Abstract
Although chronic graft-versus-host disease (cGVHD) is a major long-term complication of allogeneic hematopoietic stem cell transplantation, little is known of its pathogenesis. We have systematically examined oral mucosa among cGVHD patients and determined that the clinical severity of oral cGVHD was correlated with apoptotic epithelial cells, often found adjacent to infiltrating effector-memory T cells expressing markers of cytotoxicity and type I cytokine polarization. Accumulation of T-bet+ T-cell effectors was associated with both increased proliferation and the expression of the type I chemokine receptor CXCR3. Concurrently, in both infiltrating cells and keratinocytes, we observed increased expression of the CXCR3 ligand MIG (CXCL9) and interleukin-15 (IL-15), type I interferon (IFN)–inducible factors that support the migration, type I differentiation, and expansion of alloreactive effectors. In severely affected mucosa, we observed high levels of MxA, a protein specifically induced by type I IFN, and signal transducer and activator of transcription 1 (STAT1) phosphorylation, a critical step in the IFN-signaling pathway, along with increased numbers of plasmacytoid dendritic cells. These data challenge the current paradigm of cGVHD as a type II cytokine–driven disorder and support the model that oral cGVHD results from type I IFN–driven immigration, proliferation, and differentiation of T-bet+ type I T effectors. The clinical trials are registered at http://www.clinicaltrials.gov as NCT00331968.
Introduction
Chronic graft-versus-host disease (cGVHD) is the most important long-term complication of allogeneic hematopoietic stem cell transplantation,1 yet its pathogenesis remains poorly understood. The highly heterogeneous clinical manifestations of cGVHD and the lack of animal models adequately replicating human disease have complicated analysis.2 Although it is generally recognized to be a T cell–mediated disorder, controversies have persisted regarding the specific pathways involved. Acute GVHD has long been demonstrated to be dependent upon type I cytokine–driven CD8 effectors, whereas cGVHD has been associated with type II CD4 T cells.3 Yet evidence of Th2 involvement is limited and, depending upon the animal model used, all 3 major subtypes of CD4 cells (Th1, Th2, and Th17) have been implicated in cGVHD.3-5 Furthermore, very few studies have focused on the events occurring in the target tissues of cGVHD in humans. Retrospective case studies of biopsies performed for clinical indications have been accompanied by minimal patient information and have relied for controls on tissues from healthy donors, not non-GVHD transplant recipients.6,7 The limited understanding of disease pathogenesis has restricted the development of new therapies for cGVHD. Current treatment of cGVHD continues to focus on nonspecific immunosuppression, resulting in exacerbation of immune deficits, infectious complications, and tumor relapse.1
Clinical presentation of cGVHD is highly variable but commonly includes sclerotic or lichenoid skin involvement, oral and genital mucosal lesions, and salivary and lacrimal gland damage resulting in xerostomia and xerophthalmia, respectively. Importantly, many of these manifestations closely resemble classic autoimmune conditions such as scleroderma, lichen planus, and Sjogren syndrome.1 Involvement of the oral cavity is second only to skin in frequency, occurring in the majority of cGVHD patients.8 Lichen planus–like oral mucosal lesions are considered to be highly specific for cGVHD.9 Oral cGVHD presents a significant burden to the patients leading to pain, food intolerance, change in taste, xerostomia, and ultimately decreased quality of life.10 In addition, the risk of secondary squamous cell carcinoma is greatly increased in those affected by cGVHD.11
For these reasons, we analyzed the pathologic changes in oral mucosa in patients enrolled on a cross-sectional natural history protocol of cGVHD; patients were recruited into the study at a broad range of times after transplantation and development of cGVHD. As part of a multidisciplinary evaluation of cGVHD staging,9 biopsies were collected from the buccal mucosa in patients with both lichen planus–like and more severe ulcerative oral cGVHD. These tissues were then examined by confocal microscopy and polymerase chain reaction (PCR) analysis to characterize infiltrating immune cell populations. To reduce confounding factors, such as effects of conditioning and immunosuppressive treatment, we included biopsies from cGVHD patients lacking clinical involvement of the oral cavity as controls.
We report here that severe oral cGVHD was primarily associated with infiltrating effector-memory T cells expressing T-bet, a transcription factor marking type I cytokine polarization (type I helper T cell/type I cytotoxic T cell [Th1/Tc1]). Increased epithelial apoptosis, the distinctive feature of active oral cGVHD, was associated with an increase in TIA+granzyme B+ cytotoxic CD8 cells. Furthermore, the selective accumulation of T-cell effectors was associated with both increased proliferation and the expression of the chemokine receptor CXCR3. Concurrently, we observed increased expression of interferon (IFN)–induced factors including the CXCR3 ligand MIG (CXCL9) and interleukin-15 (IL-15) in both infiltrating cells and keratinocytes in the affected tissues; these factors support the migration, Th1/Tc1 differentiation, and expansion of the T effectors. Furthermore, up-regulation of the type I IFN–specific gene MxA in the mucosal epithelium and the presence of plasmacytoid dendritic cells (pDCs), the main producer of type I IFNs, in the infiltrate are consistent with the production of type I IFN in affected tissues. These data support a model that oral cGVHD results from the interaction of IFN-driven inflammatory processes and Th1/Tc1-differentiated effectors.
Methods
Patient population
We analyzed samples from 37 patients enrolled in a natural history study of chronic GVHD (http://www.clinicaltrials.gov no. NCT00331968). The study was approved by the Institutional Review Board of the National Cancer Institute and informed consent was obtained from all participants in accordance with the Declaration of Helsinki. Patients underwent comprehensive initial evaluation including detailed history, clinical examination, laboratory tests, and collection of research samples. Demographic and transplant characteristics of patients are represented in Tables 1 and 2. Oral cGVHD was evaluated and graded by an oral medicine specialist using the following severity scoring system: normal mucosa—no oral CGVHD; lichenoid changes with or without erythema—mild oral CGVHD; and lichenoid changes and presence of ulcerations—severe oral CGVHD. Images representative of the spectrum of clinical changes are presented in Figure 1. For patients completing the full NIH cGVHD assessment, an NIH Global cGVHD Severity Score (mild, moderate, or severe) was generated based upon a detailed multidisciplinary clinical and diagnostic evaluation of 8 organ systems, including the skin, mouth, eyes, liver, lungs, genital tract, joint fascia, and gastrointestinal systems.9 In Table 2, the global CGVHD severity and the number of organ systems involved are listed. Six patients enrolled in the cGVHD natural history study, who had no clinical evidence of oral cGVHD at the time of the evaluation (independent of mild to severe overall cGVHD based on involvement of other organ systems), served as oral cGVHD controls. In addition, biopsies were assessed from 1 long-term transplant recipient who did not develop cGVHD and from 2 patients in a separate longitudinal study before onset of any cGVHD symptoms; the latter 2 provided posttransplantation time points comparable with those developing severe oral cGVHD by 6 months after transplantation.
Demographic characteristics of patients
| No. . | Age . | Race . | Sex . | Diagnosis . | Conditioning . | Donor . | Stem cell source . |
|---|---|---|---|---|---|---|---|
| 1 | 53 | White | F | AA | ATG-Flu-Cy | Sibling | PB |
| 2 | 50 | White | F | MM | Flu-Cy | Sibling | PB |
| 3 | 47 | White | M | NHL | Flu-Cy | Sibling | PB |
| 4 | 22 | White | M | TLL | Cy-TBI | MUD | BM |
| 5 | 57 | White | M | MDS | Bu-Cy | Sibling | PB |
| 6 | 40 | White | M | AML | Bu-VP16-TBI | Sibling | PB |
| 7 | 56 | White | M | AML | Bu-Cy | MUD | PB |
| 8 | 51 | Black | M | CML | Bu-Flu-ATG | Sibling | PB |
| 9 | 67 | White | M | TLL | Flu-Cy | Sibling | PB |
| 10 | 45 | White | F | AML | Flu-Cy | Sibling | PB |
| 11 | 58 | White | M | NHL | Flu-Cy | Sibling | PB |
| 12 | 42 | White | M | NHL | Flu-Cy | Sibling | PB |
| 13 | 41 | White | M | CML | Cy-TBI | MUD | BM |
| 14 | 44 | White | M | ALL | Cy-TBI | MUD | PB |
| 15 | 39 | White | F | NHL | Flu-Cy | Sibling | PB |
| 16 | 44 | White | F | CML | MTX-Cy-AraC-TBI | Sibling | BM |
| 17 | 42 | Hispanic | M | CML | Bu-Cy | Sibling | BM |
| 18 | 59 | White | M | MF | Flu-Cy | Sibling | PB |
| 19 | 36 | White | F | CML | Bu-Cy | MUD | PB |
| 20 | 36 | White | F | HD | Flu-Cy | Sibling | PB |
| 21 | 53 | White | M | CML | Cy-TBI | Haplo | BM |
| 22 | 55 | White | M | MM | Flu-Cy | Sibling | PB |
| 23 | 39 | White | F | TLL | Cy-TBI | Sibling | PB |
| 24 | 52 | White | F | AML | Cy-TBI | Sibling | PB |
| 25 | 58 | White | M | CML | HU-Bu-Cy | Sibling | PB |
| 26 | 45 | White | M | TLL | Cy-TBI | Sibling | PB |
| 27 | 60 | White | F | NHL | Unknown | Haplo | PB |
| 28 | 33 | White | M | HD | Flu-Cy | Sibling | PB |
| 29 | 56 | White | M | MM | Flu-Cy | Sibling | PB |
| 30 | 61 | White | F | NHL | Flu-Cy | Sibling | PB |
| 31 | 39 | Hispanic | M | MDS | Bu-Cy | Sibling | PB |
| 32 | 41 | Black | F | CML | Flu-Cy | Sibling | PB |
| 33 | 49 | White | F | CML | Bu-Cy | Sibling | PB |
| 34 | 40 | White | F | AML | Bu-Flu | Sibling | PB |
| 35 | 58 | Hispanic | F | MM | Flu-Cy | Sibling | PB |
| 36 | 52 | White | M | NHL | Flu-Cy | Sibling | PB |
| 37 | 47 | Hispanic | M | MDS | Flu-Cy | Sibling | PB |
| No. . | Age . | Race . | Sex . | Diagnosis . | Conditioning . | Donor . | Stem cell source . |
|---|---|---|---|---|---|---|---|
| 1 | 53 | White | F | AA | ATG-Flu-Cy | Sibling | PB |
| 2 | 50 | White | F | MM | Flu-Cy | Sibling | PB |
| 3 | 47 | White | M | NHL | Flu-Cy | Sibling | PB |
| 4 | 22 | White | M | TLL | Cy-TBI | MUD | BM |
| 5 | 57 | White | M | MDS | Bu-Cy | Sibling | PB |
| 6 | 40 | White | M | AML | Bu-VP16-TBI | Sibling | PB |
| 7 | 56 | White | M | AML | Bu-Cy | MUD | PB |
| 8 | 51 | Black | M | CML | Bu-Flu-ATG | Sibling | PB |
| 9 | 67 | White | M | TLL | Flu-Cy | Sibling | PB |
| 10 | 45 | White | F | AML | Flu-Cy | Sibling | PB |
| 11 | 58 | White | M | NHL | Flu-Cy | Sibling | PB |
| 12 | 42 | White | M | NHL | Flu-Cy | Sibling | PB |
| 13 | 41 | White | M | CML | Cy-TBI | MUD | BM |
| 14 | 44 | White | M | ALL | Cy-TBI | MUD | PB |
| 15 | 39 | White | F | NHL | Flu-Cy | Sibling | PB |
| 16 | 44 | White | F | CML | MTX-Cy-AraC-TBI | Sibling | BM |
| 17 | 42 | Hispanic | M | CML | Bu-Cy | Sibling | BM |
| 18 | 59 | White | M | MF | Flu-Cy | Sibling | PB |
| 19 | 36 | White | F | CML | Bu-Cy | MUD | PB |
| 20 | 36 | White | F | HD | Flu-Cy | Sibling | PB |
| 21 | 53 | White | M | CML | Cy-TBI | Haplo | BM |
| 22 | 55 | White | M | MM | Flu-Cy | Sibling | PB |
| 23 | 39 | White | F | TLL | Cy-TBI | Sibling | PB |
| 24 | 52 | White | F | AML | Cy-TBI | Sibling | PB |
| 25 | 58 | White | M | CML | HU-Bu-Cy | Sibling | PB |
| 26 | 45 | White | M | TLL | Cy-TBI | Sibling | PB |
| 27 | 60 | White | F | NHL | Unknown | Haplo | PB |
| 28 | 33 | White | M | HD | Flu-Cy | Sibling | PB |
| 29 | 56 | White | M | MM | Flu-Cy | Sibling | PB |
| 30 | 61 | White | F | NHL | Flu-Cy | Sibling | PB |
| 31 | 39 | Hispanic | M | MDS | Bu-Cy | Sibling | PB |
| 32 | 41 | Black | F | CML | Flu-Cy | Sibling | PB |
| 33 | 49 | White | F | CML | Bu-Cy | Sibling | PB |
| 34 | 40 | White | F | AML | Bu-Flu | Sibling | PB |
| 35 | 58 | Hispanic | F | MM | Flu-Cy | Sibling | PB |
| 36 | 52 | White | M | NHL | Flu-Cy | Sibling | PB |
| 37 | 47 | Hispanic | M | MDS | Flu-Cy | Sibling | PB |
AA indicates aplastic anemia; aGVHD, acute graft-versus-host disease; ALL, acute lymphocytic leukemia; AML, acute myelogenous leukemia; ATG, anti-thymocyte globulin; BM, bone marrow; Bu, busulfan; CML, chronic myelogenous leukemia; Cy, cyclophosphamide; Flu, fludarabine; HD, Hodgkin lymphoma; MDS, myelodysplastic syndrome; MM, multiple myeloma; MF, myelofibrosis; NHL, non-Hodgkin lymphoma; PB, peripheral blood; TBI, total body irradiation; and TLL, T-cell leukemia/lymphoma.
Clinical characteristics of patients
| No. . | aGVHD prophylaxis . | Oral CGVHD severity . | NIH Global CGVHD Severity Score* . | Number of organ systems affected . | cGVHD onset . | Months after HSCT . | Systemic Immunosuppression at the time of evaluation . |
|---|---|---|---|---|---|---|---|
| 1 | CSA-MTX | Severe | Severe | 4 | Progressive | 13 | Prednisone, TL, MMF, ECP |
| 2 | CSA-MTX | Severe | Moderate | 4 | De novo | 9 | Prednisone, CSA |
| 3 | CSA-Sirolimus | Severe | Moderate | 3 | De novo | 6 | Prednisone |
| 4 | TL-MTX | Severe | Moderate | 4 | Progressive | 9 | Prednisone, TL, MMF |
| 5 | TL-MTX | Severe | Severe | 5 | Progressive | 46 | Prednisone, HCQ, TL, MMF, ECP |
| 6 | CSA-MMF | Severe | Severe | 4 | Progressive | 55 | Prednisone, MMF, SL, rituximab |
| 7 | CSA | Severe | Moderate | 4 | Progressive | 28 | Prednisone, TL, MMF |
| 8 | CSA | Severe | Severe | 6 | Quiescent | 64 | Hydrocortisone, CSA, MMF |
| 9 | CSA-Sirolimus | Severe | na | na | De novo | 6 | None |
| 10 | CSA-Sirolimus | Severe | Moderate | 6 | De novo | 6 | CSA |
| 11 | CSA-MTX | Severe | Moderate | 2 | Progressive | 5 | None |
| 12 | CSA-MTX | Severe | na | na | Quiescent | 7 | None |
| 13 | CSA-MTX | Mild | Severe | 5 | Quiescent | 147 | None |
| 14 | CSA-MTX | Mild | Moderate | 3 | Progressive | 33 | CSA, MMF |
| 15 | CSA | Mild | Severe | 5 | Progressive | 58 | Prednisone, TL, HCQ |
| 16 | CSA-MTX | Mild | Severe | 7 | Quiescent | 205 | MMF, CSA, ECP |
| 17 | CSA-MTX | Mild | Moderate | 3 | Quiescent | 100 | Prednisone |
| 18 | CSA-MTX | Mild | Severe | 4 | De novo | 22 | Prednisone, TL |
| 19 | ATG-unknown | Mild | Moderate | 5 | De novo | 41 | MMF |
| 20 | CSA-MTX | Mild | na | na | Quiescent | 29 | None |
| 21 | CSA-MTX | Mild | Severe | 3 | Progressive | 159 | Prednisone, CSA |
| 22 | CSA-MTX | Mild | Moderate | 6 | Progressive | 5 | Prednisone, CSA |
| 23 | CSA | Mild | Severe | 6 | Progressive | 55 | TL, MMF, ECP |
| 24 | TL-MMF | Mild | Moderate | 6 | Progressive | 12 | CSA |
| 25 | TL | Mild | Severe | 3 | Quiescent | 29 | None |
| 26 | CSA-MTX | Mild | na | na | De novo | 27 | Prednisone |
| 27 | Unknown | Mild | Moderate | 6 | Quiescent | 43 | Methylprednisolone, TL, MMF |
| 28 | CSA | Mild | na | na | De novo | 35 | Prednisone |
| 29 | CSA-MTX | None | Moderate | 3 | De-novo | 27 | None |
| 30 | CSA | None | Mild | 2 | Progressive | 39 | None |
| 31 | Unknown | None | Severe | 6 | Progressive | 59 | TL, MMF |
| 32 | CSA-Sirolimus | None | Mild | 2 | De novo | 12 | Prednisone, MMF, CSA |
| 33 | TL-MTX | None | Moderate | 3 | De novo | 28 | None |
| 34 | CSA-MMF | None | Severe | 6 | Quiescent | 22 | Hydrocortisone, CSA |
| 35 | CSA-MTX | None | No cGVHD | 0 | No cGVHD | 3 | CSA |
| 36 | CSA-MTX | None | No cGVHD | 0 | No cGVHD | 73 | None |
| 37 | CSA-MTX | None | No cGVHD | 0 | No cGVHD | 6 | CSA |
| No. . | aGVHD prophylaxis . | Oral CGVHD severity . | NIH Global CGVHD Severity Score* . | Number of organ systems affected . | cGVHD onset . | Months after HSCT . | Systemic Immunosuppression at the time of evaluation . |
|---|---|---|---|---|---|---|---|
| 1 | CSA-MTX | Severe | Severe | 4 | Progressive | 13 | Prednisone, TL, MMF, ECP |
| 2 | CSA-MTX | Severe | Moderate | 4 | De novo | 9 | Prednisone, CSA |
| 3 | CSA-Sirolimus | Severe | Moderate | 3 | De novo | 6 | Prednisone |
| 4 | TL-MTX | Severe | Moderate | 4 | Progressive | 9 | Prednisone, TL, MMF |
| 5 | TL-MTX | Severe | Severe | 5 | Progressive | 46 | Prednisone, HCQ, TL, MMF, ECP |
| 6 | CSA-MMF | Severe | Severe | 4 | Progressive | 55 | Prednisone, MMF, SL, rituximab |
| 7 | CSA | Severe | Moderate | 4 | Progressive | 28 | Prednisone, TL, MMF |
| 8 | CSA | Severe | Severe | 6 | Quiescent | 64 | Hydrocortisone, CSA, MMF |
| 9 | CSA-Sirolimus | Severe | na | na | De novo | 6 | None |
| 10 | CSA-Sirolimus | Severe | Moderate | 6 | De novo | 6 | CSA |
| 11 | CSA-MTX | Severe | Moderate | 2 | Progressive | 5 | None |
| 12 | CSA-MTX | Severe | na | na | Quiescent | 7 | None |
| 13 | CSA-MTX | Mild | Severe | 5 | Quiescent | 147 | None |
| 14 | CSA-MTX | Mild | Moderate | 3 | Progressive | 33 | CSA, MMF |
| 15 | CSA | Mild | Severe | 5 | Progressive | 58 | Prednisone, TL, HCQ |
| 16 | CSA-MTX | Mild | Severe | 7 | Quiescent | 205 | MMF, CSA, ECP |
| 17 | CSA-MTX | Mild | Moderate | 3 | Quiescent | 100 | Prednisone |
| 18 | CSA-MTX | Mild | Severe | 4 | De novo | 22 | Prednisone, TL |
| 19 | ATG-unknown | Mild | Moderate | 5 | De novo | 41 | MMF |
| 20 | CSA-MTX | Mild | na | na | Quiescent | 29 | None |
| 21 | CSA-MTX | Mild | Severe | 3 | Progressive | 159 | Prednisone, CSA |
| 22 | CSA-MTX | Mild | Moderate | 6 | Progressive | 5 | Prednisone, CSA |
| 23 | CSA | Mild | Severe | 6 | Progressive | 55 | TL, MMF, ECP |
| 24 | TL-MMF | Mild | Moderate | 6 | Progressive | 12 | CSA |
| 25 | TL | Mild | Severe | 3 | Quiescent | 29 | None |
| 26 | CSA-MTX | Mild | na | na | De novo | 27 | Prednisone |
| 27 | Unknown | Mild | Moderate | 6 | Quiescent | 43 | Methylprednisolone, TL, MMF |
| 28 | CSA | Mild | na | na | De novo | 35 | Prednisone |
| 29 | CSA-MTX | None | Moderate | 3 | De-novo | 27 | None |
| 30 | CSA | None | Mild | 2 | Progressive | 39 | None |
| 31 | Unknown | None | Severe | 6 | Progressive | 59 | TL, MMF |
| 32 | CSA-Sirolimus | None | Mild | 2 | De novo | 12 | Prednisone, MMF, CSA |
| 33 | TL-MTX | None | Moderate | 3 | De novo | 28 | None |
| 34 | CSA-MMF | None | Severe | 6 | Quiescent | 22 | Hydrocortisone, CSA |
| 35 | CSA-MTX | None | No cGVHD | 0 | No cGVHD | 3 | CSA |
| 36 | CSA-MTX | None | No cGVHD | 0 | No cGVHD | 73 | None |
| 37 | CSA-MTX | None | No cGVHD | 0 | No cGVHD | 6 | CSA |
aGVHD indicates acute graft-versus-host disease; ATG, anti-thymocyte globulin; CSA, cyclosporine; ECP, extracorporeal photopheresis; HCQ, hydroxychloroquine; HSCT, hematopoietic stem cell transplant; MMF, mycophenolate mofetil; MTX, methotrexate; na, not available; SL, Sirolimus; and TL, tacrolimus.
Scoring based upon the National Institutes of Health (NIH) Consensus Development Project on criteria for clinical trials in cGVHD.9
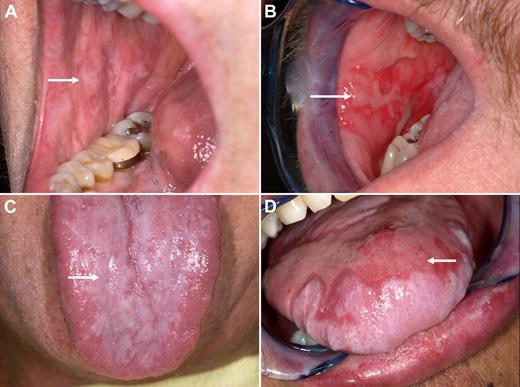
Clinical spectrum of oral GVHD, ranging from mild, predominantly lichenoid lesions to more severe, ulcerative lesions. (A) Lichenoid changes of the buccal mucosa. Note white striations (arrow). (B) Severe cGVHD of the buccal mucosa. Note pronounced erythema and the presence of ulcerations (arrow). (C,D) Mild and severe changes of the tongue.
Clinical spectrum of oral GVHD, ranging from mild, predominantly lichenoid lesions to more severe, ulcerative lesions. (A) Lichenoid changes of the buccal mucosa. Note white striations (arrow). (B) Severe cGVHD of the buccal mucosa. Note pronounced erythema and the presence of ulcerations (arrow). (C,D) Mild and severe changes of the tongue.
Six-millimeter punch biopsies were then performed from the buccal mucosa of consenting patients at the time of the comprehensive evaluation and scoring. For immunofluorescent staining, samples were fixed in 10% neutral-buffered formalin and embedded in paraffin. In a subset of 21 patients, biopsy tissue was split and half was immediately frozen in dry ice-isopentane slurry for PCR analysis. Frozen samples were stored at − 80°C until further analysis.
Immunofluorescent staining
Five-micrometer sections were prepared from formalin-fixed paraffin-embedded samples. After deparaffinization and rehydration high-temperature antigen retrieval was performed in 0.01 M ethylenediamine-tetraacetic acid (EDTA) solution (pH 8.0) in a pressure cooker. After blocking with 10% normal serum from the secondary antibody species, the primary antibodies were applied and incubated for 2 hours at room temperature or overnight at 4°C. The antibodies to the following antigens were used: CD45, clone 2B11/PD7/26; CD3, clone F7.2.38; CD3, rabbit polyclonal; CD8, clone C8/144B; granzyme B, clone GrB-7; CD68, clone PG-M1; CD20, clone L26 (all from Dako, Carpinteria, CA); active (cleaved) caspase-3, rabbit polyclonal; phospho–signal transducer and activator of transcription-1 (STAT1), clone 58D6 (all from Cell Signaling Technology, Danvers, MA); Ki-67, clone B56; CD45RO, clone UCHL1; CD45RA, clone HI100 (all from BD Biosciences, San Diego, CA); CD8, clone SP16; CD163, clone 10D6 (all from Labvision, Fremont, CA); HLA-DR, clone LN-3 (Invitrogen, Carlsbad, CA); IL-15, clone 34505; MIG, clone 49106 (all from R&D Systems, Minneapolis, MN); pancytokeratin, clone AE1/AE3 (AbD Serotec, Raleigh, NC); CD3, clone PS1 (Vision Biosystems, Norwell MA); Tia-1, clone 2G9A10F5 (Beckman Coulter, Miami, FL); T-bet, clone 4B10 (eBioscience, San Diego, CA); and CD2ap, rabbit polyclonal (Sigma-Aldrich, St Louis, MO). Mouse monoclonal antibody against MxA, clone M143, was kindly provided by Dr Otto Haller (University of Freiburg, Freiburg, Germany).12 Alexa Fluor 488–, 555–, 568–, 594–, 633–, 647–, and 680–labeled donkey and goat secondary antibodies (Invitrogen) were used for detection. CD4 T cells were identified as CD3+CD8−. Mounting medium with 4′,6′-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) or DRAQ5 (Biostatus, Shepshed, United Kingdom) was used for nuclear counterstaining.
Confocal laser-scanning microscopy and image analysis
Images were acquired on a Leica SP2 confocal system (Leica Microsystems, Bannockburn, IL) equipped with AOBS (Acousto Optical Beam Splitter) and a Leica DM RE-7 upright microscope using a 40×/1.25 NA objective. To get an estimate of cell populations in each section, 5 to 10 adjacent nonoverlapping images were obtained centering on the basement membrane region. Immune cell populations expressing markers of interest were then quantified visually or in software-assisted manner, using Photoshop CS (Adobe, San Jose, CA) or Volocity 4.2 (Improvision, Waltham, MA), respectively. CD68 cells were quantified by total area of positive pixels in the image. MxA expression was quantified by mean pixel intensity throughout the image.
Quantitative real-time PCR
Frozen samples of oral mucosa were homogenized and total RNA extracted using RNA Stat60 according to the manufacturer's protocol (Tel-Test, Friendswood, TX). One round of RNA amplification was performed on 500 ng total RNA using the MessageAmp aRNA Kit (Ambion, Austin, TX) according to the manufacturer's protocol. Synthesis of cDNA was performed using 1.5 μg aRNA and the Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science, Indianapolis, IN), also according to the manufacturer's protocol with the following modification: a mixture of oligo dT and random hexamers was used in the reaction. All PCR assays were designed using Roche Applied Science's Universal Probe Library Design Center to be intron spanning. The following primer-probe combinations and were used: t-bet (NM_013351.1): forward tgtggtccaagtttaatcagca and reverse tgacaggaatgggaacatcc (probe no. 9); IL-15 (NM_000585.2): forward caaacaacagtttgtcttctaatgg and reverse gacaatatgtacaaaactctgcaaaaa, (probe no. 65).
Amplification was performed on the LightCycler 2.0 real-time PCR instrument (Roche Applied Science). Reaction conditions were as follows: 20-μL reactions with a final concentration of 0.2 μM each primer and 0.1 μM Universal Probe in 1× LightCycler TaqMan Master Mix, 5 μL cDNA. Cycling conditions were as follows: 95°C for 10 minutes, 45 cycles of 95°C for 15 seconds, and 60°C for 30 seconds. Gene expression was analyzed using LightCycler Software v.4.0.5. Standard curves were generated using dilutions of cDNA synthesized from total RNA extracted from peripheral blood mononuclear cells (PBMCs) stimulated in culture with phorbol myristate acetate (PMA). Undiluted cDNA was assigned an arbitrary concentration number to generate standard curves. Concentration per microgram of RNA was calculated for each sample for each gene.
Statistical methods
Differences between groups and correlations were assessed using nonparametric Mann-Whitney and Spearman tests, respectively. The Fisher exact test was used for categoric variables. P values less than .05 were considered significant. All calculations were performed on MedCalc version 9.3.0.0 statistical software (Mariakerke, Belgium).
Results
Severe oral cGVHD is characterized by increased keratinocyte apoptosis and infiltration
As part of multidisciplinary cGVHD staging and evaluation, patients were classified as having severe (ulcerative), mild (nonulcerative), or no clinical oral cGVHD (controls). Patients early in the course of the disease (< 1 year after transplantation) were found to be more likely to have severe, ulcerative lesions (77% vs 36%, Fisher exact test, P = .017).
The presence of apoptotic cells in the epithelial layer with adjacent mononuclear cells (satellitosis) and subepithelial infiltrate has long been considered the histopathologic hallmark of active cGVHD (Figure 2A).13 To objectively quantify the degree of apoptosis and overall density of infiltration, we have used immunofluorescent staining for active (cleaved) caspase-3, commonly used for early detection of apoptosis14 and CD45.
Severe oral cGVHD is characterized by increased infiltration in the epithelial layer and apoptosis of keratinocytes. (A) Oral chronic GVHD is classically characterized by “dyskeratotic” epithelial cells and mononuclear cell satellitosis (small arrow) and lichenoid infiltrate (large arrow) under the basement membrane (dotted line), hematoxylin and eosin [H&E] × 40). Apoptotic cells (green) closely associated with infiltrating CD45 cells (red) are increased in the keratinocyte layer of the patient with severe oral GVHD (B) but not in the control patient lacking oral cGVHD symptoms (C). Apoptosis within epithelial layer is limited to keratinocytes. Cells within epithelial layer expressing active (cleaved) caspase-3 (D, red) also express cytokeratin (E, cyan). (F) Overlay. Both apoptotic cells (G) and CD45 cells (H) are significantly increased in the patients with severe disease (n = 8, n = 16, and n = 11 for control, mild, and severe groups, respectively; *P < .01; error bars represent SEM).
Severe oral cGVHD is characterized by increased infiltration in the epithelial layer and apoptosis of keratinocytes. (A) Oral chronic GVHD is classically characterized by “dyskeratotic” epithelial cells and mononuclear cell satellitosis (small arrow) and lichenoid infiltrate (large arrow) under the basement membrane (dotted line), hematoxylin and eosin [H&E] × 40). Apoptotic cells (green) closely associated with infiltrating CD45 cells (red) are increased in the keratinocyte layer of the patient with severe oral GVHD (B) but not in the control patient lacking oral cGVHD symptoms (C). Apoptosis within epithelial layer is limited to keratinocytes. Cells within epithelial layer expressing active (cleaved) caspase-3 (D, red) also express cytokeratin (E, cyan). (F) Overlay. Both apoptotic cells (G) and CD45 cells (H) are significantly increased in the patients with severe disease (n = 8, n = 16, and n = 11 for control, mild, and severe groups, respectively; *P < .01; error bars represent SEM).
We observed increased numbers of cleaved caspase-3–positive cells in the keratinocyte layer in patients with oral cGVHD compared with control patients (Figure 2B,C). CD45 cells were frequently closely associated with the apoptotic cells (Figure 2B). Apoptotic cell count within the epithelial layer and CD45 count correlated with clinical severity assessed using the oral cGVHD grading (r = 0.53, P = .002 and r = 0.40, P = .02, respectively). To confirm that the apoptotic cells are in fact keratinocytes, we used double staining with pancytokeratin and CD45. Apoptotic cells were keratin positive but CD45−, confirming that keratinocytes, but not infiltrating cells, undergo apoptosis in the oral cGVHD epithelium (Figure 2D-F). Patients with severe oral cGVHD had increased numbers of epithelial caspase-3–positive cells and infiltrating CD45 cells compared with both control patients and patients with mild oral cGVHD (Figure 2G,H).
Infiltrating T cells express markers of effector-memory phenotype, cytotoxicity, and type I cytokine polarization
Although prior studies demonstrated that the infiltrate of oral cGVHD consists predominantly of T cells,6,7 the quantitative relationships among various subpopulations have not been fully evaluated. Consistent with previous studies, we found that the subepithelial infiltrate in oral cGVHD consisted predominantly of CD3+ T cells and CD68+ cells of macrophage/dendritic origin (Figure 3A-E). CD3+CD8+ T cells predominated over CD3+CD8− (CD4) T cells. There were very few (< 1%) natural killer (NK) cells (CD3−CD56) or B cells (CD20) in the infiltrate (data not shown). Both CD3+CD8+ and CD3+CD8− (CD4) T cells expressed CD45RO, the surface marker of effector-memory cells (Figure 3A-D).
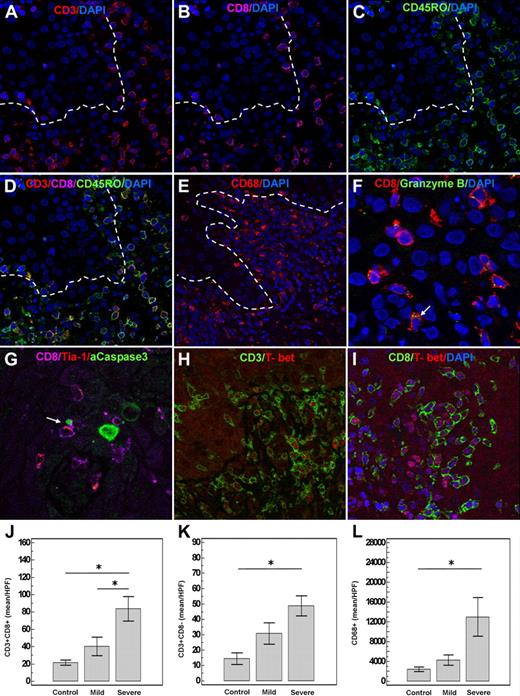
Infiltrating T cells express markers of cytotoxicity, type 1 cytokine polarization, and effector-memory phenotype. (A-D) Infiltrating T cells express CD45RO, a surface marker of the effector-memory T cells. Note predominance of the CD8 cells in the infiltrate. (E) CD68 myeloid cells are also overrepresented in oral cGVHD. CD8 cells express granzyme B (F) and Tia-1 (G) in the cytoplasm and are closely associated with the apoptotic cells (green, G). Note polarization of the cytotoxic granule (red) within the CD8 cell (cyan) in the direction of the apoptotic keratinocyte (green) shown by arrow. (B) Close-up of the same image with the arrows pointing to the granzyme B granules. (H,I) T-bet expression by the infiltrating T cells. (J-L) CD8 (n = 8, n = 16, and n = 12 for control, mild, and severe groups, respectively) and CD68 cells (n = 8, n = 15, and n = 10 for control, mild, and severe groups, respectively) are particularly prominent in severe disease (*P < .05; error bars represent SEM).
Infiltrating T cells express markers of cytotoxicity, type 1 cytokine polarization, and effector-memory phenotype. (A-D) Infiltrating T cells express CD45RO, a surface marker of the effector-memory T cells. Note predominance of the CD8 cells in the infiltrate. (E) CD68 myeloid cells are also overrepresented in oral cGVHD. CD8 cells express granzyme B (F) and Tia-1 (G) in the cytoplasm and are closely associated with the apoptotic cells (green, G). Note polarization of the cytotoxic granule (red) within the CD8 cell (cyan) in the direction of the apoptotic keratinocyte (green) shown by arrow. (B) Close-up of the same image with the arrows pointing to the granzyme B granules. (H,I) T-bet expression by the infiltrating T cells. (J-L) CD8 (n = 8, n = 16, and n = 12 for control, mild, and severe groups, respectively) and CD68 cells (n = 8, n = 15, and n = 10 for control, mild, and severe groups, respectively) are particularly prominent in severe disease (*P < .05; error bars represent SEM).
Having observed the increased apoptosis in the affected epithelium, we examined the phenotype of the infiltrating T cells in more detail. We found that CD8 cells expressed markers of activated cytotoxic effectors, such as granzyme B and TIA-1 and were often closely associated with the apoptotic keratinocytes (Figure 3F,G). Furthermore, infiltrating T cells expressed T-bet, a transcription factor essential for type I cytokine polarization and for production of granzyme B (Figure 3H,I).15,16 Although all T-bet+ cells were CD3+, some T-bet+ cells were CD8− (Figure 3H,I), suggesting that CD3+CD8−, that is, CD4+ T cells, present in the infiltrate also expressed T-bet.
Although all contributing cell populations were increased in patients with severe oral cGVHD (Figure 3J-L), CD8 T cells were particularly prevalent (Figure 3J). The relative proportion of CD4 (as assessed as CD3+CD8−) cells increased in patients late after transplantation, as indicated by positive correlation between CD4/CD8 ratio and the time after transplantation (r = 0.38, P = .03). Furthermore, the number of infiltrating CD8 cells better correlated with the epithelial apoptotic count (r = 0.69, P = .001) than that of CD4 cells (r = 0.49, P = .004). Based upon prevalence and correlation with epithelial damage, the primary effector is likely to be a T-bet+ CD8+ T cell.
Infiltrating T cells express CXCR3, and CXCR3 ligands are up-regulated in oral cGVHD
Chemokines play a key role in the pathogenesis of the autoimmune and alloimmune conditions, including GVHD, directing trafficking of the effector cells to the target tissues.17 Therefore, we examined chemokine receptor and ligand expression in the affected oral tissues. Expression of CXCR3 is characteristic of type 1 cytokine–producing T cells and is T-bet dependent.18,19 Consistent with their expression of T-bet, nearly all infiltrating T cells in oral cGVHD expressed CXCR3 (Figure 4A-C). Furthermore, CXCL9 (MIG), one of the ligands of CXCR3, was highly expressed by the oral epithelium and infiltrating cells in patients with severe oral cGVHD (Figure 4D-F).
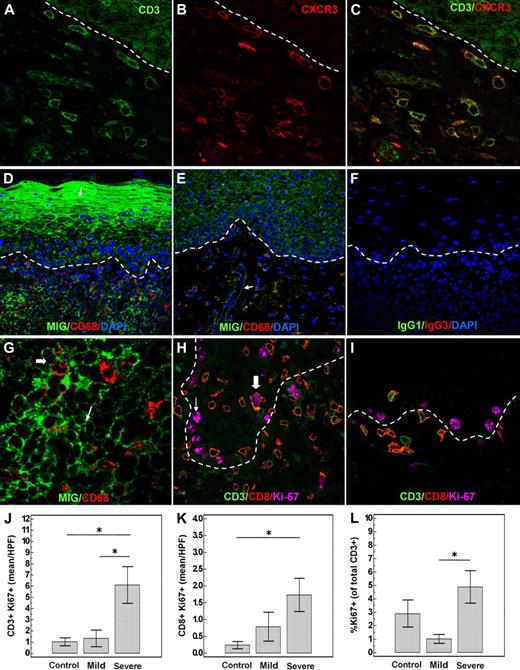
Migration and proliferation of infiltrating T cells in oral cGVHD. Infiltrating T cells (A) express the chemokine receptor CXCR3 (B). (C) Overlay of CD3 and CXCR3. (D) Increased production of the CXCR3 ligand MIG (CXCL9) by the keratinocytes (arrow) and infiltrating cells in a patient with severe oral GVHD. (E) Control patient without oral cGVHD symptoms with minimal MIG expression by the keratinocytes and endothelial cells (arrow). (F) Negative isotype staining control. (G) Enlarged image demonstrating MIG expression by CD68+ cells (large arrow), as well as CD68− cells (small arrow). Infiltrating T cells proliferate in the oral mucosa affected by cGVHD. (H) Severe oral cGVHD; close-up image showing nuclear expression of proliferation marker Ki-67 in a CD8 cell (large arrow). Basal keratinocytes (small arrow) proliferate constitutively. (I) Lack of proliferating T cells in a patient without clinical oral cGVHD. (J-L) Severe cGVHD (n = 12) is characterized by increased number and percentage of proliferating T cells compared with patients with mild (n = 16) or no (n = 8) oral cGVHD (*P < .05; error bars represent SEM).
Migration and proliferation of infiltrating T cells in oral cGVHD. Infiltrating T cells (A) express the chemokine receptor CXCR3 (B). (C) Overlay of CD3 and CXCR3. (D) Increased production of the CXCR3 ligand MIG (CXCL9) by the keratinocytes (arrow) and infiltrating cells in a patient with severe oral GVHD. (E) Control patient without oral cGVHD symptoms with minimal MIG expression by the keratinocytes and endothelial cells (arrow). (F) Negative isotype staining control. (G) Enlarged image demonstrating MIG expression by CD68+ cells (large arrow), as well as CD68− cells (small arrow). Infiltrating T cells proliferate in the oral mucosa affected by cGVHD. (H) Severe oral cGVHD; close-up image showing nuclear expression of proliferation marker Ki-67 in a CD8 cell (large arrow). Basal keratinocytes (small arrow) proliferate constitutively. (I) Lack of proliferating T cells in a patient without clinical oral cGVHD. (J-L) Severe cGVHD (n = 12) is characterized by increased number and percentage of proliferating T cells compared with patients with mild (n = 16) or no (n = 8) oral cGVHD (*P < .05; error bars represent SEM).
To better evaluate the mechanisms of effector cell accumulation in oral cGVHD, we next assessed the proliferation of the infiltrating cells by costaining with Ki-67. We found that both CD8+ and CD3+CD8− (CD4) T cells were proliferating in oral tissues of patients with oral GVHD but not control patients (Figure 4H,I). Patients with severe oral cGVHD demonstrated an increase in absolute number and percentage of proliferating T cells (Figure 4J-L).
Production of IL-15 in affected tissues in oral cGVHD
Having found that active oral cGVHD is characterized by accumulation of effector-memory cells expressing markers of type I differentiation such as T-bet and CXCR3, we examined the potential mechanisms of generation and maintenance of these cells.
IL-15 is well established as a critical cytokine for generation, proliferation, and maintenance of effector-memory CD8 cells and was recently shown to induce proliferation and tissue migration of CD4 cells.20 In addition, IL-15, similar to IL-12, has been shown to induce T-bet and drive Th1/Tc1 differentiation.21-23 In addition to its role as a homeostatic cytokine, IL-15 has been implicated in several inflammatory autoimmune conditions.24
We therefore examined IL-15 expression in affected oral tissues. IL-15 was expressed by the keratinocytes, endothelial, and infiltrating cells in patients with severe oral cGVHD but not in controls (Figure 5A-C). Because IL-15 expression was broadly distributed over the tissue, while T-bet expression was confined to scattered nuclei, we compared their mRNA levels in tissue reserved from split biopsies from 21 patients. The mRNA level of IL-15 was increased in patients with severe oral cGVHD (Figure 5D) and correlated well with that of T-bet (r = 0.59, P = .009, Figure 5E), consistent with the role of IL-15 in induction of T-bet.
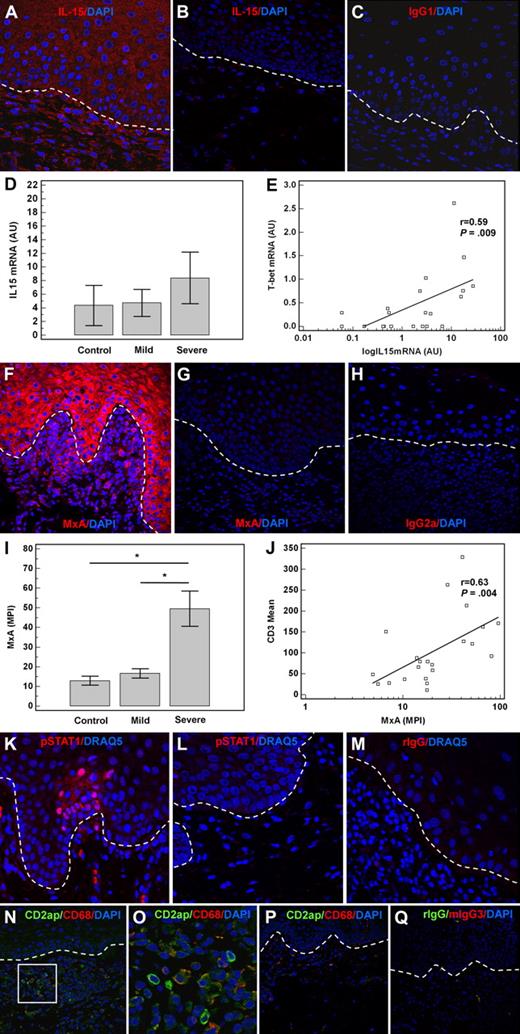
Activation of type I IFN axis in oral cGVHD. (A) IL-15 expression in the keratinocytes and infiltrating cells in a patient with severe oral cGVHD. (B) Patient without oral cGVHD. (C) Negative isotype staining control. IL-15 mRNA (mean ± SEM) is increased in severe oral GVHD (n = 6) compared with mild cGVHD (n = 10) or controls (n = 5) and correlates with that of T-bet (D-E). Increased expression of MxA, IFN α/β–inducible protein, in oral cGVHD. (F) Patient with severe disease. (G) Control patient lacking oral cGVHD. (H) Negative isotype staining control. (I) MxA (MPI ± SEM) is markedly up-regulated in patients with severe oral cGVHD (n = 9) compared with those with mild disease (n = 7) or control patients (n = 6). MxA expression in the affected tissues correlates with the number of infiltrating T cells, assessed as CD3+ cells/HPF (J). STAT1 phosphorylation and nuclear translocation is observed in the patients with severe cGVHD (K), but not control patients (L). (M) Negative isotype staining control. (N) Plasmacytoid dendritic cells identified by expression of CD2ap in the patient with severe oral cGVHD and high type I IFN activity as measured by MxA expression. (O) Close-up of the image in panel N, showing coexpression of CD2ap and CD68 in a granular peripheral pattern. (P) No CD2ap-expressing cells and few CD68 cells are present in the mucosa of a patient without oral cGVHD. (Q) Negative control. AU indicates arbitrary units; MPI, mean pixel intensity.
Activation of type I IFN axis in oral cGVHD. (A) IL-15 expression in the keratinocytes and infiltrating cells in a patient with severe oral cGVHD. (B) Patient without oral cGVHD. (C) Negative isotype staining control. IL-15 mRNA (mean ± SEM) is increased in severe oral GVHD (n = 6) compared with mild cGVHD (n = 10) or controls (n = 5) and correlates with that of T-bet (D-E). Increased expression of MxA, IFN α/β–inducible protein, in oral cGVHD. (F) Patient with severe disease. (G) Control patient lacking oral cGVHD. (H) Negative isotype staining control. (I) MxA (MPI ± SEM) is markedly up-regulated in patients with severe oral cGVHD (n = 9) compared with those with mild disease (n = 7) or control patients (n = 6). MxA expression in the affected tissues correlates with the number of infiltrating T cells, assessed as CD3+ cells/HPF (J). STAT1 phosphorylation and nuclear translocation is observed in the patients with severe cGVHD (K), but not control patients (L). (M) Negative isotype staining control. (N) Plasmacytoid dendritic cells identified by expression of CD2ap in the patient with severe oral cGVHD and high type I IFN activity as measured by MxA expression. (O) Close-up of the image in panel N, showing coexpression of CD2ap and CD68 in a granular peripheral pattern. (P) No CD2ap-expressing cells and few CD68 cells are present in the mucosa of a patient without oral cGVHD. (Q) Negative control. AU indicates arbitrary units; MPI, mean pixel intensity.
Potential role of type I IFN in oral cGVHD
Although IL-15 is constitutively produced in many tissues by myeloid cells, it is also inducible by inflammatory stimuli, in particular by type I IFN. Type I IFNs (IFN α/β) can induce bystander proliferation of CD8 cells and this effect has been shown to be in part mediated through IL-15 induction.25,26 Similarly, production of the chemokine MIG (CXCL9), observed in the same buccal mucosal keratinocytes and myeloid infiltrate as IL-15, is inducible by type I IFNs. In addition high type I IFN activity has been linked to induction of diverse autoimmune conditions, including lupus and psoriasis.27 We therefore hypothesized that type I IFN system activation may play a role in early oral cGVHD.
Because the T-bet+ Th1/Tc1 cells in the infiltrate may be producing type II IFN, which can also induce production of IL-15 and MIG, we used staining for MxA as a measure of the type I IFN activity. MxA is an antiviral protein that is potently and specifically induced by type I but not type II IFNs.12,28 MxA was highly expressed in the keratinocytes and infiltrating cells in patients with severe oral cGVHD relative to patients with mild or no oral disease (Figure 5F-I). Indeed, the overall expression of MxA, assessed as mean pixel intensity across 3 high-powered fields (HPFs), correlated well with the mean CD3 cell number per HPF (r = 0.63, P = .004, Figure 5J).
STAT1 is a critical component of the IFN-signaling pathway. Upon binding of the IFN to the receptor, STAT1 is phosphorylated and translocates to the nucleus.29 In addition to high MxA expression, patients with severe oral cGVHD demonstrated STAT1 activation in the affected tissues as evaluated by phospho-STAT1 staining (Figure 5K-M).
Plasmacytoid dendritic cells (pDCs) produce large amounts of type I IFN in response to viral infection, play a role in cross-presentation, and induce potent cytotoxic effectors and Th1 differentiation in CD4 cells.30-32 In addition to their role in innate immunity, pDCs have shown to accumulate in the affected tissues in several classic autoimmune conditions, including lupus and psoriasis.33,34 The CD68+ populations observed in the infiltrates in severely affected patients, although predominantly macrophages, include both plasmacytoid and myeloid dendritic cells.35 Recently, CD2ap has been described as a specific marker of pDCs suitable for staining of paraffin sections.36 We confirmed its specificity by costaining with other markers expressed by pDCs, such as HLA-DR, CD123, and CD45RA (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In addition, the morphology of CD2ap−CD68 myeloid cells was distinct from that of CD2ap CD68+ pDCs (Figure S1). Conversely, CD2ap was not expressed by T cells (CD3), B cells (CD20), or macrophages (CD163) (Figure S1). We found that many CD68 cells in the severe oral cGVHD expressed CD2ap (Figure 5N-Q). In contrast, patients without oral cGVHD had very few or no CD2ap+ pDCs in oral mucosa.
Discussion
In this study we have determined that in patients with severe oral cGVHD, oral mucosa is characterized by increased epithelial apoptosis (cytokeratin caspase-3 keratinocytes) associated with infiltrates of T-bet+ T cells. Among infiltrating cells, the predominately CD45RO+CD8+ T cells expressed markers of cytotoxicity such as Tia-1 and granzyme B and were located in direct proximity to the apoptotic keratinocytes, suggesting their direct involvement in epithelial cell death. This is the first report demonstrating that T cells in cGVHD infiltrates express T-bet, a transcription factor critical for Th1/Tc1 polarization and production of IFN-gamma and granzyme B.15 Importantly, in several in vivo models of alloimmunity and autoimmunity, T-bet was essential for achievement of the full pathogenic phenotype by effector CD8 cells.19,37-39 Furthermore, T-bet controls expression of CXCR3—a chemokine receptor previously shown to be critical for migration of alloreactive and autoreactive T cells to target tissues.18,19 We found that both T-bet and CXCR3 were expressed on the majority of the infiltrating T cells in oral cGVHD.
Concomitantly, keratinocytes in the mucosal epithelium and myeloid and plasmacytoid dendritic cells in the submucosal infiltrate demonstrated increased production of chemokines and cytokines that could support CD8 immigration, proliferation, and cytotoxic differentiation. The ligand for CXCR3, MIG/CXCL9, was highly expressed in these tissues in patients with active oral cGVHD. Of note, MIG/CXCL9 has been shown to be the predominant chemokine in lichen planus, an autoimmune disorder clinically and histologically very similar to chronic lichenoid GVHD.40 Moreover, in oral cGVHD the mucosal epithelia and underlying myeloid infiltrate were found to express IL-15. Keratinocytes have been previously shown to produce both IL-15 protein and IL-15R-alpha—a receptor chain necessary for cross-presentation of IL-15 to CD122-bearing effector cells.41 IL-15 is well established as a critical cytokine for proliferation, activation, and maintenance of effector-memory CD8 cells.42 Furthermore, T-bet—the Th1/Tc1-determining transcription factor expressed by most T cells in the infiltrate—has been demonstrated to enhance IL-15 responsiveness through induction of CD122.43 Consistent with the role of IL-15 in stimulating proliferation, an elevated proportion of T cells in the infiltrate was in cycle (Ki-67).
We have not ruled out the potential involvement of Th2 or Th17 cells in the inflammatory process. We would note, however, that the majority of the T cells express T-bet and CXCR3. Th1 and Th2 pathways are generally mutually exclusive and transcription factors guiding cytokine polarization (T-bet and Gata-3) are strongly antagonistic. Potential roles for Th17 cells in initiating or supporting the inflammatory process remain to be determined.
Recent analyses of autoimmune disorders have supported a key role for type I IFN in initiating and sustaining the dysfunctional inflammatory process.27,44 In the oral mucosa in cGVHD, the keratinocytes and infiltrating myeloid cells demonstrate elevated expression of IFN-inducible factors (IL-15 and MIG), and the keratinocytes show evidence of continuing IFN signaling (phosphorylation and translocation of STAT1). Furthermore, the concomitant presence in severely affected mucosa of both MxA and pDCs, that is, a specific indicator of type I IFN and a major source, is consistent with a role of this cytokine. This associated increase in type I IFN–inducible products and accumulation of plasmacytoid dendritic cells in the subepithelial infiltrate is similar to what has been previously observed in the skin of patients with lupus, psoriasis, and cutaneous lichen planus.33,34,45 Interestingly, plasmacytoid dendritic cell precursors have been reported to be decreased in the circulation in patients with lupus, presumably due to their migration into target tissues.46 Recently, decreases in the number of pDCs in the blood have been found to be associated with acute GVHD.47 We have similarly observed a reduction in pDCs in the circulation in cGVHD (data not shown).
The factors initiating type I IFN release in cGVHD are unknown and deserve further investigation. Viral infection with subsequent type I IFN activation has been shown to play a role in several models of autoimmunity.27 Conditions of immune deficiency following allogeneic hematopoietic stem cell transplantation predispose to viral reactivation. Although no direct tests of viral antigenemia were performed, cGVHD patients enrolled in this study did not have any clinical evidence of local or systemic viral infection. Reactivation of latent viral infections in the oral cavity (both clinical and subclinical), however, is well known to occur in immunosuppressed patients. In fact, such reactivation may be one of the contributors to the self-perpetuating cycle of cGVHD exacerbation. In humans, pDCs selectively express the Toll-like receptors TLR7 and TLR9, which specifically respond to single-stranded RNA (ssRNA) and ssDNA viruses commonly affecting patients following HSCT, including herpes viruses, influenza, and vesicular stomatitis virus.48 Furthermore, studies in lupus erythematosus and psoriasis have suggested that pDCs can also be activated through TLR9 by host DNA released from apoptotic cells.49 HMGB1, a nuclear DNA binding protein released by dying cells, has been shown to enhance the interaction of DNA from apoptotic cells with Toll-like receptors, resulting in activation of plasmacytoid dendritic cells.50 Increased HMGB1 release in affected tissues has been described in conditions such as lupus and Sjogren syndrome.51
Based on this analysis of an important target tissue in cGVHD, we are proposing a new model of cGVHD pathogenesis, in which the local production of type I IFN by pDCs plays a central role in the initiation and continuation of cGVHD. Secretion of type I IFN by activated pDCs induces production of IL-15 and of chemokines such as MIG by keratinocytes as well as by CD68+ myeloid cells. Type I IFN and IL-15 direct and sustain CD4 and CD8 polarization toward Th1/Tc1 phenotype, manifested in induction of T-bet and the receptor for MIG, CXCR3. Alloreactive CXCR3 T cells migrate into oral mucosal tissues along the chemokine (MIG/CXCL9) gradient. Having reached the target tissues, T effector cells proliferate in response to antigen and locally produced IL-15, enter the epithelial layer, and mediate keratinocyte apoptosis. Sustained activation by cytokines secreted by T effector cells may lead to perpetuation of the alloimmune process. This model revises the current paradigm of cGVHD as a type II cytokine–mediated disorder, but is supported by both the evidence we are presenting here and by current research in tissue-specific autoimmune disorders such as lichen planus and psoriasis. Finally, this model lays the groundwork for new approaches to cGVHD treatment focusing on the IFN-mediated inflammatory axis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all the members of the chronic GVHD protocol team for their help in data collection related to this study, and in particular Drs David Kleiner, Sandra Mitchell, Jean-Pierre Guadagnini, and Jane Atkinson, Ms Daniele Avila, and Mr Mike Krumlauf. We thank Dr Yu-Waye Chu for the paper revision and helpful suggestions. Finally, we are grateful to all the patients who volunteered for this study.
This study received funding from the National Cancer Institute (Bethesda, MD).
National Institutes of Health
Authorship
Contribution: M.M.I. designed the research, performed experiments, analyzed data, and wrote the paper; W.D.S. and S.C.L. performed experiments; and R.E.G., S.Z.P., and F.T.H. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Frances T. Hakim, Experimental Transplantation and Immunology Branch, National Cancer Institute, 10 Center Dr, 12C216, Bethesda, MD 20892; e-mail: hakimf@mail.nih.gov.
References
Author notes
*S.Z.P. and F.T.H. contributed equally to this work.

![Figure 2. Severe oral cGVHD is characterized by increased infiltration in the epithelial layer and apoptosis of keratinocytes. (A) Oral chronic GVHD is classically characterized by “dyskeratotic” epithelial cells and mononuclear cell satellitosis (small arrow) and lichenoid infiltrate (large arrow) under the basement membrane (dotted line), hematoxylin and eosin [H&E] × 40). Apoptotic cells (green) closely associated with infiltrating CD45 cells (red) are increased in the keratinocyte layer of the patient with severe oral GVHD (B) but not in the control patient lacking oral cGVHD symptoms (C). Apoptosis within epithelial layer is limited to keratinocytes. Cells within epithelial layer expressing active (cleaved) caspase-3 (D, red) also express cytokeratin (E, cyan). (F) Overlay. Both apoptotic cells (G) and CD45 cells (H) are significantly increased in the patients with severe disease (n = 8, n = 16, and n = 11 for control, mild, and severe groups, respectively; *P < .01; error bars represent SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/15/10.1182_blood-2008-07-168351/7/m_zh80160933360002.jpeg?Expires=1769673422&Signature=pJI~YEt6aTS6TQT1lwdrjvPxxmxQotI3z464YuQhbHYdz3p80gjUdf-f72mMFOfNkKyTTOyVvvXKgiSfFEzWHuwOZWdUdobkKBGVZeWY5Ly1VXRL6dD61xvEANDsMHQb5d3vTcDHoPXhZJZW2eC16-alL1OWL8XxBzTNv4nUv2CKOGKqW~vSx74p96VikIusj6L95wJcCh3ssdSOo90cd~C98zRL6IL-EMoi05LJtCIMuYyKj4FFI4dFkCiVVJAhxNp-qrfPw538WYPOCk-dangEiAq-iJHfDQhRS66JIG~~XY9mrpphQPrVyL0axbc4iXdoh44ZnRB-HFlCPtc9Mw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal