Abstract
Chronic graft-versus-host disease (cGVHD) causes significant morbidity and mortality in patients otherwise cured of malignancy after hematopoietic stem cell transplantation (HSCT). The presence of alloantibodies and high plasma B cell–activating factor (BAFF) levels in patients with cGVHD suggest that B cells play a role in disease pathogenesis. We performed detailed phenotypic and functional analyses of peripheral B cells in 82 patients after HSCT. Patients with cGVHD had significantly higher BAFF/B-cell ratios compared with patients without cGVHD or healthy donors. In cGVHD, increasing BAFF concentrations correlated with increased numbers of circulating pre–germinal center (GC) B cells and post-GC “plasmablast-like” cells, suggesting in vivo BAFF dependence of these 2 CD27+ B-cell subsets. Circulating CD27+ B cells in cGVHD comprised in vivo activated B cells capable of IgG production without requiring additional antigen stimulation. Serial studies revealed that patients who subsequently developed cGVHD had delayed reconstitution of naive B cells despite persistent BAFF elevation as well as proportional increase in CD27+ B cells in the first year after HSCT. These studies delineate specific abnormalities of B-cell homeostasis in patients with cGVHD and suggest that BAFF targeting agents may be useful in this disease.
Introduction
The limited understanding of the immune mechanisms that result in chronic graft-versus-host disease (GVHD) hinders our ability to develop effective targeted therapies that would improve the survival of patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT).1-3 In acute GVHD, tissue injury is mediated primarily by donor T cells that specifically target minor histocompatibility antigens (mHAs) in affected organs.4,5 However, despite effective prevention of acute GVHD with agents that primarily inhibit T cells (calcineurin inhibitors, mTOR inhibitors, and purine analogues),2,6 the incidence and severity of chronic GVHD (cGVHD) remain high.1,2 This suggests that immune mechanisms of cGVHD are distinct from acute GVHD. Studies in mHA-mismatched murine transplantation models demonstrate involvement of B cells in the development of cGVHD.7,8 After allogeneic HSCT in humans, alloantibodies to Y chromosome–encoded proteins are detectable in 50% of male recipients if they received hematopoietic stem cells from a female donor.9 Such alloantibodies develop 4 to 9 months after HSCT, and the presence of alloantibodies correlated significantly with clinical cGVHD development.10 HY antibodies were not associated with acute GVHD and did not develop when recipient and donor were sex matched. When studied in greater detail, patients with HY antibodies were also found to have coordinated T-cell responses to distinct epitopes in the same HY mHA (DBY).11 These studies have suggested that donor B cells play a role in the development of cGVHD in humans, and several phase 1 or 2 trials of B cell–directed therapy with rituximab in steroid-refractory cGVHD have revealed clinical responses.12-14 Although these observations provide compelling evidence that B cells play an important role in the immune pathology of human cGVHD, the mechanisms that promote and sustain B-cell involvement have not been elucidated.
HSCT conditioning depletes normal recipient B cells and the expansion of normal donor B cells is altered as reconstitution occurs in the setting of constant exposure to “foreign” minor histocompatibility antigens. Although genetic disparity between donor and recipient must exist for cGVHD to develop, sustainable autoreactivity occurs after alloreactivity in murine models. Along with a more prolonged humoral immune deficiency, a complex autoimmune disease–like phenotype is found in cGVHD.15,16 The frequent production of autoantibodies in patients with cGVHD17-19 suggests that disease pathogenesis reflects a critical breakdown in peripheral B-cell tolerance after allogeneic HSCT. Furthermore, a relative decrease in immature B-cell number in cGVHD compared with other post-HSCT patients20 suggests that altered B-cell homeostasis is a component of this disease.
B cell–activating factor (BAFF) plays a critical role in B-cell reconstitution and homeostasis after myeloablation.21 Studies of B-cell development in murine models have shown that survival of normal mature B cells depends on the relative balance of both B-cell receptor (BCR) and BAFF signaling.22,23 In addition, maintenance of B-cell homeostasis in mice depends on in vivo soluble BAFF concentration.24,25 Murine models demonstrate that a diverse B-cell pool is required for antigen-induced anergy and exclusion of autoreactive B cells from follicular niches.26,27 A set of elegant studies subsequently identified a BAFF-mediated B-cell tolerance checkpoint in which limiting amounts of BAFF are required for ongoing B-cell turnover and avoidance of B-cell autoreactivity.28,29 When the pool of normal B cells is reduced, excess BAFF promotes survival of autoreactive B cells.29 However, even in the presence of high BAFF, high numbers of nonautoreactive B cells outcompete autoreactive B cells for available soluble BAFF, causing autoreactive B cells to undergo apoptosis.28 Thus, B-cell autoimmunity in transgenic mouse models is determined by both the level of soluble BAFF and the numbers of competing naive B cells.
Defective censoring of autoreactive B cells in patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis has been observed30-32 and high BAFF has been found in patients with a variety of autoimmune diseases,33-36 suggesting that excessive BAFF stimulation in humans contributes to development of autoimmunity. Since high BAFF levels are significantly associated with presence of active cGVHD more than 1.5 years after HSCT,37,38 we hypothesized that relative B lymphopenia and high BAFF after HSCT could support potentially pathologic activated alloreactive and autoreactive B-cell populations in cGVHD patients. Examining peripheral blood after allogeneic HSCT, we found that patients with cGVHD had significantly higher “BAFF/B-cell ratio” compared with patients without cGVHD. Serial analysis of patients for 1 year after HSCT showed that, in patients without cGVHD, BAFF levels gradually normalized as B-cell numbers recovered, indicating a normal homeostatic response to B lymphopenia. In contrast, patients who subsequently developed cGVHD exhibited delayed reconstitution of naive B cells despite persistent BAFF elevation. Using CD27+ as a marker of antigen experience and ex vivo IgG production as a measure of B-cell activation, we found that circulating B cells, including pre-GC B cells, were activated in cGVHD. These results suggest that altered B-cell homeostasis and excess BAFF contribute to promotion of activated B cells in patients with cGVHD.
Methods
Patient characteristics
All patient samples were collected after written informed consent was obtained in accordance with the Declaration of Helsinki and approved by the Human Subjects Protection Committee of the Dana-Farber/Harvard Cancer Center.
Patient group 1
Blood samples for analysis of B-cell reconstitution were obtained from 57 patients who had undergone allogeneic HSCT between 1994 and 2005 and were more than 12 months after HSCT. Within this group, BAFF levels from 28 samples have been reported previously.37 Clinical characteristics of the 57 patients are summarized in Table 1. The study included patients who received nonmyeloablative or myeloablative conditioning regimens and bone marrow or mobilized peripheral blood stem cell grafts. Patients whose primary disease relapsed within 1 year of transplantation and patients receiving high-dose steroids (> 0.5 mg/kg prednisone) at the time of sample collection were excluded. Chronic GVHD status at the time of sample collection was categorized according to documented clinical examination and laboratory studies using both Seattle criteria and National Institutes of Health (NIH) cGVHD consensus criteria. Twenty-two patients had “active cGVHD” at the time of primary analysis. This included patients with either limited or extensive cGVHD based on clinical severity and target organs affected per modified Seattle criteria.39 Twenty-three patients had “inactive cGVHD” at the time of sample collection. Inactive cGVHD was used to describe patients with cGVHD who had achieved a complete response to immune-suppressive therapy at the time of analysis. Patients who never developed cGVHD after HSCT were designated as having “no cGVHD.” Unlike patients with inactive or active cGVHD, patients with no cGVHD were receiving no immune-suppressive agents, except one patient who received low-dose steroids for a non-GVHD indication (Table 1). Median time of analysis was 20.9 months after transplantation for patients with active cGVHD compared with 26.6 months after transplantation for patients with no cGVHD (P = .10). Patients with inactive cGVHD were significantly later in their post-HSCT course (30.9 months) compared with patients with active cGVHD (20.9 months, P = .007). All other clinical characteristics including age, sex, type of transplant, and underlying hematologic malignancy were similar in patients with active and inactive cGVHD. We also studied 33 healthy controls.
Clinical characteristics of 57 patients who underwent allogeneic HSCT
| Characteristic . | Chronic GVHD . | |||
|---|---|---|---|---|
| Active . | Inactive . | No . | P . | |
| No. (%) | 22 (45) | 23 (36) | 12 (18) | |
| Median age, y (range) | 46 (24-64) | 47 (19-66) | 44 (25-58) | .65 |
| Female sex (%) | 10 (45) | 11 (48) | 4 (33) | .73 |
| Conditioning regimen (%) | ||||
| Myeloablative | 14 (64) | 17 (74) | 10 (83) | .52 |
| Nonmyeloablative | 8 (36) | 6 (26) | 2 (17) | |
| Cell source (%) | ||||
| Peripheral blood stem cells | 19 (86) | 15 (65) | 9 (75) | .26 |
| Bone marrow | 3 (14) | 8 (35) | 3 (25) | |
| HLA matching (%) | ||||
| Matched, unrelated | 10 (45) | 13 (57) | 2 (17) | .11 |
| Matched, related | 11 (50) | 9 (39) | 10 (83) | |
| Mismatched | 1 (5) | 1 (4) | 0 (0) | |
| Prednisone dose (%) | ||||
| 0 mg/day | 9 (30) | 10 (42) | 11 (92) | |
| 0-30 mg/day | 13 (59) | 13 (57) | 1 (8) | |
| Cellcept (%) | 7 (32) | 5 (21) | 0 (0) | |
| Tacrolimus (%) | 8 (36) | 3 (13) | 0 (0) | |
| Rapamycin (%) | 8 (36) | 3 (13) | 0 (0) | |
| Months after transplantation, median (range) | 20.9 (13.6-79.2) | 30.9 (16.7-134.6) | 26.6 (18.7-93.2) | .01* |
| BAFF level, ng/mL, median (range) | 7.0 (1.6-24.5) | 5.5 (1.5-24.3) | 3.0 (1.4-4.9) | .001 |
| Grades II-IV aGVHD (%) | 2 (9) | 5 (22) | 2 (17) | .52 |
| Disease (%) | ||||
| AML/AML from MDS | 8 (36) | 5 (22) | 2 (17) | |
| ALL | 2 (9) | 1 (4) | 3 (25) | |
| CML | 0 (0) | 9 (39) | 3 (25) | |
| CLL | 3 (14) | 0 (0) | 0 (0) | |
| MDS | 4 (18) | 4 (17) | 0 (0) | |
| NHL | 2 (9) | 1 (4) | 4 (33) | |
| HL | 1 (5) | 0 (0) | 0 (0) | |
| MM | 0 (0) | 1 (4) | 0 (0) | |
| Other | 2 (9) | 2 (9) | 0 (0) | |
| Characteristic . | Chronic GVHD . | |||
|---|---|---|---|---|
| Active . | Inactive . | No . | P . | |
| No. (%) | 22 (45) | 23 (36) | 12 (18) | |
| Median age, y (range) | 46 (24-64) | 47 (19-66) | 44 (25-58) | .65 |
| Female sex (%) | 10 (45) | 11 (48) | 4 (33) | .73 |
| Conditioning regimen (%) | ||||
| Myeloablative | 14 (64) | 17 (74) | 10 (83) | .52 |
| Nonmyeloablative | 8 (36) | 6 (26) | 2 (17) | |
| Cell source (%) | ||||
| Peripheral blood stem cells | 19 (86) | 15 (65) | 9 (75) | .26 |
| Bone marrow | 3 (14) | 8 (35) | 3 (25) | |
| HLA matching (%) | ||||
| Matched, unrelated | 10 (45) | 13 (57) | 2 (17) | .11 |
| Matched, related | 11 (50) | 9 (39) | 10 (83) | |
| Mismatched | 1 (5) | 1 (4) | 0 (0) | |
| Prednisone dose (%) | ||||
| 0 mg/day | 9 (30) | 10 (42) | 11 (92) | |
| 0-30 mg/day | 13 (59) | 13 (57) | 1 (8) | |
| Cellcept (%) | 7 (32) | 5 (21) | 0 (0) | |
| Tacrolimus (%) | 8 (36) | 3 (13) | 0 (0) | |
| Rapamycin (%) | 8 (36) | 3 (13) | 0 (0) | |
| Months after transplantation, median (range) | 20.9 (13.6-79.2) | 30.9 (16.7-134.6) | 26.6 (18.7-93.2) | .01* |
| BAFF level, ng/mL, median (range) | 7.0 (1.6-24.5) | 5.5 (1.5-24.3) | 3.0 (1.4-4.9) | .001 |
| Grades II-IV aGVHD (%) | 2 (9) | 5 (22) | 2 (17) | .52 |
| Disease (%) | ||||
| AML/AML from MDS | 8 (36) | 5 (22) | 2 (17) | |
| ALL | 2 (9) | 1 (4) | 3 (25) | |
| CML | 0 (0) | 9 (39) | 3 (25) | |
| CLL | 3 (14) | 0 (0) | 0 (0) | |
| MDS | 4 (18) | 4 (17) | 0 (0) | |
| NHL | 2 (9) | 1 (4) | 4 (33) | |
| HL | 1 (5) | 0 (0) | 0 (0) | |
| MM | 0 (0) | 1 (4) | 0 (0) | |
| Other | 2 (9) | 2 (9) | 0 (0) | |
AML indicates acute myeloid leukemia; MDS, myelodysplastic syndrome; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; CLL, chronic lymphoblastic leukemia; NHL, non-Hodgkin leukemia; HL, Hodgkin lymphoma; and MM, multiple myeloma.
P = .101 for active group versus none; P = .007 for active versus inactive group.
Patient group 2
We prospectively followed an additional group of 25 patients from day of stem cell transplantation (day 0), with samples obtained every 3 months over a 19-month period. These patients received either myeloablative or nonmyeloablative conditioning regimens and tacrolimus and/or sirolimus as GVHD prophylaxis.6,40 Within this cohort, 8 patients did not develop cGVHD by 12 months after HSCT and 17 patients developed cGVHD between 3 and 12 months after HSCT. All patients in group 2 were analyzed, regardless of prednisone dose or treatment with other immunosuppressive agents. Six of the 17 patients who developed cGVHD had prior grades 2 to 4 acute GVHD. None of the patients without cGVHD were taking immunosuppressive agents after the 6-month time point. Control blood samples were obtained from 8 patients with multiple myeloma after the second of a planned tandem autologous transplantation.
Processing of patient plasma and peripheral blood cells
Whole blood was drawn into standard EDTA-containing collection tubes. Plasma was separated from whole blood cells by centrifugation at 600g. Plasma was stored in aliquots at −80°C and used after first thaw for BAFF measurements. Whole blood for flow cytometry studies was also collected on day of use in EDTA-containing tubes. Peripheral blood leukapheresis products were obtained from 2 cGVHD patients. Discarded leukocyte filters from anonymous healthy platelet donors were obtained from the Kraft Blood Donor Center at Dana-Farber Cancer Institute (DFCI).
BAFF enzyme-linked immunosorbent assay
Soluble BAFF in patient plasma samples was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) and the manufacturer's recommended procedures (R&D Systems, Minneapolis, MN).
Flow cytometric analysis of peripheral B cells
Antibodies used for flow cytometry were as follows: CD19 ECD or CD19 PC7 (both clone J4.119), CD20 ECD (clone B9E9), CD38 PE (clone LS198), CD27 PCD5 (clone 1A4), and IgD FITC (clone IA6-2) from BD Biosciences Pharmingen (San Diego, CA); BR3-FITC (eBioscience, San Diego, CA); and TACI-PE (R&D Systems). Whole blood was processed for flow cytometry using the Prep Plus 2 system (Beckman Coulter, Fullerton, CA). The lymphocyte gate was established using forward and side scatter. A minimum of 50 000 lymphocytes were collected for all samples to ensure adequate subset analysis. Gates positive for CD19, IgD, CD38, and CD27 were first set according to isotype controls. Only CD19+ cells were analyzed to ensure that CD3+ cells were not included in the analysis of CD38 or CD27 expression. Anti-CD19 PC7 (Beckman Coulter) was used per the manufacturer's instructions. Red blood cells (RBCs) were lysed and leukocytes were fixed using the Beckman Coulter TQPrep system before analysis. Cells were analyzed using a Cytomics FC 500 instrument and CXP Analysis 2.0 software (Beckman Coulter). To determine BAFF receptor profiles, CD19+ cells positively selected from large volume leukapheresis samples (Miltenyi Biotec, Auburn, CA) were stained with anti–BAFF-R, BR3 PE (clone 8A7; eBioscience), or biotinylated BMCA or TACI (R&D Systems) followed by streptavidin-APC antibody (Invitrogen, Carlsbad, CA).
Ex vivo purification of B-cell subsets
For purification of CD19+CD27+ and CD19+CD27− B cells, whole EDTA anticoagulated blood (10-12 mL) was obtained from post-HSCT patients or healthy individuals. For purification of IgD versus CD38 B-cell subsets (Table 2), large volume leukapheresis products from 2 cGVHD patients or leukocyte filters from 3 healthy donors were obtained. Lymphocytes were isolated in a sterile fashion using Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden). CD27+ or CD27− B cells were sorted by flow cytometry to more than 98% purity (BD FACSAria Special Order Cell-Sorting System; Becton Dickinson, San Jose, CA). Purified CD27+ or CD27− B cells (> 95%) were also obtained using the indirect magnetic labeling system B Cell Isolation Kit II, human (Miltenyi Biotec). In brief, non-B cells were retained on magnetic-activated cell sorting (MACS) columns after staining with a cocktail of biotin-conjugated antibodies against CD2, CD14, CD16, CD36, and CD43 and binding to streptavidin magnetic microbeads. B cells passed through the column and were positively selected with CD27 magnetic beads and enriched to more than 95% CD20+CD27+. IgD versus CD38 B-cell subsets were isolated using a BD FACSAria cell sorter (BD Biosciences, San Jose, CA).
“Antigen-naive” (naive and transitional) and CD27+ “antigen-experienced” B cells in patients after HSCT
| B-cell subset . | Active, n = 22 . | Inactive, n = 23 . | No, n = 12 . | Healthy, n = 33 . |
|---|---|---|---|---|
| Total naive B cells ×1000/L | 79.8 | 99.1 | 260.5 | 89.5 |
| P vs none | .001 | .001 | .001 | |
| Total transitional B cells ×1000/L | 8.1 | 8.5 | 28.7 | 14.4 |
| P vs none | .04 | .04 | .02 | |
| Total CD27+CD19+ B cells ×1000/L | 17.5 | 25.3 | 24.9 | 68.1 |
| P vs none | .14 | .37 | .01 | |
| CD27+ B cells, % | 19.0 | 8.4 | 6.05 | 27.3 |
| P vs none | .06 | .21 | < .001 |
| B-cell subset . | Active, n = 22 . | Inactive, n = 23 . | No, n = 12 . | Healthy, n = 33 . |
|---|---|---|---|---|
| Total naive B cells ×1000/L | 79.8 | 99.1 | 260.5 | 89.5 |
| P vs none | .001 | .001 | .001 | |
| Total transitional B cells ×1000/L | 8.1 | 8.5 | 28.7 | 14.4 |
| P vs none | .04 | .04 | .02 | |
| Total CD27+CD19+ B cells ×1000/L | 17.5 | 25.3 | 24.9 | 68.1 |
| P vs none | .14 | .37 | .01 | |
| CD27+ B cells, % | 19.0 | 8.4 | 6.05 | 27.3 |
| P vs none | .06 | .21 | < .001 |
IgG measurement
B cells purified to more than 98% purity as assessed by CD20 staining were incubated in vitro in complete RPMI medium with 100 ng/mL IL-10 (R&D Systems); 250 U IL-2 (BD Biosciences); and 0, 25, 250, or 2500 ng/mL BAFF (Axxora LLC, San Diego, CA). IgG was measured using the Human IgG Elisa Quantitation Kit (Bethyl Laboratories, Montgomery, TX).
Statistical analyses
For 2-sample comparison of continuous variables, a Wilcoxon rank sum test was performed. The Fisher exact test was used to compare categoric variables and the Spearman rank test was used for correlation analysis. All tests performed were 2-sided and considered significant at the .05 level.
Results
Relative B lymphopenia and high BAFF/B-cell ratio are associated with cGVHD
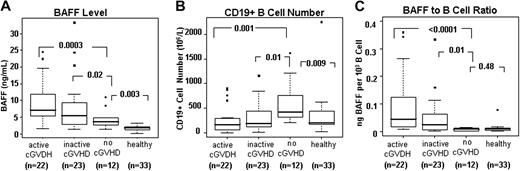
A detailed analysis of plasma BAFF levels and B-cell phenotype was carried out in 57 patients who underwent allogeneic HSCT more than 12 months ago (Table 1). As shown in Figure 1A, all patients after HSCT had significantly higher BAFF levels compared with healthy individuals. Patients with active cGVHD had BAFF levels that were significantly higher than patients without cGVHD (no cGVHD; P < .001) or healthy donors (P < .001). Patients without cGVHD also had higher than normal BAFF levels (3.0 ng/mL vs 1.9 ng/mL, P = .003). Consistent with a previously described “surge” in B-cell number after HSCT,20 B-cell numbers in patients without cGVHD were significantly higher than normal (Figure 1B). In contrast, patients with active or inactive cGVHD had significantly lower numbers of total CD19+ B cells compared with patients without cGVHD (P = .001 and P = .01, respectively). Since BAFF level per B cell has been determined in murine models to be a critical determinant of autoreactive B-cell survival,28,29 we calculated BAFF/B-cell ratios (level of BAFF per 103 B cells) for each patient in each post-HSCT group. Figure 1C shows that despite higher than normal BAFF levels, supranormal B-cell numbers in patients without cGVHD resulted in BAFF/B-cell ratios that were similar to those found in healthy individuals. In contrast, patients with cGVHD had significantly higher BAFF/B-cell ratios compared with other post-HSCT patients and healthy individuals (P < .001 each). As shown in Figure 1A and B, high BAFF/B-cell ratios in patients with cGVHD were due to both high BAFF levels and B lymphopenia.
High BAFF levels and low B-cell numbers result in high BAFF/B-cell ratios in patients with cGVHD. Plasma BAFF concentrations and numbers of CD19+ B cells were measured in 3 groups after allogeneic HSCT: 22 patients with active cGVHD, 23 with inactive cGVHD, and 12 who did not develop cGVHD. Results were compared with 33 healthy donors. (A) Plasma BAFF concentrations in each patient group after HSCT and healthy donors. (B) Total number of CD19+ B cells in each patient group and healthy donors. (C) Median BAFF/B-cell ratio for each patient group and healthy donors. The BAFF/B-cell ratio was defined as nanograms of BAFF per 103 CD19+ B cells. Box plots in each figure depict 75th percentile; median and 25th percentile values and whiskers represent maximum and minimum values.
High BAFF levels and low B-cell numbers result in high BAFF/B-cell ratios in patients with cGVHD. Plasma BAFF concentrations and numbers of CD19+ B cells were measured in 3 groups after allogeneic HSCT: 22 patients with active cGVHD, 23 with inactive cGVHD, and 12 who did not develop cGVHD. Results were compared with 33 healthy donors. (A) Plasma BAFF concentrations in each patient group after HSCT and healthy donors. (B) Total number of CD19+ B cells in each patient group and healthy donors. (C) Median BAFF/B-cell ratio for each patient group and healthy donors. The BAFF/B-cell ratio was defined as nanograms of BAFF per 103 CD19+ B cells. Box plots in each figure depict 75th percentile; median and 25th percentile values and whiskers represent maximum and minimum values.
Chronic GVHD is associated with decreased numbers of naive B cells
In murine models, high BAFF/B-cell ratios promote survival of autoreactive B-cell clones in the absence of competition for available soluble BAFF by sufficient numbers of antigen-naive B cells.28 We therefore examined the relative numbers of antigen-naive B cells and antigen-experienced B cells in post-HSCT patients (Table 1) and in healthy individuals. In this analysis, antigen-naive B cells were defined as naive (CD19+IgDCD38LoCD27−) B cells and transitional (CD19+IgD+CD38HiCD27−) B cells; antigen-experienced B cells were defined as CD19+CD27+ B cells. As shown in Table 2, patients with active cGVHD had low numbers of naive B cells compared with patients with no cGVHD (median time after HSCT, 21 months vs 27 months, respectively, P = .01). Patients without cGVHD had supranormal numbers of both naive and transitional B cells (260.5 × 106/L vs 89.5 × 106/L [P = .001] and 28.7 × 106/L in cGVHD vs 14.4 × 106/L [P = .02], respectively). High total numbers of circulating recent bone marrow emigrants or transitional cells and naive B cells in patients greater than 1 year after HSCT without cGVHD suggests increased bone marrow output and prolonged survival of naive B cells compared with healthy individuals and with patients with cGVHD. In contrast to naive B cells, numbers and frequencies of CD27+ antigen-experienced B cells were significantly decreased compared with healthy donors but these cells were not different among post-HSCT groups (Table 2). In the setting of relative naive B lymphopenia, active cGVHD patients tended to have higher frequencies of CD27+CD19+ B cells (19.0% in active cGVHD vs 6.1% in patients without cGVHD), but this difference did not reach significance (P = .06).
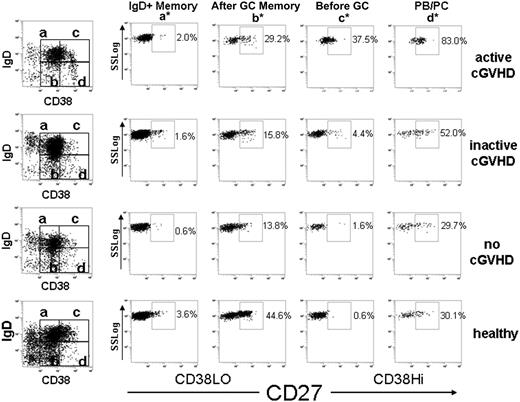
Identification of circulating pre-GC (IgD+CD38HiCD27+) B cells in cGVHD
In humans, CD27 expression identifies antigen-experienced B cells committed to plasma cell differentiation.41 CD27+ B-cell subsets include IgDLo post–germinal center (GC) memory and plasmablast-like (PB)/plasma cell–like (PC) populations. CD27+ IgD+ B-cell populations include “IgD+ memory” V region–mutated memory B cells.42 In addition, the presence of CD27 on IgD+ CD38Hi–expressing B cells allows distinction of human peripheral transitional B cells from pre-GC B cells.43 Pre–germinal center cells (GCs), also called BM2′, GC founder, or preplasmablast cells, are IgD+CD38HiCD27+. These cells are typically found in human tonsils and have also been described in peripheral blood in SLE patients.44,45 Table 3 summarizes peripheral CD27+ B-cell subset phenotypes and their corresponding functional characteristics. We used multiparameter flow cytometry to determine whether any of these distinct circulating CD27+ B-cell subsets were more prevalent in cGVHD. As described in “Flow cytometric analysis of peripheral B cells” in “Methods,” we first gated on CD19+ B cells and examined relative levels of surface IgD, CD38, and CD27+ on these cells. Figure 2 shows representative examples of flow cytometric profiles of CD27+ B-cell subgroups identified in patients after HSCT and in healthy individuals. Table 4 compares the median frequency of each CD27+ B-cell subset in patients with and without cGVHD and in healthy individuals. Consistent with previous reports, all post-HSCT patients had fewer post-GC (IgDLo).46 We found that the post-GC memory (CD27+IgDLoCD38Lo) B-cell subgroup was less frequent and lower in number in each post-HSCT group compared with healthy individuals, whereas all other CD27+ subsets were not proportionally or numerically different from normal (Table 4 and data not shown). As shown in Table 4, median IgD+ naive (CD38Lo) or pre-GC (CD38Hi) percentages in all post-HSCT groups were the same, but these populations tended to have higher CD27+ expression in cGVHD patients compared with patients without cGVHD (although differences did not reach significance).
Peripheral blood B-cell subsets analyzed in this study
| Peripheral blood B-cell subset . | Cell-surface phenotype . |
|---|---|
| CD27− subsets | |
| Naive B cells | IgD+CD38LoCD27− |
| Transitional B cells42,43 | IgD+CD38HiCD27− |
| CD27+ subsets | |
| IgD+ memory B42,45 | IgD+CD38LoCD27+ |
| Pre-GC (or “GC founder”)42 | IgD+CD38HiCD27+ |
| Post-GC memory B | IgDLo/−CD38LoCD27+ |
| Plasmablast/plasma cell (PB/PC) | IgDLo/−CD38HiCD27+ |
| Peripheral blood B-cell subset . | Cell-surface phenotype . |
|---|---|
| CD27− subsets | |
| Naive B cells | IgD+CD38LoCD27− |
| Transitional B cells42,43 | IgD+CD38HiCD27− |
| CD27+ subsets | |
| IgD+ memory B42,45 | IgD+CD38LoCD27+ |
| Pre-GC (or “GC founder”)42 | IgD+CD38HiCD27+ |
| Post-GC memory B | IgDLo/−CD38LoCD27+ |
| Plasmablast/plasma cell (PB/PC) | IgDLo/−CD38HiCD27+ |
Flow cytometric gating algorithm demonstrating circulating CD27+ B-cell subsets in patients after HSCT. Gated CD19+ B cells were first examined for IgD and CD38 expression (far left column). Subsequently, each subset (a-d) was analyzed for CD27+ expression according to Sims et al.43 (a*) IgD+ Memory B cells are IgDHiCD38LoCD27+. (b*) Post-GC memory B cells are IgDLoCD38LoCD27+. (c*) Pre–germinal center (GC) B cells are IgDHiCD38HiCD27+. (d*) Plasmablast or plasma cell–like (PB/PC) B cells are IgDLoCD38HiCD27+.
Flow cytometric gating algorithm demonstrating circulating CD27+ B-cell subsets in patients after HSCT. Gated CD19+ B cells were first examined for IgD and CD38 expression (far left column). Subsequently, each subset (a-d) was analyzed for CD27+ expression according to Sims et al.43 (a*) IgD+ Memory B cells are IgDHiCD38LoCD27+. (b*) Post-GC memory B cells are IgDLoCD38LoCD27+. (c*) Pre–germinal center (GC) B cells are IgDHiCD38HiCD27+. (d*) Plasmablast or plasma cell–like (PB/PC) B cells are IgDLoCD38HiCD27+.
Proportions of CD27+ B-cell subsets in patients after HSCT
| CD19+ subset . | Active, n = 22 . | Inactive, n = 23 . | No, n = 12 . | Healthy, n = 33 . |
|---|---|---|---|---|
| IgD+ memory | 8.4 | 2.8 | 2.3 | 4.1 |
| P vs none | .04 | .45 | .11 | |
| Post-GC memory, % | 20.4 | 18.4 | 10.8 | 32.8 |
| P vs none | .06 | .06 | < .001 | |
| Pre-GC, % | 41.3 | 9.7 | 2.6 | 4.7 |
| P vs none | .06 | .42 | .65 | |
| PB/PC, % | 86.9 | 66.4 | 36.8 | 40.7 |
| P vs none | .08 | .23 | .80 |
| CD19+ subset . | Active, n = 22 . | Inactive, n = 23 . | No, n = 12 . | Healthy, n = 33 . |
|---|---|---|---|---|
| IgD+ memory | 8.4 | 2.8 | 2.3 | 4.1 |
| P vs none | .04 | .45 | .11 | |
| Post-GC memory, % | 20.4 | 18.4 | 10.8 | 32.8 |
| P vs none | .06 | .06 | < .001 | |
| Pre-GC, % | 41.3 | 9.7 | 2.6 | 4.7 |
| P vs none | .06 | .42 | .65 | |
| PB/PC, % | 86.9 | 66.4 | 36.8 | 40.7 |
| P vs none | .08 | .23 | .80 |
Overall increased CD27+ expression on B-cell subsets in the active cGVHD group could not be attributed to time after HSCT, since the active cGVHD group was closest in time to HSCT (median, 20.9 months) compared with the patients with inactive or without cGVHD, and therefore would have been expected to have the lowest proportion of CD27+ B cells (Table 1). Numbers of each CD27+ B-cell subset were lower than those found in healthy individuals with one notable exception. Importantly, active cGVHD patients had significantly higher than normal numbers of pre-GC cells (2.5 × 106/L vs 0.89 × 106/L, respectively, P = .03) and higher frequency of pre-GC B cells in patients with active cGVHD compared with healthy individuals (41.3% vs 4.7%, P = .02). Pre-GC cells have not previously been detected in healthy individuals.42,43 In the current study, we detected circulating cells with a pre-GC phenotype in 3 (of 33 tested) healthy individuals. However, the pre-GC cells in these 3 healthy individuals were unlike cGVHD samples tested in that they did not coexpress the follicular marker CD10 (data not shown). Thus, we identified a distinct and potentially pathologic pre-GC population, similar to circulating pre-GC cells identified by other investigators in SLE patients, in patients with cGVHD.44,45
Increasing BAFF levels correlate with increasing numbers of activated pre-GC and PB/PC cells in cGVHD
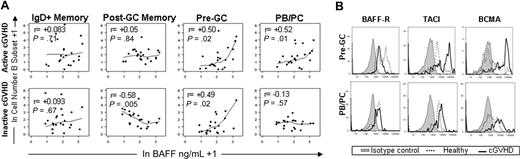
To assess whether high BAFF levels were associated with increased numbers of any circulating B-cell subsets in vivo, we examined possible correlations between the BAFF level and total B-cell number in each subset for each patient within each post-HSCT group. Figure 3A depicts the significant correlations found between cell numbers and high BAFF levels in patients with active or inactive cGVHD. Increased BAFF levels in patients with inactive disease correlated inversely with post-GC memory subset numbers (r = −0.57, P = .005), possibly reflecting reconstitution and turnover of memory B cells despite high BAFF levels in the group of patients with clinically inactive cGVHD. Only patients with active disease had a significant positive correlation between BAFF and PB/PC cell number (r = 0.52, P = .01). Active and inactive cGVHD patients had positive correlations between only BAFF and number of CD27+ pre-GC cells (r = 0.5 and r = 0.49, respectively; P = .02 each), suggesting that BAFF preferentially affected these 2 B-cell populations in vivo. Consistent with an activated state, BAFF-R expression was relatively low, whereas TACI and BCMA expression was increased47 on pre-GC cells in cGVHD (Figure 3B). PB/PC cells also expressed higher BCMA levels in cGVHD (Figure 3B). Taken together, these results suggest that high BAFF in cGVHD patients promotes survival and activation of pre-GC and PB/PC cells.
BAFF promotes CD27+ pre-GC and PB/PC subsets in cGVHD. (A) In vivo correlation between soluble BAFF and numbers of CD27+ BCR-activated B-cell subsets is shown for patients with active cGVHD (top row) and patients with inactive cGVHD (bottom row). The total number of each CD27+ B-cell subset (Table 2) and the BAFF level (ng/mL) were natural log–transformed. (B) BCMA, TACI, and BAFF-R expression on pre-GC and PB/PC cells from a patient with active cGVHD compared with healthy control. Data are representative of 2 independent experiments conducted using 2 cGVHD patients and 2 healthy donors.
BAFF promotes CD27+ pre-GC and PB/PC subsets in cGVHD. (A) In vivo correlation between soluble BAFF and numbers of CD27+ BCR-activated B-cell subsets is shown for patients with active cGVHD (top row) and patients with inactive cGVHD (bottom row). The total number of each CD27+ B-cell subset (Table 2) and the BAFF level (ng/mL) were natural log–transformed. (B) BCMA, TACI, and BAFF-R expression on pre-GC and PB/PC cells from a patient with active cGVHD compared with healthy control. Data are representative of 2 independent experiments conducted using 2 cGVHD patients and 2 healthy donors.
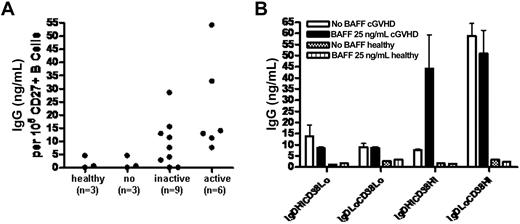
We then examined qualitative differences in CD27+ populations in patients with and without cGVHD. Human CD27+ B cells are antigen experienced and include memory B cells and antibody-secreting cells.41 To determine whether CD27+ B cells in patients with active cGVHD were functionally activated antibody-secreting cells in vivo, we measured IgG production of freshly purified CD27+ B cells from these patients compared with CD27+ B cells from patients without cGVHD and from healthy donors. As shown in Figure 4A, ex vivo–purified CD27+ B cells from patients with active and inactive cGVHD produce IgG without requiring in vitro antigen or BCR stimulation. Constitutive IgG secretion by CD27+ B cells from patients without cGVHD or from healthy donors did not occur. Increasing in vitro BAFF concentration also did not result in IgG production by CD27+ B cells (data not shown). High IgG secretion by the PB/PC population occurred without additional BAFF (Figure 4B). Although constitutive IgM production by pre-GC B cells was found (data not shown), these cells produced IgG after addition of BAFF in vitro (Figure 4B). Except for PB/PC cells, which produced consistently lower amounts of IgG compared with cGVHD patients (10-15 ng/mL), B-cell subsets from healthy individuals (n = 2) did not produce measurable IgG after 48 hours under these conditions. These data suggest that constitutive IgG production by circulating PB/PC cells in cGVHD is due to an in vivo activated state and suggests that the activated pre-GC B cells in cGVHD are capable of BAFF-induced isotype switching.48
Constitutive production of IgG by CD27+ B cells and B-cell subsets in patients with cGVHD. (A) Spontaneous IgG production by purified peripheral blood CD27+ B cells from post-HSCT patients or healthy individuals. (B) IgG production by cGVHD patient B-cell subsets after 48 hours with or without additional BAFF in vitro. Error bars indicate SD (± mean).
Constitutive production of IgG by CD27+ B cells and B-cell subsets in patients with cGVHD. (A) Spontaneous IgG production by purified peripheral blood CD27+ B cells from post-HSCT patients or healthy individuals. (B) IgG production by cGVHD patient B-cell subsets after 48 hours with or without additional BAFF in vitro. Error bars indicate SD (± mean).
Development of cGVHD is associated with delayed B-cell reconstitution and persistent BAFF elevation
Finally, we prospectively measured BAFF levels, B-cell numbers, and B-cell phenotype in a group of 25 patients followed for at least 1 year after HSCT. Median B-cell number and BAFF level are depicted for the 8 patients who did not develop cGVHD during this period (Figure 5A) and compared with 17 patients who developed cGVHD a median of 6 months after HSCT (range, 3-12 months; Figure 5B). Figure 5C shows median B-cell numbers and BAFF levels in 8 patients after autologous HSCT in whom B-cell reconstitution occurred in the absence of alloantigen exposure. In all HSCT groups, BAFF levels were elevated early (1 and 3 months) after HSCT. B-cell numbers increased more rapidly after autologous HSCT (Figure 5C) than in patients without GVHD who underwent allogeneic transplantation (Figure 5A). In both of these patient groups, BAFF levels decreased as B-cell numbers increased. In contrast, BAFF levels remained high as B-cell numbers remained low in patients who developed cGVHD (Figure 5B). In the first year after transplantation, these results suggest that BAFF levels generally reflect the degree of B-cell lymphopenia after transplantation.
Delayed reconstitution of B cells and increased proportion of CD27+ B cells in patients who develop cGVHD. CD19+ B-cell number (left y-axis) and BAFF levels (right y-axis) were measured every 3 months after HSCT in 3 patient groups. (A) Eight patients who did not develop cGVHD by 12 months. (B) Seventeen patients who developed cGVHD during this period. (C) Eight patients who underwent autologous transplantation after myeloablative conditioning. Numbers of patients measured at each time point are shown. The percentage of CD27+ B cells was measured every 3 months after HSCT. (D) Patients who did not develop cGVHD by 12 months are compared with (E) patients who developed cGVHD during this period. Absolute numbers of CD27− B-cell subsets were also identified in these patients. (F) Results in patients who do not develop cGVHD between 3 and 12 months after HSCT are compared with (G) patients who developed cGVHD during this period.
Delayed reconstitution of B cells and increased proportion of CD27+ B cells in patients who develop cGVHD. CD19+ B-cell number (left y-axis) and BAFF levels (right y-axis) were measured every 3 months after HSCT in 3 patient groups. (A) Eight patients who did not develop cGVHD by 12 months. (B) Seventeen patients who developed cGVHD during this period. (C) Eight patients who underwent autologous transplantation after myeloablative conditioning. Numbers of patients measured at each time point are shown. The percentage of CD27+ B cells was measured every 3 months after HSCT. (D) Patients who did not develop cGVHD by 12 months are compared with (E) patients who developed cGVHD during this period. Absolute numbers of CD27− B-cell subsets were also identified in these patients. (F) Results in patients who do not develop cGVHD between 3 and 12 months after HSCT are compared with (G) patients who developed cGVHD during this period.
A more detailed prospective analysis of B-cell subsets after allogeneic HSCT is shown in Figure 5D through G. In patients who do not develop cGVHD (Figure 5D), the percentage of CD27+ B cells decreased gradually as total B-cell numbers recovered and BAFF levels returned toward normal. In contrast, the percentage of CD27+ B cells remained elevated in many patients who developed cGVHD (Figure 5E). Although naive B cells (IgD+ and IgDLo CD27− populations) increased steadily in patients without cGVHD compared with patients with cGVHD. Each of the 4 antigen-naive CD27− B-cell subsets increased at a faster rate (Figure 5F) in patients who did not develop cGVHD compared with patients who developed cGVHD (Figure 5G). Circulating transitional B cells were also notably higher between 1 and 9 months after HSCT in patients who did not develop cGVHD compared patients who developed cGVHD (Figure 5G). Unlike other B-cell subsets in the early post-HSCT period, recent bone marrow emigrant or transitional B cells in patients without cGVHD reached normal levels by 9 months after HSCT (13.5 × 106/L vs 14.4 × 106/L in healthy individuals). In contrast, the transitional B-cell population was exceedingly rare in the cGVHD group, suggesting low bone marrow output of these cells in patients who would subsequently develop cGVHD (Figure 5G).
Discussion
In murine models, maintenance of B-cell homeostasis and a nonautoimmune state requires a diverse naive B-cell compartment and limiting amounts of BAFF.26-29 BAFF levels respond to alterations in B-cell homeostasis and reduced marrow output.21 Failure to compensate after disruption of B-cell homeostasis with an increase in B-cell number or decreased BAFF results in a breach of an “elastic” BAFF B-cell tolerance checkpoint.49,50 The present study is the first to demonstrate that delayed recovery of B-cell homeostasis and persistence of high BAFF/B-cell ratios are associated with an activated peripheral B-cell pool in human cGVHD.
In both murine models and patients, HSCT conditioning results in supranormal recovery of naive B cells before B-cell homeostasis is reached.20,46,51 Thus, it appears that attaining B-cell homeostasis after lymphopenia induction requires a high degree of output from bone marrow progenitor cells. Our studies demonstrated that patients who underwent allogeneic transplantation who did not develop cGVHD had supranormal naive and transitional B-cell numbers even as late as 28 months after HSCT (Table 2). These data provide evidence that the posttransplantation surge in naive B-cell number found in autologous HSCT and in studies of B-cell reconstitution after myeloablation in mice reflect a normal B-cell homeostatic compensatory mechanism that may contribute to ongoing removal of potentially alloreactive or autoreactive B cells. BAFF/B-cell ratios in patients without cGVHD after HSCT were not different from healthy individuals who had not undergone HSCT (Figure 1C). In contrast, failure to achieve a normal BAFF/B-cell ratio was associated with cGVHD. Constitutive production of the BAFF cytokine by stromal cells likely maintains B-cell pool number, but local production of BAFF by other (myeloid) cells likely promotes autoreactive B cells.52 These data suggest that decreasing BAFF levels therapeutically after HSCT may help prevent the persistent activation of B cells and the loss of B-cell tolerance associated with cGVHD.
Although treatment of steroid refractory cGVHD with rituximab appears to be effective,13,14 the mechanisms underlying B-cell involvement in this post-HSCT complication have not been elucidated. For the first time, we characterized the mature B-cell compartment in a large number of post-HSCT patients and discovered a relative preponderance of activated CD27+ B cells in individuals with cGVHD. These studies specifically identified 2 circulating IgG-producing CD27+ B-cell populations in cGVHD, a pre-GC population, and a PB/PC population. These findings suggest that exclusion of potentially pathologic, activated B cells from follicular niches that must occur in healthy individuals30 might not occur in cGVHD. Both extrafollicular and GC pathways have been implicated in the development of autoreactive B cells in murine models.53 Chronic GVHD patients with persistently elevated BAFF/B-cell ratios more than 12 months after HSCT had peripheral B-cell pools composed primarily of CD27+ peripheral B-cell subsets. In cGVHD patients, increasing BAFF levels correlated in vivo with increasing numbers of activated pre-GC and post-GC PB/PC cells that expressed high BCMA and TACI (Figure 3). Production of IgG by cGVHD pre-GC and PB/PC cells further suggested that these cells were activated in vivo. Circulating B cells with a similar phenotype have been described in SLE and Sjögren syndrome.44,45 Our studies showed that these circulating cells did not require antigen or second signal stimulation for IgG production, indicating that pre-GC and PB/PC cells were activated in secondary lymphoid or nonlymphoid sites before recirculation and subsequent homing to secondary lymphoid organs. Future examination of corresponding B-cell subsets and BAFF levels in emerging murine models possessing phenotypic changes that closely mirror those of cGVHD patients will yield important information.54 Identification of immune targets in cGVHD in further investigations is also required to determine whether IgG produced by activated B cells contributes to end organ damage by aiding in cross-presentation to T cells,55,56 forming immune complexes that deposit in tissue,57 or through binding to functional receptors.18
Naive B lymphopenia in patients with active cGVHD appeared to begin during the first year after HSCT. Despite high BAFF levels early after HSCT, patients who subsequently developed cGVHD had delayed B-cell reconstitution and lower B-cell numbers. Very few of the B cells in these individuals were antigen-naive CD27− B cells compared with patients who did not develop cGVHD. Ongoing use of immunosuppressive medications, alloimmune destruction of secondary lymphoid structures, and/or ongoing inflammation may account for decreased production of B cells.58-60 Importantly, cGVHD patients had low numbers of circulating bone marrow transitional B-cell emigrants in the early post-HSCT period and this persisted at later time points. Allogeneic HSCT resulted in more prolonged B lymphopenia compared with autologous HSCT. This suggests that restoration of B-cell homeostasis after HSCT may be disrupted by mismatch of minor histocompatibility antigens (mHAs). This delay may reflect increased B-cell turnover and ongoing selection processes in the allogeneic setting. The paucity of circulating transitional B cells in patients with cGVHD may also reflect abnormalities of the bone marrow microenvironment. Bone marrow stromal cells produce cytokines important for B-cell lymphopoiesis, such as SDF-1 and flt3L.61,62 It is also possible that decreased bone marrow output in cGVHD signals BAFF production by granulocytes, monocytes, dendritic cells, or stromal cells in as yet undetermined ways. Alternatively, high BAFF in the setting of B lymphopenia may be a result of increased free BAFF due to lack of available naive B-cell BAFF receptors that are otherwise occupied by BAFF.63
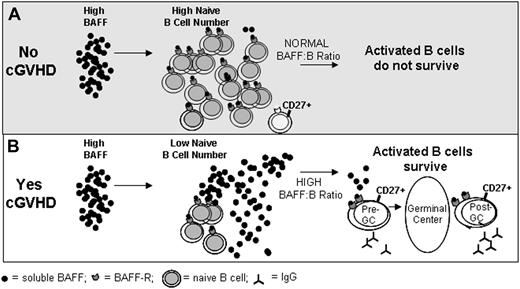
Taken together, our results highlight the importance of the BAFF/B-cell ratio after HSCT in promotion of an activated B-cell pool. We can now begin to formulate a working model for B-cell pathophysiology in cGVHD in which disrupted B-cell homeostasis and excess BAFF promote activated B cells that are most reliant on BAFF for survival (Figure 6). In the absence of cGVHD, B cells reconstitute promptly and large numbers of naive B cells are present, BAFF/B-cell ratios normalize, and insufficient BAFF is present to support survival of large numbers of activated CD27+ B cells (Figure 6A). This situation appears to be markedly different in patients with cGVHD. In these patients, inadequate reconstitution of naive B cells is associated with persistence of high BAFF levels and expansion of activated CD27+ B cells (Figure 6B). This model has several potential clinical implications. Agents that target BAFF directly would be expected to lower BAFF levels, normalize BAFF/B-cell ratio, and selectively reduce levels of activated CD27+ B cells. In contrast, pathologic B cells are depleted with rituximab, but BAFF levels would remain elevated and BAFF/B-cell ratios become more abnormal after therapy. Moreover, B-cell recovery after treatment with rituximab again occurs in the setting of very high BAFF, which would continue to support activated B cells in vivo. Thus, our studies provide a framework for future clinical development of agents that target either B cells or BAFF in the post-HSCT setting for prevention and treatment of cGVHD.
Model: Excess BAFF promotes activated B cells in cGVHD. (A) In patients who do not develop cGVHD, high BAFF levels are associated with increased numbers of naive B cells. This results in a normal BAFF/B-cell ratio and potentially autoreactive B cells are eliminated due to limiting amounts of BAFF. (B) In patients who develop cGVHD, high BAFF levels are associated with decreased numbers of naive B cells resulting in a high BAFF/B-cell ratio. In this setting, excess BAFF levels support alloreactive and autoreactive pre- and post-GC B cells that are most reliant on BAFF for their survival.
Model: Excess BAFF promotes activated B cells in cGVHD. (A) In patients who do not develop cGVHD, high BAFF levels are associated with increased numbers of naive B cells. This results in a normal BAFF/B-cell ratio and potentially autoreactive B cells are eliminated due to limiting amounts of BAFF. (B) In patients who develop cGVHD, high BAFF levels are associated with decreased numbers of naive B cells resulting in a high BAFF/B-cell ratio. In this setting, excess BAFF levels support alloreactive and autoreactive pre- and post-GC B cells that are most reliant on BAFF for their survival.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank John Daley, Suzan Lazo-Kallanian, Whitney Washel, and Sean McDonough for expert technical assistance; Doreen Hearsey for help obtaining clinical samples; Drs John Manis and Shiv Pillai for helpful discussions and critical review of the paper; Christine Canning and Dr Nicholas Souders for critical review of the paper; and Drs Roberto Bellucci and Emmanuel Zorn for helpful discussions.
This study was supported by the Leukemia & Lymphoma Society (White Plains, NY), Jock and Bunny Adams Research and Education Endowment (Boston, MA), Ted and Eileen Pasquarello Research Fund (Boston, MA), and National Institutes of Health (Bethesda, MD) grants AI29530, HL70149, and K12CA087723.
National Institutes of Health
Authorship
Contribution: S.S. designed and conceived the study, performed the research, analyzed and interpreted the data, and wrote the paper; K.E.S. and H.T.K. analyzed and interpreted data and participated in writing the paper; C.S.C., V.T.H., E.P.A., J.K., R.J.S., and J.H.A. provided vital patient samples and clinical information and edited the paper; N.S.B. and M.S. performed experiments and analyzed and interpreted the data; B.R.B. reviewed and edited the paper; and J.R. supervised the work, contributed to design and interpretation of the study, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefanie Sarantopoulos, Dana-Farber Cancer Institute, 44 Binney St, M530, Boston, MA 02115; e-mail: stefanie_sarantopoulos@dfci.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal