Abstract
Protein Z is a vitamin K–dependent plasma glycoprotein that is involved in the regulation of blood coagulation. Plasma concentrations of protein Z vary widely between subjects and are greatly reduced during warfarin therapy. We developed a sensitive and quantitative assay for protein secretion using a secretory luciferase to explore the mode of secretion of protein Z compared with that of factor X. Protein Z secretion was much less efficient than factor X and was totally dependent upon added vitamin K, while factor X secretion was not. Protein Z secretion was highly sensitive to warfarin treatment of the synthesizing cells. In contrast, although factor X secretion was not precluded by warfarin, its γ-carboxylation was completely blocked. An exchange of the propeptide and/or γ-carboxyglutamic acid domain between protein Z and factor X reproduced the inefficient and warfarin-sensitive secretion pattern of protein Z, and vice versa. Joining of the propeptide and γ-carboxyglutamic acid domain to luciferase also demonstrated that the γ-carboxyglutamic acid domain of protein Z was responsible for its warfarin-sensitive secretion. Thus, it was concluded that the difference observed in secretion patterns of protein Z and factor X was mainly based on the structure of their γ-carboxyglutamic acid domains.
Introduction
Protein Z (PZ) is a vitamin K (Vit.K)–dependent plasma glycoprotein homologous to coagulation factors VII, IX, and X (FVII, FIX, and FX), and protein C. Mature human PZ consists of 360 amino acids containing 13 γ-carboxyglutamic acid (Gla) residues, and 1 Gla, 2 epidermal growth factors, and 1 serine protease domains.1,2 PZ is not the zymogen of a serine protease, because it lacks the His and Ser residues of the catalytic triad. The human PZ gene is localized to chromosome 13q34, where the genes for FVII and FX exist side by side, and it spans approximately 14 kilobases consisting of 9 exons including 1 alternative exon.3 PZ forms a calcium ion–dependent complex with activated FX (FXa) on the phospholipid surface and thereby serves as a cofactor for the inhibition of FXa by a PZ-dependent protease inhibitor.4 Although PZ-null mice have an apparently normal phenotype, PZ deficiency dramatically enhances the thrombotic phenotype in mice carrying the factor VLeiden genotype.5 These findings indicate that PZ plays an important role in the regulation of blood coagulation.
Vit.K is required for the posttranslational formation of Gla from glutamic acid residue, which is present in several plasma proteins that are involved in hemostasis: prothrombin, FVII, FIX, and FX, and proteins C, S, and PZ.6-8 Gla-mediated Ca2+ binding in these proteins is necessary for their association with phospholipid surfaces and is critical for their hemostatic function. The conversion of glutamate to Gla is catalyzed by a microsomal membrane protein, Vit.K-dependent γ-glutamylcarboxylase (GCX).9 The formation of the GCX-substrate complex is mediated by the interaction between a propeptide (PP) in the substrate protein and the PP-binding site in GCX. Furthermore, GCX converts glutamate residues in the Gla domains of substrate proteins to Glas, in cooperation with the oxygenation of Vit.K hydroquinone to Vit.K 2,3-epoxide.10,11 Vit.K 2,3-epoxide is reduced to Vit.K hydroquinone by Vit.K-epoxide reductase, which has recently been identified as an endoplasmic reticulum membrane protein.12,13 Warfarin blocks the reduction of Vit.K 2,3-epoxide to Vit.K hydroquinone, resulting in a lack of the GCX cofactor, and thereby a lack of Gla in Vit.K-dependent proteins.14
PZ is synthesized in the liver and secreted into the circulation. Plasma concentrations of PZ vary widely among subjects (0.6-5.7 mg/L) with an average of 2.9 mg/L,15 and concentrations are low in newborn infants16 and patients with chronic liver disease.17 Warfarin treatment reduces the PZ antigen in plasma to levels much lower than those of other warfarin-treated Vit.K-dependent proteins.15 We previously identified a mutation in the PZ gene that resulted in the replacement of glutamate in the Gla domain with glutamine (E30Q).18 E30Q PZ could not be secreted when its cDNA was expressed in baby hamster kidney (BHK) cells. These findings proposed that a Vit.K-dependent γ-carboxylation system is indispensable to PZ secretion. In the present study, we performed structure-based examinations of PZ secretion in comparison with the secretion of another Vit.K-dependent protein, FX, using human embryonic kidney (HEK) 293 cells, the most common cell line currently being used for the biosynthesis of Vit.K-dependent proteins.
Methods
Constructions
An expression vector for PZ cDNA (pcDNA-PZ) was generated previously.18 FX cDNA was amplified from human liver RNA by reverse transcription polymerase chain reaction (RT-PCR) using primers FX-F and FX-R1 (Table 1). PCR-amplified FX cDNA was digested with restriction enzyme BamHI and inserted into a BamHI site of the pcDNA3 (Invitrogen, Carlsbad, CA). To generate fusion proteins with a secretory luciferase, which had been cloned from the marine copepod Metridia longa (MetLuc),19 full-length and amino-terminal–truncated MetLucs were amplified by PCR using a pMetLuc-control vector (Clontech, Mountain View, CA) as a template and primers MLUC-F1 and MLUC-F2, respectively, and MLUC-R (Table 1 and Figure 1A left). The PCR-amplified MetLuc cDNA was digested with BspHI and ligated into a BspHI site of PZ cDNA located 2 codons upstream from the translation termination codon of PZ.1,2 FX was fused with MetLuc as follows: the translation termination codon of FX was replaced with ATG (Met) containing an NcoI restriction site using a primer, FX-R2 (Table 1), and an NcoI-digested FX cDNA was ligated to a BspHI-digested MetLuc cDNA. Next, the fused cDNAs were inserted into BamHI and XbaI sites of the pcDNA3.
Primers for vector construction
| Primer . | Strand . | Sequence . | RE site* . | Constructs . |
|---|---|---|---|---|
| PZ-F | Sense | 5′-TCCGGATCCGAATGGCAGGCTGCGTCCCAC-3′ | BamHI | Full-length PZ and ZPro |
| PZ-R | Antisense | 5′-GCTGGGATCCAGTTAGTTCATGATCTGTTTAAA-3′ | BamHI | Full-length PZ and PZC |
| FX-F | Sense | 5′-CTGGGATCCCACCATGGGGCGCCCACTGCAC-3′ | BamHI | Full-length FX and XPro |
| FX-R1 | Antisense | 5′-GTGGGATCCTCACTTTAATGGAGAGGACGTTAT-3′ | BamHI | Full-length FX and FXC |
| FX-R2 | Antisense | 5′-TCACTCCATGGGAGAGGACGTTATGACCTC-3′ | NcoI | FXMetLuc |
| MLUC-F1 | Sense | 5′-CGCCATCATGAACATCAAGG-3′ | BspHI | PZMetLuc(+Sig) |
| MLUC-F2 | Sense | 5′-TGGTGCTCATGAAGAGCACCGAGTTCGA-3′ | BspHI | PZMetLuc, FXMetLuc |
| MLUC-R | Antisense | 5′-TGGCTGATTATGATCTAGAG-3′ | XbaI | PZMetLuc, FXMetLuc |
| XPPZGla-F | Sense | 5′-GGTCACGAGGGCGGGCTCCTATCTTCTGGA-3′ | XPPZGla | |
| XPPZGla-R | Antisense | 5′-AGGAGCCCGCCCTCGTGACCCTCGCCAGGA-3′ | XPPZGla | |
| ZPPXGla-F | Sense | 5′-GTGGAAGCGTGCCAATTCCTTTCTTGAAGA-3′ | ZPPXGla | |
| ZPPXGla-R | Antisense | 5′-AGGAATTGGCACGCTTCCACCTCACCAGAA-3′ | ZPPXGla | |
| ZPPmut-F | Sense | 5′-CTGGTGGGGTGGAAGGGTGCGG-3′ | PZPPmut | |
| ZPPmut-R | Antisense | 5′-CCGCACCCTTCCACCCCACCAG-3′ | PZPPmut | |
| ZGla-R | Antisense | 5′-GCACGCCATGGCCTTATATCGTCTCC-3′ | NcoI | ZPPZGlaMetLuc, XPPZGlaMetLuc |
| XGla-R | Antisense | 5′-CTGGTCCATGGCTTTGTATTTATTCCA-3′ | NcoI | ZPPXGlaMetLuc, XPPXGlaMetLuc |
| Primer . | Strand . | Sequence . | RE site* . | Constructs . |
|---|---|---|---|---|
| PZ-F | Sense | 5′-TCCGGATCCGAATGGCAGGCTGCGTCCCAC-3′ | BamHI | Full-length PZ and ZPro |
| PZ-R | Antisense | 5′-GCTGGGATCCAGTTAGTTCATGATCTGTTTAAA-3′ | BamHI | Full-length PZ and PZC |
| FX-F | Sense | 5′-CTGGGATCCCACCATGGGGCGCCCACTGCAC-3′ | BamHI | Full-length FX and XPro |
| FX-R1 | Antisense | 5′-GTGGGATCCTCACTTTAATGGAGAGGACGTTAT-3′ | BamHI | Full-length FX and FXC |
| FX-R2 | Antisense | 5′-TCACTCCATGGGAGAGGACGTTATGACCTC-3′ | NcoI | FXMetLuc |
| MLUC-F1 | Sense | 5′-CGCCATCATGAACATCAAGG-3′ | BspHI | PZMetLuc(+Sig) |
| MLUC-F2 | Sense | 5′-TGGTGCTCATGAAGAGCACCGAGTTCGA-3′ | BspHI | PZMetLuc, FXMetLuc |
| MLUC-R | Antisense | 5′-TGGCTGATTATGATCTAGAG-3′ | XbaI | PZMetLuc, FXMetLuc |
| XPPZGla-F | Sense | 5′-GGTCACGAGGGCGGGCTCCTATCTTCTGGA-3′ | XPPZGla | |
| XPPZGla-R | Antisense | 5′-AGGAGCCCGCCCTCGTGACCCTCGCCAGGA-3′ | XPPZGla | |
| ZPPXGla-F | Sense | 5′-GTGGAAGCGTGCCAATTCCTTTCTTGAAGA-3′ | ZPPXGla | |
| ZPPXGla-R | Antisense | 5′-AGGAATTGGCACGCTTCCACCTCACCAGAA-3′ | ZPPXGla | |
| ZPPmut-F | Sense | 5′-CTGGTGGGGTGGAAGGGTGCGG-3′ | PZPPmut | |
| ZPPmut-R | Antisense | 5′-CCGCACCCTTCCACCCCACCAG-3′ | PZPPmut | |
| ZGla-R | Antisense | 5′-GCACGCCATGGCCTTATATCGTCTCC-3′ | NcoI | ZPPZGlaMetLuc, XPPZGlaMetLuc |
| XGla-R | Antisense | 5′-CTGGTCCATGGCTTTGTATTTATTCCA-3′ | NcoI | ZPPXGlaMetLuc, XPPXGlaMetLuc |
Restriction enzyme (RE) site indicated italics in primer sequence.
Primers were generated to facilitate the exchange of PPs and Gla domains (PP and Gla, respectively, in Figure 1A left) between PZ and FX (Table 1). Mutations at PP-cleavage sites were introduced using a pair of primers, ZPPmut-F and ZPPmut-R (Table 1). Full-length constructs were prepared using various pairs of primers with PZ-F, PZ-R, FX-F, and FX-R1 that were digested with BamHI and inserted into a BamHI site of the pcDNA3. Short PP-Gla constructs were generated by EcoRI digestion of the full-length constructs in a pcDNA3 vector. Various combinations of PP and Gla domains were also amplified using primers PZ-F or FX-F, and ZGla-R or XGla-R (Table 1), digested with NcoI, and followed by ligation with a BspHI-digested MetLuc cDNA.
Western blot analysis
HEK 293 cells (2 × 105 cells) were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum, 1× nonessential amino acids (Sigma-Aldrich, St Louis, MO) and 1× antibiotics-antimycotics (Sigma-Aldrich). A 20-μg plasmid DNA was transfected to approximately 2 × 105 cells by the calcium phosphate precipitation method. Forty-eight hours after transfection, the cells were divided into 2 or 4 dishes and incubated for 24 additional hours in DMEM containing 0.2% bovine serum albumin with or without 5 μg/mL Vit.K1 (Sigma-Aldrich) and/or 10 μg/mL warfarin (gift from Eisai, Tokyo, Japan). Medium (10 μL) was mixed and boiled with an equal volume of 2% sodium dodecyl sulfate (SDS), 0.1 M Tris, pH 6.8, 15% glycerol, and 0.02% bromphenol blue (SDS-sample buffer). The remaining 1.0 mL medium was mixed with 50 μL 0.25 M sodium citrate, and then added to 50 μL 1 M barium chloride and rotated at 4°C for 1 hour. After centrifugation, the pellet was rinsed twice with 0.5 mL 20 mM Tris, pH 7.5, 150 mM sodium chloride, 10 mM barium chloride, and 10 mM benzamidine, dissolved with 50 μL 0.1 M ethylenediaminetetraacetate (EDTA), 20 mM Tris, pH 7.5, 150 mM sodium chloride, and 10 mM benzamidine, and boiled in an equal volume of SDS-sample buffer containing 5% β-mercaptoethanol. The cells were washed twice with phosphate-buffered saline, harvested, and lysed with 50 mM Tris, pH 7.5, 150 mM sodium chloride, 1% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride, and boiled with equal volumes of the SDS-sample buffer containing 5% β-mercaptoethanol. The samples were electrophoresed using an 8% polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was reacted with an anti-PZ (BioPure, Bubendorf, Switzerland) or an anti-FX antibody, followed by reaction with horseradish peroxidase–conjugated anti–rabbit immunoglobulin G antibody. Detection was achieved using an enhanced chemiluminescent substrate (GE Healthcare, Little Chalfont, United Kingdom).
Pulse-chase experiment
HEK 293 cells transfected with a 20-μg plasmid vector were divided into 5 dishes at 48 hours after transfection and cultured in a standard medium for 24 hours. After preincubation with methionine/cysteine-free DMEM containing 2% dialyzed fetal bovine serum for 1 hour, 50 μCi EXPRE35S35S protein labeling mix (NEN Life Science Products, Boston, MA) were added to the cells and incubated at 37°C for 1 hour; then the medium was replaced by the standard medium containing 5 μg/mL Vit.K1. Medium samples were collected, and the cells were lysed with 0.1% SDS, 0.1% Triton X-100, 10 mM Tris, pH 7.5, 150 mM sodium chloride, 1 mM benzamidine, and 1 mM phenylmethylsulfonyl fluoride. One microgram of mouse immunoglobulin G and 20 μL PANSORBIN (Calbiochem, Darmstadt, Germany) were mixed with the cell lysates and medium for 1 hour to remove nonspecific materials. After centrifugation, 5 μL anti-PZ or anti-FX antibody were reacted with the supernatant at 4°C overnight and mixed with 20 μL PANSORBIN for 1 hour. The PANSORBIN pellet was washed 3 times with 0.1% SDS, 0.1% Triton X-100, 10 mM Tris, pH 7.5, and 150 mM sodium chloride and boiled in the SDS-sample buffer containing 5% β-mercaptoethanol. After centrifugation, the supernatant was subjected to SDS polyacrylamide gel electrophoresis (PAGE) followed by fluorography using a FLA-2000 fluoroimage analyzer (Fujifilm, Tokyo, Japan).
Luciferase-based secretion assay
Fifteen micrograms of the MetLuc vector were transfected by the calcium phosphate precipitation method to HEK 293 cells together with 2 μg pGL3-control (Promega, Madison, WI). Twenty-fours hours after transfection, the cells were divided into 2 dishes and incubated an additional 24 hours in DMEM containing 10% fetal bovine serum with or without 5 μg/mL Vit.K1 and/or 10 μg/mL warfarin. A 0.2-mL aliquot of the culture medium was mixed with 10 μL 0.25 M sodium citrate and 10 μL 1 M barium chloride for 1 hour. The barium citrate precipitate was washed twice with 0.5 mL 20 mM Tris, pH 7.5, 150 mM sodium chloride, 10 mM barium chloride, and 10 mM benzamidine and then dissolved with 0.2 mL 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), pH 7.5, and 150 mM sodium chloride. MetLuc activity in the medium and barium-absorbed samples was assayed using a Ready-To-Glow Secreted Luciferase Reporter Assay (Clontech). Cells were lysed with 0.1 M Tris-acetate, pH 7.8, 10 mM magnesium acetate, 1 mM EDTA, 1 mM dithiothreitol, and 0.2% Triton X-100, and firefly luciferase activity in the cell lysates was measured using a Pikka Gene luciferase assay kit (Toyo B-Net, Tokyo, Japan) as an internal control.
Results
Development of novel PZ secretion assay
We previously demonstrated that PZ could be secreted from BHK cells stably transformed with a high copy number of PZ cDNA, in the presence of Vit.K.18 Western blot analysis failed to detect the presence of any PZ in the culture medium when an expression vector for PZ was transiently transfected into BHK cells. Accordingly, we developed a sensitive and quantitative assay to effectively detect PZ secretion and to compare the PZ and FX secretions under the same experimental conditions. In addition, the PZ secretion was examined using HEK 293 cells, the most common cell line currently being used for the biosynthesis of Vit.K-dependent proteins.
A secretory form of luciferase, cloned from the marine copepod Metridia longa,19 was fused to PZ. When a full-length Metridia luciferase (MetLuc),19 including its signal sequence (Figure 1A a line with “1-18”) that had been fused to the carboxyl terminus of PZ (PZMetLuc(+Sig)), was expressed, a high activity of luciferase was detected in the medium even in the absence of Vit.K (Figure 1A), the condition under which natural PZ is not secreted.18 This is because the MetLuc region was released from the PZ part of PZMetLuc(+Sig) by cleavage at the signal peptide, as shown by the presence of a PZ band in the same position as unfused PZ (Figure 1B middle lane); such cleavage of a signal sequence at an internal position was previously reported in a chimera of α-globin and preprolactin.20 Accordingly, the amino-terminal signal peptide of MetLuc was removed to express the fusion protein of PZ and MetLuc (PZMetLuc). Western blot analysis of the barium citrate adsorbate (Figure 1B right lane) confirmed the attachment of MetLuc to PZ in PZMetLuc, by the fact of its migration in SDS-PAGE being slower than PZ alone. The newly developed assay revealed Vit.K-dependent secretion of active luciferase into the medium of HEK 293 cells (Figure 1A bottom) as with natural PZ in BHK cells.18
Construction and secretion of PZ fused with MetLuc from HEK 293 cells. (A) MetLuc cDNA encodes a 219-amino acid polypeptide containing an amino-terminal signal peptide of 17 amino acid residues for secretion (dark lines left panel). PZ cDNA was fused with MetLuc cDNA with (PZMetLuc(+Sig)) or without (PZMetLuc) the signal peptide of MetLuc. Expression vectors were transfected to HEK 293 cells with pGL3-control, and the cells were incubated without (□) or with (■) Vit.K for 24 hours. MetLuc activity in the culture medium was measured. The data were normalized to the firefly luciferase activity in cell lysates (relative light unit [RLU] against pGL3-control) and were shown as the mean (± SD) for 3 independent experiments (right panel). The numbers depict the residue numbers of the first and last amino acid residues of regions or domains in PZ (in parentheses) or MetLuc (without parentheses). PP, Gla, and PZC stand for the propeptide, Gla domain, and C-terminal region of PZ, respectively. (B) PZ in the medium was collected by barium citrate adsorption and subjected to SDS-PAGE under reducing conditions, followed by Western blot analysis using an anti-PZ antibody. The position of human PZ is indicated by an arrow; molecular weight standards are shown by numbers with kilodaltons (kDa).
Construction and secretion of PZ fused with MetLuc from HEK 293 cells. (A) MetLuc cDNA encodes a 219-amino acid polypeptide containing an amino-terminal signal peptide of 17 amino acid residues for secretion (dark lines left panel). PZ cDNA was fused with MetLuc cDNA with (PZMetLuc(+Sig)) or without (PZMetLuc) the signal peptide of MetLuc. Expression vectors were transfected to HEK 293 cells with pGL3-control, and the cells were incubated without (□) or with (■) Vit.K for 24 hours. MetLuc activity in the culture medium was measured. The data were normalized to the firefly luciferase activity in cell lysates (relative light unit [RLU] against pGL3-control) and were shown as the mean (± SD) for 3 independent experiments (right panel). The numbers depict the residue numbers of the first and last amino acid residues of regions or domains in PZ (in parentheses) or MetLuc (without parentheses). PP, Gla, and PZC stand for the propeptide, Gla domain, and C-terminal region of PZ, respectively. (B) PZ in the medium was collected by barium citrate adsorption and subjected to SDS-PAGE under reducing conditions, followed by Western blot analysis using an anti-PZ antibody. The position of human PZ is indicated by an arrow; molecular weight standards are shown by numbers with kilodaltons (kDa).
Inefficient and Vit.K-dependent secretion of PZ
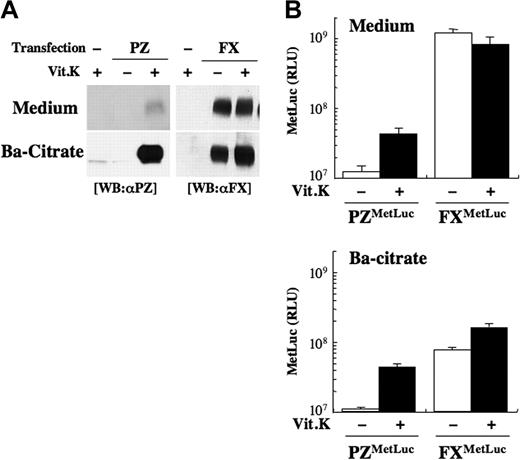
PZ was faintly detected in the medium, even in the presence of Vit.K by Western blot analysis (Figure 2A left panel). On the other hand, a large amount of FX was detected in the medium independent of the treatment of those cells with Vit.K (Figure 2A right panel).
Secretion of PZ and FX from HEK 293 cells. (A) An expression vector for PZ (left) or FX (right) without the MetLuc region was transfected to HEK 293 cells, and the cells were incubated with (+) or without (−) Vit.K for 24 hours. γ-Carboxylated PZ or FX in 1 mL medium was collected into a 50-μL solution by barium citrate adsorption, 10-μL aliquots of the medium (Medium, top panel) or the barium citrate adsorbate PZ/FX (Ba-citrate, bottom panel) were subjected to SDS-PAGE followed by Western blot analysis using an anti-PZ (left) or anti-FX (right) antibody. (B) HEK 293 cells transfected with 15 μg pcDNA-PZMetLuc or -FXMetLuc and 2 μg pGL3-control were incubated with (+, ■) or without (−, □) Vit.K for 24 hours, and luciferase activity was measured in the medium and in the barium citrate–adsorbed fraction. Values were normalized to the firefly luciferase activity in cell lysates for transfection efficiecy. The data shown are the mean (± SD) for 3 independent experiments.
Secretion of PZ and FX from HEK 293 cells. (A) An expression vector for PZ (left) or FX (right) without the MetLuc region was transfected to HEK 293 cells, and the cells were incubated with (+) or without (−) Vit.K for 24 hours. γ-Carboxylated PZ or FX in 1 mL medium was collected into a 50-μL solution by barium citrate adsorption, 10-μL aliquots of the medium (Medium, top panel) or the barium citrate adsorbate PZ/FX (Ba-citrate, bottom panel) were subjected to SDS-PAGE followed by Western blot analysis using an anti-PZ (left) or anti-FX (right) antibody. (B) HEK 293 cells transfected with 15 μg pcDNA-PZMetLuc or -FXMetLuc and 2 μg pGL3-control were incubated with (+, ■) or without (−, □) Vit.K for 24 hours, and luciferase activity was measured in the medium and in the barium citrate–adsorbed fraction. Values were normalized to the firefly luciferase activity in cell lysates for transfection efficiecy. The data shown are the mean (± SD) for 3 independent experiments.
FX as well as PZ fused with MetLuc (FXMetLuc and PZMetLuc, respectively) were also expressed in HEK 293 cells. The levels of luciferase activity of FXMetLuc secreted into the medium did not vary between treatments of cells with and without Vit.K and was 20-fold higher than that of Vit.K-treated PZMetLuc (Figure 2B top). However, at the beginning of Vit.K treatment, intracellular MetLuc activity did not differ between PZMetLuc and FXMetLuc (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), indicating that their levels of synthesis per se were similar to each other.
Barium citrate adsorption would be expected to collect and condense the γ-carboxylated PZ as much as 20 times by volume (vol/vol = 50 μL/1000 μL) in a culture medium of HEK 293 cells that had been transfected with the PZ cDNA and grown in the presence of Vit.K. In fact, a band with an intensity roughly 20 times that of the PZ in the uncondensed medium was obtained (Figure 2A), confirming Vit.K-dependent secretion of γ-carboxylated PZ (Gla-form) from these cells.
Nearly all the luciferase activity of PZMetLuc in the medium could be recovered by barium citrate adsorption (88.7% ± 9.2% and 108% ± 38% under treatment with and without Vit.K, respectively; ratio of the values of top and bottom in Figure 2B). In contrast, only 6.3% (± 0.1%) and 20.8% (± 4.0%) of the FXMetLuc activity in the medium under no treatment and Vit.K treatment, respectively, were collected by the same barium citrate adsorption procedure. Thus, the new sensitive and quantitative luciferase-based assay could clearly demonstrate that practically all secreted PZ was γ-carboxylated and the majority of secreted FX was noncarboxylated; in addition, these results were consistent with the qualitative results determined by Western blot analysis as shown in Figure 2A.
A pulse-chase experiment of unfused PZ and FX was carried out to measure the rate and efficiency of the PZ secretion. Vit.K was added at the beginning of the chase. When PZ was transiently expressed in HEK 293 cells, less than 10% of the synthesized PZ was secreted into the medium after 8 hours even in the presence of Vit.K (Figure 3A), although the newly synthesized PZ decreased to 40% inside the cells. On the other hand, 50% of the synthesized FX was secreted from HEK 293 cells within 2 hours (Figure 3B). Thus, the secretion of PZ was much slower and less efficient than that of FX, resulting in a low level of PZ in the medium.
Pulse-chase analysis of PZ and FX secretion. HEK 293 cells transiently transfected with expression vectors were divided into 5 dishes and were labeled with a 50 μCi [35S]protein labeling mixture for 1 hour. The chase was then initiated by changing the medium containing Vit.K, and the samples were incubated for 1, 2, 4, and 8 hours. PZ (A) or FX (B) in the medium (●) and in the cell lysates (○) were collected by immunoprecipitation using an anti-PZ or anti-FX antibody. Their intensity under fluorography was calculated by setting the signal in the cell lysates at chase-time 0 to 100%. The data shown are the mean (± SD) for 3 independent experiments.
Pulse-chase analysis of PZ and FX secretion. HEK 293 cells transiently transfected with expression vectors were divided into 5 dishes and were labeled with a 50 μCi [35S]protein labeling mixture for 1 hour. The chase was then initiated by changing the medium containing Vit.K, and the samples were incubated for 1, 2, 4, and 8 hours. PZ (A) or FX (B) in the medium (●) and in the cell lysates (○) were collected by immunoprecipitation using an anti-PZ or anti-FX antibody. Their intensity under fluorography was calculated by setting the signal in the cell lysates at chase-time 0 to 100%. The data shown are the mean (± SD) for 3 independent experiments.
Warfarin sensitivity of PZ secretion
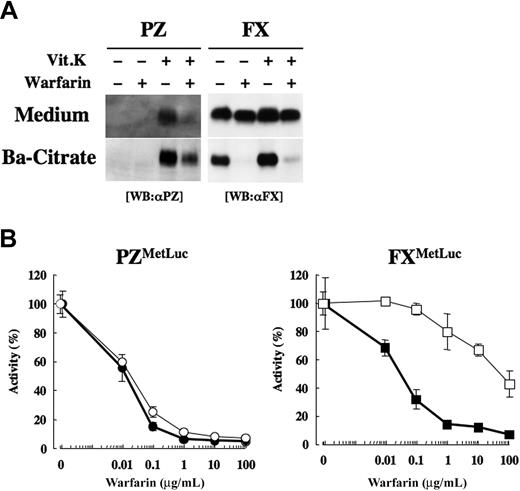
PZ secretion appeared to be dependent on Vit.K, as described in the preceding section. In humans, warfarin treatment reduces the PZ antigen in plasma to levels much lower than those of other warfarin-treated Vit.K-dependent proteins.15 To assess how much warfarin affected the secretion of PZ and FX, transfected HEK 293 cells were treated with warfarin, and the release of PZ and FX into the medium was examined. PZ secretion into the medium was minimal in the absence of Vit.K, and treatment of the cells with Vit.K enabled the release of γ-carboxylated PZ into the medium (Figure 4A left panel). When the cells were treated with 10 μg/mL warfarin together with Vit.K, PZ secretion was greatly restricted. On the other hand, FX could be secreted into the medium even in the absence of Vit.K (Figure 4A right panel); furthermore, the secretion of FX was only slightly suppressed by warfarin treatment of the synthesizing cells, although the adsorption of FX to barium citrate (ie, its γ-carboxylation) was almost completely halted.
Effect of warfarin on the secretion of PZ and FX in HEK 293 cells. (A) HEK 293 cells transiently transfected with a PZ or FX cDNA were treated with or without 10 μg/mL warfarin and/or 5 μg/mL Vit.K for 24 hours. γ-Carboxylated PZ or FX in 0.2 mL medium was collected by barium citrate adsorption in a 30-μL solution. Medium or barium citrate adsorbate (10 μL) was subjected to SDS-PAGE/Western blot analysis. (B) HEK 293 cells transfected with PZMetLuc (left, circles) or FXMetLuc (right, squares) were divided into 6 wells on a 48-well plate at 48 hours after transfection and treated with 0, 0.01, 0.1, 1, 10, or 100 μg/mL warfarin in the presence of 5 μg/mL Vit.K for 24 hours. Luciferase activity in the medium (open symbols) and barium citrate-adsorbate (closed symbols) were calculated relative to the activity of the samples without warfarin treatment (arbitrarily set at 100%).
Effect of warfarin on the secretion of PZ and FX in HEK 293 cells. (A) HEK 293 cells transiently transfected with a PZ or FX cDNA were treated with or without 10 μg/mL warfarin and/or 5 μg/mL Vit.K for 24 hours. γ-Carboxylated PZ or FX in 0.2 mL medium was collected by barium citrate adsorption in a 30-μL solution. Medium or barium citrate adsorbate (10 μL) was subjected to SDS-PAGE/Western blot analysis. (B) HEK 293 cells transfected with PZMetLuc (left, circles) or FXMetLuc (right, squares) were divided into 6 wells on a 48-well plate at 48 hours after transfection and treated with 0, 0.01, 0.1, 1, 10, or 100 μg/mL warfarin in the presence of 5 μg/mL Vit.K for 24 hours. Luciferase activity in the medium (open symbols) and barium citrate-adsorbate (closed symbols) were calculated relative to the activity of the samples without warfarin treatment (arbitrarily set at 100%).
When the cells transfected with PZMetLuc were treated with various doses of warfarin, active luciferase that was secreted into the medium was diminished by the treatment of warfarin at as low as 0.01 μg/mL and reached a minimum by warfarin treatment of more than 1 μg/mL (Figure 4B left panel). An identical inhibition pattern was observed in the PZMetLuc barium citrate adsorbate. On the other hand, the secretion of FXMetLuc into the medium was only moderately inhibited by warfarin treatment of the transfected cells, as 43% luciferase activity remained even in the presence of warfarin at 100 μg/mL (Figure 4B right panel), although the activity recovered by barium citrate adsorption drastically decreased, to an extent similar to that seen with PZMetLuc. These results confirm warfarin sensitivity in PZ secretion and warfarin insensitivity in FX secretion.
Contribution of PP and Gla domain in PZ to the Vit.K-dependent/warfarin-sensitive secretion
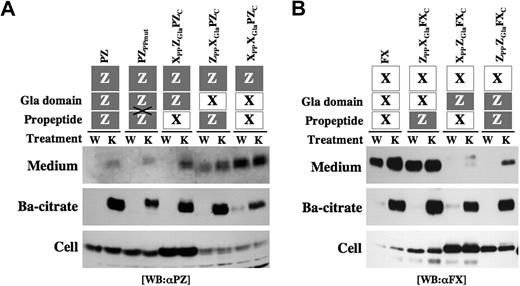
The interaction of Vit.K-dependent proteins with GCX is mediated primarily by a PP in one of the substrate proteins and the PP-binding site in GCX.10,11 Thus, both the PP and Gla domain of Vit.K-dependent proteins are involved in the γ-carboxylation system. To assess whether the PP and/or Gla domain in PZ contributed to its Vit.K-dependent/warfarin-sensitive secretion, various types of chimeric proteins were examined in which the PPs and/or Gla domains were exchanged between PZ and FX. The form of PZ having the PP of FX (XPPZGlaPZC) showed inefficient and warfarin-sensitive secretion similar to that of PZ (Figure 5A). A chimeric PZ having the Gla domain of FX (ZPPXGlaPZC) showed increased secretion partly resistant to warfarin, which was characteristic of FX. A chimera of the PP and Gla domain of FX and the C-terminal PZ (XPPXGlaPZC) demonstrated substantially efficient and warfarin-resistant secretion like FX.
Secretion of PP/Gla domain-chimeric PZ and FX. Construction of the chimeric proteins is illustrated on the top: domains in PZ and FX are shown as gray and white boxes, respectively. In PZPPmut, PZ was mutated at the cleavage site of its PP. Twenty micrograms expression vector for each construct were transfected to HEK 293 cells, and the cells were incubated with warfarin (W) or Vit.K (K). Barium citrate adsorption was carried out from 0.5 mL medium, and the precipitate was dissolved in a 50-μL 0.1 M EDTA solution. The medium, barium citrate adsorbate, and cell lysates were analyzed by Western blot analysis using an anti-PZ (A) or anti-FX (B) antibody.
Secretion of PP/Gla domain-chimeric PZ and FX. Construction of the chimeric proteins is illustrated on the top: domains in PZ and FX are shown as gray and white boxes, respectively. In PZPPmut, PZ was mutated at the cleavage site of its PP. Twenty micrograms expression vector for each construct were transfected to HEK 293 cells, and the cells were incubated with warfarin (W) or Vit.K (K). Barium citrate adsorption was carried out from 0.5 mL medium, and the precipitate was dissolved in a 50-μL 0.1 M EDTA solution. The medium, barium citrate adsorbate, and cell lysates were analyzed by Western blot analysis using an anti-PZ (A) or anti-FX (B) antibody.
Although the PZ antigen levels in the medium varied widely among test chimeras (Figure 5A Medium), similar amounts of PZs were collected by barium citrate adsorption in the presence of Vit.K (Ba-citrate), indicating similar efficiencies of γ-carboxylation among chimeras. PZs remaining inside cells diminished symmetrically for ZPPXGlaPZC and XPPXGlaPZC, which is consistent with their efficient secretion.
A chimeric FX having the PP of PZ (ZPPXGlaFXC) showed efficient and warfarin-resistant secretion similar to that of FX (Figure 5B). In contrast, inefficient and warfarin-sensitive secretion was observed in FX chimeras having the Gla domain of PZ (XPPZGlaFXC and ZPPZGlaFXC). As with the cases of PZ chimeras, similar amounts of FXs were collected by barium citrate adsorption, although the antigen levels in the medium varied widely among chimeras. Large amounts of FXs remained inside cells in XPPZGlaFXC and ZPPZGlaFXC, reflecting their inefficient secretion like PZ.
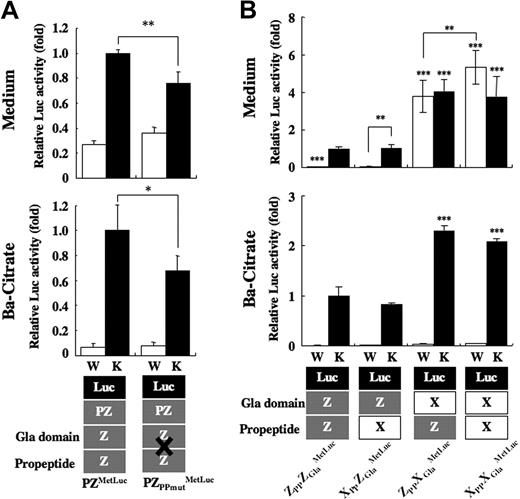
Incidentally, PPs in Vit.K-dependent proteins are generally cleaved inside cells before secretion. To determine whether PP-cleavage is essential for PZ secretion, Arg-(−4) and Arg-(−1) residues at the PP cleavage site in PZ were replaced with 2 Gly residues (PZPPmut). PZPPmut was detected in the medium and precipitated with barium citrate like the wild-type PZ (Figure 5A). The migration of PZPPmut on electrophoresis was slower than that of PZ, indicating that PZPPmut was secreted without cleavage of its PP. When the mutations were introduced into PZMetLuc (PZPPmutMetLuc), the luciferase activity in the medium decreased to 80% of wild-type (Figure 6A), indicating that PP cleavage is partly involved, but not essential, in the process of PZ secretion.
Secretion of PP/Gla domain of PZ and/or FX fused to MetLuc. (A) PZPPmut was fused with MetLuc, and its expression vector (PZPPmutMetLuc) was transfected to HEK 293 cells with a pGL3-control. Twenty-four hours after incubation with warfarin (W, □) or Vit.K (K, ■), MetLuc activity in the medium (top panel) and barium citrate-adsorbate (bottom panel) were assayed. Values of means (± SD) for 3 transfections were calculated relative to the activity of PZMetLuc treated with Vit.K (arbitrarily set at 1-fold; *P = .01; **P < .001). (B) Combinations of PP and Gla domains of PZ (gray boxes) and FX (white boxes) were fused to amino-terminal–truncated MetLuc (black boxes), as illustrated at the bottom. Fifteen micrograms of expression vector for each construct were transfected to HEK 293 cells together with the pGL3-control, and the cells were incubated with warfarin (W, □) or Vit.K (K, ■) for 24 hours. MetLuc activity in the medium (top panel) and barium citrate adsorbate (bottom panel) was measured and normalized to the activity of firefly luciferase in the cell lysates. Values of means (± SD) for 3 transfections were calculated relative to the activity of ZPPZGlaMetLuc treated with Vit.K (arbitrarily set at 1-fold; **P < .005; ***P < .001).
Secretion of PP/Gla domain of PZ and/or FX fused to MetLuc. (A) PZPPmut was fused with MetLuc, and its expression vector (PZPPmutMetLuc) was transfected to HEK 293 cells with a pGL3-control. Twenty-four hours after incubation with warfarin (W, □) or Vit.K (K, ■), MetLuc activity in the medium (top panel) and barium citrate-adsorbate (bottom panel) were assayed. Values of means (± SD) for 3 transfections were calculated relative to the activity of PZMetLuc treated with Vit.K (arbitrarily set at 1-fold; *P = .01; **P < .001). (B) Combinations of PP and Gla domains of PZ (gray boxes) and FX (white boxes) were fused to amino-terminal–truncated MetLuc (black boxes), as illustrated at the bottom. Fifteen micrograms of expression vector for each construct were transfected to HEK 293 cells together with the pGL3-control, and the cells were incubated with warfarin (W, □) or Vit.K (K, ■) for 24 hours. MetLuc activity in the medium (top panel) and barium citrate adsorbate (bottom panel) was measured and normalized to the activity of firefly luciferase in the cell lysates. Values of means (± SD) for 3 transfections were calculated relative to the activity of ZPPZGlaMetLuc treated with Vit.K (arbitrarily set at 1-fold; **P < .005; ***P < .001).
Combinations of the PPs and Gla domains of PZ and FX were fused to MetLuc and expressed in HEK 293 cells. MetLuc, which had fused with the PP and Gla domain of PZ (ZPPZGlaMetLuc), was secreted into the medium in the presence of Vit.K but not after having been treated with warfarin (Figure 6B). A similar secretion pattern of luciferase activity was observed in a combination consisting of the PP of FX and the Gla domain of PZ (XPPZGlaMetLuc). However, MetLuc with the PP of PZ and the Gla domain of FX (ZPPXGlaMetLuc) was secreted into the medium 4-fold more than ZPPZGlaMetLuc, even in the presence of warfarin. XPPXGlaMetLuc, a MetLuc fusion protein with the PP and Gla domain of FX, was secreted more efficiently than ZPPXGlaMetLuc in the presence of warfarin, although its secretion in the presence of Vit.K did not differ from that of ZPPXGlaMetLuc. All fusion proteins could be collected from the medium of Vit.K-treated cells by barium citrate adsorption: the luciferase activity for MetLuc chimeras having the Gla domain of FX (ZPPXGlaMetLuc and XPPXGlaMetLuc) was 2-fold higher than in those having the Gla domain of PZ (ZPPZGlaMetLuc and XPPZGlaMetLuc). These results indicate that the Gla domain of PZ is responsible for the inefficient, Vit.K-dependent, and warfarin-sensitive secretion of PZ.
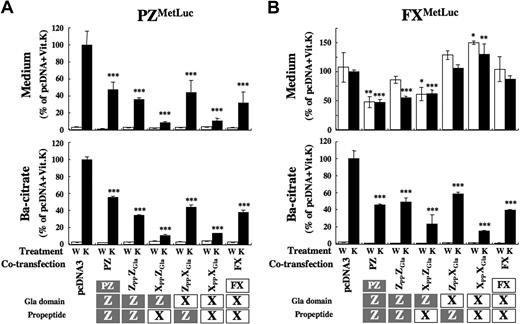
The contribution of the PP/Gla domain to the secretion of PZ and FX was further confirmed by experiments in which PZ, FX, or MetLuc-fusion cDNAs were cotransfected with various mini-constructs of a PP and a Gla domain. Secretion of PZ and PZMetLuc into the medium was suppressed to less than 10% by the cotransfection of constructs having the PP of FX (XPPZGla and XPPXGla; Figure 7A and Figure S2 left). Mini-constructs having the PP of PZ (ZPPZGla and ZPPXGla) showed a moderate effect on the secretion of PZ. The cotransfection of constructs had no effect on the warfarin sensitivity of PZ secretion.
Effect of cotransfection of the PP/Gla domain on secretion of PZ and FX. Ten micrograms of each PP-Gla construct were cotransfected with 2 μg pcDNA-PZMetLuc (A) or -FXMetLuc (B). Twenty-four hours after incubation with warfarin (W, □) or Vit.K (K, ■), MetLuc activity in the medium (top panel) and barium citrate-adsorbate (bottom panel) were measured. Values of means (± SD) for 3 transfections were calculated relative to the activity of cotransfected samples by empty pcDNA treated with Vit.K (arbitrarily set at 100%; *P < .05; **P < .005; ***P < .001).
Effect of cotransfection of the PP/Gla domain on secretion of PZ and FX. Ten micrograms of each PP-Gla construct were cotransfected with 2 μg pcDNA-PZMetLuc (A) or -FXMetLuc (B). Twenty-four hours after incubation with warfarin (W, □) or Vit.K (K, ■), MetLuc activity in the medium (top panel) and barium citrate-adsorbate (bottom panel) were measured. Values of means (± SD) for 3 transfections were calculated relative to the activity of cotransfected samples by empty pcDNA treated with Vit.K (arbitrarily set at 100%; *P < .05; **P < .005; ***P < .001).
When the FX or FXMetLuc cDNA was cotransfected with either of 2 constructs carrying the Gla domain of PZ (ZPPZGla and XPPZGla), the secretion of FX was significantly decreased even in the presence of warfarin (Figure 7B and Figure S2 right). The cotransfection of either FX or FXMetLuc and mini-constructs having the FX-Gla domain (ZPPXGla and XPPXGla) revealed rather enhanced FX secretion into the medium. The coexpression of full-length PZ or FX also inhibited the secretion of another protein. Although the inhibitory profile by mini-constructs for secretion differed markedly between PZ and FX (PZMetLuc and FXMetLuc), the effects of these mini-constructs on the secretion of γ-carboxylated PZ and FX, collected by barium citrate adsorption, were similar (Figure 7AB bottom).
Discussion
The secretion of Vit.K-dependent proteins is the end point of a very complex process which, although poorly understood,21 includes various posttranslational modification reactions, such as pre- and propeptide processing, N- and O-glycosylation, aspartyl β-hydroxylation, and carboxylation as well. The latter reaction itself is complex and has recently been explored in-depth by several investigators using FIX and FX in particular.21,22 In addition, an intracellular quality control system complicates the analysis of secreted Vit.K-dependent proteins, because the extent of carboxylation also affects the susceptibility of these proteins to degradation through the proteasome pathway, where poorly carboxylated Vit.K-dependent proteins are preferentially degraded.23
Novel assay to detect inefficient PZ secretion
In the present study, we developed an assay system for PZ secretion using a secretory form of luciferase (MetLuc) to measure its extent sensitively, because the PZ secretion was not efficient. Fusion of PZ with MetLuc lacking the amino-terminal signal peptide successfully reproduced Vit.K-dependent secretion of luciferase activity into the medium, which could be recovered by barium citrate adsorption, as natural PZ itself. This system enabled us to estimate the ratio of carboxylated PZ secreted into the medium and, more importantly, to compare its mode of secretion with that of FX under uniform conditions; this constitutes a powerful tool for the analysis of protein secretion, including the identification of functional residues and domains, the characterization of disease-related mutations, and so on.
Unique secretion mode of PZ
Vit.K is required for the conversion of glutamates to Gla residues in Vit.K-dependent proteins.6-8 We show in the present study that Vit.K is also required for the secretion of PZ but not for FX. Plasma concentrations of PZ vary widely among subjects.15 Concentrations of Vit.K in plasma, on the other hand, are influenced by dietary intake and vary among subjects24 ; PZ levels in plasma may be partly associated with dietary intake and plasma concentrations of Vit.K, although this idea remains unproven. We also demonstrated that PZ secretion is sensitive to warfarin, while FX secretion is resistant to warfarin's influence. Warfarin treatment drastically decreases plasma concentrations of PZ in vivo (1% to ∼16% of normal),15 which plausibly reflects the marked suppression of its secretion, at least in part, as observed in our in vitro studies.
Accordingly, γ-carboxylation is essential for the secretion of PZ, because practically all PZ secreted into the medium was recovered by barium citrate adsorption (ie, γ-carboxylated). In addition, using an established cell line stably expressing PZ, we previously demonstrated that approximately 30% of the synthesized PZ converted to a Gla form inside cells before secretion, and most of it was then released into the medium.18
These findings indicate that secretory processing is not necessarily the same for all Vit.K-dependent proteins and that the mode of secretion of PZ is unique. The amino acid sequence of PZ, which is substantially different from other Vit.K-dependent proteins,2 must be responsible for the differential secretion.
Common γ-carboxylation–dependent secretory mode of PZ and FX
While PZ and FX secretion showed differences both in efficiency and Vit.K dependency/warfarin sensitivity, the cotransfection of full-length PZ with FX suppressed the secretion of each of these proteins, implying that they shared and/or competed a common machinery for secretion. Expression of various chimeric proteins (eg, PZ and FX containing an exchanged PP and/or a Gla domain, and MetLuc fused with combinations of a PP and a Gla domain) clearly indicated that the Gla domain of PZ is responsible for its inefficient and warfarin-sensitive secretion.
In addition, FX secretion in the presence of Vit.K was significantly inhibited by mini-constructs having the PZ-Gla domain, suggesting that the Gla domain of PZ takes dominance over that of FX in interacting with the common secretion machinery. Furthermore, mini-constructs having the FX-PP strongly inhibited the PZ secretion, suggesting that there is a contribution of the FX-PP to the interaction with the common secretion machinery.
The secretion of PZ was exclusively γ-carboxylation–dependent, and not only the secretion but also the γ-carboxylation of both PZ and FX was inhibited by mini-constructs of their PP and Gla domains. Accordingly, the common secretory machinery for these Vit.K-dependent proteins must be closely related to or directly involved in the γ-carboxylation system; the most plausible candidate for this machinery is GCX itself, since GCX directly interacts with both the PPs and Gla domains of Vit.K-dependent proteins by forming an enzyme-substrate complex.10,11 It is also reported that the Gla domain of FIX contributes allosterically to the interaction between its PP and GCX.25
The affinities of PPs for GCX vary among Vit.K-dependent proteins by more than 100-fold, and the PP of FX has the highest affinity to GCX of all, at least in vitro.26 The inhibition of PZ secretion by mini-constructs having the PP of FX may have been due to the more dominant interaction of the FX-PP with the PP-binding site of GCX over PZ, by competing the binding of the PZ-PP to GCX. In fact, PZ binds relatively tightly to GCX but not as tight as FX (Prof D. Stafford, School of Medicine, University of North Carolina, e-mail communication, July 17, 2007).
The moderate suppression of FX secretion observed with mini-constructs containing the Gla domain of PZ is likely to have been caused by competition of the Gla domain-binding to GCX. The Gla domain of PZ may form a stable complex with and remain bound to GCX even after its γ-carboxylation is completed; and/or the Gla domain of FX may be released from GCX more readily despite its being poorly γ-carboxylated, especially in the presence of a protein substrate containing PZ's Gla domain. Alternatively, PZ may tightly bind to GCX via a second site of the Vit.K-dependent protein-GCX interaction of unknown identity.19
However, the mini-constructs containing the Gla domain of PZ and the PP of FX also diminished the warfarin-resistant secretion of FX; thus, at this writing, it is uncertain whether or not GCX is truly involved in a common secretory mechanism for PZ and FX. It also remains distinctly possible that unidentified molecular chaperons may participate in the secretion of Vit.K-dependent proteins.
γ-carboxylation–independent secretory mode of FX
In contrast to PZ, it would appear that FX does not always require γ-carboxylation for secretion, because a large amount of FX appeared in the medium even in the presence of as high as 10 μg/mL warfarin, and more than 80% of it was not adsorbed to barium citrate (ie, not γ-carboxylated). These results are consistent with an earlier study by Camire et al, in which 2 pools of FX were secreted, one being completely uncarboxylated and the other being fully γ-carboxylated.27 This is also true for FIX, which is secreted essentially at the same rate both in the absence and presence of Vit.K.28 Hallgren et al also showed that carboxylation of FIX is not obligatory for secretion.29,30 In addition, the secretion of various Vit.K-dependent proteins without γ-carboxylation has been reported in vivo in population studies of human plasma under warfarin treatment,15,31-33 as well as in vitro in expression studies on prothrombin, FVII, FIX, FX, protein S, etc, in the presence of warfarin.34-36
It remains unexplained why a considerable amount of FX, as well as FIX, can be secreted without carboxylation. Uncarboxylated FX may readily dissociate from GCX and/or bypass the carboxylation system in the absence of reduced Vit.K and transfer to the conventional/classical secretory pathway during its exit from the endoplasmic reticulum (ER) to the Golgi apparatus. Thus, it is possible that FX, by nature, can be secreted regardless of carboxylation, while uncarboxylated PZ is retained inside the ER,18 where the carboxylation reaction occurs.37 For PZ, only carboxylated molecules may be transported out of the ER.
PP cleavage of PZ
A PP in Vit.K-dependent proteins is removed inside cells before secretion. Mutations within a PP lead to a marked reduction in or elimination of γ-carboxylation but no change in the secretion of Vit.K-dependent proteins.33-35,38,39 PZPPmut, having mutations at the PP cleavage site, was able to be γ-carboxylated and secreted into the medium only slightly less efficiently than the wild-type PZ. Accordingly, PP cleavage is partly involved in, but not essential for, the secretion of PZ.
In conclusion, the secretion of PZ is Vit.K-dependent and warfarin-sensitive, and these features are determined by the protein's PP and Gla domain. GCX is a possible candidate for the secretory mechanism for PZ. Direct evidence for the interaction of PZ with GCX has not been reported yet, underscoring the need for further research into the effect of GCX on the secretion of PZ and other Vit.K-dependent proteins.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are greatly indebted to Drs H. Sugawara of Yamagata University and F. Tokunaga of Osaka Municipal University for invaluable discussion; to Prof D. Stafford of the University of North Carolina for personal communication regarding the affinity of PZ to GCX; to Dr M. Adam of Servio (France) for providing an anti–human protein Z antibody; and to Mrs L. B. Joshi for her assistance in preparation of the manuscript.
This study was supported in part by research grants from the Japanese Society for the Promotion of Sciences and the Naito Foundation (Japan).
Authorship
Contribution: M.S. performed research, analyzed data, and wrote the paper; H.I. and W.G.Z. performed research; and A.I. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Akitada Ichinose, Department of Molecular Patho-Biochemistry and Patho-Biology, Yamagata University School of Medicine, Iida-Nishi 2-2-2, Yamagata 990-9585 Japan; e-mail: aichinos@med.id.yamagata-u.ac.jp.

![Figure 1. Construction and secretion of PZ fused with MetLuc from HEK 293 cells. (A) MetLuc cDNA encodes a 219-amino acid polypeptide containing an amino-terminal signal peptide of 17 amino acid residues for secretion (dark lines left panel). PZ cDNA was fused with MetLuc cDNA with (PZMetLuc(+Sig)) or without (PZMetLuc) the signal peptide of MetLuc. Expression vectors were transfected to HEK 293 cells with pGL3-control, and the cells were incubated without (□) or with (■) Vit.K for 24 hours. MetLuc activity in the culture medium was measured. The data were normalized to the firefly luciferase activity in cell lysates (relative light unit [RLU] against pGL3-control) and were shown as the mean (± SD) for 3 independent experiments (right panel). The numbers depict the residue numbers of the first and last amino acid residues of regions or domains in PZ (in parentheses) or MetLuc (without parentheses). PP, Gla, and PZC stand for the propeptide, Gla domain, and C-terminal region of PZ, respectively. (B) PZ in the medium was collected by barium citrate adsorption and subjected to SDS-PAGE under reducing conditions, followed by Western blot analysis using an anti-PZ antibody. The position of human PZ is indicated by an arrow; molecular weight standards are shown by numbers with kilodaltons (kDa).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/16/10.1182_blood-2008-07-171884/7/m_zh80170934010001.jpeg?Expires=1765893747&Signature=Q293Bs2~LcxqH4rjBOM2ELCyhJnkxWktXQnMTqebf7D1gufuJblgnvZviZl091zRwd4SBN2O2GaLe2KILyHvfhS4s79VCymItA-xxMUIvowj2-MuBuzvcy5CBM~3FNsGA7hFO8IRR7Tk5~zjDcK6Par-snLGmuR5Hs5koZdNfbi2nb6-PcllnnclAYwugUEai6CXb-CwVD4CUm8ouGD6NM0N4aPHujoUU2Tui1zPs8LH7NrZwv-4JTwFWHTSILcH7kksMU2N7rcGK1xs9W2zSNbC7Z3KEXYUQjf5GCItbpsuMxUT-K3LgDFpfp6SOHrukyZwRyh6LnVE7A-gxLCOCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Pulse-chase analysis of PZ and FX secretion. HEK 293 cells transiently transfected with expression vectors were divided into 5 dishes and were labeled with a 50 μCi [35S]protein labeling mixture for 1 hour. The chase was then initiated by changing the medium containing Vit.K, and the samples were incubated for 1, 2, 4, and 8 hours. PZ (A) or FX (B) in the medium (●) and in the cell lysates (○) were collected by immunoprecipitation using an anti-PZ or anti-FX antibody. Their intensity under fluorography was calculated by setting the signal in the cell lysates at chase-time 0 to 100%. The data shown are the mean (± SD) for 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/16/10.1182_blood-2008-07-171884/7/m_zh80170934010003.jpeg?Expires=1765893747&Signature=q0IWjspDRc2P-h4vdGdXrzwDHf~i1fgNwxvCeY9wDqF~nF3DZZCg9nzN1mN2R6Bh58hgufgbwfuTTnT7wKPUoDgk-QHZdILU8qVr5WepgI0TzGDJWONYYLmWrEFtdnvpeVRuFLPbU0E8wvWhm2eXK799RxwU00Xm~UOTm3UWXQkWzd5o4ZhxmEa4RK3axuyrO5dtaj8-9aWvwHI6h3tvCrUwApzU4XBN~fS~xqfrkfSaGZN1XXqw73p1eVveKMCvcbhwbkeSRoneyh6Nv5xGG97m2sSmB-phwog-hoZE35mWUC-lChd5dXdihQIKO4Pi-KFZh16kKYX~sJ1-2cyJKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal