Abstract
APO866 inhibits nicotinamide phosphoribosyltransferase (NMPRTase), a key enzyme involved in nicotinamide adenine dinucleotide (NAD) biosynthesis from the natural precursor nicotinamide. Intracellular NAD is essential for cell survival, and NAD depletion resulting from APO866 treatment elicits tumor cell death. Here, we determine the in vitro and in vivo sensitivities of hematologic cancer cells to APO866 using a panel of cell lines (n = 45) and primary cells (n = 32). Most cancer cells (acute myeloid leukemia [AML], acute lymphoblastic leukemia [ALL], mantle cell lymphoma [MCL], chronic lymphocytic leukemia [CLL], and T-cell lymphoma), but not normal hematopoietic progenitor cells, were sensitive to low concentrations of APO866 as measured in cytotoxicity and clonogenic assays. Treatment with APO866 decreased intracellular NAD and adenosine triphosphate (ATP) at 24 hours and 48 to72 hours, respectively. The NAD depletion led to cell death. At 96 hours, APO866-mediated cell death occurred in a caspase-independent mode, and was associated with mitochondrial dysfunction and autophagy. Further, in vivo administration of APO866 as a single agent prevented and abrogated tumor growth in animal models of human AML, lymphoblastic lymphoma, and leukemia without significant toxicity to the animals. The results support the potential of APO866 for treating hematologic malignancies.
Introduction
Although existing anticancer agents induce cell death by a variety of mechanisms, development of resistance commonly occurs. Novel approaches to circumvent tumor cell resistance mechanisms are therefore required and, in this context, several anticancer agents have recently been described that induce cell death by manipulating cellular energy stores. Indeed, intracellular depletion of nicotinamide adenine dinucleotide (NAD) and adenosine triphosphate (ATP) exerts strong cytolytic effects,1-8 and the antitumor agent APO866 (previously called FK866 or WK175) is reported to induce cell death by reducing intracellular NAD content.9,10
NAD plays a crucial role as a cofactor/substrate in numerous biochemical and biologic processes, including those catalyzed by poly(ADP-ribose) polymerase 1 (PARP1), sirtuins, and ADP-ribosyl cyclase 1-6. Since nicotinamide phosphoribosyltransferase (NMPRTase) activity is essential for replenishing cellular NAD levels, it represents an attractive therapeutic target for the development of new anticancer agents.9,10 Furthermore, since cancer cells have a higher rate of NAD turnover compared with normal cells due to genomic instability and persistent PARP1-dependent DNA repair,11,12 targeting NAD synthesis is expected to selectively kill cancer cells.
Published data have shown that APO866: (1) kills tumor cells in vitro from lines mostly originating from solid tumors,9,13-15 (2) exhibits antiangiogenic properties that may contribute to limiting tumor growth and metastasis development in a human solid renal cell carcinoma xenograft model,16 and (3) demonstrates a capacity to enhance the effectiveness of radiation therapy.17 A phase 1 clinical study of APO866 has recently been completed.18
Based on the reported effects of APO866, we sought to investigate both its in vitro cytotoxic effects using a cell panel of human hematologic malignancies, and its in vivo effects on early and established human hematologic cancers, areas that have not been studied to date. We show that APO866 induces cell death at low concentrations in various human hematologic malignancies, including leukemias (acute myeloid leukemia [AML], acute lymphoblastic leukemia [ALL], chronic lymphocytic leukemia [CLL]), and various T- and B-cell lymphomas, whereas it has no significant effect on normal hematopoietic progenitor cells (HPCs). Furthermore, in vivo administration of APO866 at well-tolerated doses prevents and eradicates tumor growth in animal models of several human hematologic malignancies.
Methods
Chemical reagents and monoclonal antibodies
Clinical grade APO866 and Fas agonist (MegaFasL) were provided by TopoTarget (Lausanne, Switzerland). The pan caspase inhibitor (zVAD-fmk) was obtained from BachemAG (Bubendorf, Switzerland). Caspase 3 (zDEVD-fmk), caspase 8 (zIETD-fmk), and caspase 9 inhibitors (zLEHD-fmk) were purchased from R&D Systems (Minneapolis, MN). The 5,5′,6,6′–tetrachloro-1,1′,3,3′–tetraethylbenzimididazolyl-carbocyanine iodide (JC-1) was purchased from Calbiochem (San Diego, CA). β-Nicotinamide adenine dinucleotide (β-NAD), phenazine ethosulfate (PES), alcohol deshydrogenase (ADH), thiazolyl blue tetrazolium bromide (MTT), autophagy inhibitors (wortmannin, LY294002, 3-methyladenine, bafilomycin A1), and cycloheximide were purchased from Sigma-Aldrich (St Louis, MO). Micro BCA Protein Assay Kit was purchased from Pierce (Rockford, IL). Annexin V–fluorescein isothiocyanate (FITC) and 7-aminoactinomycin D (7AAD) were purchased from Becton Dickinson Biosciences Pharmingen (San Diego, CA).
The following monoclonal antibodies were purchased from Immunotech (Marseille, France): phycoerythrin (PE)–conjugated mouse anti–human CD5; energy coupled dye (ECD)–conjugated mouse anti–human CD3; CD10; phycoerythrin covalently linked to cyanine 7 (PC7)–conjugated purified mouse anti–human CD19. PE-conjugated mouse anti–human CD33 was purchased from Becton Dickinson Biosciences Pharmingen.
Cell lines, primary cells, and culture conditions
A panel of 45 hematologic cancer cell lines was evaluated. The following cell lines were purchased from DSMZ (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) or ATCC (Manassas, VA): ML-2, NOMO-1, MV4–11, Mutz-2, NB-4, MOLM-13, HEL, OCI-M1, Kasumi-1, SKM-1, UT-7, THP-1, HL-60 (AML); CCRF-CEM, MOLT4 (B-acute lymphoblastic leukemia [B-ALL]); Jurkat (T-ALL); Mec-2, EHEB, Mec-1 (B-CLL); K562 (chronic myelogenous leukemia [CML]); Ramos, Namalwa, Daudi, Raji (Burkitt lymphoma [BL]); DOHH-2, SC-1 (follicular lymphoma [FL]); and OPM-2, MOLP-8, KSM-12-BM, EJM, RPMI-8226, ARH-77, U266 (multiple myeloma [MM]). The following cell lines were kindly supplied by Professor Pascal Schneider (University of Lausanne, Lausanne, Switzerland): Ramos (BL); L428 (Hodgkin lymphoma); IM9, SKW6.4 (B-lymphoblastoid lymphoma); Hut78 (cutaneous T-cell lymphoma [CTCL]); U937 (histiocytic lymphoma); L363 (MM); Jurkat cells and Jurkat cells deficient in FADD (clone I.21), caspase 8 (clone I.92), and RIP. Raji-GFP cells were a gift from Dr Peter J. Sims (The Scripps Research Institute, La Jolla, CA).
Primary cells from 32 consenting patients were also analyzed. Study protocols were approved by the ethics committee at the University of Lausanne, and informed consent was obtained in accordance with the Declaration of Helsinki. Primary cells were collected from either bone marrow or peripheral blood (purity > 80%) from patients with AML (n = 10; AML 1-8, M5; AML 9, M2; and AML 10, secondary AML); ALL (n = 4; ALL 1, early pre-B, CALLA+; ALL 2-4, pre-B, CALLA−); CLL (n = 11); T-cell lymphoma (n = 2); marginal zone lymphoma (n = 3); mantle cell lymphoma (n = 1); and 1 follicular lymphoma (n = 1). Cells were frozen in medium containing 10% dimethyl sulfoxide [DMSO] within 24 hours of harvesting. Unpurified human CD34+ cells (enriched for HPCs) from mobilized peripheral blood (mPB) were obtained from AllCells (San Mateo, CA), and purified using an anti-CD34 magnetic bead column (Miltenyi Biotec, Bergisch Gladbach, Germany). All cells were cultured in RPMI (Gibco, Paisley, United Kingdom) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco) and 1% penicillin/streptomycin at 37°C (Bioconcept, Allschwil, Switzerland) in a humidified atmosphere of 95% air and 5% CO2.
Measurement of cell survival using MTT assay and trypan blue dye exclusion staining
For MTT assays, 0.5 × 106 cells/mL was plated in triplicate on 96-well plates. APO866 (0.01 nM-100 nM) was added in 50 μL of culture medium, with culture medium alone serving as control. After 72 or 96 hours of incubation, 15 μL of dye solution was added to each well and cells were incubated for an additional 4 hours. Stop solution (100 μL/well) was added for 1 hour and the absorbance was read at 570 nm on a spectrophotometer.
For trypan blue dye exclusion staining, 0.5 × 105 cells/well was grown in 6-well plates with 1 mL media in the absence or presence of APO866 for 96 hours. Cells from each sample were incubated with 10 μL trypan blue solution (at a 1:1 ratio [vol/vol] for 1 minute). Cell survival was determined by calculating proportion of live (unstained) cells.
Characterization of cell death
Cells were cultured with APO866 (0.01 nM-100 nM) for 96 hours. For caspase inhibition assays, cells were pretreated with zVAD-fmk (100 μM), zDEVD-fmk, zIETD-fmk, or zLEHD-fmk (50 μM) for at least 2 hours before addition of APO866. For inhibition of autophagy, cells were incubated with the inhibitors wortmannin (0.25 μM), LY294002 (5 μM), 3-methyladenine (3MA; 10 mM), and bafilomycin A1 (10 nM) for at least 30 minutes before APO866 treatment. APO866-induced cell death was assessed using annexin V and 7AAD stainings as described by the manufacturer and analyzed using a Beckman Coulter Cytomics FC500 flow cytometer. Dead cells were identified as annexin V+ and/or 7AAD+.
Determination of intracellular NAD content
Intracellular NAD content was measured in cell lysates using a biochemical assay described elsewhere.9 Cells (106/mL) in log growth phase were seeded in 24-well plates with or without APO866, and incubated for 24 hours. Cells were then centrifuged at 900g (2000 rpm) for 5 minutes. Supernatant was discarded and cells were resuspended in 100 μL lysis buffer and kept at −80°C for at least 4 hours before analysis. Cell lysate (20 μL) was plated in a 96-well flat-bottom plate. A standard curve was generated using a 1:3 serial dilution in lysis buffer of a β-NAD stock solution. Cycling buffer (160 μL) was added into each well and the plate was incubated for 5 minutes at 37°C. Ethanol (20 μL), prewarmed at 37°C, was added into each well and the plate was incubated for an additional 5 minutes at 37°C. Absorbance at 570 nm was read after 5, 10, 15, 20, and 30 minutes at 37°C on a spectrophotometer. The amount of NAD in each sample was normalized to the protein content for each test sample.
Determination of intracellular ATP content
Intracellular ATP content was measured according to the manufacturer's instructions (Invitrogen, Eugene, OR). In brief, logarithmically growing cells (106/mL) were seeded in 24-well plates with or without increasing concentrations of APO866. Cells were subsequently centrifuged at 900g (2000 rpm) for 5 minutes at different time points (24-96 hours), resuspended in 100 μL lysis buffer and kept at −80°C for at least 4 hours before further manipulations. The standard reaction solution (90 μL) was distributed into each well of the luminometer plate and the background luminescence was measured. Cell lysate (10 μL) or ATP standard curve samples were distributed in the wells containing 90 μL reaction buffer, and luminescence was measured using the luminometer. The amount of ATP in each sample was normalized to protein content.
Assessment of mitochondrial membrane potential
Mitochondrial membrane depolarization was determined using the JC-1 lipophilic cation fluorescent dye. JC-1 accumulates in the mitochondria, showing green fluorescence at a low mitochondrial membrane potential (MMP) and forming red fluorescent J-aggregates at higher membrane potential. Drop-in mitochondrial membrane potential is indicated by a decrease in the ratio of the red signal to the green signal. Briefly, cells were cultured in the absence or presence of various concentrations of APO866 for 24 to 96 hours. Cells were centrifuged, resuspended in culture medium containing 5 μM JC-1, and were then incubated at 37°C for 15 minutes in the dark. The cells were washed twice with phosphate-buffered saline (PBS), and immediately analyzed using flow cytometry.
Clonogenic assay
Tumor cells (Namalwa, Raji-GFP, ML-2, Molt-4, EHEB) or normal human HPCs incubated for 96 hours with or without 10 nM APO866 were evaluated for their capacity to subsequently form colonies on methylcellulose medium. After 3 washes, 5 × 102, 5 × 103, or 5 × 104 tumor cells, or 2 × 103 HPCs, were plated in 1 mL methylcellulose medium (Methocult GF+ H4435; StemCell Technologies, Vancouver, BC). Alternatively, mice were killed with CO2 and bone marrow cells obtained by flushing the femoral bone cavity with Iscove modified Dulbecco medium with 10% fetal bovine serum at 37°C. Viable murine cells (2.5 × 104) were plated in triplicate with APO866 concentrations ranging from 3 nM to 300 nM on 35 mm petri dishes with MethoCult M3534 methylcellulose medium (StemCell Technologies). Colonies (> 50 cells) were scored under an inverted microscope after 14 to 18 days at 37°C in a humidified atmosphere supplemented with 5% CO2.
Therapeutic efficacy evaluation of APO866 in xenograft models
The in vivo evaluation of APO866 was carried out using xenograft models of 3 human hematologic malignancies. BALB/c nude or nonleaky C.B.-17 SCID mice (6 to 10 weeks old; Iffa Credo, L'Arbresle, France) were bred and housed in micro-isolator cages in a specific pathogen-free room within the animal facilities at the University Hospital of Lausanne or TopoTarget. Animals were allowed to acclimatize to their new environment for 1 week prior to use. All animals were handled according to the respective institutional regulations after approval of the animal ethic committee of the University of Lausanne. Manipulations were performed under a laminar flow hood under sterile conditions. Mice were administered intraperitoneally with APO866 (20 mg/kg body weight) in 200 μL 0.9% saline, twice a day for 4 days, repeated weekly over 3 weeks. Control groups were treated similarly with vehicle only (200 μL 0.9% saline).
Xenograft model of human AML.
ML-2 (AML-M4) cells (2 × 107) were conditioned to grow subcutaneously on the back of BALB/c nude mice. Subsequently, tumors were excised and tumor fragments inserted through a small incision into the left flank of BALB/c nude mice. Once tumors became palpable (approximately 150 mm3 ) at day 18, mice were randomized into control and treated groups.
Xenograft model of Namalwa human Burkitt lymphoma.
Namalwa cells (107) were injected subcutaneously into the back of SCID mice. Mice were subdivided into 3 groups (n = 10): (1) early-stage disease, in which APO866 administration was initiated 1 week (day 7) after cell injection; (2) advanced-stage disease, in which treatment was initiated once the tumor reached a volume of 100 mm3 (day 14); and (3) vehicle control groups.
Xenograft model of Raji human Burkitt leukemia.
Raji-GFP cells (5 × 104) were injected into the tail vein of SCID mice in 200 μL sterile saline. One week after cell injection (day 7), animals were divided into 2 groups that received either APO866 (n = 10) or vehicle (n = 8).
All animals were monitored daily for signs of illness and killed immediately if tumor size reached a diameter of 15 mm, or if respiratory distress or hind limb paralysis were noted. Alternatively, animals surviving 180 days of observation were killed.
In vivo procedures for APO866 toxicity and pharmacokinetic studies
NMRI female mice were obtained from Taconic (Ry, Denmark). Experiments were approved by the Danish Animal Experimentation Board, Ministry of Justice. Peripheral blood counts (white blood cell, red blood cell, and platelet counts) were determined in 20 μL EDTA stabilized tail vein blood samples before treatment and one day after a 4-day treatment cycle of 20 mg/kg APO866 2 times per day using the CA530VET hematology analyzer (Boule Medonic, Stockholm, Sweden). Blood smears were prepared and stained with Hemacolor (Merck, Darmstadt, Germany); differential counts of the white blood cells were made manually. In the pharmacokinetic study, the mice were given an intraperitoneal dose of 20 mg/kg APO866 in saline. Li-Heparin stabilized blood samples were obtained at the outlined time points and placed on ice and centrifuged as fast as possible after sampling.
Bioanalysis
Plasma samples (50 μL) were protein precipitated by addition of 150 μL acetonitrile in 96-well plates and filtered. A quantity of 20 μL of the filtrate was analyzed by liquid chromatographic using a Waters Alliance 2795 system connected to a Quattro Premier MS-MS system (Waters, Milford, MA). The sample compartment was maintained at 7°C. Separation was performed using a Sunfire C18 column (2.1 mm × 50 mm, 3.5 μm; Waters) operated at 40°C. The following gradient was used: 0.05% vol/vol formic acid (A) and acetonitrile (B): 0 to 1 minute 30% B, 1 to 1.4 minutes linear gradient to 50% B, 1.4 to 1.5 minutes linear gradient to 30% B, 1.5 to 1.8 minutes at 30% B. The flow rate was 0.4 mL/min. MRM detection of APO866 using electro spray ionization in the positive mode was performed at transitions of 364.2 m/z > 105.1 m/z. The limit of quantification was 2 ng/mL (5 nM).
Polymerase chain reaction
The detection of any residual disease was carried out by amplifying t(8;14)(q24;q32) present in Burkitt lymphoma Raji cells. DNAs were extracted from xenochimeric mouse BM cells and from Raji cells using the high pure polymerase chain reaction (PCR) template preparation kit (Roche Diagnostics, Mannheim, Germany). DNAs were subjected to long-distance PCR using MYC/04 and Cγ/02 primers and an expanded long template PCR system (Roche Diagnostics) exactly as described.19
Statistical analysis
Data are expressed as mean plus or minus standard error of the mean (SEM) unless otherwise noted. Values between groups were compared using one-way analysis of variance (ANOVA), nonparametric test. Paired t tests were performed to test differences in pre- and posttreatment white blood cell counts. The Kaplan-Meier method using long rank test was applied for the analyses of animal survival studies. GraphPad Prism version 4.00 (GraphPad Software, San Diego, CA) was used for statistical analysis. P values less than .05 were considered statistically significant.
Results
APO866 selectively induces cytotoxicity in hematologic malignant cells, but not normal hematopoietic progenitor cells
The APO866 sensitivity of a panel of 41 human hematologic cancer cell lines was determined using an MTT assay. As summarized in Table 1, most hematologic cancer cells were sensitive to low concentrations of APO866 (half maximum effective concentration [EC50] between 0.09 nM and 27.2 nM). AML cells were generally most sensitive, with an EC50 between 0.09 nM and 5.80 nM. Cell viability, as measured by trypan blue dye exclusion, was confirmed in 3 cell lines; 10 nM APO866 reduced cell viability to 5%, 4%, and 8% compared with 97%, 94%, and 95% in untreated controls in ML-2 (AML), Namalwa (Burkitt lymphoma), and Jurkat (ALL) cell lines, respectively.
APO866 induces cell death in various hematologic malignant cell lines
| Disease diagnosis/Cell line name . | 96 hours APO866 . | |
|---|---|---|
| EC50 MTT, nM . | FDC, 10 nM, % . | |
| Acute myeloid leukemia | ||
| ML-2 (AML-M4) | 0.13 ± 0.03 | 99 |
| THP-1 (AML-M5) | 0.30 ± 0.05 | 99 |
| NOMO-1 (AML-M5) | 0.09 ± 0.02 | 98 |
| NB4 (AML-M3) | 2.88 ± 0.58 | 93 |
| MV4–11 (AML-M5) | 0.33 ± 0.12 | 92 |
| MOLM-13 (AML-M5) | 1.69 ± 0.35 | 92 |
| Mutz-2 (AML-M2) | 5.80 ± 0.51 | 89 |
| HEL (AML-M6) | 1.20 ± 0.16 | 74 |
| SKM-1 (AML-M5) | N/A | 73 |
| OCI-M1 (AML-M6) | N/A | 72 |
| Kasumi-1 (AML-M2) | 2.27 ± 0.15 | 68 |
| HL-60 (AML-M2) | 3.62 ± 1.33 | 67 |
| UT-7 (AML-M7) | 22.72 ± 5.15 | 47 |
| Acute lymphoblastic leukemia | ||
| Molt-4 | 1.14 ± 0.14 | 87 |
| Jurkat | 0.24 ± 0.06 | 80 |
| CCRF-CEM | 0.39 ± 0.10 | 23 |
| B-chronic lymphocytic leukemia | ||
| Mec-2 | 8.43 ± 2.89 | 73 |
| EHEB | 5.68 ± 0.65 | 65 |
| Mec-1 | 4.32 ± 0.74 | 55 |
| CML | ||
| k562 | 7.20 ± 0.92 | 36 |
| Burkitt lymphoma | ||
| Bjab | 1.34 ± 0.16 | 89 |
| Ramos | 10.64 ± 1.06 | 89 |
| Namalwa | 6.33 ± 4.45 | 87 |
| Daudi | 13.21 ± 5.26 | 36 |
| Raji | 2.22 ± 0.12 | 30 |
| Follicular lymphoma | ||
| DOHH-2 | 0.86 ± 0.03 | 89 |
| SC-1 | 2.03 ± 0.48 | 85 |
| Hodgkin lymphoma | ||
| L428 | 14.64 ± 2.12 | 57 |
| Histiocytic lymphoma | ||
| U937 | 8.35 ± 0.49 | 83 |
| Cutaneous T-cell lymphoma | ||
| Myla | 1.20 ± 0.18 | 97 |
| Hut78 | 2.83 ± 0.37 | 82 |
| Multiple myeloma | ||
| OPM-2 | 3.11 ± 0.17 | 97 |
| MOLP-8 | N/A | 90 |
| RPMI8226 | 8.98 ± 0.20 | 77 |
| IM9 | 27.22 ± 5.4 | 77 |
| ARH-77 | 12.19 ± 1.58 | 65 |
| KMS-12-BM | N/A | 63 |
| EJM | N/A | 63 |
| L-363 | 15.6 ± 1.97 | 33 |
| U266 | 26.9 ± 5.3 | 26 |
| EBV-transformed B-lymphocyte | ||
| SKW6.4 | 8.17 ± 0.7 | 81 |
| Disease diagnosis/Cell line name . | 96 hours APO866 . | |
|---|---|---|
| EC50 MTT, nM . | FDC, 10 nM, % . | |
| Acute myeloid leukemia | ||
| ML-2 (AML-M4) | 0.13 ± 0.03 | 99 |
| THP-1 (AML-M5) | 0.30 ± 0.05 | 99 |
| NOMO-1 (AML-M5) | 0.09 ± 0.02 | 98 |
| NB4 (AML-M3) | 2.88 ± 0.58 | 93 |
| MV4–11 (AML-M5) | 0.33 ± 0.12 | 92 |
| MOLM-13 (AML-M5) | 1.69 ± 0.35 | 92 |
| Mutz-2 (AML-M2) | 5.80 ± 0.51 | 89 |
| HEL (AML-M6) | 1.20 ± 0.16 | 74 |
| SKM-1 (AML-M5) | N/A | 73 |
| OCI-M1 (AML-M6) | N/A | 72 |
| Kasumi-1 (AML-M2) | 2.27 ± 0.15 | 68 |
| HL-60 (AML-M2) | 3.62 ± 1.33 | 67 |
| UT-7 (AML-M7) | 22.72 ± 5.15 | 47 |
| Acute lymphoblastic leukemia | ||
| Molt-4 | 1.14 ± 0.14 | 87 |
| Jurkat | 0.24 ± 0.06 | 80 |
| CCRF-CEM | 0.39 ± 0.10 | 23 |
| B-chronic lymphocytic leukemia | ||
| Mec-2 | 8.43 ± 2.89 | 73 |
| EHEB | 5.68 ± 0.65 | 65 |
| Mec-1 | 4.32 ± 0.74 | 55 |
| CML | ||
| k562 | 7.20 ± 0.92 | 36 |
| Burkitt lymphoma | ||
| Bjab | 1.34 ± 0.16 | 89 |
| Ramos | 10.64 ± 1.06 | 89 |
| Namalwa | 6.33 ± 4.45 | 87 |
| Daudi | 13.21 ± 5.26 | 36 |
| Raji | 2.22 ± 0.12 | 30 |
| Follicular lymphoma | ||
| DOHH-2 | 0.86 ± 0.03 | 89 |
| SC-1 | 2.03 ± 0.48 | 85 |
| Hodgkin lymphoma | ||
| L428 | 14.64 ± 2.12 | 57 |
| Histiocytic lymphoma | ||
| U937 | 8.35 ± 0.49 | 83 |
| Cutaneous T-cell lymphoma | ||
| Myla | 1.20 ± 0.18 | 97 |
| Hut78 | 2.83 ± 0.37 | 82 |
| Multiple myeloma | ||
| OPM-2 | 3.11 ± 0.17 | 97 |
| MOLP-8 | N/A | 90 |
| RPMI8226 | 8.98 ± 0.20 | 77 |
| IM9 | 27.22 ± 5.4 | 77 |
| ARH-77 | 12.19 ± 1.58 | 65 |
| KMS-12-BM | N/A | 63 |
| EJM | N/A | 63 |
| L-363 | 15.6 ± 1.97 | 33 |
| U266 | 26.9 ± 5.3 | 26 |
| EBV-transformed B-lymphocyte | ||
| SKW6.4 | 8.17 ± 0.7 | 81 |
FDC indicates fraction of dead cells at 10 nM APO866, corresponding to percentage of annexin V–stained positive cells; and N/A, not analyzed
To confirm whether the cytotoxicity exerted by APO866 involved programmed cell death and that the latter is not cell-type dependent, cells from 3 selected cell lines and primary malignant cells from various hematologic malignancies were incubated with APO866 for 96 hours, and subsequently double stained using annexin V/7AAD. APO866 elicited high levels of early (annexin V+7AAD−) and late (annexin V+7AAD+) cell death phenotypes at concentrations between 0.1 nM and 10 nM (Figure 1A-G). A time course analysis showed that cell death appeared 72 hours after exposure to 10 nM APO866 and was maximal at 96 hours (Figure 2A-G). The same analysis using 10 nM APO866 was subsequently expanded and performed on the panel of 41 hematologic malignant cells lines and on primary cells from 32 patients with various hematologic malignancies (Tables 1 and 2). Primary cells from all hematologic malignancies tested were highly sensitive to APO866. Among cell lines, those from AML-M5 were the most sensitive, with more than 90% of cells dead after 96 hours of drug exposure to 10 nM APO866. An attempt to identify cell biomarkers associated with APO866 sensitivity in hematologic cancer cells failed to detect any correlation between APO866 sensitivity and expression of key player enzymes involved in either NAD biosynthesis (indoleamine 2,3-dioxygenase and NMPRTase) or NAD metabolism (CD38 and poly-ADP-ribose polymerase-1; data not shown).
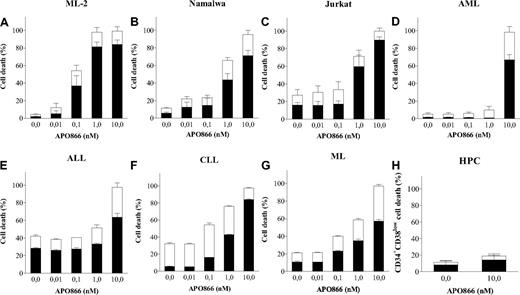
APO866 induces dose-dependent cell death in malignant cells from various hematologic malignancies but not in normal HPCs. Dose-dependent analysis of cell death induced by APO866 on cancer cells from cell lines ML-2 (A), Namalwa (B), and Jurkat (C); primary cells from patients with various hematologic malignancies, AML (D), ALL (E), CLL (F), ML (G); and from normal HPCs (H). Cell death was assessed by flow cytometry using annexin V and 7AAD double staining after 96 hours. The percentage of early apoptotic cells (annexin V+7AAD−) are shown as white columns and that of late apoptotic cells (annexin V+7AAD+) are shown as solid black columns. Data are derived from at least 3 independent experiments. AML indicates acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; ML, marginal zone lymphoma; and HPCs, hematopoietic progenitor cells.
APO866 induces dose-dependent cell death in malignant cells from various hematologic malignancies but not in normal HPCs. Dose-dependent analysis of cell death induced by APO866 on cancer cells from cell lines ML-2 (A), Namalwa (B), and Jurkat (C); primary cells from patients with various hematologic malignancies, AML (D), ALL (E), CLL (F), ML (G); and from normal HPCs (H). Cell death was assessed by flow cytometry using annexin V and 7AAD double staining after 96 hours. The percentage of early apoptotic cells (annexin V+7AAD−) are shown as white columns and that of late apoptotic cells (annexin V+7AAD+) are shown as solid black columns. Data are derived from at least 3 independent experiments. AML indicates acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; ML, marginal zone lymphoma; and HPCs, hematopoietic progenitor cells.
APO866 induces time-dependent cell death in malignant cells from several hematologic malignancies. Time course analysis of cell death induced by 10 nM APO866 on cancer cells from cell lines ML-2 (A), Namalwa (B), and Jurkat (C); and primary cells from patients with various hematologic malignancies, AML (D), ALL (E), CLL (F), and ML (G). Cell death was assessed as in Figure 1. Data are derived from at least 3 independent experiments.
APO866 induces time-dependent cell death in malignant cells from several hematologic malignancies. Time course analysis of cell death induced by 10 nM APO866 on cancer cells from cell lines ML-2 (A), Namalwa (B), and Jurkat (C); and primary cells from patients with various hematologic malignancies, AML (D), ALL (E), CLL (F), and ML (G). Cell death was assessed as in Figure 1. Data are derived from at least 3 independent experiments.
APO866 is potent killing agent for most primary cells from patients with various hematologic malignancies
| Disease diagnosis . | Patient sample no. . | FDC, 10 nM, % . |
|---|---|---|
| Acute myeloid leukemia (AML) | ||
| AML-M5 | 1 | 100 |
| AML-M5 | 2 | 99 |
| AML-M5 | 3 | 98 |
| AML-M6 | 4 | 98 |
| AML-M5 | 5 | 96 |
| AML-M5 | 6 | 96 |
| AML-M5 | 7 | 84 |
| Secondary AML | 8 | 83 |
| AML-M3 | 9 | 73 |
| AML-M5 | 10 | 69 |
| Acute lymphoblastic leukemia (ALL) | ||
| ALL-early pre-B | 11 | 100 |
| ALL-pre-B | 12 | 97 |
| ALL -pre-B | 13 | 88 |
| B-chronic lymphocytic leukemia (B-CLL) | ||
| B-CLL | 14 | 100 |
| B-CLL | 15 | 100 |
| B-CLL | 16 | 100 |
| B-CLL | 17 | 99 |
| B-CLL | 18 | 99 |
| B-CLL | 19 | 98 |
| B-CLL | 20 | 98 |
| B-CLL | 21 | 97 |
| B-CLL | 22 | 96 |
| B-CLL | 23 | 95 |
| B-CLL | 24 | 94 |
| B-CLL | 25 | 88 |
| T-large granular lymphocyte leukemia (T-LGL) | ||
| T-LGL | 26 | 93 |
| T-lymphoma (TL) | ||
| TL | 27 | 98 |
| Marginal zone lymphoma (ML) | ||
| ML | 28 | 97 |
| ML | 29 | 96 |
| ML | 30 | 82 |
| Mantle cell lymphoma (MCL) | ||
| MCL | 31 | 97 |
| Follicular lymphoma (FL) | ||
| FL | 32 | 98 |
| Disease diagnosis . | Patient sample no. . | FDC, 10 nM, % . |
|---|---|---|
| Acute myeloid leukemia (AML) | ||
| AML-M5 | 1 | 100 |
| AML-M5 | 2 | 99 |
| AML-M5 | 3 | 98 |
| AML-M6 | 4 | 98 |
| AML-M5 | 5 | 96 |
| AML-M5 | 6 | 96 |
| AML-M5 | 7 | 84 |
| Secondary AML | 8 | 83 |
| AML-M3 | 9 | 73 |
| AML-M5 | 10 | 69 |
| Acute lymphoblastic leukemia (ALL) | ||
| ALL-early pre-B | 11 | 100 |
| ALL-pre-B | 12 | 97 |
| ALL -pre-B | 13 | 88 |
| B-chronic lymphocytic leukemia (B-CLL) | ||
| B-CLL | 14 | 100 |
| B-CLL | 15 | 100 |
| B-CLL | 16 | 100 |
| B-CLL | 17 | 99 |
| B-CLL | 18 | 99 |
| B-CLL | 19 | 98 |
| B-CLL | 20 | 98 |
| B-CLL | 21 | 97 |
| B-CLL | 22 | 96 |
| B-CLL | 23 | 95 |
| B-CLL | 24 | 94 |
| B-CLL | 25 | 88 |
| T-large granular lymphocyte leukemia (T-LGL) | ||
| T-LGL | 26 | 93 |
| T-lymphoma (TL) | ||
| TL | 27 | 98 |
| Marginal zone lymphoma (ML) | ||
| ML | 28 | 97 |
| ML | 29 | 96 |
| ML | 30 | 82 |
| Mantle cell lymphoma (MCL) | ||
| MCL | 31 | 97 |
| Follicular lymphoma (FL) | ||
| FL | 32 | 98 |
FDC indicates fraction of dead cells at 10 nM APO866.
We next assessed the selectivity of APO866. Normal human HPCs from mPB were treated with 10 nM APO866 for 96 hours and cell death monitored in early HPCs (CD34+CD38low). In contrast to the high sensitivity of hematologic cells, HPCs were resistant to APO866 treatment (Figure 1H). CD34+CD38low cells represent a subpopulation of HPCs responsible for repopulating the hematopoietic system in human HPC transplantation models.20 The APO866 concentration of 10 nM was chosen as the test concentration nearest to the steady-state plasma level of 14 nM measured at the maximum tolerated dose in patients in the phase 1 clinical trial.18 Collectively, these results demonstrate that APO866 induces potent and selective effects on human hematologic malignant cells without adversely effecting normal HPCs.
APO866 kills cells independently of caspase activation, induces mitochondrial dysfunction, and triggers autophagy
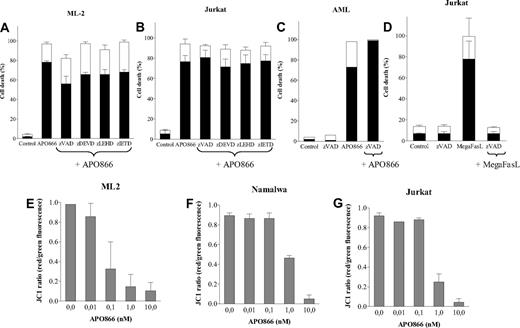
APO866-treated malignant cells underwent PS externalization as revealed by annexin V staining, suggesting apoptotic cell death. To determine whether caspase activation is involved in APO866-induced cell death, cells from different hematologic malignancies were treated with either a broad-spectrum caspase inhibitor (zVAD-fmk) or specific inhibitors of caspases 3, 8, and 9 prior to treatment with APO866. As shown in Figure 3A through C, none of the caspase inhibitors prevented APO866-mediated apoptosis, suggesting a caspase-independent pathway.
APO866-induced cell death is independent of caspase activation, and is associated with depolarization of mitochondrial membrane. Caspase inhibitors failed to prevent APO866-induced cell death in ML-2 cells (A), Jurkat cells (B), or primary AML cells (C). Cells were exposed to APO866 (10 nM) for 4 days in the presence or absence of zVAD-fmk (100 μM), zDEVD-fmk, zIETD-fmk, or zLEHD-fmk (50 μM). Controls consisted of untreated cells similarly analyzed (A-C). Control of the activity of the pan-caspase inhibitor (D) included wild-type Jurkat cells incubated for 4 days with 100 ng/ml MegaFasL alone (“MegaFasL”) or with zVAD (100 μM, “zVAD-fmk + MegaFasL”). ML-2 (E), Namalwa (F), and Jurkat (G) cells were incubated without or with various concentrations of APO866 for 96 hours. Mitochondrial potential was measured using JC-1 staining red versus green fluorescence as described in “Methods.” Data are derived from at least 3 independent experiments.
APO866-induced cell death is independent of caspase activation, and is associated with depolarization of mitochondrial membrane. Caspase inhibitors failed to prevent APO866-induced cell death in ML-2 cells (A), Jurkat cells (B), or primary AML cells (C). Cells were exposed to APO866 (10 nM) for 4 days in the presence or absence of zVAD-fmk (100 μM), zDEVD-fmk, zIETD-fmk, or zLEHD-fmk (50 μM). Controls consisted of untreated cells similarly analyzed (A-C). Control of the activity of the pan-caspase inhibitor (D) included wild-type Jurkat cells incubated for 4 days with 100 ng/ml MegaFasL alone (“MegaFasL”) or with zVAD (100 μM, “zVAD-fmk + MegaFasL”). ML-2 (E), Namalwa (F), and Jurkat (G) cells were incubated without or with various concentrations of APO866 for 96 hours. Mitochondrial potential was measured using JC-1 staining red versus green fluorescence as described in “Methods.” Data are derived from at least 3 independent experiments.
A time course analysis demonstrated that caspase inhibition early after APO866 contact (48 hours) partially inhibited apoptosis, even though the inhibitory effect was no longer observed after 96 hours (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). As a control, the pan caspase inhibitor similarly applied prevented cell death induced by MegaFasL, a potent Fas-ligand that exerts cytotoxicity through caspase activation21 (Figure 3D). To gain insight on the cellular events involved in APO866-induced cell killing, the sensitivities of cell lines deficient in key proteins (FADD, RIP, or caspase-8) implicated in the extrinsic apoptotic pathway were compared with wild-type (wt) Jurkat cells. APO866 sensitivity in Jurkat cells deficient in FADD, RIP, or caspase 8 was comparable to wt cells (Figure S2), indicating that the cell death pathway triggered by APO866 is independent of FADD, RIP, and caspase-8. As a control, wt Jurkat cells and FADD-, RIP-, or caspase-8–deficient cells were treated with MegaFasL. While wt and RIP-deficient Jurkat cells were killed by MegaFasL, FADD- and caspase-8–deficient Jurkat cells were completely resistant to MegaFasL-induced apoptosis (Figure S2). These findings indicate that the integrity of the main extrinsic death-receptor components is not necessary for APO866-induced cell death.
Since mitochondrial outer membrane permeabilization (MMP) is often involved in cell death, its involvement in APO866-mediated cell death was evaluated. Malignant cells from different hematologic cell lines were exposed to various concentrations of APO866 and loss of MMP was measured using fluorescent dye and flow cytometry analysis. Treatment with APO866 induced a potent, dose-dependent mitochondrial membrane depolarization at 96 hours (Figure 3E-G), but not at earlier time points (24-48 hours; Figure S3). The timing of MMP loss correlated with APO866-mediated cell death.
A recent study by Billington et al reported that NAD synthesis inhibition induced autophagy (macroautophagy) in neuroblastoma cells.15 Autophagy can function as a cytoprotective mechanism, but also has the capacity to induce cell death.22 To delineate which function autophagy plays in APO866-induced cell death in hematologic cancer cells, cells from various hematologic malignancies incubated with autophagy inhibitors were subsequently treated with APO866. Inhibitors of autophagy included wortmannin and LY294002 (inhibitors of phosphatidylinositol-3 kinase, an essential component of the core machinery involved in autophagic vesicle formation23 ); 3MA (the blocker of autophagy formation24 ), or bafilomycin A1 (an inhibitor of H+-ATPase responsible for acidification of autophagolysosomal vacuoles25 ). PI3-kinase inhibitors attenuated APO866-induced cell death in malignant cell lines at 72 hours, but not at 96 hours (Figure 4A-H). In the case of primary malignant cells, the PI3-kinase inhibitors appeared to sensitize cells to APO866 at 72 hours (Figure 4D), but not at 96 hours (Figure 4H). Pretreatment with 3MA or bafilomycin A1 significantly decreased APO866-induced cell death at 96 hours (Figure 4E-G). Treatment with the protein synthesis inhibitor cycloheximide also decreased APO866-mediated cell death (Figure 4A-H), suggesting that APO866-induced cell death is dependent on de novo protein synthesis.
APO866-mediated cell death involves autophagy. ML-2, Namalwa, Jurkat, or primary AML cells pretreated with either inhibitors of autophagy (wortmannin [WMN], LY294002, 3-methyladenine [3-MA], or bafilomycin A1 [BAFA]) or inhibitor of translation cycloheximide (CHX) were exposed to 10 nM APO866 for either 72 hours (A-D) or 96 hours (E-H). APO866-induced cell death was assessed as described in Figure 1. Percent cell death induced by drug = [(S-C) / (100-C)] × 100; where S = treated sample cell death and C = untreated sample cell death. Data are derived from at least 3 independent experiments.
APO866-mediated cell death involves autophagy. ML-2, Namalwa, Jurkat, or primary AML cells pretreated with either inhibitors of autophagy (wortmannin [WMN], LY294002, 3-methyladenine [3-MA], or bafilomycin A1 [BAFA]) or inhibitor of translation cycloheximide (CHX) were exposed to 10 nM APO866 for either 72 hours (A-D) or 96 hours (E-H). APO866-induced cell death was assessed as described in Figure 1. Percent cell death induced by drug = [(S-C) / (100-C)] × 100; where S = treated sample cell death and C = untreated sample cell death. Data are derived from at least 3 independent experiments.
These results indicate that APO866-induced cell death (1) involves a caspase-independent pathway, (2) does not require the integrity of the main death-receptor components, (3) is associated with mitochondrial dysfunction, (4) involves autophagy, and (5) requires new protein synthesis.
APO866 induces intracellular NAD and ATP depletion in hematologic tumor cells
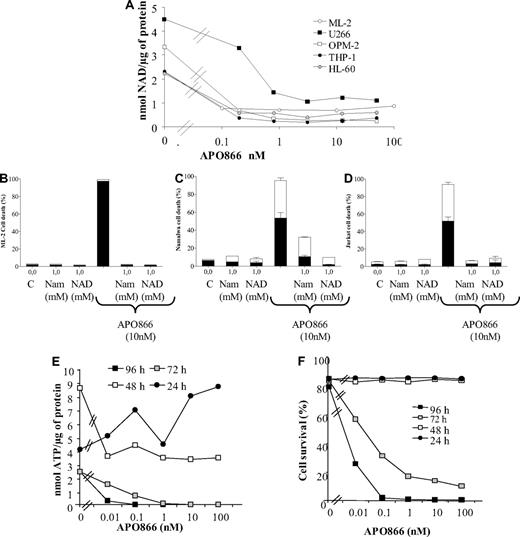
To confirm whether NAD depletion is involved in APO866-induced hematologic cell death, we determined the concentrations of intracellular NAD contents upon treatment with APO866 in 5 cell lines (3 AML [ML-2, THP-1, and HL-60] and 2 MM [U266 and OPM-2]). As shown in Figure 5A, APO866 consistently induced NAD depletion within 24 hours. Low concentrations of APO866 (0.1 nM-0.5 nM) were sufficient to deplete NAD below detectable levels in most cell lines (ML-2, OPM-2, THP-1, HL-60). We next evaluated the capacity of nicotinamide, a precursor of NAD biosynthesis and a competitive inhibitor of APO86626 as well as NAD to protect APO866-induced cell death in various cell lines. As shown in Figure 5B through D and Figure S4, nicotinamide and NAD prevented APO866-induced cell death in various cell lines in a concentration-dependent manner.
APO866 induces depletion of intracellular NAD and ATP contents and cell death in various hematologic cancer cells, and extracellular addition of nicotinamide or NAD prevents APO866-mediated cell death. Intracellular NAD content in 5 different cell lines plated with or without various concentrations of APO866 as indicated (A). After 24 hours of incubation, intracellular NAD content was measured and normalized relative to protein content. ML-2, Namalwa, or Jurkat cells were incubated with or without nicotinamide /NAD in presence or absence of 10 nM APO866 for 96 hours (B-D). Cell death was monitored as described in Figure 1. After 24, 48, 72, and 96 hours of exposure to increasing concentrations of APO866, intracellular ML-2 ATP levels were measured (E) and ML-2 cell survival was assessed by annexin V staining (F).
APO866 induces depletion of intracellular NAD and ATP contents and cell death in various hematologic cancer cells, and extracellular addition of nicotinamide or NAD prevents APO866-mediated cell death. Intracellular NAD content in 5 different cell lines plated with or without various concentrations of APO866 as indicated (A). After 24 hours of incubation, intracellular NAD content was measured and normalized relative to protein content. ML-2, Namalwa, or Jurkat cells were incubated with or without nicotinamide /NAD in presence or absence of 10 nM APO866 for 96 hours (B-D). Cell death was monitored as described in Figure 1. After 24, 48, 72, and 96 hours of exposure to increasing concentrations of APO866, intracellular ML-2 ATP levels were measured (E) and ML-2 cell survival was assessed by annexin V staining (F).
Since NAD depletion reduces intracellular ATP levels,3,9 the effect of APO866 on the intracellular ATP content of hematologic tumor cells was evaluated. Incubation of ML-2 cells with increasing concentrations of APO866 (0.01 nM-10 nM) resulted in dose- and time-dependent ATP depletion, starting at 72 hours and reaching a maximum at 96 hours (Figure 5E). Additionally, timing of ATP depletion correlated with the apoptosis marker annexin V expression on the ML-2 cell surface (Figure 5F). In agreement with a previous study,9 these data demonstrate that APO866 depletes NAD cell content, followed by ATP depletion, and that NAD depletion was responsible for cell death.
APO866 inhibits the clonogenic capacity of hematologic tumor cells but not normal hematopoietic progenitor cells
To further investigate the tumor cell growth inhibitory effect of APO866, we tested the capacity of malignant cells or normal human HPCs treated with APO866 to form colonies on methylcellulose medium containing proliferation factors (CFU assays). As shown in Table 3, APO866 inhibited (> 3 logs) the clonogenic capacity of a wide range of tumor cells. In contrast, the clonogenic capacity of HPCs was not affected by APO866. These data further support the selectivity of APO866, and highlight its potent cytotoxic effect on human hematologic malignant cells.
APO866 inhibits the capacity of hematologic cancer cells to form colonies
| Colonies formed at 14 to 18 days . | ||
|---|---|---|
| Cells plated after 96-hour incubation . | Untreated cells . | Cell exposure to 10 nM APO866 . |
| Namalwa | ||
| 5 × 102 | 73 ± 11 | 0 |
| 5 × 103 | ND | 0 |
| 5 × 104 | ND | 0 |
| RajiGFP | ||
| 5 × 102 | 95 ± 4 | 0 |
| 5 × 103 | ND | 0 |
| 5 × 104 | ND | 0 |
| ML-2 | ||
| 5 × 102 | 134 ± 21 | 0 |
| 5 × 103 | ND | 0 |
| 5 × 104 | ND | 0 |
| Molt-4 | ||
| 5 × 102 | 120 ± 19 | 0 |
| 5 × 103 | ND | 0 |
| 5 × 104 | ND | 0 |
| EHEB | ||
| 5 × 102 | 374 ± 11.3 | 0 |
| 5 × 103 | ND | 0 |
| 5 × 104 | ND | 0 |
| HPCs | ||
| 2 × 103 | ||
| BFU-Es | 158 ± 62 | 103 ± 64 |
| CFU-GMs | 125 ± 67 | 169 ± 69 |
| Colonies formed at 14 to 18 days . | ||
|---|---|---|
| Cells plated after 96-hour incubation . | Untreated cells . | Cell exposure to 10 nM APO866 . |
| Namalwa | ||
| 5 × 102 | 73 ± 11 | 0 |
| 5 × 103 | ND | 0 |
| 5 × 104 | ND | 0 |
| RajiGFP | ||
| 5 × 102 | 95 ± 4 | 0 |
| 5 × 103 | ND | 0 |
| 5 × 104 | ND | 0 |
| ML-2 | ||
| 5 × 102 | 134 ± 21 | 0 |
| 5 × 103 | ND | 0 |
| 5 × 104 | ND | 0 |
| Molt-4 | ||
| 5 × 102 | 120 ± 19 | 0 |
| 5 × 103 | ND | 0 |
| 5 × 104 | ND | 0 |
| EHEB | ||
| 5 × 102 | 374 ± 11.3 | 0 |
| 5 × 103 | ND | 0 |
| 5 × 104 | ND | 0 |
| HPCs | ||
| 2 × 103 | ||
| BFU-Es | 158 ± 62 | 103 ± 64 |
| CFU-GMs | 125 ± 67 | 169 ± 69 |
Values represent the mean plus or minus the standard deviation (SD) of 3 independent experiments. BFU-Es are burst-forming units (erythroid colonies); CFU-G/Ms are granulocyte/macrophage colonies; HPCs are normal hematopoietic progenitor cells. ND indicates not done.
APO866 exhibits antitumor activity in animal models of human hematologic malignancies without significant toxicity in animals
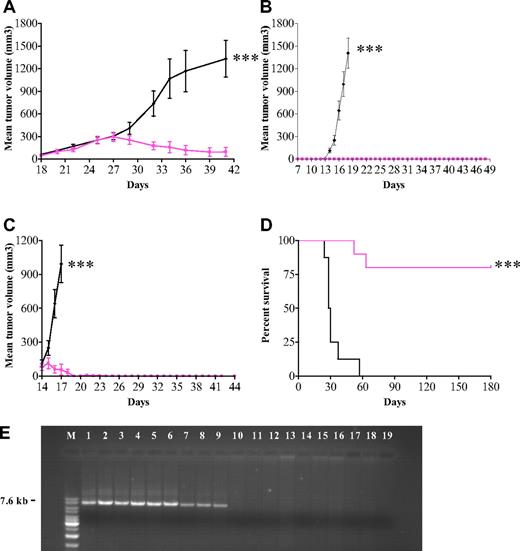
The in vitro data prompted us to test APO866 in 3 in vivo models. We first evaluated the anticancer activity of APO866 in mice xenografted subcutaneously with AML-M4 (ML-2) cells. Treatment of mice with established tumors (n = 8) with 20 mg/kg APO866 resulted in 95% inhibition of tumor growth compared with controls (n = 7) (Figure 6A). Second, we evaluated the in vivo efficacy of APO866 in a xenochimeric mouse model of human Burkitt lymphoma using mice xenografted subcutaneously with Namalwa cells. Early treatment with APO866 (early-stage disease group, n = 10) prevented tumor development and significantly prolonged mouse survival, whereas all mice (n = 10) in the control group developed tumors within 15 days (Figure 6B). To evaluate the therapeutic efficacy in mice with advanced human disease, APO866 was administered to mice with well-established Namalwa tumors. As compared with control, APO866 treatment abrogated tumor growth and significantly prolonged median survival times over those of controls (Figure 6C). Third, the long-term preventive effect of APO866 was investigated in SCID mice xenografted intravenously with Raji (Burkitt lymphoma) cells. This xenochimeric model is characterized by hind leg paralysis due to central nervous system involvement and bone marrow infiltration. Treatment with APO866 cleared tumor cells to below detectable levels (Figure 6E) and resulted in 80% survival for more than 180 days of observation. A bone marrow flush available from 1 of the 2 treated animals that died revealed no sign of tumor cells when analyzed by FACS, CFU followed by FACS, and PCR, indicating that the animal was cured. In contrast, all 8 mice from the control group died within less than 2 months (Figure 6D). Moreover, no trace of t(8;14)(q24;q32) DNA fragment (7.6 kb) specific for Raji cells was detected in bone marrow cells using long-distance PCR amplification from 9 available of 10 APO866-treated mice. In contrast, this fragment was amplified in samples from all 8 control (untreated) paretic mice (Figure 6E).
In vivo antitumor activity of APO866 in mouse xenograft models of human leukemia and lymphoma. (A) In vivo efficacy of APO866 on mice xenografted with ML-2 cells. Fifteen 6- to 8-week-old Balb/c nude mice received subcutaneous transplants of 2 × 107 ML-2 cells. Once tumors became palpable (approximately 150 mm3, at day 18), mice were randomized into a control group (n = 7, black line) and a treated group (n = 8, colored line). APO866 administration and tumor volume assessment were conducted as described in “Methods.” (B)Treatment with APO866 at early stage of disease prevents tumor growth and prolongs survival in a mouse model of human Burkitt lymphoma. Twenty 6- to 8-week-old C.B.-17 SCID mice were each injected subcutaneously with 107 Namalwa cells and mice were subdivided into 2 groups. APO866 administration was initiated as described in “Methods” starting 1 week after cell injection (n = 10), whereas mice in the control group (n = 10) received intraperitoneal injection with saline solution. (C) APO866 applied on established human Burkitt lymphoma tumors abrogates tumor growth. Mice underwent transplantation with Namalwa cells as described in panel B. When tumors reached a volume of 100 mm3 (at day 14), mice were divided in 2 groups. Mice from APO866 group were treated with APO866 as described above (n = 10) and control animals (n = 10) received vehicle only. (D) APO866 treatment eradicates tumor growth and prolongs the overall survival in a mouse model of human Burkitt leukemia. Raji-GFP cells (5 × 104) were injected into the tail vein of each SCID mouse (D0). Starting 1 week after cell injection, animals were treated with APO866 (n = 10, colored line) or received vehicle (n = 8, black line). All animals were monitored daily, and the end point of the study was survival defined as the time point where hind limb paresis was noted. Animals that reached end point or survived after 180 days of observation were killed. ***P < .001. (E) Detection of t(8;14)(q24;q32) DNA fragment specific of Raji cells (Burkitt lymphoma). DNA was extracted from 17 bone marrow cells from either vehicle-injected (lanes 2-9) or APO866-treated mice (lanes 10-18). Positive and negative controls for PCR included DNA from pure Raji cells (lane 1) and buffer (lane 19), respectively. M: DNA ladder mix marker (Fermentas Life Science, Madison, WI).
In vivo antitumor activity of APO866 in mouse xenograft models of human leukemia and lymphoma. (A) In vivo efficacy of APO866 on mice xenografted with ML-2 cells. Fifteen 6- to 8-week-old Balb/c nude mice received subcutaneous transplants of 2 × 107 ML-2 cells. Once tumors became palpable (approximately 150 mm3, at day 18), mice were randomized into a control group (n = 7, black line) and a treated group (n = 8, colored line). APO866 administration and tumor volume assessment were conducted as described in “Methods.” (B)Treatment with APO866 at early stage of disease prevents tumor growth and prolongs survival in a mouse model of human Burkitt lymphoma. Twenty 6- to 8-week-old C.B.-17 SCID mice were each injected subcutaneously with 107 Namalwa cells and mice were subdivided into 2 groups. APO866 administration was initiated as described in “Methods” starting 1 week after cell injection (n = 10), whereas mice in the control group (n = 10) received intraperitoneal injection with saline solution. (C) APO866 applied on established human Burkitt lymphoma tumors abrogates tumor growth. Mice underwent transplantation with Namalwa cells as described in panel B. When tumors reached a volume of 100 mm3 (at day 14), mice were divided in 2 groups. Mice from APO866 group were treated with APO866 as described above (n = 10) and control animals (n = 10) received vehicle only. (D) APO866 treatment eradicates tumor growth and prolongs the overall survival in a mouse model of human Burkitt leukemia. Raji-GFP cells (5 × 104) were injected into the tail vein of each SCID mouse (D0). Starting 1 week after cell injection, animals were treated with APO866 (n = 10, colored line) or received vehicle (n = 8, black line). All animals were monitored daily, and the end point of the study was survival defined as the time point where hind limb paresis was noted. Animals that reached end point or survived after 180 days of observation were killed. ***P < .001. (E) Detection of t(8;14)(q24;q32) DNA fragment specific of Raji cells (Burkitt lymphoma). DNA was extracted from 17 bone marrow cells from either vehicle-injected (lanes 2-9) or APO866-treated mice (lanes 10-18). Positive and negative controls for PCR included DNA from pure Raji cells (lane 1) and buffer (lane 19), respectively. M: DNA ladder mix marker (Fermentas Life Science, Madison, WI).
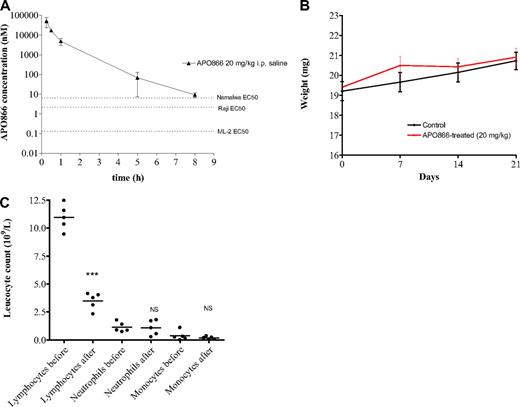
While twice daily treatment with APO866 is not expected to result in accumulation of APO866 based on pharmacokinetic parameters, a plasma concentration above the relevant in vitro EC50 value was achieved for at least 8 hours after injection of a 20 mg/kg dose (Figure 7A), which is sufficient to explain the therapeutic effect in the xenograft studies. In terms of safety, no evident signs of toxicity were observed in mice treated with 20 mg/kg APO866 using the present schedule. APO866 was well tolerated with no premature deaths, and 8 of 10 mice treated with APO866 survived over 180 days of observation without signs of toxicity including loss of body weight (Figure 7B), lethargy or rough coat. This confirmed and extended a previous report where animals received APO866 at lower doses.27 Biochemical analyses of liver enzymes and kidney markers such as creatinine and urea were not performed in the present study, but data obtained in regulatory toxicity studies in rats and monkeys have shown that these organs are not affected by APO866 treatment at equivalent doses (data not shown). Hematologic variables were determined in the present study, and a nadir in white blood cell counts was found on day 4 compared with pretreatment (12.4 ± 1.3 vs 4.7 ± 1.4 × 109/L), which was due to a decrease in the lymphocyte fraction of leucocytes, whereas neutrophil, and monocytes were unaffected (Figure 7C). This finding is consistent with data from a colony-forming unit–granulocyte-macrophage assay using stem cells from mouse bone marrow, which demonstrated relative resistance to APO866 (IC50 value of 50 nM). Red blood cell counts also showed a nadir following APO866 treatment (12.4 ± 0.3 decreased to 11.2 ± 0.3 × 1012/L, P < .001), and platelet counts showed a slight increase (756.2 ± 49.4 vs 830.6 ± 59.6 × 109/L, P = .06). Sensitivity of lymphocyte development to changes in NMPRTase activity has been described in the literature: lymphocyte viability is dependent on the nicotinamide pathway of NAD synthesis and inhibition of NMPRTase using 20 nM APO866 for 24 or 48 hours affected the viability of mouse spleen cells.28
In vivo pharmacokinetic and absence of toxicity effect of APO866 in mice. (A) Plasma concentration (nM) of APO866 following a single intraperitoneal dose of 20 mg/kg is above the EC50 values of the cell lines used for xenograft studies for at least 8 hours. Three mice were killed at each time point, and a Li-Heparin stabilized blood sample was obtained and analyzed for APO866 concentration using HPLC-MS/MS as described in “Methods.” Error bars represent SD. (B) APO866 treatment did not induced any weight loss in mice. Test SCID mice (n = 10) were treated intraperitoneally with 20 mg/kg APO866, twice daily for 4 days, repeated weekly 3 times. Control mice (n = 10) received vehicle only. Groups of animals were compared with respect to variation of their weight by statistical analysis using the one-way ANOVA. (C) Counts of lymphocytes, neutrophils, and monocytes in tail vein blood samples of mice treated intraperitoneally with 20 mg/kg APO866 twice daily for 4 days. Blood samples were taken before treatment (pre) and on the day after the last treatment with APO866 (post). The P values of paired t tests comparing the pre- and posttreatment lymphocyte, neutrophil, and monocyte counts, respectively, are indicated on the figure. ***P < .001; NS indicates difference not statistically significant.
In vivo pharmacokinetic and absence of toxicity effect of APO866 in mice. (A) Plasma concentration (nM) of APO866 following a single intraperitoneal dose of 20 mg/kg is above the EC50 values of the cell lines used for xenograft studies for at least 8 hours. Three mice were killed at each time point, and a Li-Heparin stabilized blood sample was obtained and analyzed for APO866 concentration using HPLC-MS/MS as described in “Methods.” Error bars represent SD. (B) APO866 treatment did not induced any weight loss in mice. Test SCID mice (n = 10) were treated intraperitoneally with 20 mg/kg APO866, twice daily for 4 days, repeated weekly 3 times. Control mice (n = 10) received vehicle only. Groups of animals were compared with respect to variation of their weight by statistical analysis using the one-way ANOVA. (C) Counts of lymphocytes, neutrophils, and monocytes in tail vein blood samples of mice treated intraperitoneally with 20 mg/kg APO866 twice daily for 4 days. Blood samples were taken before treatment (pre) and on the day after the last treatment with APO866 (post). The P values of paired t tests comparing the pre- and posttreatment lymphocyte, neutrophil, and monocyte counts, respectively, are indicated on the figure. ***P < .001; NS indicates difference not statistically significant.
Taken together, these results strongly support a therapeutic efficacy of APO866 at preventing or eradicating tumor growth in xenochimeric models of human hematologic malignancies.
Discussion
The present study has identified hematologic malignancies as being highly sensitive to treatment with the NAD synthesis inhibitor (APO866), and has characterized cellular metabolic events involved in APO866-mediated cell death. These in vitro and in vivo data demonstrate that APO866 is effective in a wide range of human hematologic malignancies, including leukemias (AML, ALL, CLL), MM, and various T- and B-cell lymphomas, but has no adverse effects on normal human HPCs. The in vivo administration of APO866 as a single agent prevented tumor formation and abrogated tumor growth in animal models of human hematologic malignancies (AML or Burkitt leukemia/lymphoma), and significantly prolonged median survival, thereby underlining its curative potential. APO866 inhibited the clonogenic potential of tumor cells, but not human and mouse HPCs, and induced cell death in hematologic cancer cells. Primary cells from patients were also highly sensitive to APO866 regardless of type of hematologic malignancy.
The mechanisms of APO866-induced cell death involved a rapid decrease in intracellular NAD content (within 24 hours), and a later decrease in ATP content (at 72 hours), the latter coinciding with the detection of cell death. APO866-induced cell death was independent of caspase activation and did not require the integrity of the extrinsic apoptotic pathway. However, APO866-mediated cell death involved mitochondrial dysfunction, as revealed by the loss of MMP, and also autophagy as proposed previously based on morphologic data from APO866-treated cells.15 Although we found no role for caspase activation in APO866-induced cell death, a previous study provided such evidence.10 This apparent discrepancy could be explained by the different cell lines used. An alternative possibility is that cell death triggering mechanisms are differently expressed with time. Indeed, a time course analysis demonstrated that caspase inhibition moderately inhibited apoptosis after 2 days of APO866 contact, but this effect was no longer evidenced after 4 days. Further experiments to fully understand the molecular mechanisms of action involved in APO866-mediated toxicity are warranted. Such understanding may also offer potential for combination studies with agents already in use in cancer therapy.
The novel mode of action and the potent antitumor activity of APO866 make it attractive as an antitumor agent and it is noteworthy that a phase 1 clinical trial has already been conducted.18 The APO866 dose used in the present in vivo studies was selected based on pharmacokinetic data from the clinical trial, possibly explaining the potent anticancer activity of APO866 observed without evident sign of toxicity in mice. An additional point of interest concerning the NAD depleting mode of action of APO866 is that infusion of niacinamide within 72 hours of a lethal dose of APO866 prevents death in all animals tested, indicating its potential as an antidote to protect patients from APO866 side effects.18
In summary, our in vitro and in vivo data demonstrate that APO866 is a potent anticancer agent in numerous hematologic malignancies, providing justification for clinical development of APO866 in the treatment of hematologic malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof P. Schneider and Dr Peter J. Sims for providing cell lines. We also thank Dr Steven Butcher for critical reading of the manuscript and Kuen-Mooi Béguin for animal care. We are indebted to all members of the bone marrow laboratory, University Hospital of Lausanne (CHUV), for kindly helping us in collecting clinical samples.
This work was supported by a contract (no. 8368.2 LSPP-LS) from the innovation promotion agency at the Swiss Federal Office for Professional Education and Technology.
Authorship
Contribution: A.N. designed, executed, and analyzed experiments, coordinated the project, and wrote the paper; A.A., D.A., P.G., C.I., A.V.T, and J.T. designed, executed, and analyzed experiments; K.M.D. analyzed results and wrote the paper; and M.D. and M.A.D. designed and analyzed experiments, coordinated the project, and wrote the paper.
Conflict-of-interest disclosure: A.A., P.G., C.I., K.M.D., and M.D. were employed by Apoxis (now TopoTarget Switzerland S.A.). A.V.T. and J.T. are employed by TopoTarget, Denmark S.A. TopoTarget has licensed the worldwide development and marketing rights to APO866 from Astellas. The remaining authors declare no competing financial interests.
Correspondence: Michel A. Duchosal, Service of Hematology, University Hospital of Lausanne (CHUV), 46, rue du Bugnon, 1011 Lausanne, Switzerland; e-mail: michel.duchosal@chuv.ch.


![Figure 4. APO866-mediated cell death involves autophagy. ML-2, Namalwa, Jurkat, or primary AML cells pretreated with either inhibitors of autophagy (wortmannin [WMN], LY294002, 3-methyladenine [3-MA], or bafilomycin A1 [BAFA]) or inhibitor of translation cycloheximide (CHX) were exposed to 10 nM APO866 for either 72 hours (A-D) or 96 hours (E-H). APO866-induced cell death was assessed as described in Figure 1. Percent cell death induced by drug = [(S-C) / (100-C)] × 100; where S = treated sample cell death and C = untreated sample cell death. Data are derived from at least 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/14/10.1182_blood-2008-08-173369/4/m_zh80170934250004.jpeg?Expires=1765889830&Signature=oYpERJe5FgIKtmUQK6CVlPyYALC5PP2YTWgV5LyjktQdMRwm68G6O-peFgqoa2y~a1UIzigUW2zHEHoEb1q-MpuHTwO8P91TQMvrdwEJJqJpnMgodAIzeZWnIFCxuPxvA~js-1V7Y2WFtMgGfBmZyHUpck-0QFMphNKG6w3WY9E7uc-~9tU2Tea9WFbYElGihLLucgdqHnODfNOVQvnS~ji8jnVSJFR-uJ-O1eeRy1W-VAv1782m6uRocqsI4fBGnb5dBbXl-0lntZcnHEbKqDU1rm2e-SaGIZ1EgqG2RRVYqeyysbQh3JH47CvIgVhAlWRpBVss~IZSJGT1ErbnPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal