Abstract
Hepcidin, a key regulator of iron metabolism, is a small antimicrobial peptide produced by the liver that regulates intestinal iron absorption and iron recycling by macrophages. Hepcidin is stimulated when iron stores increase and during inflammation and, conversely, is inhibited by hypoxia and augmented erythropoiesis. In many pathologic situations, such as in the anemia of chronic disease (ACD) and iron-loading anemias, several of these factors may be present concomitantly and may generate opposing signaling to regulate hepcidin expression. Here, we address the question of dominance among the regulators of hepcidin expression. We show that erythropoiesis drive, stimulated by erythropoietin but not hypoxia, down-regulates hepcidin in a dose-dependent manner, even in the presence of lipopolysaccharide (LPS) or dietary iron-loading, which may act additively. These effects are mediated through down-regulation of phosporylation of Stat3 triggered by LPS and of Smad1/5/8 induced by iron. In conclusion, hepcidin expression levels in the presence of opposing signaling are determined by the strength of the individual stimuli rather than by an absolute hierarchy among signaling pathways. Our findings also suggest that erythropoietic drive can inhibit both inflammatory and iron-sensing pathways, at least in part, via the suppression of STAT3 and SMAD4 signaling in vivo.
Introduction
Body iron stores are kept in balance by controlling the level of absorption from the diet because iron is not efficiently excreted in mammals.1 Thus, considerable work in the past was directed at identifying factors that influence intestinal iron absorption. Those studies established that both the size of iron stores and the rate of erythropoiesis influence the amount of iron that is absorbed in the intestine.2 Information about the amount of iron in the body is transmitted to the intestine where iron absorption takes place such that, if the body is iron-depleted, the amount of daily iron absorption is augmented, whereas if there is an excess of iron, absorption is diminished to prevent iron overloading (referred to as the store regulator). Iron absorption is also independently regulated by erythropoiesis: it increases when erythropoietic activity rises (erythroid regulator).2 Additional systemic stimuli that also affect intestinal iron absorption include tissue oxygenation (hypoxia regulator)3 and inflammatory cytokines (inflammatory regulator).4 During hypoxia, iron absorption increases,5 while, conversely, it may decline during inflammation.4
More recent work identified hepcidin, a small antimicrobial peptide,6,7 as an effector molecule common to these 4 regulators of iron absorption.8,9 Hepcidin expression is stimulated when iron stores increase8 as well as during inflammation.9 Conversely, it is inhibited by anemia/hypoxia and heightened erythropoiesis drive.9,10
In many pathologic situations, however, antagonistic signaling for hepcidin regulation can co-occur. For example, anemia may co-occur with inflammation, as seen in the anemia of chronic disease (ACD)11 arising in chronic inflammatory conditions such as autoimmune diseases, chronic infectious diseases, and cancer. ACD is associated with excessive production of cytokines produced by macrophages and T-lymphocytes, which ultimately leads to iron withdrawal from circulation and iron sequestration in reticuloendothelial macrophages and thus, to impaired iron mobilization from stores.11 This may explain why patients with ACD may become iron-deficient when inhibition of intestinal iron absorption by high hepcidin levels is sustained for a long period. When treating the underlying disease is not feasible, erythropoietin (EPO) agents may help stimulate erythropoiesis and improve hemoglobin levels in patients with ACD.11 However, whether the therapeutic effect of EPO also involves suppression of hepcidin expression in inflammatory conditions has not yet been demonstrated. Previously, we showed that hepcidin expression is induced in iron-deficient mice after lipopolysaccharide (LPS) challenge, even in iron-depleted mice.12 Similarly, in humans treated with endotoxin, serum hepcidin levels also increased despite the presence of slightly decreased serum iron levels.13
Anemia may also co-occur with iron overload, as observed in disorders characterized by robust but inefficient erythropoiesis.14 These disorders, collectively called iron-loading anemias, include, among others: thalassemia syndromes, congenital dyserythropoietic anemias, and sideroblastic anemias that have ineffective erythropoiesis in common, leading to the development of anemia and consequent hypoxia.15 The second common characteristic of these disorders is the development of iron overload, which can be severe as a consequence of inappropriately increased intestinal iron absorption.2 Indirect evidence suggests that serum factors in patients with β-thalassemia might override the potential effect of iron overload on hepcidin expression and thus explain the inappropriate intestinal iron absorption levels. In fact, serum from individuals with β-thalassemia has been reported to inhibit hepcidin mRNA expression in hepatoma cells.16
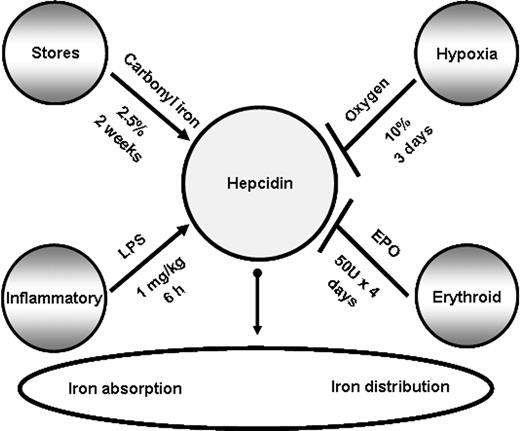
Because of the central role of hepcidin in iron metabolism and its direct contribution to the pathogenesis of several common disorders, it is important to understand how different factors influence hepcidin expression in vivo and lead to its misregulation when present concomitantly. In this study, we examined how hepcidin is regulated in the presence of opposing stimuli in vivo in mice as summarized in Figure 1, and further investigated the contribution of mediators and downstream signaling pathways of hepcidin expression.
Factors affecting mRNA hepcidin expression in the liver. Hepcidin levels are regulated by iron levels (store regulator), immune mediators (inflammatory regulator), hypoxia (hypoxia regulator), and erythropoietic demand (erythroid regulator). Pointed arrows indicate up-regulation of hepcidin, and blunt arrows inhibition of its mRNA expression. For each regulator, the treatments used in this study to stimulate or suppress hepcidin expression are shown.
Factors affecting mRNA hepcidin expression in the liver. Hepcidin levels are regulated by iron levels (store regulator), immune mediators (inflammatory regulator), hypoxia (hypoxia regulator), and erythropoietic demand (erythroid regulator). Pointed arrows indicate up-regulation of hepcidin, and blunt arrows inhibition of its mRNA expression. For each regulator, the treatments used in this study to stimulate or suppress hepcidin expression are shown.
Methods
Animals
All procedures were performed in accordance with Canadian Council on Animal Care guidelines after approval by the institutional Animal Care Committee of the Centre de Recherche, Centre Hospitalier de l'Université de Montréal (CRCHUM). C57BL/6 female mice aged 6 weeks were purchased from Charles River Laboratories (Wilmington, MA).
Animal treatments
Erythroid regulator.
Erythropoiesis was induced by treating mice with 50 U of human biosynthetic EPO (Ortho Biotech, Bridgewater, NJ) dissolved in phosphate-buffered saline (PBS). Mice were injected intraperitoneally daily for 4 days and were killed on day 5. Control mice were similarly injected with an equivalent volume of PBS.
Immune regulator.
Acute inflammation was produced by a single dose of LPS (Escherichia coli serotype 055:B5-1 mg/kg intraperitoneally; Sigma-Aldrich, St Louis, MO). In other experiments, mice were injected with recombinant mouse IL-6 (1 μg intraperitoneally; Cedarlane Laboratories, Hornby, ON). Control mice were similarly injected with an equivalent volume of sterile saline solution (0.09% NaCl). The animals were killed 6 hours after the LPS injection and 3 hours after the IL-6 injection.
Stores regulator.
Control mice were given a commercial diet containing approximately 226 mg of iron per kilogram (Teklad Global 18% protein rodent diet; Harlan Teklad, Madison, WI). Dietary iron overload was produced by giving 8-week-old mice the same commercial diet supplemented with 25 g carbonyl iron (2.5% wt/wt carbonyl iron; Sigma-Aldrich) for 2 weeks.
Hypoxia.
Normobaric hypoxia was established by diluting ambient air with nitrogen in a special ventilated chamber in which N2-enriched air supply was controlled with an O2 sensor-driven inlet valve. Mice were maintained in the chamber and exposed to normobaric hypoxia (10% O2) for 3 days. An oxygen analyzer was used to monitor the oxygen concentration in the hypoxic chamber. Age-matched control mice were kept under normoxia (room air) in the same room in which the hypoxic chamber was placed.
Hematologic measurements and transferrin saturation
Red blood cell (RBC) count, hemoglobin (Hb), hematocrit (HCT), and mean corpuscular volume (MCV) were measured with an automated cell counter calibrated for murine samples (ABC vet counter; ABX Hématologie, Montpellier, France). Serum iron, total iron-binding capacity, and transferrin saturation were assessed by colorimetric assay with the Kodak Ektachem DT60 system (Johnson & Johnson, Ortho Clinical Diagnostics, Mississauga, ON).
Measurement of tissue iron concentration
Liver iron concentrations were assessed by acid digestion of tissue samples, followed by iron quantification with atomic absorption spectroscopy.17
Quantitative RT-PCR
Total RNA was isolated with Trizol reagent (Invitrogen, Burlington, ON), and reverse transcription was performed with the Thermoscript reverse transcriptase–polymerase chain reaction (RT-PCR) system (Invitrogen). Hepcidin and β-actin mRNA levels were measured by real-time PCR in a Rotor Gene 3000 Real Time DNA Detection System (Montreal Biotech, Kirkland, QC) with QuantiTect SYBRGreen I PCR kits (QIAGEN, Mississauga, ON) as described.18 The primers used were: β-actin, 5′-TGTTACCAACTGGGACGACA-3′ and 5′-GGTGTTGAAGGTCTCAAA-3′; hepcidin, 5′-AGAGCTGCAGCCTTTGCAC-3′ and 5′-GAAGATGCAGATGGGGAAGT-3′; and IL-6, 5′-TGTGCAATGGCAATTCTGAT-3′ and 5′-CCAGAGGAAATTTTCAATAGGC-3′. Expression levels were normalized to the housekeeping gene β-actin.
IL-6 assay
IL-6 was measured in serum and in supernatants of liver homogenates with an enzyme-linked immunosorbent assay (ELISA) kit as per manufacturer's instructions (mouse IL-6 ELISA kit, catalog no. M6000B; R&D Systems, Minneapolis, MN). Snap-frozen liver samples were thawed, weighed, and homogenized in solutions containing 1 mL protease inhibitor cocktail (Complete; Boehringer Mannheim, Indianapolis, IN). The resulting supernatants were analyzed and standardized to the weight of the liver sample.
SDS–polyacrylamide gel electrophoresis and Western blot analysis
Livers were removed, rinsed in ice-cold PBS, and used to prepare liver nuclear extracts with Nuclear Extract Kits (Active Motif, Carlsbad, CA). Nuclear protein extracts were separated on 10% SDS–polyacrylamide gel and blotted onto nitrocellulose membranes (GE Healthcare, Little Chalfont, United Kingdom). The membranes were immunoblotted with the following antibodies: phospho-Stat3, Stat3, phospho-Smad1/5/8 (Cell Signaling, Danvers, MA), Smad1/5/8 (Santa Cruz Biotechnology, Santa Cruz, CA) and β-actin (Abcam, Cambridge, MA). As a secondary antibody, anti–rabbit IgG (Cell Signaling) or anti–mouse IgG (GE Healthcare) were used. Antigen-antibody complexes were visualized with the ECL Western Blotting Detection Reagent (GE Healthcare).
Statistical analysis
All statistics were calculated with SigmaStat 3.1 (Systat Software, Richmond, CA). Multiple comparisons were evaluated statistically by 1-way analysis of variance (ANOVA) followed by the Bonferroni multiple comparison test. When data failed the equal variance test, the Kruskal-Wallis 1-way ANOVA on ranks was used followed by Student-Newman-Keuls or the Dunn post-hoc test. Correlation coefficients were determined by Spearman correlation coefficients.
Results
Erythropoietin but not hypoxia blocks hepcidin induction by LPS
EPO.
Previous studies have demonstrated that when erythropoiesis is stimulated by EPO, hepcidin mRNA expression is inhibited,10,19 and conversely, LPS induces it.8 To assess whether increased erythropoiesis could affect the induction of hepcidin by LPS, mice were treated for 4 consecutive days with EPO to stimulate erythropoiesis, followed by a single injection of LPS on day 5, and were then examined after 6 hours. EPO treatment stimulated erythropoiesis, as revealed by the elevation of hematologic indices (RBCs, 12.4%; HGB, 11.5%; HCT, 19.9% increase). As shown in Figure 2A, EPO alone led to a significant inhibition of mRNA hepcidin expression (92% reduction). When compared with LPS-treated controls, EPO pretreatment considerably reduced the ability of LPS to evoke hepcidin mRNA expression (LPS vs EPO + LPS; P < .001). However, the observed reduction was not absolute, because hepcidin levels remained higher in mice with the combined EPO and LPS treatments than in mice treated with EPO alone (EPO vs EPO + LPS; P < .0001).
Erythropoietin but not hypoxia inhibits hepcidin induction through the inflammatory pathway. (A) Hepcidin mRNA levels in the liver of mice treated with saline (control [CTL]), EPO, LPS, and mice with combined treatments (EPO + LPS). (B) Hepcidin mRNA levels in the liver of mice treated with saline (CTL), mice subjected to 10% oxygen (hypoxia [Hpx]), LPS, and mice with combined treatments (Hpx + LPS). Hepatic hepcidin expression was quantified by real-time RT-PCR and normalized to β-actin. The hepcidin/β-actin ratios are shown, each symbol representing 1 mouse. Statistical analysis was performed by 1-way ANOVA; **P < .001 for comparison with control mice. n.s. indicates not significant.
Erythropoietin but not hypoxia inhibits hepcidin induction through the inflammatory pathway. (A) Hepcidin mRNA levels in the liver of mice treated with saline (control [CTL]), EPO, LPS, and mice with combined treatments (EPO + LPS). (B) Hepcidin mRNA levels in the liver of mice treated with saline (CTL), mice subjected to 10% oxygen (hypoxia [Hpx]), LPS, and mice with combined treatments (Hpx + LPS). Hepatic hepcidin expression was quantified by real-time RT-PCR and normalized to β-actin. The hepcidin/β-actin ratios are shown, each symbol representing 1 mouse. Statistical analysis was performed by 1-way ANOVA; **P < .001 for comparison with control mice. n.s. indicates not significant.
Hypoxia.
Hypoxia has been identified as an independent regulator of iron absorption20 and, consistently, exposure to hypoxia has been shown to suppress hepcidin mRNA both in mice in vivo and in isolated hepatocytes in vitro,9 suggesting that hypoxia may directly modulate hepcidin expression. To test whether hypoxia could, similarly to EPO, block LPS induction of hepcidin expression, mice were exposed to normobaric hypoxia (10% O2) for 3 days and received a single LPS injection 6 hours before being analyzed. Hypoxia stimulated erythropoiesis, as judged by the elevation of hematologic indices when compared with control, normoxic mice (RBCs, 23%; Hb, 21.8%; HCT, 23% increase). As expected,9 hypoxia alone led to suppression of hepcidin mRNA levels in the liver (∼72% inhibition) but unlike EPO, was insufficient to block induction by LPS (approximately 112% induction compared with control, normoxic mice; Figure 2B).
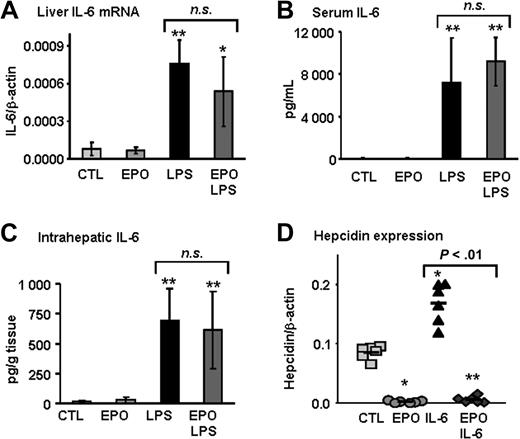
Erythropoietin inhibits LPS-mediated hepcidin induction independently of IL-6 production
LPS injection leads to the production of inflammatory cytokines, including IL-6,21 which has been identified as a major hepcidin inducer.22 Because EPO has been shown to inhibit LPS-induced secretion of IL-6 in cell lines,23 we next set out to determine whether the increase in IL-6 that occurs in vivo after LPS injection would be blocked in animals previously treated with EPO. We found that IL-6 mRNA as well as serum and intrahepatic IL-6 levels were similar in mice treated with LPS alone and mice treated with both EPO and LPS (Figure 3A-C). Furthermore, EPO treatment was able to completely inhibit IL-6–induced hepcidin expression (Figure 3D). These data reveal that in vivo, an IL-6 deficit is unlikely to explain EPO inhibition of hepcidin expression induced by LPS.
EPO inhibits LPS-mediated hepcidin induction independently of IL-6 production. (A) IL-6 mRNA levels in liver, (B) IL-6 levels in serum, and (C) intrahepatic IL-6 protein levels in mice treated with saline (CTL), EPO, LPS, and mice with combined treatments (EPO + LPS). (D) Hepcidin mRNA levels in the liver of mice treated with saline (CTL), EPO, mouse recombinant IL-6 (IL-6), and mice with combined treatments (EPO + IL-6). IL-6 and hepcidin mRNA levels were quantified by real-time RT-PCR and normalized to β-actin. The IL-6/β-actin and hepcidin/β-actin ratios are shown. IL-6 protein levels were measured by ELISA. Statistical analysis was performed by 1-way ANOVA; *P < .01 and **P < .001 for comparison with control mice. Data are presented as means plus or minus SD with n = 6 mice per group in panels A through C and as individual mice in panel D. n.s. indicates not significant.
EPO inhibits LPS-mediated hepcidin induction independently of IL-6 production. (A) IL-6 mRNA levels in liver, (B) IL-6 levels in serum, and (C) intrahepatic IL-6 protein levels in mice treated with saline (CTL), EPO, LPS, and mice with combined treatments (EPO + LPS). (D) Hepcidin mRNA levels in the liver of mice treated with saline (CTL), EPO, mouse recombinant IL-6 (IL-6), and mice with combined treatments (EPO + IL-6). IL-6 and hepcidin mRNA levels were quantified by real-time RT-PCR and normalized to β-actin. The IL-6/β-actin and hepcidin/β-actin ratios are shown. IL-6 protein levels were measured by ELISA. Statistical analysis was performed by 1-way ANOVA; *P < .01 and **P < .001 for comparison with control mice. Data are presented as means plus or minus SD with n = 6 mice per group in panels A through C and as individual mice in panel D. n.s. indicates not significant.
Erythropoietin but not hypoxia partially inhibits phosphorylation of Stat3 mediated by LPS
IL-6 production causes the activation of STAT3 in hepatocytes, which in turn induces hepcidin.24,25 To analyze whether EPO would inhibit Stat3 activation by LPS in mice, we undertook phosphoimmunoblotting of Stat3 in liver nuclear extracts (Figure 4A), followed by chemiluminescence quantification and normalization to β-actin as loading control (Figure 4B). We found that EPO treatment by itself reduced basal phosphorylation levels of Stat3 in the liver (P < .01), whereas LPS induced both total Stat3 and its phosphorylation in the liver. Importantly, the phosphorylated Stat3/β-actin ratio was 54% lower in mice pretreated with EPO before LPS injection compared with animals treated with LPS alone.
Stat3 phosphorylation induced by LPS is partially inhibited by EPO but not by hypoxia. (A,C) Liver nuclear extracts from mice treated with saline (CTL), EPO, hypoxia, LPS, and mice with combined treatments (EPO + LPS and hypoxia + LPS) were analyzed by Western blotting with an antibody to phosphorylated Stat3 and total Stat3. Blots were stripped and reprobed with an antibody to β-actin as loading control. A representative Western blot is shown. Lane “+” is a positive control consisting of total cell extracts from serum-starved HeLa cells prepared with interferon-α treatment. (B,D) Quantification of chemiluminescence to calculate the ratio of phosphorylated Stat3 relative to β-actin (pStat3/β-actin). This experiment was repeated twice, and the combined results are shown as means plus or minus SD with n = 7. Statistical analysis was performed by 1-way ANOVA; *P < .01, **P < .001; and ***P < .0001 for comparison with control mice. n.s. indicates not significant.
Stat3 phosphorylation induced by LPS is partially inhibited by EPO but not by hypoxia. (A,C) Liver nuclear extracts from mice treated with saline (CTL), EPO, hypoxia, LPS, and mice with combined treatments (EPO + LPS and hypoxia + LPS) were analyzed by Western blotting with an antibody to phosphorylated Stat3 and total Stat3. Blots were stripped and reprobed with an antibody to β-actin as loading control. A representative Western blot is shown. Lane “+” is a positive control consisting of total cell extracts from serum-starved HeLa cells prepared with interferon-α treatment. (B,D) Quantification of chemiluminescence to calculate the ratio of phosphorylated Stat3 relative to β-actin (pStat3/β-actin). This experiment was repeated twice, and the combined results are shown as means plus or minus SD with n = 7. Statistical analysis was performed by 1-way ANOVA; *P < .01, **P < .001; and ***P < .0001 for comparison with control mice. n.s. indicates not significant.
We similarly examined activation levels of Stat3 in hypoxic mice treated with LPS. As shown in Figure 4C and 4D, hypoxia alone led to a reduction of basal Stat3 phosphorylation levels in the liver (P < .01). However, unlike EPO, hypoxia was insufficient in influencing LPS-induced Stat3 activation. Taken together, these data suggest that mechanistically, EPO pretreatment may result in deficits in LPS-induced Stat3-signaling pathways without affecting IL-6 production.
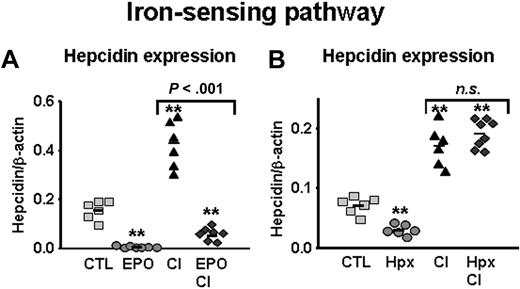
Erythropoietin but not hypoxia blocks hepcidin induction by dietary iron supplementation
EPO.
Next, we tested whether EPO treatment could similarly block the induction of hepcidin by iron, another well-established hepcidin inducer.8 Mice were placed on a carbonyl-iron (CI)–supplemented diet and treated with EPO for 5 days before being analyzed, as described in “Methods.” As expected, iron-loaded mice presented a 5.2-fold increase in liver iron concentrations compared with mice kept on the standard diet, reflecting a significant rise in iron stores (from 233 ± 8 μg iron/g dry weight in control mice to 1207 ± 303 μg iron/g dry weight in iron-loaded mice; P < .001). Hepcidin elevation evoked by iron-loading was substantially blocked by EPO treatment (∼86% inhibition; Figure 5A; CI vs EPO + CI; P < .001). However, as similarly observed with LPS, the reduction was not complete since hepcidin levels remained higher in mice with the combined EPO and CI treatments than in mice treated with EPO alone (EPO vs EPO + CI; P < .001).
EPO but not hypoxia inhibits hepcidin induction through the iron-sensing pathway. (A) Hepcidin mRNA levels in the liver of mice treated with saline (CTL), EPO, LPS, carbonyl iron–supplemented diet (2.5% CI), and mice with combined treatments (EPO + CI). (B) Hepcidin mRNA levels in the liver of mice treated with saline (CTL), mice subjected to 10% oxygen (Hpx), 2.5% CI, and mice with combined treatments (Hpx + CI). Hepatic hepcidin expression was quantified by real-time RT-PCR and normalized to β-actin. The hepcidin/β-actin ratios are shown, each symbol representing one mouse. Statistical analysis was performed by 1-way ANOVA; **P < .001 for comparison with control mice. n.s. indicates not significant.
EPO but not hypoxia inhibits hepcidin induction through the iron-sensing pathway. (A) Hepcidin mRNA levels in the liver of mice treated with saline (CTL), EPO, LPS, carbonyl iron–supplemented diet (2.5% CI), and mice with combined treatments (EPO + CI). (B) Hepcidin mRNA levels in the liver of mice treated with saline (CTL), mice subjected to 10% oxygen (Hpx), 2.5% CI, and mice with combined treatments (Hpx + CI). Hepatic hepcidin expression was quantified by real-time RT-PCR and normalized to β-actin. The hepcidin/β-actin ratios are shown, each symbol representing one mouse. Statistical analysis was performed by 1-way ANOVA; **P < .001 for comparison with control mice. n.s. indicates not significant.
Hypoxia.
To test whether hypoxia could also inhibit the induction of hepcidin expression in dietary iron-loading, mice were placed on an iron-enriched diet (2.5% CI) for 2 weeks. For combined treatment, the animals were simultaneously exposed to hypoxia for the last 3 days of the experiment. Again, feeding mice the iron-enriched diet led to a 5.5-fold increase in iron stores. As shown in Figure 5B, suppression of hepcidin mRNA levels by hypoxia was completely inhibited in iron-loaded mice, as hepcidin rose to similar levels as in mice exposed to the iron-enriched diet alone (2.7- and 2.4-fold induction, respectively).
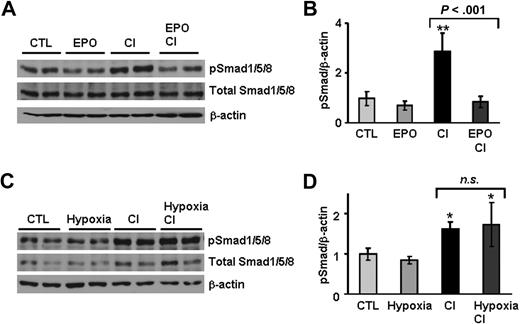
EPO but not hypoxia inhibits phosphorylation of Smad1/5/8 mediated by iron
Regulation of hepcidin expression by the iron-sensing pathway involves signaling through the bone morphogenetic protein and Sma- and Mad-related protein 4 (BMP/SMAD4) pathway that regulates hepcidin via the Smad1, Smad5, and Smad8 set of Smad proteins.26 To ascertain the potential role of this signaling pathway in the regulation of hepcidin by antagonistic stimuli, we examined the effects of dietary iron-loading on hepatic Smad1/5/8 activation. As shown in Figure 6, iron-loading led to a 3-fold induction of phosphorylated Smad1/5/8 in the liver compared with basal levels in control mice. Most importantly, EPO treatment of iron-loaded mice completely inhibited this increase in Smad1/5/8 phosphorylation (Figure 6A,B). In contrast, activation levels of the SMAD4 pathway in hypoxic mice challenged with iron-loading were similar to those induced by dietary iron-loading alone, indicating that hypoxia is insufficient to block activation of the SMAD4 pathway by iron (Figure 6C,D).
Smad1/5/8 phosphorylation induced by dietary iron-loading is partially inhibited by erythropoietin, but not by hypoxia. (A,C) Liver nuclear extracts from mice treated with saline (CTL), EPO, 2.5% CI, hypoxia, and mice with combined treatments (EPO + CI and Hypoxia + CI) were analyzed by Western blotting with an antibody to phosphorylated Smad1/5/8 and total Smad1/5/8. Blots were stripped and reprobed with an antibody to β-actin as loading control. A representative Western blot is shown. (B,D) Quantification of chemiluminescence to calculate the ratio of phosphorylated Smad1/5/8 relative to β-actin (pSmad/β-actin). This experiment was repeated twice, and the combined results are shown as means plus or minus SD with n = 7. Statistical analysis was performed by 1-way ANOVA; *P < .01; and **P < .001 for comparison with control mice. n.s. indicates not significant.
Smad1/5/8 phosphorylation induced by dietary iron-loading is partially inhibited by erythropoietin, but not by hypoxia. (A,C) Liver nuclear extracts from mice treated with saline (CTL), EPO, 2.5% CI, hypoxia, and mice with combined treatments (EPO + CI and Hypoxia + CI) were analyzed by Western blotting with an antibody to phosphorylated Smad1/5/8 and total Smad1/5/8. Blots were stripped and reprobed with an antibody to β-actin as loading control. A representative Western blot is shown. (B,D) Quantification of chemiluminescence to calculate the ratio of phosphorylated Smad1/5/8 relative to β-actin (pSmad/β-actin). This experiment was repeated twice, and the combined results are shown as means plus or minus SD with n = 7. Statistical analysis was performed by 1-way ANOVA; *P < .01; and **P < .001 for comparison with control mice. n.s. indicates not significant.
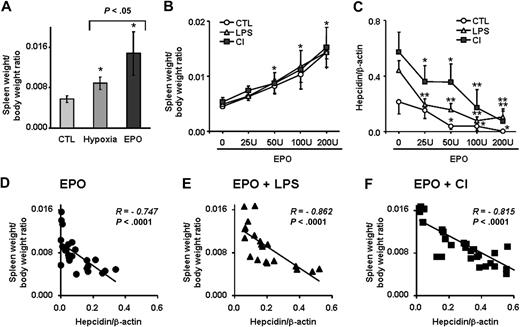
Suppression of hepcidin by the erythroid regulator depends on the degree of erythropoiesis activity
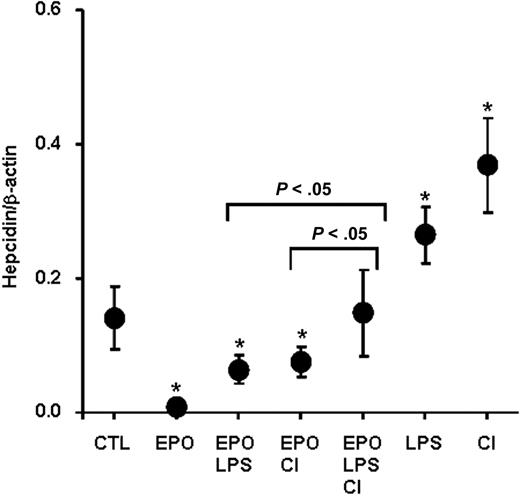
While EPO significantly blocked hepcidin induction by LPS and dietary iron, this inhibitory effect was not total, as shown in Figures 2A and 5A. Furthermore, we observed that spleen–body weight ratio, as an indicator of extramedullary erythropoiesis activity, was significantly higher in mice treated with EPO than in hypoxic mice (Figure 7A). Because EPO production is also stimulated during hypoxia,27 we questioned whether dominance of the erythroid over the immune and stores regulator as opposed to hypoxia depends on the extent of erythropoietic activity. To this end, we treated mice with increasing amounts of EPO for 4 days, from a total of 25 U (4 × 6.25 U) to 200 U (4 × 50 U), alone or in combination with LPS injections or with the CI-supplemented diet. We found a dose-dependent increase in the spleen–body weight ratio that was indistinguishable between mice treated with EPO alone and mice treated with EPO in combination with LPS or CI (Figure 7B), indicating that discrete degrees of erythropoietic activity were attained in all treatment groups. Importantly, the capacity of EPO to block hepcidin induction by LPS or CI was strongly dependent on EPO dosage, with lower EPO dosages being significantly less effective in suppressing hepcidin expression compared with higher dosages (Figure 7C). The dose-dependent effect of erythropoietic activity on the inhibition of hepcidin in the presence of opposing stimuli was further supported by the strong inverse correlation of hepcidin mRNA concentration with the spleen–body weight ratio found in each treatment group (Figure 7D-F; Spearman R = −0.747 for EPO alone; R = −0.862 for EPO + LPS; and R = −0.815 for EPO + CI; P < .0001), indicating that dominance of the erythroid regulator over inflammatory and store regulators depends on the degree of erythropoiesis activity. Finally, when both LPS and dietary iron treatments were combined to antagonize EPO-mediated hepcidin suppression, an additive effect of LPS and dietary iron counteracting EPO treatment was observed, with significantly higher hepcidin levels found in mice with the triple treatment (ie, EPO, LPS, and CI) compared with double treatments (ie, mice treated with EPO + LPS or EPO + CI [P < .05; Figure 8]).
Relationship between erythropoiesis rate and hepatic hepcidin expression. (A) Spleen weight–body weight ratio in control, hypoxic, and EPO-treated mice (50 U for 4 days). (B) Spleen weight–body weight ratio and (C) hepatic hepcidin expression in mice treated with increasing amounts of EPO (total dosage over 4 days is shown) alone (CTL) and in combination with LPS or CI-supplemented diet (CI). (A-C) Data are presented as means plus or minus SD with n = 5 to 6 mice per group. Statistical analysis was performed by 1-way ANOVA; *P < .01 and **P < .001 for comparison with control mice. (D-F) Negative correlation between spleen weight–body weight ratio and hepatic hepcidin expression in mice treated with increasing amounts of EPO: (D) alone (EPO); (E) in combination with LPS (EPO + LPS); and (F) in combination with CI-supplemented diet (EPO + CI). Hepatic hepcidin expression was quantified by real-time RT-PCR and normalized to β-actin. The hepcidin/β-actin ratios are shown.
Relationship between erythropoiesis rate and hepatic hepcidin expression. (A) Spleen weight–body weight ratio in control, hypoxic, and EPO-treated mice (50 U for 4 days). (B) Spleen weight–body weight ratio and (C) hepatic hepcidin expression in mice treated with increasing amounts of EPO (total dosage over 4 days is shown) alone (CTL) and in combination with LPS or CI-supplemented diet (CI). (A-C) Data are presented as means plus or minus SD with n = 5 to 6 mice per group. Statistical analysis was performed by 1-way ANOVA; *P < .01 and **P < .001 for comparison with control mice. (D-F) Negative correlation between spleen weight–body weight ratio and hepatic hepcidin expression in mice treated with increasing amounts of EPO: (D) alone (EPO); (E) in combination with LPS (EPO + LPS); and (F) in combination with CI-supplemented diet (EPO + CI). Hepatic hepcidin expression was quantified by real-time RT-PCR and normalized to β-actin. The hepcidin/β-actin ratios are shown.
Additive effect of LPS and dietary iron on EPO-mediated hepcidin suppression. Hepatic hepcidin expression in control mice (CTL), mice treated with EPO alone (50 U for 4 days; EPO) and in combination with LPS (EPO + LPS), with CI-supplemented diet (EPO + CI), with both LPS and CI (EPO + LPS + CI), with LPS alone (LPS), and CI-supplemented diet alone (CI). Data are presented as means plus or minus SD with n = 6 mice per group. Statistical analysis was performed by 1-way ANOVA; *P < .05 for comparison with control mice.
Additive effect of LPS and dietary iron on EPO-mediated hepcidin suppression. Hepatic hepcidin expression in control mice (CTL), mice treated with EPO alone (50 U for 4 days; EPO) and in combination with LPS (EPO + LPS), with CI-supplemented diet (EPO + CI), with both LPS and CI (EPO + LPS + CI), with LPS alone (LPS), and CI-supplemented diet alone (CI). Data are presented as means plus or minus SD with n = 6 mice per group. Statistical analysis was performed by 1-way ANOVA; *P < .05 for comparison with control mice.
Discussion
Hepcidin has emerged as a central negative regulator of intestinal iron absorption and distribution that can be induced through at least 2 major pathways, namely the inflammatory pathway and the iron-sensing pathway.28,29 In this study, we investigated its regulation through these 2 pathways in the presence of antagonistic stimuli.
Inflammatory pathway
In the first set of experiments, we compared the capacity of erythropoietic drive stimulated by EPO and hypoxia to suppress hepcidin induction by inflammation, which we triggered by LPS injections in our model. Of note, LPS appears to be a relatively weak inflammatory stimulus in mice compared with other inflammatory conditions,9,30 since in our experiments, it induced only a 2-fold increase in hepcidin mRNA levels. Regardless, our results show that hypoxia alone may be insufficient to inhibit hepcidin induction by LPS. In contrast, EPO led to inhibition of hepcidin induction by LPS, an effect that we found to be dependent on the degree of erythropoietic activity. The induction of hepcidin during inflammatory and infectious states can be mediated, at least in hepatocytes, by cytokines, mainly IL-6,22 and subsequent STAT3 signaling. This pathway may be initiated by Toll-like receptor signaling leading to the induction of inflammatory cytokines,12 including IL-6, which binds to its membrane-bound receptor (gp80) on hepatocytes and interacts with gp130, resulting in Stat3 activation and binding to a regulatory element in the hepcidin promoter.24,25,31 Recent studies have demonstrated that EPO potentially has antiinflammatory properties (eg, against brain injury,32 myocardial dysfunction induced by ischemia/reperfusion,33 and chronic heart failure),34 and that EPO protection involves the suppression of inflammatory cytokines, including IL-6.34,35 In our models, IL-6 production after LPS injection remained unaffected by EPO pretreatment, indicating that an IL-6 deficit is unlikely to explain EPO suppression of hepcidin in an inflammatory context. However, we found that EPO inhibits downstream signaling of both basal and LPS-induced levels in the liver by suppressing Stat3 phosphorylation. To our knowledge, this is the first evidence that EPO might modulate hepatic hepcidin expression via suppression of STAT3 signaling in the liver in vivo.
Iron-sensing pathway
In the second set of experiments, we evaluated the ability of hypoxia and EPO to block the up-regulation of hepcidin induced by dietary iron-loading, thus counteracting the iron-sensing pathway. We found that hypoxia was insufficient to block hepcidin up-regulation by iron. However, stimulation of erythropoiesis by EPO partially suppressed hepcidin expression in iron-loaded mice, indicating that the iron-sensing pathway can be blocked by erythropoietic drive.
The iron-sensing pathway involves the activation of BMP/SMAD4 signaling, which is initiated after binding of BMP cytokines to BMP receptors, leading to the generation of phosphorylated RSmads, which dimerize with Smad4. The RSmad/Smad4 heterodimers translocate into the nucleus and presumably activate transcription of the hepcidin gene.26 We found that dietary iron-loading in mice results in activation of the SMAD4 pathway, as judged by the induction of Smad1/5/8 phosphorylation. Similar results have been reported with parenteral iron challenge.36 Importantly, we show that EPO pretreatment inhibits the activation of this pathway by iron, providing evidence that in vivo, EPO effects on hepcidin expression are mediated by the suppression of SMAD4 signaling.
EPO has been shown to be able to modulate hepcidin expression directly in hepatocytes or indirectly through serum mediators. In fact, in vitro, EPO may directly suppress hepcidin expression in a dose-dependent manner in the human hepatocyte cell line HepG2.37 In vivo, however, it has been demonstrated that during erythropoiesis stimulated by EPO, phlebotomy, or phenylhydrazine, hepcidin suppression is blocked in mice in which erythropoiesis is simultaneously inhibited, indicating that the regulation of hepcidin by EPO is indirect and that it requires increased erythropoiesis and possibly the release of some mediator(s).38,39 Alternatively, EPO may interfere with signal transduction at the IL-6/gp80 receptor level and downstream signaling via gp130, which has been shown to be essential for hepcidin activation through STAT3.31
Dominance between regulators for hepcidin expression
Recent studies in patients with thalassemia syndromes revealed that, despite elevated iron parameters present in patients with thalassemia intermedia, their urinary hepcidin levels are severely depressed, indicating that the erythropoietic drive may have a dominant effect over the iron signal.40,41 However, several confounding factors, including the effect of iron chelation, inflammatory conditions such as hepatitis C, presence or absence of splenectomy, and the presence of iron-induced end-organ damage may render the assessment of the influence of opposing stimuli for hepcidin regulation more difficult in these patients.40 In animal models, assessment of the influence of opposing signals for hepcidin expression has been performed by using only single dosages of the antagonistic stimuli. These experiments found that iron-loaded mice subjected to experimentally induced anemia showed reduced hepcidin expression,9 while iron-deficient mice injected with LPS up-regulated hepcidin expression,12 suggesting dominant effects of the erythroid and inflammatory regulators over iron stores.
Our present results further advance those previous studies by showing that hepcidin expression levels in the presence of opposing signals, namely LPS and dietary iron, depend on the dosage of EPO used. Furthermore, we found that LPS and dietary iron can act additively to induce hepcidin in vivo, a finding that is in agreement with recently reported data of a synergistic induction of hepcidin expression by BMPs and IL-6 in vitro.42 Taken together, these data indicate that final hepcidin levels are determined by the individual strength of the regulators rather than by an absolute hierarchy among the pathways. This notion is further supported by the finding that these effects are mediated, at least partially, through inhibition of STAT3 and BMP/SMAD4 signaling in vivo, and thus offer an explanation as to how EPO suppression of hepcidin expression in the presence of antagonistic stimuli is dose dependent. Dose dependency also may explain why hypoxia seems to be unable to antagonize LPS or dietary iron, since much lower endogenous EPO levels are elicited by hypoxia compared with the high pharmacologic doses of EPO used in our experiments.
In summary, the present data contribute to the elucidation of dominance among the regulators of hepcidin expression and provide insights into the mechanisms by which erythropoietic drive, stimulated by EPO, affects both inflammatory and iron-sensing pathways via suppression of STAT3 and SMAD4 signaling in vivo.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge the technical help of Dongwang Mei and Alexandre Reuben. We also thank Dr Alexandre Grassino for his help with the hypoxia model.
This work was supported by grants from the Canadian Institutes of Health Research (grant no. MOP44045) and the Natural Sciences and Engineering Research Council of Canada (grant no. 298518-06). M.M.S. is the recipient of a Research Scholarship (Junior 2) from the FRSQ (Fonds de la Recherche en Santé du Québec).
Authorship
Contribution: H.H. designed and performed research, analyzed data, and wrote the paper; M.C. performed research and analyzed data; A.L. performed research and analyzed data; and M.M.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Manuela Santos, Centre de Recherche, CHUM—Hôpital Notre-Dame, Pavillon De Sève Y5625, 1560 rue Sherbrooke est, Montréal, QC H2L 4M1, Canada; e-mail: manuela.santos@umontreal.ca.

![Figure 2. Erythropoietin but not hypoxia inhibits hepcidin induction through the inflammatory pathway. (A) Hepcidin mRNA levels in the liver of mice treated with saline (control [CTL]), EPO, LPS, and mice with combined treatments (EPO + LPS). (B) Hepcidin mRNA levels in the liver of mice treated with saline (CTL), mice subjected to 10% oxygen (hypoxia [Hpx]), LPS, and mice with combined treatments (Hpx + LPS). Hepatic hepcidin expression was quantified by real-time RT-PCR and normalized to β-actin. The hepcidin/β-actin ratios are shown, each symbol representing 1 mouse. Statistical analysis was performed by 1-way ANOVA; **P < .001 for comparison with control mice. n.s. indicates not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/15/10.1182_blood-2008-08-173641/7/m_zh80180934400002.jpeg?Expires=1769091888&Signature=KRn4hMviJER6iuwrr4RKbAWUaGNmTtPYE9i7iVd8itb5YGr7yhOP6A4iN81Zs5fxMNJFqEcr2nuc8YJq4p1GjaQun3F5QJMrw84YYXBUjGGnOWcyOK9GwmhHYme1KbdoIH0DI8ZW2jRElRyhUpM06R1P0u1WSsmqw49UE9DDUwgeKfmieAL7tWaTclSpB8lpzoRCJPYfBGlA~b2lEaZqTahshoGYOGHYq9HVSIQy5Ehs0jzvUpt~nuzHUl-6v1QiCAvp4BoOb1JDRP5NIat~ycPfCE0aUvB3u-TkqS-2tPsQxggsViInugdLu66ZBFJKef2qhdwUDJg-7CMFRGM7dA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal