Abstract
The CD31+ subset of human naive CD4+ T cells is thought to contain the population of cells that have recently emigrated from the thymus, while their CD31− counterparts have been proposed to originate from CD31+ cells after homeostatic cell division. Naive T-cell maintenance is known to involve homeostatic cytokines such as interleukin-7 (IL-7). It remains to be investigated what role this cytokine has in the homeostasis of naive CD4+ T-cell subsets defined by CD31 expression. We provide evidence that IL-7 exerts a preferential proliferative effect on CD31+ naive CD4+ T cells from adult peripheral blood compared with the CD31− subset. IL-7–driven proliferation did not result in loss of CD31 expression, suggesting that CD31+ naive CD4+ T cells can undergo cytokine-driven homeostatic proliferation while preserving CD31. Furthermore, IL-7 sustained or increased CD31 expression even in nonproliferating cells. Both proliferation and CD31 maintenance were dependent on the activation of phosphoinositide 3-kinase (PI3K) signaling. Taken together, our data suggest that during adulthood CD31+ naive CD4+ T cells are maintained by IL-7 and that IL-7–based therapies may exert a preferential effect on this population.
Introduction
Human naive CD4+ T cells have recently been shown to contain 2 subpopulations distinguished by the expression of CD31 (platelet endothelial cell adhesion molecule-1, PECAM-1). The CD31+ subset is thought to incorporate the population of cells recently emigrated from the thymus, whereas the CD31− subset has been proposed to derive from CD31+ after homeostatic cell division.1 During T-cell development in the thymus, rearrangement of the T-cell receptor (TCR) genes generates stable episomal DNA excision circles (TRECs) that are progressively diluted with cell division.2-4 Accordingly, CD31+ naive CD4+ T cells have higher TREC content compared with the CD31− naive subset.1 Moreover, the progressive age-associated decline in naive CD4+ T cells is mainly due to a reduction in the CD31+ naive subset while the CD31− subset persists,5,6 further supporting the contribution of thymic output to the maintenance of CD31+ cells. However, the decrease in TREC levels observed during aging is disproportionally greater compared with the decline in CD31+ naive T cells, implicating other mechanisms, in addition to thymic output, in the persistence of these cells into old age.4
Cytokine-driven expansion has been proposed to significantly contribute to a low level of homeostatic proliferation that maintains naive T-cell numbers.7 Besides its established importance in thymopoiesis, interleukin-7 (IL-7) is considered to play a key role in naive T-cell survival and proliferation in the periphery.2,7 In vitro studies of human naive CD4+ T cells cultured in the presence of IL-7 revealed, alongside with its antiapoptotic properties, an ability to induce proliferative responses without a switch to a memory phenotype.8 IL-7 seems to exert a preferential effect on umbilical cord blood (CB) naive T cells that proliferate significantly more than adult peripheral blood naive T cells in response to IL-7.8,9 Despite this, a considerable reliance upon IL-7 in naive T-cell homeostasis after T-cell depletion has been established.7,10 IL-7 was able to promote T-cell reconstitution after bone marrow transplantation in mice acting not only at the thymic but also at the peripheral level,11-13 and to expand naive and memory T cells in uninfected14 and simian immunodeficiency virus (SIV)–infected nonhuman primates.15 Furthermore, IL-7 serum levels were shown to increase in different lymphopenic settings in humans in strong inverse correlation with naive CD4+ T-cell counts, suggesting a feedback mechanism to counteract T-cell depletion.16-19 IL-7 administration to patients with metastatic melanoma led to CD4+ and CD8+ T-cell expansion, particularly of CD45RA+ naive T cells,20 and further clinical trials are currently exploring its therapeutic potential.
The possibility of IL-7 having distinct effects on human CD31+ and CD31− naive subsets has not yet been investigated. These data are relevant not only to further clarify the mechanisms involved in the maintenance of these 2 naive populations during aging, but also to better characterize the potential cellular targets of therapeutic interventions involving IL-7 administration. In this respect, a recently published phase 1 clinical trial with IL-7 in refractory cancer shows a preferential expansion of the CD31+ naive CD4+ subset.21 This was associated with a decrease in TREC content in this population consistent with the induction of proliferation by IL-7 in this subset.21
Here, we report that IL-7 exerted a selective proliferative effect on CD31+ naive CD4+ T cells from adult peripheral blood compared with their CD31− counterparts. We further observed that proliferation of adult CD31+ naive CD4+ T cells was dependent on the activation of phosphoinositide 3-kinase (PI3K) signaling pathway and was not associated with loss of CD31 expression. IL-7 also promoted the preservation of CD31 levels in nonproliferating naive T cells through PI3K activation. Taken together, our data suggest that IL-7 may play a preferential role in the maintenance of CD31+ naive CD4+ T cells during adult life.
Methods
Cell isolation
This study was approved by the Ethics Board of the Faculty of Medicine of Lisbon. Mononuclear cells were isolated from heparinized adult peripheral blood of healthy volunteers, and from umbilical cord blood (CB) obtained immediately after delivery of full-term infants, with informed consent obtained in accordance with the Declaration of Helsinki, by Ficoll-Hypaque density gradient (Amersham Pharmacia Biotech, Uppsala, Sweden). CD4+ T cells were negatively selected using the EasySep Human CD4+ T-Cell Enrichment Kit (StemCell Technologies, Vancouver, BC) and subsequently sorted into CD31+ and CD31− naive subsets using a FACSAria flow cytometer (BD Biosciences, San Jose, CA) after staining for CD45RA, CD45RO, CD4, and CD31 as described below.
Cell culture
Cells were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated human AB serum (Sigma-Aldrich, St Louis, MO), 100 U/mL penicillin, 100 mg/mL streptomycin, and 2 mM L-glutamine (Invitrogen), in the presence or absence of recombinant human IL-7 (10 ng/mL; R&D Systems, Minneapolis, MN) or recombinant human IL-2 (10 U/mL; obtained through the National Institutes of Health (NIH)/AIDS Research and Reference Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH [IL-2] from Hoffman-La Roche). PI3K and mitogen-activated protein kinase (MEK)–extracellular signal-regulated kinase (ERK) activity were respectively blocked by incubation of cells for 1 hour at 37°C before IL-7 stimulation with either 10 μM LY294002 or 10 μM PD98059 (both from Calbiochem, Merck Biosciences, Nottingham, United Kingdom) or the equivalent volume of the vehicle control dimethyl sulfoxide (DMSO; Sigma-Aldrich) alone. LY294002, PD98059, and DMSO were readded to the culture at day 4.
Phenotypic analysis
Cells resuspended in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA; Sigma-Aldrich) and 0.1% sodium azide (Sigma-Aldrich) were stained for 20 minutes at room temperature with the following anti–human monoclonal antibodies: CD4-phycoerythrin (PE)–cyanin 7 (PE-CY7; clone, L3T4), CD45RA–fluorescein isothiocyanate (FITC) or allophycocyanin (APC; clone, HL100), CD45RO-PE (clone; UCHL1), CD62L-APC-cyanin 7 (APC-Cy7; clone, DREG 56) and CD31 PE or APC (clone, WM59) from eBioscience (San Diego, CA); CD38 PE (clone, HB7) and CD3–peridinin chlorophyll protein (PerCP; clone, SK7) from BD Biosciences; and CD127 PE (IL-7Rα; clone 40131; R&D Systems). Intracellular staining for Bcl-2 FITC (clone 124; Dako, Glostrup, Denmark) and Ki67 FITC (clone B56; BD Biosciences) was performed using fixation and permeabilization reagents from eBioscience. Cells were labeled with 0.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes-Invitrogen, Carlsbad, CA) at 37°C for 15 minutes in the dark, quenched with ice-cold culture medium at 4°C for 5 minutes, and washed 3 times before culture. Apoptosis was assessed using 7-amino-actinomycin D (7-AAD) viability Staining Solution (eBioscience) or by annexin V/propidium iodide (PI) detection kit (BD Biosciences). Samples were acquired on a BD FACSCanto flow cytometer (BD Biosciences) after fixation with 1% formaldehyde (Sigma-Aldrich). Data were analyzed using FlowJo software version 8.1.1 (TreeStar, Ashland, OR).
STAT5 tyrosine phosphorylation analysis
Cells were surface stained and stimulated with 50 ng/mL IL-7 for 15 minutes, fixed with 2% formaldehyde at 37°C for 10 minutes, and placed on ice. Cells were then permeabilized with ice-cold 90% methanol (Sigma-Aldrich) at 4°C for 30 minutes and incubated with anti–phospho-STAT5 (pY694) antibody coupled to Alexa Fluor 488 (BD Biosciences) at room temperature for 1 hour. Samples were immediately acquired on FACSCanto.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 4.00 (GraphPad Software, San Diego, CA). Data are presented as mean plus or minus standard error of mean (SEM). P less than .05 was considered significant.
Results
IL-7–induced proliferation of adult naive CD4+ T cells is restricted to the CD31+ subset
IL-7 is known to induce proliferation of naive CD4+ T cells,8,22,23 but the possibility of distinct effects on naive subsets defined by CD31 expression has not been determined. Our preliminary data from the culture of adult total naive CD4+ T cells (CD4+CD45RA+CD45RO−) with recombinant human IL-7 for 7 days suggested that the proliferative response was confined to CD31+ cells (data not shown). Of note, in agreement with previous reports,23,24 similar results were obtained when the concentration of IL-7 was increased from 10 to 50 ng/mL. Proliferation was assessed using the cell-cycle entry marker Ki67, because we found it to be the most reliable method to quantify low levels of proliferation. Although we cannot guarantee that all Ki67+ cells complete the proliferative cycle, we were able to confirm the proliferation using CFSE staining in adult cells upon IL-7 stimulation (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Moreover, IL-7 has previously been shown to induce similar levels of cell division in adult naive CD4+ T cells.9,25
To exclude a gain of CD31 upon proliferation, we proceeded by investigating the ability of purified CD31+ and CD31− naive (CD45RA+CD45RO−) CD4+ T cells from adult peripheral blood as well as umbilical CB to proliferate in response to IL-7 after 7 days of in vitro culture. Figure 1 illustrates representative flow cytometry dot plots of CD31/CD45RA profiles of freshly isolated CD4+ T cells from adult and CB as well as the gating strategy used to purify the subsets. We confirmed that proliferative responses from adult naive CD4+ T cells were only observed within the CD31+ subset (Figure 2). In agreement with previous reports,8 CB naive T cells showed consistently stronger proliferative responses to IL-7 stimulation than adult naive T cells. Only 12 of the 22 studied adult samples proliferated in response to IL-7, whereas all 12 CB samples proliferated. Purified CD31+ naive CD4+ T cells from adults also proliferated significantly less than CD31+ from CB (2.82% ± 1.11% vs 26.7% ± 3.22% Ki67+ cells, respectively; P = .001). Of note, both CD31+ and CD31− naive CD4+ T-cell subsets isolated from CB were found to proliferate in response to IL-7, while in all analyzed adults proliferation was restricted to the CD31+ subset, as illustrated in Figure 2A. Adult cells able to proliferate in response to IL-7 did not significantly differ from nonresponders with respect to the proportion of males and females, the percentage of naive (CD45RA+) or CD31+ naive within CD4+ cells, or the percentage of CD31+ within the naive CD4+ subset (data not shown). We also did not find any differences comparing the expression of the alpha chain of the IL-7 receptor (IL-7Rα) within the total naive CD4+ gate, or within the CD4+CD45RA+CD31+ and CD4+CD45RA+CD31− gates (data not shown). Interestingly, responders tended to be younger than nonresponders, although this did not reach statistical significance (28.9 ± 2.42 years and 36.4 ± 3.41 years, respectively; P = .109).
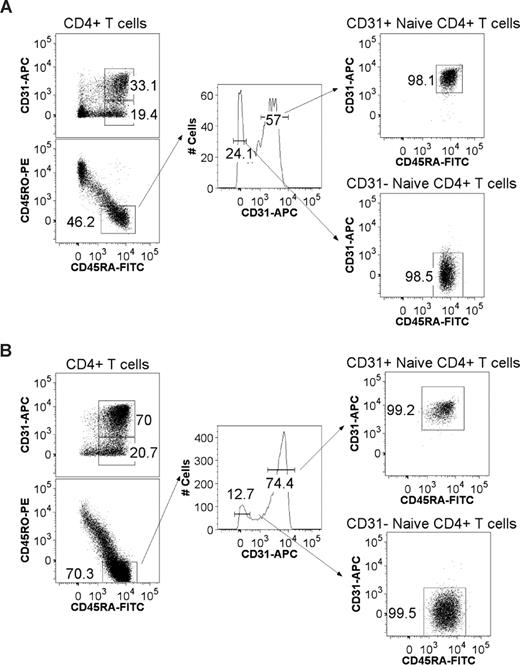
CD31 expression profiles and gating strategy used to purify CD31+ and CD31− naive CD4+ T-cell subsets from adult and cord blood. CD4+ T cells were negatively selected using the EasySep Human CD4+ T-cell Enrichment Kit and stained using monoclonal antibodies for CD45RA, CD45RO, CD4, and CD31. Flow cytometry profiles of CD4+ T cells stained for CD45RA and CD31 are shown for representative adult (A) and cord blood (B) samples. Also shown is the gating strategy used for FACS sorting. After gating on viable lymphocytes and CD4+ T cells, cells were gated on CD45RA+ and CD45RO− expression followed by tight gates on CD31+ and CD31− cells as illustrated by the resulting postsorting profiles.
CD31 expression profiles and gating strategy used to purify CD31+ and CD31− naive CD4+ T-cell subsets from adult and cord blood. CD4+ T cells were negatively selected using the EasySep Human CD4+ T-cell Enrichment Kit and stained using monoclonal antibodies for CD45RA, CD45RO, CD4, and CD31. Flow cytometry profiles of CD4+ T cells stained for CD45RA and CD31 are shown for representative adult (A) and cord blood (B) samples. Also shown is the gating strategy used for FACS sorting. After gating on viable lymphocytes and CD4+ T cells, cells were gated on CD45RA+ and CD45RO− expression followed by tight gates on CD31+ and CD31− cells as illustrated by the resulting postsorting profiles.
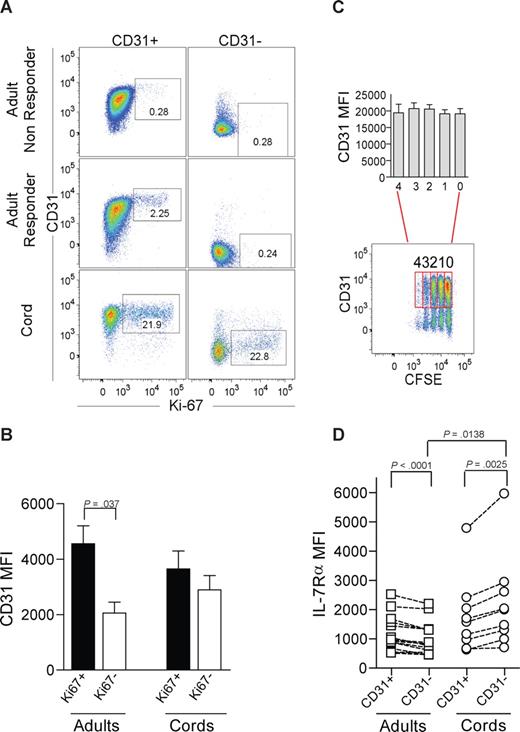
IL-7–induced proliferation of adult naive CD4+ T cells is restricted to the CD31+ subset. (A) Representative dot-plots of CD31 and Ki67 flow cytometry analysis after 7-day culture in the presence of IL-7 of purified CD31+ and CD31− naive CD4+ T-cell subsets from adult peripheral blood, for an IL-7 “nonresponder” (top panel), an IL-7 “responder” (middle panel), and CB (bottom panel). Cells were successively gated on a viable lymphogate, CD3+, CD4+, and CD45RA+. (B) CD31 MFI was assessed within the purified CD31+ naive subset further gated on Ki67+ or Ki67− cells after 7-day culture with IL-7. Three adults and 4 CB samples were studied. (C) Representative dot-plot illustrating CD31 expression plotted against CFSE labeling of CB CD4+CD45RA+ T cells cultured with IL-7 for 7 days. CD31+ cells were further gated according to the number of cell divisions, and bars show CD31 MFI from 4 experiments. (D) Ex vivo analysis of IL-7Rα MFI on freshly isolated mononuclear cells from adult and CB samples sequentially gated on CD3+, CD4+, CD45RA+, and CD31+ or CD31− lymphocytes. Each symbol represents one individual. Bars represent mean plus or minus SEM. Data were compared using paired or unpaired t test as appropriate and significant P values are shown.
IL-7–induced proliferation of adult naive CD4+ T cells is restricted to the CD31+ subset. (A) Representative dot-plots of CD31 and Ki67 flow cytometry analysis after 7-day culture in the presence of IL-7 of purified CD31+ and CD31− naive CD4+ T-cell subsets from adult peripheral blood, for an IL-7 “nonresponder” (top panel), an IL-7 “responder” (middle panel), and CB (bottom panel). Cells were successively gated on a viable lymphogate, CD3+, CD4+, and CD45RA+. (B) CD31 MFI was assessed within the purified CD31+ naive subset further gated on Ki67+ or Ki67− cells after 7-day culture with IL-7. Three adults and 4 CB samples were studied. (C) Representative dot-plot illustrating CD31 expression plotted against CFSE labeling of CB CD4+CD45RA+ T cells cultured with IL-7 for 7 days. CD31+ cells were further gated according to the number of cell divisions, and bars show CD31 MFI from 4 experiments. (D) Ex vivo analysis of IL-7Rα MFI on freshly isolated mononuclear cells from adult and CB samples sequentially gated on CD3+, CD4+, CD45RA+, and CD31+ or CD31− lymphocytes. Each symbol represents one individual. Bars represent mean plus or minus SEM. Data were compared using paired or unpaired t test as appropriate and significant P values are shown.
CD31− naive CD4+ T cells are thought to represent a subpopulation that has undergone peripheral expansion.1 Thus, we next addressed whether in vitro IL-7–induced proliferation resulted in loss of CD31 expression. We found that proliferating CD31+ naive CD4+ T cells did not lose CD31, and that the CD31 median fluorescence intensity (MFI) was significantly higher in Ki67-expressing than in noncycling CD31+ cells (Figure 2B). Furthermore, we were able to monitor cell divisions using CFSE labeling in CB naive CD4+ T cells given their strong proliferative responses to IL-7, and observed that cells that divided up to 4 times during the culture period maintained CD31 expression. Statistical analysis using paired t test showed no statistically significant differences in CD31 expression levels between undivided populations and those that had undergone proliferation (Figure 2C).
We next evaluated whether the different levels of proliferative responses could be attributed to a distinct basal expression of IL-7Rα. We measured ex vivo IL-7Rα expression levels by flow cytometry in freshly isolated lymphocytes, and found that adult CD31− naive CD4+ T cells expressed lower levels than their CD31+ counterparts (Figure 2D). The opposite was found in CB subsets where CD31− cells showed higher IL-7Rα expression than CD31+. Although the levels of IL-7Rα expression were significantly higher in the CD31− subset of CB compared with adults, they were similar in adult CD31− and CB CD31+ subsets (Figure 2D). Thus, the proliferative outcome of IL-7 stimulation is unlikely to rely solely on IL-7Rα expression levels.
These data suggest that in adulthood, the ability of naive CD4+ T cells to proliferate in response to IL-7 is restricted to the CD31+ subset and show that CD31 is not lost after IL-7–induced proliferation.
IL-7–induced proliferation of adult CD31+ naive CD4 T cells is dependent on the PI3K pathway
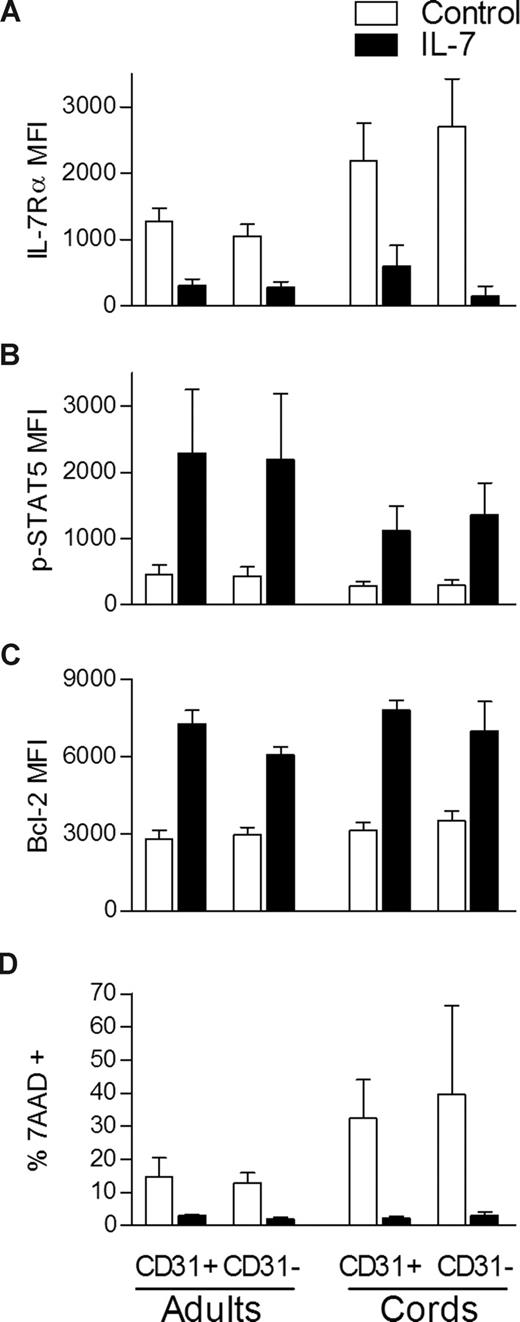
We next investigated whether the decreased proliferation of the CD31− naive CD4+ subset was associated with a general inability to respond to IL-7. A consequence of IL-7 binding is the down-regulation of its own receptor which has been shown to be controlled at the transcriptional level.9,26 We found a clear down-regulation of the IL-7Rα in all populations compared with freshly isolated cells (Figure 3A). IL-7–mediated signaling is known to induce signal transducer and activator of transcription-5 (STAT5) phosphorylation that promotes not only cell cycling but also cell survival through Bcl-2 up-regulation.8,27,28 We found induction of STAT5 phosphorylation (Figure 3B) and Bcl-2 up-regulation (Figure 3C) in both adult and CB CD31+ and CD31− naive CD4 subsets after IL-7 stimulation in comparison with freshly isolated cells. In agreement, similar levels of inhibition of apoptosis (ranging from 60%-70%) were observed in all subsets, using 7-AAD incorporation to compare unstimulated with IL-7 stimulated cells after 7 days of culture (Figure 3D). These data show that despite exerting distinct proliferative effects, IL-7 is able to induce STAT5 phosphorylation, to up-regulate Bcl-2 expression, to prevent apoptosis, and to down-regulate IL-7Rα in both CD31+ and CD31− naive CD4+ subsets.
IL-7 stimulation leads to STAT5 phosphorylation, Bcl-2 up-regulation, and IL-7Rα down-modulation in both CD31+ and CD31− naive CD4+ subsets. IL-7Rα expression (A), STAT5 phosphorylation (B), Bcl-2 expression (C), and 7-AAD incorporation (D) were evaluated by flow cytometry within gated CD31+ and CD31− naive CD4 subsets. p-STAT5 was assessed on freshly isolated mononuclear cells from adult (n = 5) and CB (n = 3) samples either unstimulated or stimulated with IL-7 for 15 minutes. Bcl-2 and IL-7Rα MFI were evaluated ex vivo in adult PBMC (n = 6 and n = 9, respectively) and CB cells (n = 4 and n = 6, respectively) and in the corresponding purified CD31+ and CD31− naive subsets cultured in the presence of IL-7 for 7 days. 7-AAD incorporation was measured in purified CD31+ and CD31− subsets after 7 days of culture in the presence of IL-7 and in its absence (control). Bars represent mean MFI values plus or minus SEM.
IL-7 stimulation leads to STAT5 phosphorylation, Bcl-2 up-regulation, and IL-7Rα down-modulation in both CD31+ and CD31− naive CD4+ subsets. IL-7Rα expression (A), STAT5 phosphorylation (B), Bcl-2 expression (C), and 7-AAD incorporation (D) were evaluated by flow cytometry within gated CD31+ and CD31− naive CD4 subsets. p-STAT5 was assessed on freshly isolated mononuclear cells from adult (n = 5) and CB (n = 3) samples either unstimulated or stimulated with IL-7 for 15 minutes. Bcl-2 and IL-7Rα MFI were evaluated ex vivo in adult PBMC (n = 6 and n = 9, respectively) and CB cells (n = 4 and n = 6, respectively) and in the corresponding purified CD31+ and CD31− naive subsets cultured in the presence of IL-7 for 7 days. 7-AAD incorporation was measured in purified CD31+ and CD31− subsets after 7 days of culture in the presence of IL-7 and in its absence (control). Bars represent mean MFI values plus or minus SEM.
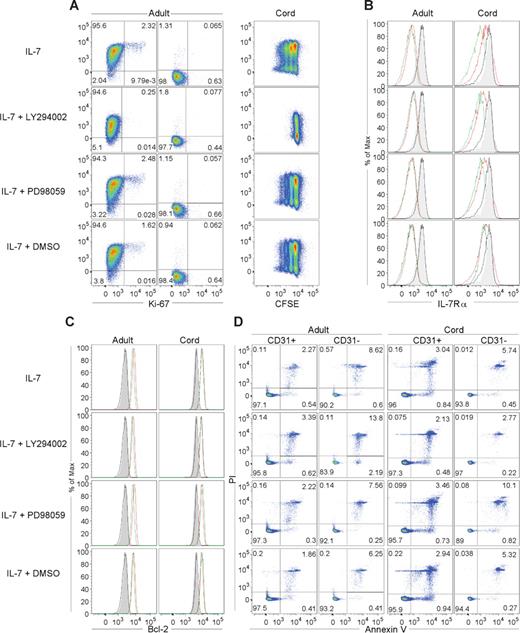
IL-7–mediated signaling leads to PI3K activation, a pathway that regulates cell proliferation and metabolism.23,27 In particular, IL-7–induced proliferation and glucose uptake of naive CD4+ T cells from CB was shown to be dependent upon the PI3K pathway.23 Through the use of the cell-permeable PI3K-specific inhibitor LY294002, we investigated whether the PI3K pathway was required for IL-7–mediated proliferation of adult and CB CD31+ and CD31− subsets. As shown in Figure 4A, LY294002 was very effective at blocking proliferation of adult CD31+ naive CD4+ T cells cultured in IL-7 for 7 days. IL-7Rα down-modulation was found to be PI3K independent (Figure 4B). Despite blocking proliferation, LY294002 did not affect Bcl-2 levels, showing a dissociation of these pathways in these cells (Figure 4C). As previously reported,23,29 we observed a minor increase in apoptosis in the presence of LY294002 in adult naive CD4+ T-cell subsets that was not observed in CB cultures (Figure 4D). Although the possibility of a contribution of apoptosis to the observed block in proliferation induced by PI3K inhibition in the adult CD31+ subset cannot be excluded, this is unlikely to be the case because LY294002 completely blocked proliferation in CB cultures (Figure 4A) without an increase in apoptosis (Figure 4D).
The IL-7–induced proliferation of adult CD31+ naive CD4+ T cells is dependent on the PI3K pathway. CD31+ and CD31− naive CD4+ T cells were purified from adult peripheral blood and CB, cultured in the presence of IL-7 with or without the PI3K inhibitor LY294002 or the MEK-ERK inhibitor PD98059 as indicated, and harvested at day 7 of culture. DMSO was used as a vehicle control. Representative examples of the 6 adults and 4 CBs studied are shown. (A) Assessment of proliferation using Ki67 in an adult sample. Representative analysis of a CB (1 of 4) is also shown illustrating the blocking effects of LY294002 on whole naive CD4+ T-cell subset proliferation as assessed by CFSE labeling. CD31 staining is shown on the y-axis. (B) IL-7Rα and (C) Bcl-2 expression analyzed at day 0 within CD31+ (gray filled histograms) and CD31− cells (black line). Analysis at day 7 within CD31+ (red line) and CD31− (green line) purified populations cultured in the presence of IL-7 and the indicated inhibitors are also shown. (D) Evaluation of apoptosis by annexin V and PI staining after 7 days of culture of the purified CD31+ and CD31− naive subsets.
The IL-7–induced proliferation of adult CD31+ naive CD4+ T cells is dependent on the PI3K pathway. CD31+ and CD31− naive CD4+ T cells were purified from adult peripheral blood and CB, cultured in the presence of IL-7 with or without the PI3K inhibitor LY294002 or the MEK-ERK inhibitor PD98059 as indicated, and harvested at day 7 of culture. DMSO was used as a vehicle control. Representative examples of the 6 adults and 4 CBs studied are shown. (A) Assessment of proliferation using Ki67 in an adult sample. Representative analysis of a CB (1 of 4) is also shown illustrating the blocking effects of LY294002 on whole naive CD4+ T-cell subset proliferation as assessed by CFSE labeling. CD31 staining is shown on the y-axis. (B) IL-7Rα and (C) Bcl-2 expression analyzed at day 0 within CD31+ (gray filled histograms) and CD31− cells (black line). Analysis at day 7 within CD31+ (red line) and CD31− (green line) purified populations cultured in the presence of IL-7 and the indicated inhibitors are also shown. (D) Evaluation of apoptosis by annexin V and PI staining after 7 days of culture of the purified CD31+ and CD31− naive subsets.
The ability of IL-7 to activate the MEK-ERK pathway in T cells remains controversial. Although IL-7 is able to induce ERK1/2 phosphorylation in human leukemia T-cell precursors,27 it does not appear to do so in some mouse T-cell lines,30 in normal human thymocytes,31 or in human peripheral blood T cells.32 We used the MEK-specific inhibitor PD98059 to test the involvement of this pathway in IL-7–mediated effects on human adult CD31+ naive CD4+ subset. As illustrated in Figure 4, PD98059 did not impair any of the IL-7–dependent effects assessed, indicating that the MEK-ERK pathway does not play a critical role in the overall effects of IL-7 in human naive CD4+ T cells.
The same findings were observed for CB CD31+ and CD31− naive CD4+ T cells. Namely, proliferation was blocked by LY294002 but not PD98059, while all the other IL-7 readouts assessed were unaffected by PI3K or MEK-ERK inhibition (Figure 4).
Overall, we show that despite their inability to proliferate in response to IL-7, adult CD31− naive CD4+ T cells are not refractory to IL-7–mediated signaling as measured by STAT5 phosphorylation, Bcl-2 up-regulation or IL-7Rα down-modulation. These data suggest a selective inability of IL-7 to activate the signaling pathways that lead to proliferation in these cells. Moreover, we show for the first time that adult CD31+ naive CD4+ T-cell proliferation is dependent on PI3K activation.
IL-7 promotes the maintenance of CD31 expression in both adult and CB naive CD4+ T cells in a PI3K-dependent manner
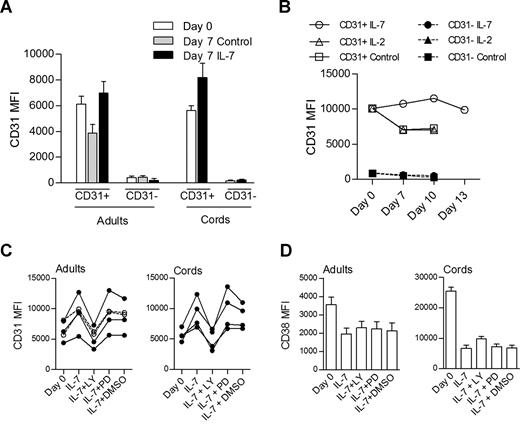
As shown in Figure 2B, cells actively proliferating in response to IL-7 do not lose CD31 expression. We further assessed whether CD31− cells could reexpress CD31 after culture in the presence of IL-7. As shown in Figure 5A, purified CD31− cells from either adult or CB did not acquire CD31 during the culture period. In addition, Figure 5A clearly shows that the levels of CD31 expression were maintained or even increased in CD31+ naive CD4+ cells after in vitro culture with IL-7, whereas cells cultured in medium alone showed reduced CD31 expression (P = .008, paired t test comparison of adult CD31+ cells cultured in the presence of IL-7 and in its absence). This was also the case when cells were cultured for up to 13 days, where CD31 levels were maintained in the presence of IL-7, while cells cultured in medium alone or in the presence of IL-2 exhibited decreased CD31 expression (Figure 5B).
IL-7 promotes the maintenance of CD31 expression on both adult and CB naive CD4 T cells through the PI3K pathway. (A) CD31 MFI on CD31+ and CD31− sorted subpopulations of naive CD4+ T cells from adult (n = 13) and CB (n = 5) at day 0 and day 7 in the presence or absence (control) of IL-7. Analysis of CB subsets cultured in the absence of IL-7 was precluded by the high rate of cell death. (B) Longitudinal analysis of CD31 MFI of adult naive CD4+ subsets cultured in the presence of IL-7, IL-2, or medium alone (control) for up to 13 days (data representative of 3 individuals). Open symbols represent CD31+ purified cells while closed symbols correspond to the CD31− fraction. (C) CD31 MFI assessed on purified CD31+ naive CD4+ T cells at day 0 and after 7-day culture in the presence of IL-7 alone or in addition to LY294002, PD98059, or DMSO. Each symbol represents one individual. Filled symbols refer to individuals with a proliferative response to IL-7 and open symbols to those that did not proliferate. (D) CD38 MFI are shown in the same culture conditions in adult (n = 6) and CB (n = 4) samples, respectively. Bars represent mean values plus or minus SEM.
IL-7 promotes the maintenance of CD31 expression on both adult and CB naive CD4 T cells through the PI3K pathway. (A) CD31 MFI on CD31+ and CD31− sorted subpopulations of naive CD4+ T cells from adult (n = 13) and CB (n = 5) at day 0 and day 7 in the presence or absence (control) of IL-7. Analysis of CB subsets cultured in the absence of IL-7 was precluded by the high rate of cell death. (B) Longitudinal analysis of CD31 MFI of adult naive CD4+ subsets cultured in the presence of IL-7, IL-2, or medium alone (control) for up to 13 days (data representative of 3 individuals). Open symbols represent CD31+ purified cells while closed symbols correspond to the CD31− fraction. (C) CD31 MFI assessed on purified CD31+ naive CD4+ T cells at day 0 and after 7-day culture in the presence of IL-7 alone or in addition to LY294002, PD98059, or DMSO. Each symbol represents one individual. Filled symbols refer to individuals with a proliferative response to IL-7 and open symbols to those that did not proliferate. (D) CD38 MFI are shown in the same culture conditions in adult (n = 6) and CB (n = 4) samples, respectively. Bars represent mean values plus or minus SEM.
We next asked whether the preservation of CD31 expression in cells cultured with IL-7 was dependent on the PI3K pathway. For this purpose, we monitored CD31 levels in the presence of IL-7 alone or with the PI3K inhibitor (Figure 5C), and found that blocking the PI3K pathway led to a statistically significant decrease in CD31 expression levels in both adult and CB naive cells (P = .002 and P = .009, for adults and CB, respectively, paired t test comparison of IL-7 culture with and without LY294002). DMSO, used as a vehicle control, and PD98059 had no effect on CD31 MFI compared with IL-7 alone (Figure 5C).
As mentioned above, CB samples always proliferated in response to IL-7, while approximately one-half of the adults studied exhibited proliferative responses in vitro. Importantly, blocking the PI3K pathway prevented CD31 maintenance in all adults tested regardless of their ability to proliferate in response to IL-7. This is shown in Figure 5C, where individuals who proliferated in response to IL-7 and those who did not are represented. These data suggest that the preservation or increase of CD31 expression is independent of proliferation.
We also assessed the possible effects of blocking PI3K signaling on the expression of the CD31 ligand, CD38.33 This molecule has been shown to decrease on naive CB T cells cultured in the presence of IL-7.22 As shown in Figure 5D, we observed that adult and CB CD31+ naive CD4+ T cells exhibited a significant reduction of CD38 expression after culture with IL-7 (P < .001, paired t test), and this was not altered by the presence of PI3K or MEK-ERK inhibitors. These data show that LY294002 is unable to recover the reduction of CD38 expression associated with IL-7 culture, suggesting that IL-7 may regulate CD31 expression independently of its ligand.
Overall, we report a role for IL-7 not only in the proliferation of adult CD31+ naive CD4+ T cells, but also in the maintenance or increase of CD31 expression levels in a PI3K-dependent manner.
Discussion
Our data indicate that IL-7 preferentially promotes proliferation of CD31+CD4+ naive T cells in adults, while preventing the loss of CD31 expression in both cycling and noncycling cells. The 2 mechanisms appear to depend upon the activation of the PI3K pathway and likely contribute to the maintenance of CD31+ naive CD4+ T cells promoted by IL-7. In contrast, the CD31− subset appears to rely on other homeostatic cues.
The selective ability of IL-7 to induce proliferation of the CD31+ subset during adulthood, and in this way contribute to the maintenance of a population that is known to incorporate recent thymic emigrants,1 is expected to have a physiologic role in the preservation of the TCR repertoire diversity within naive CD4+ T cells.
Thus, as thymic output is reduced during aging, IL-7 may contribute to the persistence of the CD31+ population through low-level proliferation. This is in agreement with recent data showing that both TREC content and telomere length decrease in CD31+ naive CD4+ T cells during aging, implying that their persistence is dependent on proliferation in the periphery.6 The persistence of relatively high TREC content in the CD31+ subset can be attributed to both residual thymic output and to a low rate of peripheral cell division.
Importantly, we have previously associated IL-7 serum levels and preservation of CD31+ naive subset during aging in lymphopenic settings, and suggested that this positive correlation may contribute to the slower rate of CD4+ T-cell decline in HIV-2 compared with HIV-1 infection.16
Our observation that the adult CD31− subset did not proliferate in response to IL-7 in vitro does not exclude the possibility of IL-7 acting as a costimulus to other homeostatic proliferation mechanisms, such as self-peptide–major histocompatibility complex (MHC) interactions.34 However, our findings suggest that the CD31− subset may be preferentially regulated by mechanisms other than direct IL-7–driven proliferation.
The maintenance of CD31 expression upon IL-7 stimulation raises questions regarding the mechanisms underlying the loss of CD31 in naive CD4+ T cells. CD31 expression has been shown to be lost after TCR stimulation of naive CD4+ T cells,35 and therefore low-affinity self-peptide–MHC interactions may be implicated in the generation as well as maintenance of the CD31− subset.34 Our observation of a restricted IL-7 proliferative effect on adult CD31+ naive CD4+ T cells further support this possibility. In agreement with this, Kholer et al5 reported that the CD31− subset expresses increased levels of BFL-1/A1 ex vivo compared with the CD31+ subset. BFL-1/A1 has been described as a marker of recent TCR engagement whose expression is not induced by cytokine stimulation,36 further implying that the CD31− subset is likely to be maintained by mechanisms that rely on TCR engagement rather than cytokine-induced proliferation. On the other hand, the presence of CD31 may impair TCR-mediated maintenance of CD31+ cells, since there are data supporting an inhibitory function for CD31 in TCR activation through its cytoplasmic immunoreceptor tyrosine-based inhibitory motifs (ITIMs).37,38 Our data further support the view that CD31 expression may impact on the homeostatic mechanisms involved in the maintenance of the adult naive CD4+ T-cell pool.
We also demonstrated that the distinct responses of the CD31+ and CD31− subsets to IL-7 could not be solely attributed to differences in IL-7Rα expression. Interestingly, a previous study addressing the effects of IL-7 in human B-cell progenitors comparing pro-B and pre-B cells reported that only pro-B cells proliferate in response to IL-7 despite similar levels of IL-7Rα in both subsets.39 In addition, this study demonstrated that in contrast to the pre–B-cell subset, pro-B cells expressed CD31, further demonstrating an association between CD31 expression and the ability to proliferate in response to IL-7.39
In addition, we show that IL-7–induced proliferation of CD31+ naive CD4+ T cells from adults is dependent on PI3K activation, in agreement with what was previously reported for umbilical cord blood naive T cells.23 Furthermore, we show for the first time that IL-7 induces maintenance or an increase of CD31 expression in a PI3K-dependent manner and that this occurs irrespectively of the induction of proliferation. The biologic significance of this finding is further emphasized by the absence of changes in the expression of the CD31 ligand (CD38) upon PI3K inhibition.
While contributing to the understanding of the role of IL-7 in the maintenance of naive CD4+ subsets in humans, our data further imply that the CD31+ subset is likely to be the main target of IL-7–driven proliferation during its therapeutic use. This is in agreement with data recently published of a phase 1 trial using recombinant IL-7.21 A clear induction of T-cell proliferation was shown, whereby naive CD4+ expansion was accounted by proliferation of the CD31+ naive CD4+ T-cell subset that was associated with a decrease in TREC content which is highly suggestive of IL-7–driven peripheral expansion.21
In conclusion, our data support the view that the adult naive CD4+ T-cell subset identified by the CD31 marker, besides including the recent thymic emigrants,1 represents a population with a unique ability to proliferate in response to IL-7. Moreover, we show that IL-7 sustains CD31 expression in naive CD4+ T cells in a PI3K-dependent manner. This preferential effect of IL-7 on the CD31+ population provides a biologic rationale for the use of IL-7 therapy in clinical settings where the expansion of the T-cell repertoire diversity is required.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Professor Arne N. Akbar (University College London) and Russell B. Foxall and Helena Cabaço (both from Instituto de Medicina Molecular, Lisboa) for critical review of this manuscript and for scientific discussions. We acknowledge Dr Helena Ferreira (Hospital Universitário de Santa Maria, Lisboa) for providing umbilical cord blood samples.
This work was supported by grant POCI/BIA-BCM/61079/2004 from Fundação para a Ciência e a Tecnologia (FCT) and by Programa Operacional Ciência e Inovação 2010 (POCI2010; to M.V.D.S.) R.I.A., M.V.D.S., and R.T. received scholarships from FCT cofinanced by POCI 2010 and FSE.
Authorship
Contribution: R.I.A. and M.V.D.S. designed and performed research, analyzed and interpreted data, and wrote the paper; J.T.B. designed research and discussed data; R.T. and A.S.-C. performed research; R.M.M.V. discussed data; and A.E.S. designed research, supervised the work, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maria Vieira D. Soares, Unidade de Imunologia Clínica, Instituto de Medicina Molecular, Faculdade de Medicina de Lisboa, Av Prof Egas Moniz, 1649-028 Lisboa, Portugal; e-mail: msoares@fm.ul.pt.
References
Author notes
*R.I.A. and M.V.D.S. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal