Antithymocyte globulin (ATG) has been used in allogeneic stem-cell transplantation to prevent graft rejection and graft-versus-host disease (GvHD). Its use, however, has been associated with delayed T-cell reconstitution and prolonged susceptibility to opportunistic infections (OIs) especially in patients undergoing T cell–depleted (TCD) transplantation. Recently, a prospective trial was conducted in 52 adult patients (median age, 47 years) with various hematologic malignancies undergoing TCD transplantation from HLA-matched related donors without the use of ATG. The cytoreductive regimen consisted of hyperfractionated total body irradiation (HFTBI), thiotepa, and fludarabine. The preferred source of the graft was peripheral blood stem cells (PBSCs). No additional graft rejection or GvHD prophylaxis was given. All evaluable patients engrafted without any immune-mediated graft rejections. Disease-free survival (DFS) at 3 years was 61% in all patients, and 70% in patients with standard-risk disease. Acute GvHD was limited to grade 2 in 8% and chronic GvHD in 9% of patients. Life-threatening OIs occurred in 3 of 52 patients and was fatal in 1. This study demonstrates durable engraftment with a low incidence of GvHD despite the lack of ATG, as well as the curative potential of this regimen.
Introduction
In earlier studies at this center,1,2 the efficacy of allogeneic bone marrow (BM) transplantation with allografts depleted of T cells by soybean lectin agglutination (SBA) and sheep red blood cell (sRBC) rosetting was demonstrated in patients receiving sibling donor grafts for acute myelogenous leukemia (AML) in first and second remission (CR1 and CR2). A nearly complete elimination of acute and chronic graft-versus-host disease (GvHD) was achieved without compromising the antileukemic effect of the allograft (disease-free survival [DFS] at 4 years of 77% and 50%, AML CR1 and CR2, respectively). The early observation of immune-mediated graft rejection was eliminated by the addition of antithymocyte globulin (ATG), but at the expense of delayed immune reconstitution in adults compared with children,3 or adults receiving conventional grafts. In an effort to improve these results and to address the complexities of an expanding older patient population, a clinical trial testing a modified regimen for T cell–depleted (TCD) grafts was undertaken. The conditioning regimen consisted of hyperfractionated total body irradiation (HFTBI) combined with thiotepa and the immunosuppressive drug fludarabine. Granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cells (PBSCs) were the preferred source for the graft, and TCD was performed by automated CD34+ selection and sRBC rosetting. ATG was removed from the conditioning regimen and we sought to determine first if consistent engraftment could be achieved, and second, if T-cell reconstitution could be improved with a reduction in the incidence of opportunistic infections (OIs). We now report the results of this trial in 52 patients who received HLA-identical related donor grafts with this modified regimen.
Patients, materials, and methods
Patient characteristics
Fifty-two patients with a variety of malignant hematologic diseases and treatment backgrounds were enrolled on an IRB-approved protocol and underwent transplantation from July 1, 2001, to December 31, 2005. Follow-up was until March 1, 2007, with a median follow-up of 45 months. Eligibility included availability of an HLA-identical related donor; age older than 18 years; Karnofsky performance status (KPS) of 70 or greater; absence of active infection; absence of active central nervous system or cutaneous disease; and lack of serious coexisting cardiac, hepatic, or renal dysfunction that would preclude safe administration of the regimen. Satisfactory cardiac and renal function required a left ventricular ejection fraction of 50% or more and a normal serum creatinine or a clearance of more than 60 mL/min/1.73 m2. For patients with AML, only those with intermediate- or high-risk disease, based on cytogenetics,4 could undergo transplantation in CR1. Patients with t(8:21), t(15:17), or inversion 16 were excluded. Only patients with high-risk acute lymphocytic leukemia (ALL) by cytogenetics (generally Philadelphia chromosome–positive [Ph+] or t(4;11)) were eligible in CR1. HLA matching was established by DNA sequence-specific oligonucleotide typing for HLA-A, -B, -Cw, -DQB1, and -DRB1 loci. All patients and donors signed informed consent in accordance with the Declaration of Helsinki on a Memorial Sloan-Kettering Cancer Center (MSKCC) Institutional Review and Privacy Board–approved protocol prior to participation in the study.
Preparative regimen
The cytoreduction consisted of HFTBI followed by thiotepa and fludarabine. HFTBI was administered in 11 fractions of 125 cGy over 4 days, to a total dose of 1375 cGy. All patients had protective lung shielding and overlying ribs received an additional 600 cGy boost. Male patients with acute leukemia received an additional 400 cGy testicular boost in a single fraction.5
After completion of HFTBI, thiotepa 5 mg/kg per day was administered over 4 hours on each of 2 consecutive days, with no adjustment for weight. Fludarabine 25 mg/m2 per day was administered over 30 minutes for 5 days beginning on the first day of thiotepa.
Stem-cell collection, T-cell depletion, and transplantation
Mobilization of PBSCs from 48 donors was performed by administration of G-CSF 8 μg/kg subcutaneously every 12 hours, for 11 doses. Two 3- to 4-hour aphereses were performed on the 5th and 6th days after the 9th and 11th doses with a minimum target of 109 mononuclear cells (MNCs)/kg (3 × 106 CD34+/kg) of recipient weight. Selection of CD34+ stem cells was accomplished using the ISOLEX 300i Magnetic Cell Separator (Baxter, Deerfield, IL), followed by sRBC-rosette depletion of T cells. This method achieved an approximate 5 log10 depletion of CD3+ cells.6 Bone marrow was used for transplantation in 4 patients because of donor preference. T cells were removed from the BM by sequential SBA and sRBC-rosette depletion.7 Fresh BM or PBSC grafts were infused through a central venous catheter 24 to 48 hours after completion of fludarabine.
GvHD evaluation and management
The diagnosis of GvHD was made clinically, and confirmed pathologically with skin, mucosal, or liver biopsy whenever possible. It was classified according to standard criteria.8,9 Patients who engrafted and survived more than 30 days were evaluable for acute GvHD, unless it had already been diagnosed before a terminal event. Patients surviving more than 100 days were evaluable for chronic GvHD.
Supportive care
Patients were managed clinically according to MSKCC standard guidelines including infection prophylaxis for Pneumocystis carinii, herpes viruses, and fungus. In addition, atovaquone was prescribed for prevention of toxoplasmosis infections after transplantation in seropositive patients or those with seropositive donors. Patients received no cytomegalovirus (CMV)–specific prophylaxis, but CMV-seronegative patients received seronegative blood products regardless of the donor's serologic status. Monitoring of CMV reactivation by CMV pp65 antigenemia assay of peripheral blood was performed regularly through day 100 when either the patient or donor was CMV seropositive. Preemptive therapy was instituted in patients with documented CMV viremia. Epstein-Barr virus (EBV) was monitored by qualitative polymerase chain reaction (PCR) until 2003 and thereafter by real-time PCR of the BNRF1-p143 locus (Roche, Indianapolis, IN).10
Prophylactic antibacterial agents were not used except in 3 patients who received vancomycin at day −2. Patients received G-CSF beginning at or past day +7 after transplantation, only if clinically indicated.
Engraftment, donor chimerism, and immune reconstitution
Myeloid engraftment was defined as an absolute neutrophil count (ANC) of 0.5 × 109/L (500/μL) or more on 3 consecutive days after transplantation. Platelet engraftment was defined as a platelet count of 20 × 109/L (20 000/μL) or more without transfusion for at least 3 consecutive days. Bone marrow aspirates were obtained at regular intervals after transplantation to assess for cellularity, disease status, and donor chimerism. Immunophenotyping and T-cell proliferative responses to phytohemagglutinin-P (PHA) mitogen were evaluated every 3 to 6 months until normal. Immunofluorescence analysis and in vitro lymphocyte proliferation assay with PHA were performed as previously described.11 OIs were identified from the patient database and cross-checked with computerized medical and microbiology records. Life-threatening OIs were defined as pneumonia due to viral, fungal, and/or parasitic pathogens, invasive viral disease (eg, CMV, adenovirus), refractory herpes simplex or zoster infections, EBV–lymphoproliferative disease (EBV-LPD), toxoplasmosis, organ-based fungal infections, and progressive multifocal leukoencephalopathy, JC-virus induced.
Biostatistics
Analyses were performed as of March 1, 2007. DFS was defined as the interval from transplantation to death, hematologic or clinical relapse, or last follow up, and was estimated using the method of Kaplan-Meier. Estimates of the probability of relapse and nonrelapse mortality (NRM) were calculated with the cumulative incidence function.12 The Wilcoxon rank sum test was used to compare immune reconstitution results between groups of patients.
Results
Patient characteristics
Details of patient and donor characteristics before transplantation are presented in Table 1. The median age of patients was 47.3 years; 19 of the 52 were 50 years or older. Patients were classified as standard or poor risk based on their disease status at time of transplantation. Those with AML-CR1, AML-CR2, ALL-CR1, and CML-CP1 were classified as standard risk. All others were poor risk. As noted in Table 1, 9 patients with AML-CR1 underwent transplantation for intermediate-risk (n = 6) or high-risk (n = 3) cytogenetics, and 5 with ALL-CR1, for high-risk cytogenetics, predominantly Ph+. Three of 10 patients with non-Hodgkin lymphoma (NHL) were in remission at the time of transplantation. All others had residual disease that had demonstrated a recent response to chemotherapy and/or radiotherapy. Forty-seven of the 52 patients received PBSC grafts, with 1 patient receiving both BM and PBSC due to inadequate stem-cell mobilization by the donor. All donors were HLA-matched siblings, except one who was an HLA-identical cousin. The sex pairings were evenly distributed, and the median age of the donors was similar to that of the patients, 45 years. KPS ranged from 70 to 100 before transplantation.
Patient demographics
| Characteristics . | No. . |
|---|---|
| Total number of patients | 52 |
| KPS, range (median) | 70-100 (90) |
| M:F | 30:22 |
| Age, range (median) y | 19.5-62 (47.3) |
| Diseases | |
| AML CR1 | 9 |
| Intermediate* | 6 |
| High risk* | 3 |
| AML CR2 | 7 |
| AML CR1 after MDS | 5 |
| ALL CR1 | 5 |
| Ph+ | 3 |
| Ph+, mono 7 | 1 |
| t(9;17), mono 7 | 1 |
| ALL CR2 | 1 |
| ALL CR3 or greater | 1 |
| AL, other | 3 |
| CML CP1 | 4 |
| CML, advanced | 2 |
| MDS | 2 |
| RA after AML | 1 |
| RAEB-IT | 1 |
| NHL | 11 |
| B cell | |
| Low | 1 |
| Intermediate | 5 |
| High | 4 |
| T cell | 1 |
| T-PLL | 2 |
| Donors | |
| M → F | 11 |
| M → M | 16 |
| F → M | 14 |
| F → F | 11 |
| Age, range (median), y | 14-71 (45) |
| Grafts | |
| PBSC | 47 |
| BM | 4 |
| Both | 1 |
| Characteristics . | No. . |
|---|---|
| Total number of patients | 52 |
| KPS, range (median) | 70-100 (90) |
| M:F | 30:22 |
| Age, range (median) y | 19.5-62 (47.3) |
| Diseases | |
| AML CR1 | 9 |
| Intermediate* | 6 |
| High risk* | 3 |
| AML CR2 | 7 |
| AML CR1 after MDS | 5 |
| ALL CR1 | 5 |
| Ph+ | 3 |
| Ph+, mono 7 | 1 |
| t(9;17), mono 7 | 1 |
| ALL CR2 | 1 |
| ALL CR3 or greater | 1 |
| AL, other | 3 |
| CML CP1 | 4 |
| CML, advanced | 2 |
| MDS | 2 |
| RA after AML | 1 |
| RAEB-IT | 1 |
| NHL | 11 |
| B cell | |
| Low | 1 |
| Intermediate | 5 |
| High | 4 |
| T cell | 1 |
| T-PLL | 2 |
| Donors | |
| M → F | 11 |
| M → M | 16 |
| F → M | 14 |
| F → F | 11 |
| Age, range (median), y | 14-71 (45) |
| Grafts | |
| PBSC | 47 |
| BM | 4 |
| Both | 1 |
CR indicates complete remission; AL, acute leukemia multilineage; CML, chronic myelogenous leukemia; CP, chronic phase; MDS, myelodysplastic syndrome; RA, refractory anemia; RAEB-IT, refractory anemia with excess blasts in transformation; and T-PLL, T-cell prolymphocytic leukemia.
By cytogenetics.
Engraftment and donor chimerism
All 51 evaluable patients engrafted following TCD transplantation. One patient died of infection on day +8 and was not evaluable for engraftment. The median and range of the CD34+ and CD3+ cell doses infused, as well as time to neutrophil and platelet engraftment, are given in Table 2. The median CD34+ dose was within the range targeted by the protocol (3 × 106 CD34+ cells/kg). Eight patients underwent transplantation with grafts containing less than the targeted CD34+ cell dose, due to collection, not processing. Three of the latter 8 patients received TCD BM grafts, 1 received both BM plus PBSCs, and 4 received PBSCs. Three patients who received only BM grafts demonstrated the longest time to neutrophil recovery, 21 to 22 days, and 2 also had the longest time to platelet recovery, 36 and 54 days.
Outcome parameters of transplantation
| Outcome . | No. . |
|---|---|
| Median follow-up (range), mo | 45 (15.5-66.3) |
| Engraftment | 51 (evaluable) |
| Median transplant dose CD34+ × 106/kg (range) | 5.75 (0.37-23.9) |
| Median transplant dose CD3+ × 103/kg (range) | 0.99 (0-297) |
| Patients engrafting neutrophils | 51/51 |
| Median days to ANC 0.5 × 109/L or more (range) | 11 (9-22) |
| Patients engrafting platelets | 47/51 |
| Median days to platelets 20 × 109/L or more (range) | 16 (13-54) |
| Posttransplantation EBV-LPD | 2 |
| GvHD | 8 |
| Acute, grades II-IV | 4/49; 4 overall grade 2 |
| Chronic | 4/43; 1 limited; 3 extensive |
| Relapses | 9 |
| AML CR1 | 2 |
| AML, 2° MDS | 3 |
| ALL CR1 Ph+ | 1 |
| ALL CR2 | 1 |
| NHL | 2 |
| Deaths | 19 |
| Cause of death | |
| Relapse | 8 |
| AML CRI after MDS | 3 |
| AML CR1 | 2 |
| ALL CR2 | 1 |
| Burkitt lymphoma, PR | 1 |
| Diffuse large cell lymphoma, PR | 1 |
| Infection | 8 |
| GVHD and secondary complications | 2 |
| Other, complications of VATS | 1 |
| Outcome . | No. . |
|---|---|
| Median follow-up (range), mo | 45 (15.5-66.3) |
| Engraftment | 51 (evaluable) |
| Median transplant dose CD34+ × 106/kg (range) | 5.75 (0.37-23.9) |
| Median transplant dose CD3+ × 103/kg (range) | 0.99 (0-297) |
| Patients engrafting neutrophils | 51/51 |
| Median days to ANC 0.5 × 109/L or more (range) | 11 (9-22) |
| Patients engrafting platelets | 47/51 |
| Median days to platelets 20 × 109/L or more (range) | 16 (13-54) |
| Posttransplantation EBV-LPD | 2 |
| GvHD | 8 |
| Acute, grades II-IV | 4/49; 4 overall grade 2 |
| Chronic | 4/43; 1 limited; 3 extensive |
| Relapses | 9 |
| AML CR1 | 2 |
| AML, 2° MDS | 3 |
| ALL CR1 Ph+ | 1 |
| ALL CR2 | 1 |
| NHL | 2 |
| Deaths | 19 |
| Cause of death | |
| Relapse | 8 |
| AML CRI after MDS | 3 |
| AML CR1 | 2 |
| ALL CR2 | 1 |
| Burkitt lymphoma, PR | 1 |
| Diffuse large cell lymphoma, PR | 1 |
| Infection | 8 |
| GVHD and secondary complications | 2 |
| Other, complications of VATS | 1 |
EBV-LPD indicates Epstein-Barr virus lymphoproliferative disease; GvHD, graft-versus-host disease; PR, partial remission; and VATS, video-assisted thoracic surgery.
No immune-mediated graft rejections occurred despite the absence of ATG. Thirty-seven of the 44 patients who had BMs assessed within the first 2 months were 100% donor, and 7 had 90% or more donor chimerism. Twenty-three of the 37 patients retained complete donor chimerism on subsequent evaluations through the last follow up or death. Nine of the 37 patients suffered hematologic relapses. There was no correlation between relapse and mixed chimerism. In fact, 7 of the 9 patients who relapsed had 100% donor chimerism on day +30 marrows. The median CD34+ and CD3+ cell doses for the 21 patients who maintained 100% donor chimerism, compared with the 9 patients who relapsed or the 7 patients whose first BM chimerism was less than 100% donor, were not significantly different.
Although there were no late graft rejections, 2 patients received a single donor leukocyte infusion (DLI), providing 1 × 105 CD3+ cells/kg, for treatment of mixed donor chimerism in the bone marrow, one at 3 and the other at 12 months after transplantation. The former patient had demonstrated 92% and the latter patient 90% donor chimerism on their first BM evaluations, but subsequent BM examinations exhibited increasing host components. The CD34+ and CD3+ cell doses in the allografts for these 2 patients were not significantly different from those of patients who were 100% donor at day +30. Both patients were older than 50 years, with older female donors: 71 and 59 years. Following the DLI, both patients achieved complete donor chimerism. The patient treated at 3 months developed extensive chronic GvHD of the skin and oral mucosae, which responded to topical therapy only.
Twenty-nine of 52 patients had peripheral blood T-cell chimerism determined during the first 4 months after transplantation. The range of donor cells was 0% to 100% (median, 60%). Six of the 9 patients who relapsed were not studied. The remaining 3 were 70%, 100%, and 100% donor. Two patients with the lowest PB T-cell donor chimerism (0% and 10%) remain in remission at 4 and 5 years after transplantation. One of the latter patients had received a small dose of DLI for mixed marrow chimerism at 12 months, as previously noted.
GvHD
The median CD3+ cell dose for the 52 patients was 1 × 103/kg, which was far lower than the threshold dose of 1 × 105/kg associated with an increased risk of GvHD following an HLA-identical TCD BM sibling transplantation.13 Forty-nine of the 52 patients survived more than 30 days, and were thus evaluable for acute GvHD (Table 2). The incidence of grade 2 acute GvHD was 8%, and no patient developed grade III or IV acute GvHD. Forty-six patients survived more than 100 days and were evaluable for chronic GvHD. Four patients developed chronic GvHD (9%), 1 with limited and 3 with extensive disease. Two of the latter 3 patients eventually died from complications of GvHD. The one additional patient with extensive disease had received DLI for mixed chimerism 3 months after transplantation. The overall incidence of chronic GvHD was therefore 9%. There was no difference in the CD3+ T-cell doses infused into patients who developed GvHD compared with those who did not.
DFS and relapse
The patients treated on this study had a variety of diagnoses and treatment histories. The majority were in remission or had stable disease at the time of transplantation. Patients were almost equally divided between standard- and poor-risk disease groups (Table 1), with 25 and 27 patients, respectively. The estimated 3-year probability of DFS for the entire group was 61%, with 62% overall survival (OS) (median follow up, 45 months). As shown in Figure 1, the probability of DFS at 3 years for patients with standard-risk disease was 70%, in contrast to 52% for patients with poor-risk disease (P = .17). The OS for standard-risk disease was 72%, and 52% for poor-risk disease (P = .09). The majority of patients who underwent transplantation for poor-risk disease were 50 years or older, and thus their outcomes were nearly superimposable on those for the entire group of patients with poor-risk disease. Furthermore, although the number of older patients in the standard-risk group was small (n = 7), their outcomes were not statistically different from the outcome of younger patients with standard-risk disease (Figure 2). The median KPS of surviving patients is 100.
Kaplan-Meier estimates of the probability of DFS based on status of disease.
Kaplan-Meier estimates of the probability of DFS at 2 years after transplantation for patients younger than 50 years and 50 years or older with standard-risk disease.
Kaplan-Meier estimates of the probability of DFS at 2 years after transplantation for patients younger than 50 years and 50 years or older with standard-risk disease.
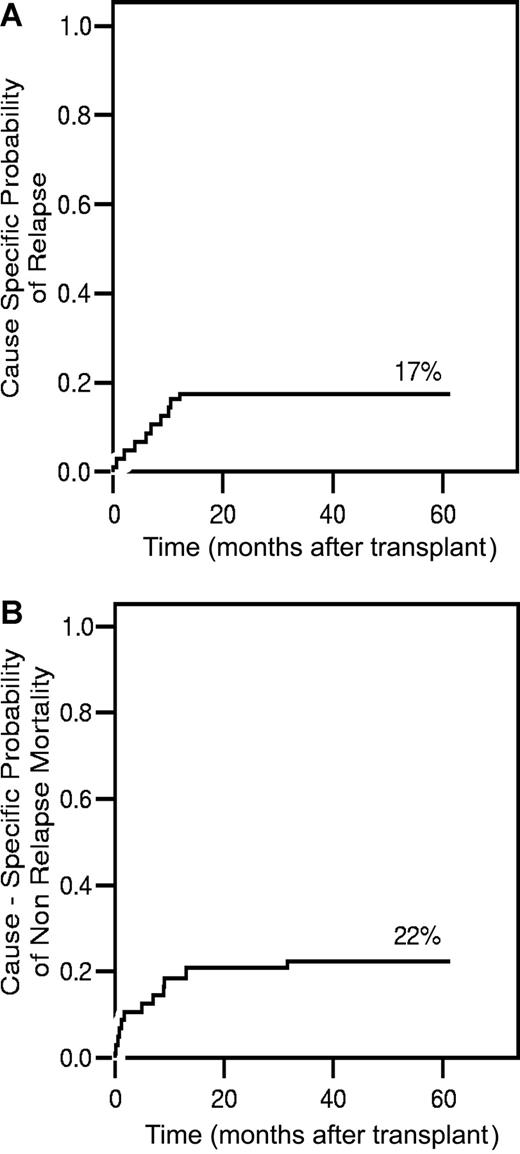
Nine patients had hematologic or clinical evidence of relapse or disease progression after transplantation (Table 2). Eight have died, and 1 patient underwent an unmodified second transplantation in remission but subsequently relapsed and was lost to follow up. Relapses occurred at a median of 6.9 months (all ≤ 12 months) after transplantation. The cumulative incidence of relapse at 3 years was 17% (Figure 3A). Three of the 25 standard-risk patients (2 AML-CR1 and 1 ALL-CR1, Ph+) have relapsed.
Cause-specific analysis of relapse and mortality. (A) Cause-specific probability of relapse, controlling for the competing risks of treatment failure by nonrelapse causes. (B) Cause-specific probability of death, adjusting for the competing risk of relapse.
Cause-specific analysis of relapse and mortality. (A) Cause-specific probability of relapse, controlling for the competing risks of treatment failure by nonrelapse causes. (B) Cause-specific probability of death, adjusting for the competing risk of relapse.
Five additional patients (CML = 3, AML after MDS = 1, AL [biphenotypic] = 1) developed molecular or cytogenetic relapse. Three patients each received one dose of DLI (0.1–3 × 106 CD3+ cells/kg), and 2 patients received 2 doses each (0.3 × 107 and 1 × 107 CD3+ cells/kg). The DLI was administered between 2 and 21 months after transplantation, and all 5 patients achieved sustained cytogenetic and molecular remissions. None of these patients developed GvHD.
Posttransplantation EBV-LPD
Two patients (4%) were diagnosed with an EBV-LPD, at 60 days and 2 years after transplantation. The former patient was successfully treated with rituximab alone. The latter patient, who was receiving treatment for chronic GvHD, responded to rituximab and reduction in immunosuppression.
Causes of death
Nineteen (35%) of the 52 patients have died. The causes of death are summarized in Table 2. Eight patients (15%) died from infection, and 8 (15%) from relapse/progression. Streptococcal viridans bacteremia, which was diagnosed on days −5 to +5, and occurred with the first neutropenic fever, was the cause of death in 4 of the 8. Despite appropriate management and sterilization of blood cultures, these patients developed acute respiratory distress syndrome (ARDS) and all died at a median of day +22 (range, days +8-37). One patient died of early CMV reactivation and pneumonia. The remaining 3 patients, who died of infection, were 57 to 62 years old. Two of these patients had abnormal chest CT scans prior to transplantation and eventually died with a pulmonary process for which a specific pathogen could not be identified. The 57-year-old patient died of polymicrobial sepsis while on treatment for GvHD. The cause-specific probability of NRM, adjusted for the competing risk of disease relapse, is 10% at 100 days and 22% at 3 years (Figure 3B).
Eight patients (15%) died from relapse or progression of disease. These included 2 patients in partial remission (PR) of NHL (1 Burkitt and 1 diffuse large cell) and 6 patients who underwent transplantation for acute leukemia. Three of these patients had a history of MDS evolving to AML, 2 had de novo AML-CR1, and 1 had ALL-CR2.
Immune reconstitution and opportunistic infections
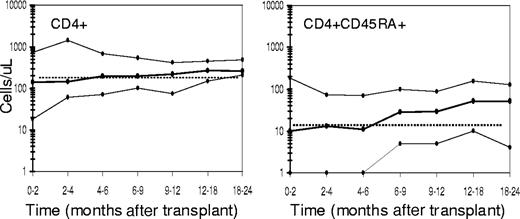
Table 3 summarizes the recovery of an absolute CD4+ count of 200 cells/μL or more and of T-cell response to PHA mitogen stimulation in vitro within at least 25% of the lower limit of normal (LLN). Because delayed immune reconstitution has been associated with older age, the results are also presented for patients younger than 50 years and 50 years or older. The majority of patients (66%) achieved a CD4+ count of 200 cells/μL or more by 1 year. Although the greater number of patients younger than 50 years who demonstrated more rapid recovery skewed these results, the incidence of a CD4+ count less than 100 cells/μL was very low for both age groups, even at the earliest evaluation. Figure 4 graphically illustrates the recovery of CD4+ and CD4+CD45RA+ cells. The median time to reach a CD4+ count of 200 cells/μL or more was 4 to 6 months after transplantation. The median time to reach a CD4+CD45RA+ cell count within the 10th percentile of normal for adults (13 cells/μL) was 6 to 9 months after transplantation.
Laboratory parameters monitored for immune recovery
| Time after transplantation . | Patients younger than 50 y, n = 33 . | Patients 50 y or older, n = 19 . | All, n = 52 . |
|---|---|---|---|
| 5 to 7 mo., no. evaluated/eligible | 24/27 | 16/17 | 40/44 |
| CD4, % | |||
| 200 or more cells/μL | 38 | 44 | 40 |
| Less than 100 cells/μL | 25 | 13 | 20 |
| PHA, % | |||
| Median | 52 | 13 | 32 |
| Less than 25% LLN | 33 | 69 | 48* |
| 11 to 12 mo., no. evaluated/eligible | 21/24 | 11/13 | 32/35 |
| CD4, % | |||
| 200 or more cells/μL | 71 | 55 | 66 |
| Less than 100 cells/μL | 10 | 9 | 9 |
| PHA, % | |||
| Median | 113 | 14 | 71 |
| Less than 25% LLN | 0 | 64 | 22† |
| 15 to 18 mo., no. evaluated/eligible | 17/23 | 10/10 | 27/33 |
| CD4, % | |||
| 200 or more cells/μL | 71 | 90 | 82 |
| Less than 100 cells/μL | 0 | 0 | 0 |
| PHA, % | |||
| Median | 129 | 37 | 100 |
| Less than 25% LLN | 6 | 44 | 20* |
| 24 mo., no. evaluated/eligible | 12/21 | 4/7 | 16/28 |
| CD4, % | |||
| 200 or more cells/μL | 83 | 75 | 81 |
| Less than 100 cells/μL | 8 | 0 | 6 |
| PHA, % | |||
| Median | 126 | 22 | 103 |
| Less than 25% LLN | 9 | 50 | 20‡ |
| Time after transplantation . | Patients younger than 50 y, n = 33 . | Patients 50 y or older, n = 19 . | All, n = 52 . |
|---|---|---|---|
| 5 to 7 mo., no. evaluated/eligible | 24/27 | 16/17 | 40/44 |
| CD4, % | |||
| 200 or more cells/μL | 38 | 44 | 40 |
| Less than 100 cells/μL | 25 | 13 | 20 |
| PHA, % | |||
| Median | 52 | 13 | 32 |
| Less than 25% LLN | 33 | 69 | 48* |
| 11 to 12 mo., no. evaluated/eligible | 21/24 | 11/13 | 32/35 |
| CD4, % | |||
| 200 or more cells/μL | 71 | 55 | 66 |
| Less than 100 cells/μL | 10 | 9 | 9 |
| PHA, % | |||
| Median | 113 | 14 | 71 |
| Less than 25% LLN | 0 | 64 | 22† |
| 15 to 18 mo., no. evaluated/eligible | 17/23 | 10/10 | 27/33 |
| CD4, % | |||
| 200 or more cells/μL | 71 | 90 | 82 |
| Less than 100 cells/μL | 0 | 0 | 0 |
| PHA, % | |||
| Median | 129 | 37 | 100 |
| Less than 25% LLN | 6 | 44 | 20* |
| 24 mo., no. evaluated/eligible | 12/21 | 4/7 | 16/28 |
| CD4, % | |||
| 200 or more cells/μL | 83 | 75 | 81 |
| Less than 100 cells/μL | 8 | 0 | 6 |
| PHA, % | |||
| Median | 126 | 22 | 103 |
| Less than 25% LLN | 9 | 50 | 20‡ |
CD4 indicates the percentage of patients with an absolute CD4 count 200 or more or less than 100 cells/μL.
Each patient's PHA response was calculated as percentage = (patient response/LLN).
Less than 25% LLN indicates the percentage of patients with PHA responses less than 25% LLN.
P = .05
P < .01
P = .14
T-cell recovery. Shown are the 10th, 50th, and 90th percentiles (solid lines, ●) of CD4+ and CD4+ CD45RA+ cells per microliter obtained over 24 to 36 months after transplantation in patients on the current study.
T-cell recovery. Shown are the 10th, 50th, and 90th percentiles (solid lines, ●) of CD4+ and CD4+ CD45RA+ cells per microliter obtained over 24 to 36 months after transplantation in patients on the current study.
The median PHA response was 71% of the LLN, for the entire group at 1 year. In contrast to the CD4+ cell counts, the differences between the younger and older groups in the median PHA response and the proportion of patients with a PHA response less than 25% LLN were more dramatic (Table 3; see P values). For patients younger than 50 years, the median response was within the normal range, and none had a response 25% LLN or less by 1 year. In contrast, 64% of patients 50 years or older had a response less than 25% LLN (median, 14% LLN) at 1 year. Although the numbers of evaluable patients in the older age group are small, their pattern of slower recovery is evident across all time points. Furthermore, the P values comparing the medians for PHA recovery of the younger with the older groups show a statistically significant difference throughout the first year. The incidence of OIs, as defined in “Engraftment, donor chimerism, and immune reconstitution,” was low, however, and included only 2 cases of EBV-LPD and 1 CMV interstitial pneumonitis. Only the latter OI was fatal.
Discussion
The benefits of TCD BM transplantation for patients with AML-CR1 and AML-CR2 with respect to DFS and complications of GvHD14 have been abrogated not by relapse, but by the incidence of OIs associated with prolonged immune deficiency after transplantation. Older age, and the use of ATG, to reduce the incidence of graft rejection,1,14,15 were identified as risk factors.
The current trial therefore addressed the challenge of facilitating T-cell recovery and decreasing regimen-related toxicity with several modifications to the previous transplantation regimen. Fludarabine, a relatively nontoxic but well-described immunosuppressive agent, replaced cyclophosphamide; and ATG was eliminated. G-CSF–mobilized PBSCs replaced BM as the source of the TCD graft, affording higher doses of CD34+ cells, which studies had suggested could provide durable engraftment and better immune reconstitution.16,–18 The method of TCD was also changed to accommodate the larger volume of cells, using an automated CD34+ selection in addition to sRBC rosetting.
The median age of the patients in this study was 47 years, more than 10 years older than that of our previous study,2 with 36% at least 50 years old. The distribution of diseases was representative of the older age of the patients and included many treated at advanced stages. Despite the high-risk nature of the transplantations and the extensive modifications to the regimen, the current study has demonstrated promising results. Engraftment was excellent, with a low incidence of GvHD. Only 3 life-threatening OIs developed, resulting in 1 death. DFS in patients undergoing TCD transplantation for standard-risk disease was also comparable with our previous results and those reported by others.19,–21
A goal of the current study was to determine whether ATG could be eliminated without incurring an increase in graft rejection or GvHD. Based on previously identified predictors of this complication using TCD grafts,14,15 all but 2 patients on this study were at high risk for rejection. With the addition of fludarabine and the use of PBSC grafts, all of the patients engrafted without the use of ATG; and no immune-mediated graft rejections were seen. Notably, 48 of the 52 patients received PBSC grafts containing a median CD34+ cell dose of 5.8 × 106/kg. This was more than 3-fold higher than that usually provided by TCD BMs, and similar to that provided by conventional PBSC transplantations.22 This probably contributed to engraftment.
Despite this high CD34+ cell content, however, the dose of T cells was very low. The TCD PBSC grafts contained more than a 1 log lower concentration of T cells compared with the TCD BM grafts (median, 0.11 vs 4.5 × 104/kg)7 and, for comparison purposes, a 4 log lower concentration than conventional PBSC grafts with similar CD34+ cell doses.22 The incidence and severity of GvHD experienced by patients in the current study, 8% grade 2 acute and 9% chronic GvHD, compare favorably to 5% grade 1 acute and 3% chronic GvHD observed in our previous trial of TCD BM. Such low incidences without increased relapse rates also compare very favorably to reported results with conventional PBSC grafts.23,–25
A major benefit observed in the current study relates to the T-cell recovery, measured by CD4+ counts and PHA responses in vitro, and its apparent impact on OIs. We have previously reported our results of T-cell recovery in 142 adult recipients of TCD HLA-matched related BM grafts following cytoreduction with HTBI, thiotepa, cyclophosphamide, and ATG before or after transplantation.11 Comparison of the earlier and current data demonstrates faster recovery to a median CD4+ cell count of 200/μL or more (4-6 versus 12-18 months), with CD4+ cell counts significantly higher (P ≤ .05) during the first 6 months in the current group of patients. At later time points, there was no significant difference between the 2 groups. Despite the older age of the current cohort of patients (47 versus 41 years), CD4+CD45RA+ recovery was also more rapid compared with adult recipients of the TCD BM transplants.
There were also age-dependent differences in PHA responses in the current trial. All patients younger than 50 years had a PHA response more than 25% LLN and a median of more than 100% LLN by 1 year. Patients 50 years or older demonstrated slower recovery with 64% showing a response less than 25% LLN at one year, similar to the pattern reported for the patients receiving TCD BM transplants. The recovery of PHA response was statistically significantly better for patients younger than 50 years versus those 50 years or older at 3 of the 4 time points studied (P < .05). Our prior studies have demonstrated a correlation between life-threatening OIs and CD4+ cell counts less than 200/μL. In this study, only 1 (2%) of the 51 engrafted patients died of an OI. This is lower than the 5% to 23% incidence of fatal OIs following HLA-matched sibling transplantation currently reported in the literature.26,,,,–31
Although the immunosuppression provided by ATG in the setting of TCD has also been associated with an increased risk of EBV-LPD,32 its removal from the conditioning regimen did not completely eliminate this complication, despite a low incidence. Two cases (4%) occurred in 49 patients on this trial, compared with 7.7% observed in our previous study that used ATG and TCD BM allografts.2 The incidence was 1.9% in a series of 106 patients who received TCD BM allografts without ATG (R.J.O., unpublished data, March 2006). There is no statistical difference between these outcomes.
Despite consistent engraftment and earlier T-cell recovery, very early bacterial infections accounted for at least half the infectious deaths. Recently, we reported our experience of Streptococcal viridans bacteremia in patients receiving TBI-containing regimens, especially those that include fludarabine.33 In the 52 patients on this trial, 4 of the 8 infectious deaths were due to S viridans sepsis occurring before day +7, despite appropriate treatment and rapid sterilization of blood cultures. As a consequence, all patients undergoing hematopoietic stem cell transplantation (HSCT) with similar TBI-containing regimens now receive vancomycin prophylaxis at the onset of neutropenia or at day −2. Although the pathophysiology of these bacteremias remains unclear, the removal of ATG and steroids from the conditioning did not reduce their occurrence.
Patients with a variety of hematologic malignancies were included in this trial, with limited numbers of patients in each pathologic disease group. To analyze transplantation outcomes related to disease, the patients were categorized according to standard- and poor-risk disease as defined in “Patients, materials, and methods; Patient characteristics.” Despite the substitution of fludarabine for cyclophosphamide, the DFS for the 25 patients with standard-risk disease remained excellent. Six of the 8 relapses, however, occurred in the 27 patients with poor-risk disease, including 3 of 5 patients with AML evolving from MDS. Although the number of patients was small, this raises concern that a regimen using HFTBI, thiotepa, and fludarabine may not be as effective as more intensive myeloablative regimens containing high-dose cyclophosphamide for disease control in such poor-risk patients with matched related donors. The lack of consolidation in most patients with secondary AML may also have compounded the relapse risk of this less intensive conditioning regimen. This argues that, as an alternative to T-replete transplantation, the poor-risk patients receiving TCD transplants may require more intensive chemotherapy and/or an alternative transplantation approach that includes cell therapy after transplantation.
Transplantation in the older population has always presented a challenge, which has grown in magnitude as the age of eligibility for transplantation reaches 70 years. Interestingly, in the current study, patients younger than 50 years and 50 years or older achieved similar outcomes when they were segregated by disease risk (Figure 2). Notably as well, none of the patients 50 years or older in this trial developed GvHD de novo. These results can be compared with those from the nonmyeloablative (NMA) and reduced-intensity conditioning (RIC) transplantation regimens that use unmodified HSCTs and are routinely being offered to patients older than 50 years23,34,,–37 In those studies treating patients with MDS, AML derived from MDS, or treatment-related AML, with DFS and OS similar to this trial, acute GvHD (II-IV) has been reported in 0% to 50% of patients and chronic GvHD, in less than 10% to more than 50%. The use of alemtuzumab in vivo as part of a RIC regimen as reported by Ho et al38 resulted in the lowest incidence of GvHD and the best DFS in such a population. The patients in that trial, however, generally received more chemotherapy before their transplantations than patients in this study. This requirement for minimal tumor burden echoes throughout most of the NMA and RIC trials, as the beneficial effect of GvHD on relapse risk, OS, and NRM has remained a point of debate. Although some investigators have noted a benefit39 from GvHD, others have actually found it to be detrimental with respect to NRM and progression-free survival.37
Based on the results in this trial and the excellent quality of life in the surviving patients, we propose that myeloablative TCD transplantation should be considered a potential alternative to unmodified sibling transplantation in future studies. Despite the elimination of ATG, this regimen achieved durable engraftment, without significant acute or chronic GvHD, and with a very limited incidence of life-threatening OIs. The relapse rates comparable with those of unmodified grafts, over a range of diagnoses, reaffirmed the findings of our prior studies with respect to the antimalignancy potential of the TCD transplantation strategy. Furthermore, this study confirms the curative potential of this treatment approach even in older patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by P01 CA23766 from the National Cancer Institute, National Institutes of Health.
We are grateful to Drs Suzanne Wolden and Joachim Yahalom who oversaw the radiation therapy, to Katherine Smith for assistance in studies monitoring immune recovery, and to Anne Thomas for assistance in data management.
National Institutes of Health
Authorship
Contribution: A.A.J. wrote the protocol, performed research as principal investigator, and coordinated collection of and analyzed data; N.A.K. wrote the protocol, acted as coprincipal investigator, analyzed data, and edited the paper; A.A.J., E.B.P., and R.J.O. analyzed data and cowrote the paper; T.N.S., J.W.Y., H.C.-M., K.C.H., M.-A.P., N.C., M.R.M.B., R.J.O., and E.B.P. were coinvestigators in performing research, reviewing data, and editing the paper; M.C. and C.C. coordinated collection and analysis of data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ann Jakubowski, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065; e-mail:jakubowa@mskcc.org.