0.8 (0.04-4.0) | 1 | 1.6 (0.1-9.4) | 1 | 0.7 (0.04-3.5) |
| ATG, selective T-cell depletion, or both† | 30 | 5.7 (2.8-12) | 17 | 2.8 (1.2-6.4) | 8 | 3.9 (1.2-14) | 0 | 0.0 (0.0-2.2) |
| Acute GVHD grade II-IV | 27 | 1.9 (1.1-3.5) | 19 | 1.8 (0.9-3.5) | 7 | 1.6 (0.5-4.2) | 9 | 1.2 (0.5-3.1) |
| Chronic GVHD moderate/severe or clinical extensive | 1 | 2.0 (0.1-9.4) | 8 | 2.3 (0.9-5.2) | 3 | 1.1 (0.2-3.8) | 10 | 3.0 (1.2-7.4) |
| Second transplantation§ | 0 | 0.0 (0.0-1.2) | 7 | 10.3 (3.9-24) | 3 | 7.0 (1.6-23) | 1 | 1.8 (0.1-9.3) |
| 50 y or older at transplantation | 3 | 2.7 (0.6-7.3) | 7 | 8.1 (3.2-18) | 2 | 3.9 (0.6-14) | 2 | 4.5 (0.7-16) |
| Total PTLD cases (person-years) | 48 | (5878) | 38 | (4560) | 19 | (7659) | 22 | (61 939) |
| Variable . | Time since transplantation, mo* . | |||||||
|---|---|---|---|---|---|---|---|---|
| Less than 3 mo . | 3 to less than 6 mo . | 6 to less than 12 mo . | More than 12 mo . | |||||
| No. PTLDs . | RR (95% CI) . | No. PTLDs . | RR (95% CI) . | No. PTLDs . | RR (95% CI) . | No. PTLDs . | RR (95% CI) . | |
| T-cell depletion methods | ||||||||
| Broad lymphocyte depletion† | 1 | 1.9 (0.1-9.3) | 0 | 0.0 (0.0-3.4) | 3 | 14.0 (2.8-59) | 2 | 3.4 (0.5-12) |
| Selective T-cell depletion† | 30 | 10.3 (5.1-21) | 24 | 12.3 (5.4-28) | 11 | 15.4 (4.2-58) | 3 | 4.2 (0.96-13) |
| ATG (preventative or acute GVHD treatment) | 23 | 5.4 (2.8-10) | 10 | 3.8 (1.6-8.2) | 4 | 3.2 (0.8-10) | 2 | 1.8 (0.3-6.9) |
| 2+ HLA antigen–mismatched related or unrelated donor | ||||||||
| No ATG, no selective T-cell depletion†‡ | 1 | 1.0 (0.1-5.1) | 1 | 0.8 (0.04-4.0) | 1 | 1.6 (0.1-9.4) | 1 | 0.7 (0.04-3.5) |
| ATG, selective T-cell depletion, or both† | 30 | 5.7 (2.8-12) | 17 | 2.8 (1.2-6.4) | 8 | 3.9 (1.2-14) | 0 | 0.0 (0.0-2.2) |
| Acute GVHD grade II-IV | 27 | 1.9 (1.1-3.5) | 19 | 1.8 (0.9-3.5) | 7 | 1.6 (0.5-4.2) | 9 | 1.2 (0.5-3.1) |
| Chronic GVHD moderate/severe or clinical extensive | 1 | 2.0 (0.1-9.4) | 8 | 2.3 (0.9-5.2) | 3 | 1.1 (0.2-3.8) | 10 | 3.0 (1.2-7.4) |
| Second transplantation§ | 0 | 0.0 (0.0-1.2) | 7 | 10.3 (3.9-24) | 3 | 7.0 (1.6-23) | 1 | 1.8 (0.1-9.3) |
| 50 y or older at transplantation | 3 | 2.7 (0.6-7.3) | 7 | 8.1 (3.2-18) | 2 | 3.9 (0.6-14) | 2 | 4.5 (0.7-16) |
| Total PTLD cases (person-years) | 48 | (5878) | 38 | (4560) | 19 | (7659) | 22 | (61 939) |
RR indicates relative risk; PTLD, posttransplantation lymphoproliferative disorder; CI, confidence interval; HLA-2Ag+/unrelated, 2+ HLA antigen–mismatched sibling/relative, or unrelated donor; ATG, antithymocyte globulin; and GVHD, graft-versus-host disease.
Latency is defined as time since first transplantation. Models are stratified by latency in 13 categories.
Broad lymphocyte depletion methods are alemtuzumab monoclonal antibody or elutriation/density gradient centrifugation; selective T-cell depletion methods include anti-T or anti-T+NK monoclonal antibodies, SRBC rosetting, or lectins with/without SRBC or anti-T monoclonal antibodies.
All PTLDs in this group were from HLA unrelated donors.
Second allogeneic transplantation among patients with a first allogeneic transplantation.
Calendar period.
Further analyses examined the risk of PTLDs among patients who underwent transplantation in the calendar period 1991 to 1996; most of these data were not included in our previous report.4 These patients had significant elevations in risk of PTLDs for most of the major risk factors shown in Table 3. Importantly, there was no evidence to suggest that the RRs differed from those in patients who underwent transplantation before 1991. Consistent with trends in allogeneic HCT use, data from the calendar years 1991 to 1996 contributed strongly to the high risks associated with age at transplantation 50 years or older (1991-1996: RR = 6.4; 95% CI = 3.0-13; 11 PTLD cases) and occurrence of a second allogeneic transplantation (1991-1996: RR = 4.7; 95% CI = 1.7-11; 6 PTLD cases).
Risk of PTLDs by subtype.
All variables that significantly increased overall PTLD risk (from Table 3A) were also found to significantly elevate the risk of polymorphic PTLDs when analyzed separately (data not shown). The risk for monomorphic PTLDs was significantly increased only by selective T-cell depletion and among patients who underwent transplantation at 50 years or older; however, relative risks for most other variables were nonsignificantly elevated and were compatible with those for polymorphic PTLD. An exception was that patients with URD/HLA-mismatched donors and T-cell depletion, ATG therapy, or both, were at little increased risk of developing monomorphic PTLDs (RR = 1.2; 95% CI = 0.4-3.6), whereas the risk of polymorphic PTLDs for this group was increased 4-fold (RR = 4.5; 95% CI = 2.5-8.5; P value for difference = .044).
Cumulative incidence of PTLDs by risk group
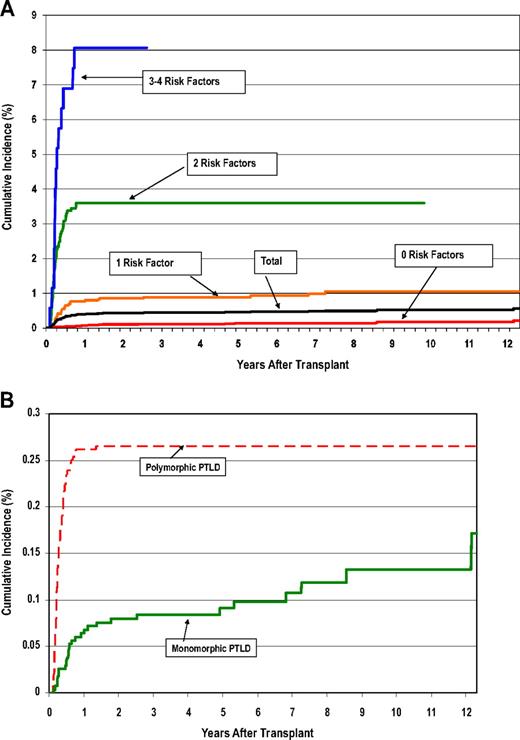
For the purpose of evaluating cumulative risks, we grouped the transplant recipients by the number of major risk factors for PTLDs that would have been known at the time of the first transplantation: (1) T-cell depletion using selective methods; (2) ATG therapy to prevent or treat acute GVHD; (3) URD/HLA-mismatched donors accompanied by selective T-cell depletion or ATG therapy; and (4) age 50 years or older at transplantation. Table 6 shows both relative risks and cumulative incidence (using methods that take account of competing risks) according to the number of major risk factors. Patients with URD/HLA-mismatched donors but no other major risk factors did not have a significantly elevated PTLD risk (RR = 1.6; 95% CI = 0.6-4.7), and were grouped with patients with no major risk factor to form the referent group (RR = 1.0). Both the RR and cumulative incidence rose steeply with increasing number of risk factors: cumulative incidence at 12 years, 0.2%, 1.2%, 3.6%, and 8.1% for 0, 1, 2, and more than 3 major risk factors, respectively (Table 6; Figure 1A). Figure 1B contrasts the early rise in cumulative incidence of polymorphic PTLDs within the first year after transplantation (last event, 1.5 years after transplantation), with the more gradual increase in the rate of monomorphic PTLDs (last event, 12.2 years after transplantation).
Risk of PTLDs by all possible categories of 4 major PTLD risk factors
| No. of major PTLD risk factors* . | No. of patients . | No. of PTLDs . | RR† (95% CI) . | Cumulative incidence (%)‡ (95% CI) . | Time of last PTLD, y . |
|---|---|---|---|---|---|
| No risk factors | 21 686 | 29 | 1.0 (Reference) | 0.2 (0.1-0.4) | 12.2 |
| One risk factor | 3670 | 35 | 8.6 (5.3-14) | 1.1 (0.7-1.5) | 7.2 |
| Two risk factors | 1371 | 49 | 43 (27-69) | 3.6 (2.7-4.7) | 0.8 |
| Three or 4 risk factors | 174 | 14 | 110 (56-206) | 8.1 (4.6-12.7) | 0.7 |
| Total | 26 901 | 127 |
| No. of major PTLD risk factors* . | No. of patients . | No. of PTLDs . | RR† (95% CI) . | Cumulative incidence (%)‡ (95% CI) . | Time of last PTLD, y . |
|---|---|---|---|---|---|
| No risk factors | 21 686 | 29 | 1.0 (Reference) | 0.2 (0.1-0.4) | 12.2 |
| One risk factor | 3670 | 35 | 8.6 (5.3-14) | 1.1 (0.7-1.5) | 7.2 |
| Two risk factors | 1371 | 49 | 43 (27-69) | 3.6 (2.7-4.7) | 0.8 |
| Three or 4 risk factors | 174 | 14 | 110 (56-206) | 8.1 (4.6-12.7) | 0.7 |
| Total | 26 901 | 127 |
RR indicates relative risk; CI, confidence interval; ATG, antithymocyte globulin; and GVHD, graft-versus-host disease.
Four major PTLD risk factors are defined as follows: (1) selective T-cell depletion methods (see definitions in Table 3); (2) use of ATG therapy to prevent or treat acute graft-versus-host disease; (3) 2+ HLA antigen–mismatched siblings, or unrelated donors, accompanied by selective T-cell depletion methods or ATG therapy; (4) age 50 years or older at allogeneic HCT.
Risk of PTLDs relative to the patients with no major risk factors.
Cumulative incidence (calculated using competing risk methods) at the time of the last event (PTLD).
Cumulative incidence of PTLDs. (A) Cumulative incidence of posttransplantation lymphoproliferative disorders (PTLDs) after hematopoietic cell transplantation (HCT), by patient risk group. (B) Cumulative incidence of polymorphic versus monomorphic PTLDs after HCT.
Cumulative incidence of PTLDs. (A) Cumulative incidence of posttransplantation lymphoproliferative disorders (PTLDs) after hematopoietic cell transplantation (HCT), by patient risk group. (B) Cumulative incidence of polymorphic versus monomorphic PTLDs after HCT.
Immunosuppressive therapy for GVHD and risk of late-onset PTLDs (case-control study)
The nest case-control study (35 PTLDs 6 or more months after transplantation, 97 matched controls) evaluated the risk of PTLDs associated with duration of GVHD therapy and specific immunosuppressive drugs used for chronic GVHD therapy. Based on a detailed medical record review, we confirmed the increased risk of late-onset PTLDs associated with the occurrence of chronic GVHD (RR = 3.17; 95% CI = 1.45-8.30). In a model accounting for chronic GVHD, prolonged duration of immunosuppressive drug therapy for GVHD (12+ months compared with < 12 months) did not significantly increase the risk (9 PTLD cases, 15 controls, RR = 1.62; 95% CI = 0.47-5.54). Patients treated for chronic GVHD with cyclosporine had a 3-fold increased risk of late-onset PTLDs compared with other immunosuppressive agents (primarily including steroids and azathioprine; 16 PTLDs, 23 controls, RR = 3.18; 95% CI = 1.25-8.06), with risk increasing to 7-fold for those receiving combined azathioprine and cyclosporine therapy (4 PTLDs, 3 controls, RR = 7.81; 95% CI = 1.18-51.94).
Discussion
Based on the current WHO classification,10 PTLD is defined as a lymphoid proliferation or lymphoma that develops as a consequence of immunosuppression in a recipient of a solid organ or bone marrow allotransplantation. The entity comprises a spectrum of tumors ranging from early EBV-driven polyclonal proliferations to EBV-positive or EBV-negative lymphomas of predominantly B-cell or, less often, T-cell type.10,19 Similar to other immunodeficiency-related lymphomas, PTLDs often present clinically with tumor involvement of extranodal and unusual sites, high-grade histopathology, and an aggressive clinical behavior.10,20,–22 Consistent with the temporal pattern of immune reconstitution after allogeneic HCT, nearly 70% of PTLDs are known to develop in the first 6 months after transplantation followed by a steep decline in incidence.4
This expansion of our earlier multicenter study increased the number of patients who underwent allogeneic HCT who could be evaluated from 18 014 to 27 000 and the number of PTLD cases from 78 to 127. This is the largest study to date and the first with sufficient statistical power to evaluate the joint effects of various PTLD risk factors. Our results confirmed previous studies showing that the strongest risk factors for PTLDs were T-cell depletion through methods that selectively targeted T cells and ATG given as prophylaxis of or treatment for GVHD. The development of acute or chronic GVHD, or both, resulted in a less pronounced 2-fold increase in PTLD risk. As a new finding we showed that older age at transplantation (50+ years) and the occurrence of a second allogeneic transplantation were strongly linked to increased risk of PTLD. In contrast to our earlier findings, which were based on a small number of nonirradiated patients,4 we found no significant effect of TBI-based conditioning regimens. Our evaluation of joint effects revealed a strong interaction between URD/HLA-mismatched transplantations and T-cell depletion/ATG, indicating that only patients with URD/HLA-mismatched donors and T-cell depletion or ATG therapy were at increased risk of PTLD. Finally, most polymorphic PTLDs occurred within a year of transplantation, whereas monomorphic PTLDs continued to occur 5 or more years after transplantation.
Allogeneic HCT at age 50 years or older has been limited in the past by the high rate of transplantation-related complications and an increased incidence and severity of both acute and chronic GVHD.23 As a result of improvements in supportive care and approaches to GVHD, several studies have shown that allogeneic HCT can be carried out successfully in select groups of older patients.24 Age at transplantation has not been identified as a risk factor for PTLDs in previous investigations,4,7,19 possibly due to small numbers of older patients undergoing transplantation in earlier time periods. There was a 5-fold increased risk of developing PTLDs among patients who underwent transplantation when 50 years or older compared with younger patients, with significantly elevated risks in most patient subgroups, including patients who did not develop acute or chronic GVHD. Previous investigators have speculated that age-related EBV-associated B-cell lymphoproliferative disorders occurring in persons without predisposing immunodeficiency may be a distinct clinical entity due to depression of the immune system with aging.25 Others have reported that allogeneic HCT patients receiving T cell–depleted grafts at ages older than 50 years show delayed immune reconstitution compared with younger patients.26 Taken together, our results suggest that transplantation at older ages may be an independent risk factor for PTLD. Future research is needed to confirm our findings and, if replicated, to clarify underlying mechanisms.
We confirmed prior investigations showing that selective T-cell depletion methods (including anti–T-cell monoclonal antibodies or sheep red blood cell [SRBC] rosetting) are associated with an 8- to 15-fold increased risk of PTLD, and that this risk factor remains the strongest contributor to PTLD occurrence.4 We found a relatively modest 3-fold increase in PTLD risk associated with alemtuzumab and elutriation lymphocyte depletion (methods that remove both B and T cells). These latter results are consistent with the hypothesis that the reduction in risk (compared with selective T-cell depletion methods) may be due to a reduction in EBV viral load, or, alternatively, to a decrease in the number of EBV-infected B cells available for transformation.27 The new finding of a high PTLD risk after grafts depleted with soybean lectin agglutination (± monoclonal antibodies or sheep red blood cell rosetting), with most of the cases receiving ATG at the time of transplantation, is consistent with other small series reporting high PTLD risks associated with lectin T-cell depletion methods, when ATG was added in the period immediately after transplantation to prevent graft rejection.28,29
Data on risk factors for PTLDs occurring several years after allogeneic HCT are sparse. Our study, with more than 14 000 1-year survivors, shows that rates of PTLDs declined sharply over time, but continued to be significantly increased compared with the general population for 5+ years after transplantation. The rapid decline in PTLD incidence over the first year after HCT is in accord with prior investigations showing that levels of cytotoxic T-lymphocyte precursors are decreased at 3 months after allogeneic HCT, but appear to normalize at 9 to 12 months.30,31 The higher incidence of monomorphic compared with polymorphic PTLDs occurring among 1-year survivors suggests differences in clinicopathologic features among the PTLD subtypes. Risks associated with most risk factors remained elevated in 1+ year survivors, but were significantly elevated only for chronic GVHD; however, there was no evidence that the increased risk was due to prolonged immunosuppressive drug therapy for GVHD. Selective T-cell depletion appeared to increase the risk of late-onset PTLD, consistent with the known delayed immune reconstitution of T-cell function among patients given T cell–depleted marrow.32,33 The declining trend over time in PTLD risk for patients who had both URD/HLA-mismatched donors and ATG use/selective T-cell depletion might indicate that HLA disparity is a risk factor mainly for the early-onset polymorphic PTLDs.
Further, the use of HLA-mismatched or unrelated donors led to a 4-fold increase in the already heightened PTLD risk among patients given selectively T cell–depleted marrow or ATG. This strong interaction suggests that the combination of these factors further impairs immune function. However, the use of URD/HLA-mismatched donors (without T-cell depletion or ATG use) did not significantly increase the risk of PTLD. Although a second allogeneic transplantation has been shown to be an effective treatment option for selected patients who had undergone a previous allogeneic HCT,34 our study showed that the prolonged immune suppression associated with second allogeneic HCT increased the risk of PTLD.
We used newer cumulative incidence methods that accounted for the high competing risk of transplant-related mortality,16 and, thus, our estimates were lower in magnitude than reported in earlier investigations4 and other single-center studies.3,7 The incidence of PTLDs was very low in the more than 20 000 patients who underwent transplantation at 50 years or younger who did not receive a T cell–depleted transplant or ATG (0.2% at 12 years). However, small subgroups of patients showed high cumulative incidences of PTLD, ranging from 3.6% to 8%. Thus, these data identify high-risk features that should trigger enhanced EBV surveillance efforts and early treatment intervention.
Strengths of our study include its large size and extended follow up based on high-quality data from 271 transplant centers worldwide. The study size and follow-up period provided reasonable statistical power to evaluate less common factors associated with risk, the joint effects of different risk factors, and predictors of both early- and late-onset PTLDs by subtype (polymorphic versus monomorphic). Further, the feature of a centralized pathology review of PTLD cases was unique given the size of this study. A limitation of our investigation is that we included only patients who underwent transplantation through 1996. However, patients who underwent allogeneic HCT in the calendar period (1991-1996) had very similar relative risks for various PTLD risk factors as patients who underwent transplantation in earlier periods, suggesting that at least over that interval no substantial changes in the pattern of PTLD development occurred.
It is important to note that patients included in this study reflect an era in which radiation was a mainstay of conditioning (> 70% received radiation), and the stem cell source was bone marrow. Newer developments in allogeneic HCT include the higher frequency of nonradiation conditioning regimens and reduced intensity preparative regimens.35 Further, use of peripheral blood progenitor cells as a source of stem cells has been increasing, and has been associated with an increased risk of chronic GVHD.36 HCT with cord blood as a source of hematopoietic cells is also being used more frequently, although immune reconstitution is delayed.37
Over the last decade, there has been a “shift” in the diagnostic criteria for PTLD. During the time period covered by this report, PTLD was diagnosed based on lymph node enlargement, biopsy findings consistent with lymphoma, and immunohistochemical staining positive for EBV. The biopsy-based identification of PTLDs may have resulted in an underreporting of outcomes, and, thus, incidence may be underestimated. The tools to monitor patients for reactivation of EBV and the development of PTLDs have changed over time. For example, many transplant centers currently use sequential quantitative polymerase chain reaction (PCR) tests to determine EBV DNA titers, and if the titer rises above a certain level (eg, > 1000 copies/mL) preemptive therapy is instituted, typically with anti-CD20 antibody (rituximab). As a result of this change in policy and diagnostic criteria, the incidence of clinically apparent PTLDs may well have declined. Whether rises in EBV titer should be viewed as indicative of a developing PTLDs remains a matter of scientific evaluation and debate.
In summary, the establishment of early diagnosis (eg, the relationship between EBV viral load, immune response, and PTLD development), and early treatment based on serologic testing before established tissue involvement, represent a dramatic shift in the management of EBV-related lymphoproliferation, which requires further study. These changes will presumably be increasingly important in the clinical monitoring and modulation aimed at preventing PTLDs in patients who undergo transplantation.38,39 Our data suggest that patients with 2 or more major risk factors (as identified in Table 6) should be given the highest priority for close monitoring of EBV titers, at least for the first 6 months after HCT, and receive preemptive treatment if there is evidence of sequential rising titers. Future research is needed to evaluate the risk of PTLDs after newer methods of allogeneic HCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to the physicians and data professionals at participating transplant centers who contributed high-quality data to this study. We are especially grateful to Dr Catherine Metayer from the University of California, Berkeley, for detailed data review and statistical analyses for the case-control study. We thank Gary Schoch from FHCRC, Seattle, and Diane Knutson and Sharon Nell from the CIBMTR, Milwaukee, for support in data collection. We acknowledge Linda Kaufman, Kathy Chimes, and Diane Fuchs from Westat (Rockville, MD) for coordination of field studies, and Nathan Appel, David Hacker, Heather Bath, and George Geise from Information Management Services (Silver Spring, MD) for computing support.
This research is supported by contract nos. CP-51027 and CP-51028 from the NCI; and by the Intramural Research Program of the NIH and the NCI. The FHCRC investigators are supported by grants P01-HL36444, P01-CA18029, P01-CA102542, and P01-CA15704 from the NIH. The CIBMTR is supported by Public Health Service Grant U24-CA76518 from the NCI, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; Office of Naval Research; Health Services Research Administration (HHS); K23 CA82350-05 grant from the NIH; and grants from Abbott Laboratories; Aetna; American International Group; American Red Cross; Amgen; anonymous donation to the Medical College of Wisconsin; AnorMED; Astellas Pharma US; Baxter International; Berlex Laboratories; Biogen IDEC; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bristol-Myers Squibb Company; BRT Laboratories; Cangene; Celgene; CellGenix; Cell Therapeutics; CelMed Biosciences; Cylex; Cytonome; CytoTherm; DOR BioPharma; Dynal Biotech, an Invitrogen Company; Enzon Pharmaceuticals; Gambro BCT; Gamida Cell; Genzyme; Gift of Life Bone Marrow Foundation; GlaxoSmithKline; Histogenetics; HKS Medical Information Systems; Kirin Brewery; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals; Miller Pharmacal Group; Milliman USA; Miltenyi Biotec; MultiPlan; National Marrow Donor Program; Nature Publishing Group; Novartis Pharmaceuticals; Osiris Therapeutics; Pall Medical; Pfizer; Pharmion; PDL BioPharma; Roche Laboratories; Sanofi-aventis; Schering Plough; StemCyte; StemSoft Software; SuperGen; Sysmex; The Marrow Foundation; THERAKOS; University of Colorado Cord Blood Bank; ViaCell; ViraCor Laboratories; Wellpoint; and Zelos Therapeutics.
The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the US government.
National Institutes of Health
Authorship
Contribution: R.E.C., H.J.D., K.A.S., M.M.H., and G.S. designed research; R.E.C., K.A.S., M.M.H., and H.J.D. collected data; E.S.G. and R.E.C. performed statistical analysis; O.L., R.E.C., and E.S.G. drafted the paper; and all authors interpreted data and critically revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ola Landgren, Medical Oncology Branch, Center for Cancer Research, National Cancer Institute, 9000 Rockville Pike, Bldg 10/Rm 13N240F, Bethesda, MD 20892; e-mail: landgreo@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal