In this issue of Blood, Roccaro and colleagues evaluate the crucial role of miRNAs in regulating the biology and prognosis of Waldenström macroglobulinemia, providing in vitro and in vivo evidences for miRNA-based targeted therapies in this disease.
Epigenetic modifications at the level of microRNAs (miRNAs) have recently gained considerable attention in the field of cancer research. The miRNAs are short, noncoding RNAs that negatively regulate gene expression by binding to the 3′ untranslated region of the target mRNAs, leading to mRNA degradation or inhibition of translation.1,2 They have been described as playing crucial roles in regulating physiological processes as well as tumor pathogenesis. Indeed, much evidence has clearly demonstrated that miRNA expression profiles differ between normal and tumor tissues, both in solid and hematologic malignancies.3-6
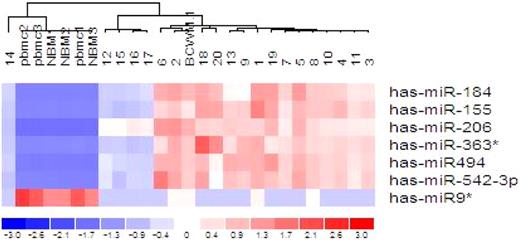
Supervised hierarchical clustering analysis demonstrates differential expression of miRNA patterns in WM patients(1-20) as compared with healthy subjects (NBM1-3; pbmc1-3), shown by the intensity of red (up-regulation) versus blue (down-regulation). See the complete figure in the article beginning on page 4391.
Supervised hierarchical clustering analysis demonstrates differential expression of miRNA patterns in WM patients(1-20) as compared with healthy subjects (NBM1-3; pbmc1-3), shown by the intensity of red (up-regulation) versus blue (down-regulation). See the complete figure in the article beginning on page 4391.
In this issue, Roccaro et al evaluate for the first time the miRNA signature in Waldenström macroglobulinemia (WM).7 They identify increased expression of miRNAs-363*, -206, -494, -155, -184, -542–3p, and decreased expression of miRNA-9* in primary bone marrow–derived WM tumor cells. Based on this first observation, they wondered next whether the miRNA signature could be linked to prognosis in these patients, and how miRNAs could functionally contribute to WM pathogenesis. The authors show that increased expression of the 6 miRNAs significantly correlated with a poorer outcome as predicted by the International Prognostic Staging System. Moreover, in vitro and in vivo studies clearly demonstrated that, among those deregulated miRNAs, miRNA-155 is likely involved in WM biology. The studies showed that miRNA-155 specifically targets WM cells even in the context of a bone marrow milieu by inhibiting MAPK/ERK, PI3/AKT, and NF-kB pathways, which are known to be constitutively activated in WM as well as in other B-cell malignancies.8
The importance of the findings put forth by Roccaro et al is substantial. If cytogenetic and molecular studies on gene expression analysis at the miRNA level have demonstrated minimal changes in WM cells,9 the described significant differences in WM miRNA expression profiling improve our understanding of the underlying molecular changes that lead to the initiation and progression of this rare disease. Also, miRNA-155 may be regarded as a sufficiently restricted therapeutic target in refractory-resistant WM.
These studies raise a few questions. It is well known that WM represents a rare B-cell malignancy, with an incidence of 3 cases per 1 000 000 persons each year, accounting for approximately 1% to 2% of all hematologic malignancies. Roccaro et al have collected and studied 20 primary bone marrow WM samples, and they did not observe differences after supervised clustering analysis between untreated and treated patients, indicating that samples had similar expression patterns. It would thus be interesting to enlarge the 2 cohorts of samples in further studies to see if any patterns correspond to disease relapse or progression. In addition, while the authors showed that miRNA-155 negatively regulates the canonical NF-kB pathway, it would be worthwhile to understand the possible effects of miRNA-155 on the noncanonical NF-kB pathway as well.
In conclusion, these innovative and very well conducted studies represent a major achievement in the field of hematologic malignancies. They bring miRNAs nearly from the bench to the bedside because they provide the preclinical evidence for the development of novel miRNA-based prognostic and therapeutic options in WM.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal