In this issue of Blood, Guo and colleagues elucidate molecular details of a novel mechanism, linking the degradation of fibrin (but not fibrinogen) by plasmin to apoptosis of placental trophoblast cells.
There is convincing evidence from the literature that disorders of the maternal coagulation system are associated with complications of pregnancy including fetal loss. In many studies, an association between fibrin deposition in the placenta and apoptosis of trophoblast cells has been observed.1 The group of Weiler and collaborators have shown previously that mouse embryos deficient in the thrombin receptor thrombomodulin (Thbd−/−) die before the development of a functional cardiovascular system because of a defect in the placenta.2 In a more recent study that analyzed the mechanisms responsible for the placental defect in these mice, they found that activated coagulation factors induce growth inhibition and apoptosis of placental trophoblast cells.3 While the growth-inhibiting effect can be attributed to the activation of protease-activated receptors, cell death is caused by degradation products of fibrin. Neither fibrinogen nor intact fibrin nor degradation products of fibrinogen caused apoptosis.
In this issue of Blood, Guo et al perform a study based on these previous findings that discloses the structural requirements and molecular mechanisms involved in fibrin degradation product-mediated cell death.4 They show that the apoptosis-inducing activity is not restricted to the mouse trophoblast system but is also seen with human fibrin degradation products and a variety of cell types. They can furthermore assign the apoptosis-inducing activity to a sequence within the Aα-chain of fibrin fragment E, which has to be cleaved by thrombin as well as by plasmin to gain apoptosis-inducing activity. Part of the proapoptotic activity can be attributed to an RGD-motif, but the majority is RGD-independent. Induction of apoptosis by fibrin fragment E requires its uptake by the cell. Uptake, but not apoptosis, is mediated via a motif located within the sequence Aα52-81. Apoptosis-inducing activity itself is located in Aα17-37. The internalization of fibrin fragment E, but not the intracellular mechanism mediating apoptosis, is caveolin-1–dependent. Intracellular pathways have not yet been analyzed in detail, but data presented suggest activation of the mitochondrial pathway and involvement of caspases 9 and 3.
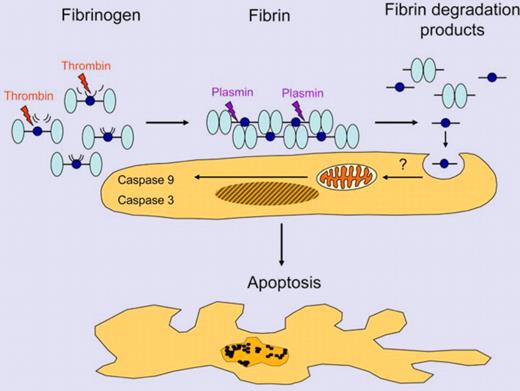
Fibrin is formed by the action of thrombin on fibrinogen releasing fibrinopeptides A and B from the Aα- and Bβ-chains of fibrinogen. Plasmin generated by the plasminogen activators uPA or tPA cleaves fibrin into fibrin degradation products (shown here: D-dimer, light blue, and fragment E, dark blue). Fibrin fragment E is internalized by cells and induces apoptosis of trophoblast cells. Illustration by Thomas Nardelli.
Fibrin is formed by the action of thrombin on fibrinogen releasing fibrinopeptides A and B from the Aα- and Bβ-chains of fibrinogen. Plasmin generated by the plasminogen activators uPA or tPA cleaves fibrin into fibrin degradation products (shown here: D-dimer, light blue, and fragment E, dark blue). Fibrin fragment E is internalized by cells and induces apoptosis of trophoblast cells. Illustration by Thomas Nardelli.
This newly described pathway linking fibrin degradation to apoptosis may play a role in several physiologic and pathophysiologic situations. It may lead to trophoblast cell death, causing placental insufficiency and pregnancy loss in situations of increased fibrin deposition and degradation. Local fibrin deposition and fibrinolysis are found at sites of wound healing and tissue remodeling. At these sites, the induction of apoptosis by fibrin degradation products could facilitate the clearance of damaged cells. Cross-linked fibrin is also a component of the matrix of most cancers,5 and degradation of fibrin by plasmin generated in the vicinity of tumor cells could support tumor cell apoptosis. The work of Guo et al, which focuses mainly on structural requirements of the proapoptotic molecule, not only raises many new questions related to the intracellular pathway–mediating apoptosis but also represents the basis for many new studies analyzing the physiologic relevance of apoptosis induction by fibrin degradation products.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal