Abstract
Although well characterized in the mouse, the role of Notch signaling in the human T-cell receptor αβ (TCR-αβ) versus TCR-γδ lineage decision is still unclear. Although it is clear in the mouse that TCR-γδ development is less Notch dependent compared with TCR-αβ differentiation, retroviral overexpression studies in human have suggested an opposing role for Notch during human T-cell development. Using the OP9-coculture system, we demonstrate that changes in Notch activation are differentially required during human T-cell development. High Notch activation promotes the generation of T-lineage precursors and γδ T cells but inhibits differentiation toward the αβ lineage. Reducing the amount of Notch activation rescues αβ-lineage differentiation, also at the single-cell level. Gene expression analysis suggests that this is mediated by differential sensitivities of Notch target genes in response to changes in Notch activation. High Notch activity increases DTX1, NRARP, and RUNX3 expression, genes that are down-regulated during αβ-lineage differentiation. Furthermore, increased interleukin-7 levels cannot compensate for the Notch dependent TCR-γδ development. Our results reveal stage-dependent molecular changes in Notch signaling that are critical for normal human T-cell development and reveal fundamental molecular differences between mouse and human.
Introduction
T-cell development is a well-ordered spatiotemporal process that occurs in the thymus and during which T-cell precursors must make several developmental lineage decisions. These decisions are influenced by different site-specific signals, derived from cytokines, chemokines, T-cell receptors (TCRs), Wnt signaling, Notch ligands, and others that T-cell precursors encounter during their migration within the thymic microenvironment.1 Although all these signals must integrate appropriately, studying individual pathways is vital for understanding their role in this complex process
In the thymus, one of the earliest developmental choices that T-cell precursors make is the TCR-αβ versus TCR-γδ lineage decision. Whereas this T-cell branching point is obviously influenced by the nature of the rearranged β, γ, and δ TCR genes, also TCR signal strength, as well as epigenetic phenomena, cytokines and transcription factors can influence the developmental outcome.2 In addition, Notch signaling has been shown to be involved in regulating the TCR-αβ/-γδ lineage decision. Notch receptors are activated by ligands of the Jagged and Delta families, resulting in activation of a CBF1/suppressor of Hairless/Lag-1–dependent (CSL) transcriptional complex that also involves proteins of the Mastermind family, thereby activating the transcription of several target genes.3 In the mouse, several lines of evidence suggest that TCR-αβ T cells require higher levels of Notch activation compared with TCR-γδ T cells. Reducing Notch1 gene dosage in vivo favored γδ T-cell development over αβ-lineage cells,4 and also mice deficient for CSL showed increased γδ T cells but reduced αβ-lineage cells.5 In addition, in vitro studies with well-defined thymocyte subsets6-8 have shown that γδ T-cell development is less stringently dependent on Delta-like (DL)–dependent Notch signaling compared with early αβ-lineage cells, which require DL-Notch interactions to pass through the β-selection checkpoint.7,9,10 Instead, γδ T cells seem more dependent on Jagged ligands,11 which are considered to induce lower Notch activation in comparison with DL ligands.12
In human, the involvement of Notch signaling in the αβ/γδ lineage decision is less clear. Studies performed in our laboratory showed that increased Notch activation results in increased γδ T-cell development at the cost of TCR-αβ lineage cells,13 and these results were later confirmed by Garcia-Peydro et al.14 Although these results suggest an opposing role for Notch signaling in the human αβ/γδ lineage decision compared with the mouse, it is still unclear whether these effects are the result of unphysiologic levels of Notch activation because of the technical approach that was used in both sets of experiments.
For these reasons, we have further dissected the role of Notch signaling during human T-cell development, using the OP9-DL1 coculture system,15 in combination with various levels of γ-secretase inhibition.16,17 Our results, which include clonal cell cultures, reveal an unexpected early requirement for a reduction in Notch activation during human TCR-αβ T-cell development, which is fundamentally different from the mouse. Furthermore, we provide a molecular mechanism through which small differences in Notch-signaling intensities are translated into downstream activation events, resulting in profound changes in developmental outcome. These mechanisms seem also critical for understanding Notch-dependent cellular transformations, which frequently occur in T-acute lymphoblastic leukemia (T-ALL).18
Methods
Monoclonal antibodies
The following monoclonal antibodies were from BD Biosciences (San Jose, CA): fluorescein isothiocyanate (FITC)–conjugated CD3, CD7, CD8, CD19, CD56, TCR-γδ; phycoerythrin (PE)–conjugated CD4, CD38, CD45, TCR-γδ; allophycocyanin (APC)–conjugated CD3, CD34. The following monoclonal antibodies were from Miltenyi Biotec (Auburn, CA): PE-conjugated TCR-αβ and APC-conjugated CD4 and CD45. CD8β-PE was from Beckman Coulter (Fullerton, CA) and CD1a-FITC was obtained from ATCC (Manassas, VA).
Tissues, cell sorting, and flow cytometry
Pediatric thymuses and cord blood (CB) samples were obtained and used according to the guidelines of the Medical Ethical Commission of Ghent University Hospital (Ghent, Belgium), which approved this study, and informed consent was obtained in accordance with the Declaration of Helsinki. Flow cytometric data were generated of FACSCalibur using CellQuest software (BD Biosciences). Cell sorting of populations was performed on a FACSVantage using CellQuest software.
CD34+CD38−Lin− CB and CD34+ thymocytes were enriched using magnetic-activated cell sorting with CD34 microbeads (Miltenyi Biotec) as described.19 CB cells were sorted as CD34+CD38−CD19−CD56−CD3−CD8− (CD34+CD38−Lin−) cells. Enriched CD34+ thymocytes were, where necessary, sorted after CD34-APC, CD1-FITC, and CD4-PE labeling into CD34+CD1−CD4− and CD34+CD1+CD4− fractions. To obtain ISP4+ cells, thymocytes were depleted for CD3/CD8 using Dynal beads sheep anti–mouse IgG (Invitrogen, Carlsbad, CA) and afterward labeled with CD34-FITC, CD3-FITC, CD8α-FITC, and CD4-PE to sort CD34−CD3−CD8α−CD4+ cells. Total thymus was stained with CD3-FITC, CD8β-PE, and CD4-APC to sort CD4+CD8β+CD3− cells. TCR-γδ and TCR-αβ populations were enriched using anti-PE beads (Miltenyi Biotec) after labeling of total thymus with TCR-γδ-PE and TCR-αβ-PE antibodies and subsequently sorted to achieve more than 98% purity. The TCR-αβ population was composed of double-positive (DP) cells (60%-73%), CD4+ single-positive cells (18%-29%), and CD8+ single-positive cells (7%-10%). For limiting dilution and single-cell experiments, Clonecyte software (BD Biosciences) was used on the FACSVantage to deposit 1, 3, 10, or 30 cells in the desired wells (BD Biosciences).
OP9 cocultures
OP9-DL1 and OP9-GFP cells have been described previously.15,20 OP9 cells expressing human DL1 (called OP9-hDL1) or human DL4 (called OP9-hDL4) were generated through retroviral transduction of OP9 cells, followed by sorting for EGFP+ transduced cells. Retrovirus encoding for human DL1 and human DL4 was generated with previous described methods21 with LZRS plasmids that encode for human DL122 (a generous gift from Dr L. Parreira [Instituto de Histologia e Embriologia, Lisboa, Portugal]) or human DL423 (a generous gift from Dr A. L. Harris [University of Oxford, Oxford, United Kingdom] and Dr G. Tosato [National Institutes of Health, Bethesda, MD]). OP9 cocultures with human CD34+CD38−Lin− cells and various subsets of postnatal thymocytes were all performed as described20 in α–minimum essential medium containing 20% fetal calf serum, 2.5 ng/mL stem cell factor (a kind gift of Amgen, Thousand Oaks, CA), and 5 ng/mL of both interleukin-7 (IL-7) and Flt3L (R&D Systems, Minneapolis, MN), with the exception of 1 set of experiments in which the effect of IL-7 concentrations was evaluated as indicated. For γ-secretase inhibition (GSI) experiments, various concentrations of 7 N-[N-(3,5-difluorophenyl-L-alanyl)]-S-phenyl-glycine t-butyl ester (DAPT; Peptides International, Louisville, KY), diluted in dimethyl sulfoxide (DMSO), were added as indicated and an equal concentration of DMSO was used as control (0 μM GSI).19 Cocultures were harvested by forceful pipetting at the indicated time points. For CB experiments, cultures were initiated with 4 to 10 × 103 cells; whereas for CD34+CD1− and CD34+CD1+ thymocytes, cocultures were initiated with 6 to 15 × 103 cells. The resulting cell numbers for these experiments are presented per 103 cells and per 10 × 103 cells, respectively, to allow comparison across all experiments.
Gene expression analysis
Cells were resuspended in RNeasy lysis buffer (RLT; QIAGEN, Valencia, CA) and stored at −70°C before RNA extraction. RNA was isolated using RNeasy (QIAGEN) and converted into cDNA using Superscript RT II (Invitrogen). Real-time polymerase chain reactions (PCRs) were performed using qPCR Core Kit for SYBR Green I (Eurogentec, Seraing, Belgium) on a 7300 Real-time PCR system (Applied Biosystems, Foster City, CA). Primer sequences are available on request. Relative expression was calculated for each gene by the Δ cycle threshold (Ct) method. For OP9-DL1–cocultured CD34+ thymocytes, cultures were labeled for CD45-PE and human CD45+ thymocytes were sorted before RNA extraction to exclude contaminating OP9 cells.
Statistical analysis
Statistical analysis was performed using the nonparametric paired Wilcoxon test from the SPSS 15.0 software package (Chicago, IL).
Results
Gene expression analysis of the Notch pathway during human T-cell development
Because little information is available on the expression levels of Notch receptors and Notch target genes during human T-cell development, we first thoroughly characterized the expression pattern of Notch-signaling components in well-defined human thymocyte subsets. Detailed gene expression analysis through quantitative real-time PCR revealed sharp changes in the expression of Notch-related genes during these distinct stages of human T-cell development (Figure 1).
Gene expression analysis of human T-cell development. Quantitative real-time RT-PCR gene expression analysis of Notch and T cell–related genes in human CB CD34+ and postnatal thymocyte subsets, ordered according to developmental progression along the T-cell pathway. Data shown are the mean plus or minus SEM from 2 to 5 sets of independent samples, relative to β-actin levels.
Gene expression analysis of human T-cell development. Quantitative real-time RT-PCR gene expression analysis of Notch and T cell–related genes in human CB CD34+ and postnatal thymocyte subsets, ordered according to developmental progression along the T-cell pathway. Data shown are the mean plus or minus SEM from 2 to 5 sets of independent samples, relative to β-actin levels.
A first major step during T-cell development involves the entry of precursors into the thymus. NOTCH1 is expressed in extrathymic CD34+Lin− CB progenitors but at a higher level in the most immature CD34+CD1− thymocytes. NOTCH2 is expressed at a similar level in CB precursors and immature thymocytes, whereas NOTCH3 is barely expressed in extrathymic precursors but induced in thymocytes. Interestingly, expression of the Notch inhibitor ZBTB7A (coding for LRF) is low at this stage. As expected, the Notch target genes HES1, DTX1, and MYC are strongly induced in the most immature thymocytes (Figure 1). In contrast, NRARP expression is high in CB precursors and slightly lower in CD34+CD1− early thymocytes. In addition, the T-cell transcription factors TCF7 (coding for TCF1) and GATA3 are expressed at a higher level in immature thymocytes. Thus, these data suggest that a strong induction of the Notch pathway occurs when precursor cells arrive in the thymus.
The next step during T-cell development is the commitment phase, which in humans occurs at the CD34+CD1− to CD34+CD1+ transition. Although NOTCH1, NOTCH2, and the target genes HES1 and MYC maintain their expression, an increase in NOTCH3 expression is observed as well as a sharp down-regulation of DTX1 and NRARP (Figure 1). The expression of GATA3, TCF7, and TCF3 (coding for E2A) is further up-regulated on T-cell commitment, whereas RUNX3 is significantly down-regulated. This developmental transition is also characterized by the induction of genes required for T-cell receptor formation and signaling, such as PTCRA, RAG1, and RAG2, whose expression further increases in the CD4+CD3−CD8− ISP stage where most of the TCR-β rearrangements occur.24 Thus, the T-cell commitment phase is characterized by differential changes in Notch target gene expression.
During the transition from the CD4+CD3−CD8− ISP stage into the CD4+CD8β+CD3− DP stage, NOTCH1, NOTCH3, HES1, and MYC are now all down-regulated, indicating a severe reduction of Notch-signaling activity for further development along the TCR-αβ lineage (Figure 1). Transcription of PTCRA is turned off but TCR-Cα expression is activated, whereas RAG transcripts remain expressed to allow TCR-α rearrangement. Thus, a shutdown of the Notch pathway occurs at the DP stage.
Comparing αβ- and γδ-lineage cells in the thymus, we observed higher Notch activation in γδ T cells as shown by the increased HES1, MYC, DTX1, and NRARP expression. In addition, RUNX3 expression is higher in TCR-γδ compared with TCR-αβ T cells.
These results show that important changes in Notch-signaling activity occur at different developmental stages of human T-cell development and suggest that these are critical for efficient T-cell generation.
Generation of T/NK-cell precursors and the induction of human T-cell development from human stem cells requires high levels of Notch signaling
Notch signaling is absolutely required for inducing T-cell development, in both mouse and human,19,25 and our gene expression analysis suggests that strong Notch-signaling events occur after thymic entry of precursor cells (Figure 1). To investigate dose-dependent Notch effects on the generation of T/NK-cell precursors and the induction of human T-cell development, we cultured CD34+CD38− hematopoietic CB stem cells on OP9-DL1 cells to strongly induce Notch signaling, and manipulated Notch activation with various concentrations of the GSI DAPT.
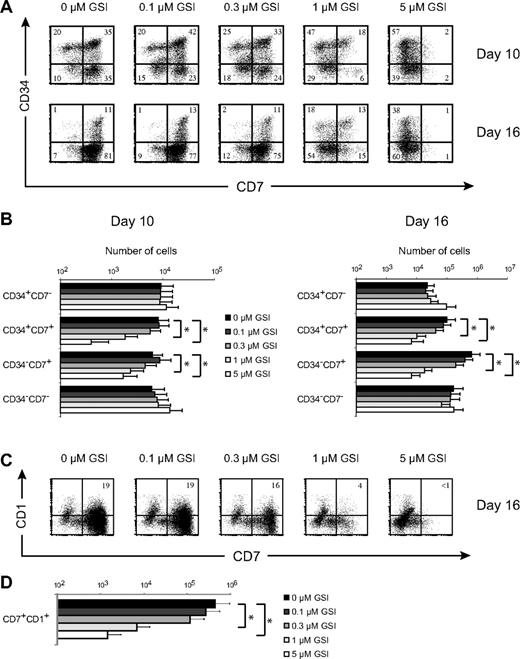
DL1 induces high CD7 expression in human CD34+CD38− CB precursors (Figure 2A), consistent with the phenotype of human T/NK progenitors13,26 and intrathymic T-cell precursors.27,28 After 10 and 16 days of culture, a gradual decrease in the frequency of CD34+CD7+ and more differentiated CD34−CD7+ T/NK-cell precursors was observed with increasing doses of GSI (Figure 2A). As a result, CD34+CD7+ and CD34−CD7+ T/NK-cell precursors27 were most efficiently generated in OP9-DL1 cultures with the highest Notch activation (Figure 2B). Consequently, analysis of T-cell commitment by fluorescence-activated cell sorter analysis for CD7 and CD1 expression showed a corresponding decrease in the frequency (Figure 2C) and number (Figure 2D) of T cell–committed CD7+CD1+ precursors as Notch signaling decreased. Thus, the optimal generation of human T/NK-cell precursors and the induction of human T-cell development from HSCs require strong Notch signals, in agreement with our gene expression analysis (Figure 1).
Generation of T/NK-cell precursors and the induction of human T-cell development from human stem cells requires high levels of Notch signaling. (A) Flow cytometric analysis of CD34 and CD7 expression in human CD34+CD38−Lin− CB precursors after 10 or 16 days of coculture on OP9-DL1 stromal cells in the presence of various concentrations of the γ-secretase inhibitor DAPT (GSI). Numbers in quadrants indicate the percentage of the corresponding population. Results shown are representative for 5 independent experiments. (B) Corresponding cellularity of CD34+CD7−, CD34+CD7+, CD34−CD7+, and CD34−CD7− subsets for cultures depicted in panel A. Data are presented on a log scale and shows mean plus or minus SEM (n = 5). (C) Flow cytometric analysis of CD7 and CD1 expression at day 16 of the same cultures as in panel A. (D) Corresponding cellularity of CD7+CD1+ T cell–committed precursors as depicted in panel C. Data are presented on a log scale and show mean plus or minus SEM (n = 5). *Significant difference (P < .05).
Generation of T/NK-cell precursors and the induction of human T-cell development from human stem cells requires high levels of Notch signaling. (A) Flow cytometric analysis of CD34 and CD7 expression in human CD34+CD38−Lin− CB precursors after 10 or 16 days of coculture on OP9-DL1 stromal cells in the presence of various concentrations of the γ-secretase inhibitor DAPT (GSI). Numbers in quadrants indicate the percentage of the corresponding population. Results shown are representative for 5 independent experiments. (B) Corresponding cellularity of CD34+CD7−, CD34+CD7+, CD34−CD7+, and CD34−CD7− subsets for cultures depicted in panel A. Data are presented on a log scale and shows mean plus or minus SEM (n = 5). (C) Flow cytometric analysis of CD7 and CD1 expression at day 16 of the same cultures as in panel A. (D) Corresponding cellularity of CD7+CD1+ T cell–committed precursors as depicted in panel C. Data are presented on a log scale and show mean plus or minus SEM (n = 5). *Significant difference (P < .05).
Human TCR-γδ T cells are highly sensitive to reduced Notch signaling
Next, we investigated the effect of different Notch signal strengths on human TCR-αβ and TCR-γδ T-cell development. Therefore, human postnatal uncommitted CD34+CD1− and T-lineage committed CD34+CD1+ T-cell precursors, which both have αβ and γδ potential,29 were subjected to OP9-DL1 cocultures with the same concentrations of GSI as used for the prethymic CB progenitors.
For both uncommitted and committed human T-cell precursors, kinetic analysis of CD4 and CD8β expression indicated that developmental progression toward the DP stage was enhanced with increasing amounts of GSI (Figure 3A). Consistent with this observation, both the frequency (Figure 3B) and number (Figure 3C) of TCR-αβ T cells increased as Notch activation was reduced with increased concentrations of GSI. In contrast, the highest percentage (Figure 3B) and absolute number (Figure 3C) of TCR-γδ T cells was observed in the absence of GSI for both CD34+CD1− and CD34+CD1+ thymocytes, and their frequency and number decreased with increasing amounts of GSI, suggesting a differential dependence of Notch activity between both T-cell lineages. Cultures were analyzed weekly, but data shown in Figure 3B and C are after 3 weeks of culture as no TCR-αβ T cells could be detected after 1 or 2 weeks (data not shown). In the presence of 5 μM DAPT and after 3 weeks of culture, virtually no TCR-γδ and TCR-αβ T cells could develop (data not shown), consistent with previous findings.19
Human TCR-γδ T cells are highly sensitive to reduced Notch signaling. Flow cytometric analysis of human CD34+CD1− and CD34+CD1+ postnatal thymocytes on OP9-DL1 stromal cells in the presence of various concentrations of the γ-secretase inhibitor DAPT (GSI), showing CD4+CD8β+ expression after 6, 10, and 16 days of coculture (A) or TCR-αβ+CD3+ and TCR-γδ+CD3+ expression after 3 weeks of coculture. (B) Numbers in quadrants indicate the percentage of cells for the corresponding population. Results shown are representative for 5 independent experiments. (C) Corresponding total, TCR-γδ, and TCR-αβ cellularity for cultures depicted in panel B, showing mean plus or minus SEM (n = 5). *Significant difference (P < .05).
Human TCR-γδ T cells are highly sensitive to reduced Notch signaling. Flow cytometric analysis of human CD34+CD1− and CD34+CD1+ postnatal thymocytes on OP9-DL1 stromal cells in the presence of various concentrations of the γ-secretase inhibitor DAPT (GSI), showing CD4+CD8β+ expression after 6, 10, and 16 days of coculture (A) or TCR-αβ+CD3+ and TCR-γδ+CD3+ expression after 3 weeks of coculture. (B) Numbers in quadrants indicate the percentage of cells for the corresponding population. Results shown are representative for 5 independent experiments. (C) Corresponding total, TCR-γδ, and TCR-αβ cellularity for cultures depicted in panel B, showing mean plus or minus SEM (n = 5). *Significant difference (P < .05).
Because it has been shown that Delta-like-4 is responsible for inducing T-cell development in vivo,30,31 we investigated whether TCR-αβ and TCR-γδ differentiation was similarly influenced by the addition of GSI when precursor cells were cocultured on OP9 stromal cells that express human DL4, in comparison with OP9 cells that express human DL1. As shown in Figure 4, addition of 1 μM GSI enhanced the differentiation into CD4+CD8+ DP cells and TCR-αβ T cells but reduced the development of human TCR-γδ T cells on both OP9-hDL1 and OP9-hDL4 after 21 days of coculture, both with respect to frequency (Figure 4A) and absolute cell numbers (Figure 4B). At day 29, we observed that the number of TCR-αβ T cells was reduced in the presence of 1 μM GSI compared with cocultures with 0 μM GSI for both OP9-hDL1 and OP9-hDL4 (Figure 4B), although the frequency was still increased with reduced Notch activation (Figure 4A). Nevertheless, the ratio of TCR-αβ to TCR-γδ T cells was still increased after 29 days in the presence of 1 μM GSI (22 to 1 for OP9-hDL1 and 15 to 1 for OP9-hDL4) compared with in the presence of 0 μM GSI (6 to 1 for OP9-hDL1 and 5 to 1 for OP9-hDL4) (Figure 4C).
DL1 and DL4 similarly affect human αβ/γδ T-cell differentiation. (A) Flow cytometric analysis of human CD34+ postnatal thymocytes after 21 and 29 days of coculture on OP9-hDL1 and OP9-hDL4 stromal cells in the presence of 0 or 1 μM of the γ-secretase inhibitor DAPT (GSI). Numbers in quadrants indicate the percentage of TCR-αβ+CD3+, TCR-γδ+CD3+, or CD4+CD8β+ cells. Results shown are representative for 2 independent experiments. (B) Corresponding TCR-γδ and TCR-αβ cellularity for cultures depicted in panel A, showing mean plus or minus SEM (n = 2). (C) Ratio of TCR-αβ to TCR-γδ T cells after 29 days of culture, derived from data shown in panel B.
DL1 and DL4 similarly affect human αβ/γδ T-cell differentiation. (A) Flow cytometric analysis of human CD34+ postnatal thymocytes after 21 and 29 days of coculture on OP9-hDL1 and OP9-hDL4 stromal cells in the presence of 0 or 1 μM of the γ-secretase inhibitor DAPT (GSI). Numbers in quadrants indicate the percentage of TCR-αβ+CD3+, TCR-γδ+CD3+, or CD4+CD8β+ cells. Results shown are representative for 2 independent experiments. (B) Corresponding TCR-γδ and TCR-αβ cellularity for cultures depicted in panel A, showing mean plus or minus SEM (n = 2). (C) Ratio of TCR-αβ to TCR-γδ T cells after 29 days of culture, derived from data shown in panel B.
IL-7 cannot compensate Notch-dependent γδ T-cell development
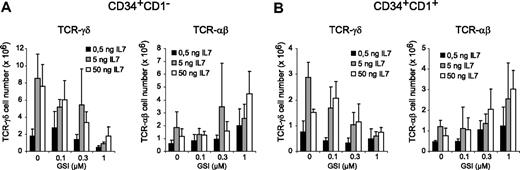
Because IL-7 is critical for T-cell development,32 especially for γδ cells,33 we investigated whether high IL-7 concentrations could rescue γδ T-cell development in OP9-DL1 cocultures with 1 μM GSI. Cultures were initiated with CD34+CD1− (Figure 5A) and CD34+CD1+ (Figure 5B) thymocytes in the presence of 0.5 or 50 ng/mL IL-7 and compared with normal culture conditions that contain 5 ng/mL. A 10-fold reduction in IL-7 reduced the number of TCR-αβ and especially TCR-γδ T cells (Figure 5). At 50 ng/mL, IL-7 was capable of increasing the number of TCR-αβ and TCR-γδ T cells in the presence of 1 μM GSI, but for TCR-γδ T cells, the absolute number was still significantly reduced compared with control cultures containing no GSI and 5 ng/mL IL-7 (Figure 5). Thus, the generation of human γδ T cells depends on high levels of Notch signaling and cannot be rescued by IL-7.
IL-7 cannot rescue Notch-dependent TCR-γδ T-cell development. Flow cytometric analysis of human CD34+CD1− (A) and CD34+CD1+ (B) postnatal thymocytes after 3 weeks of coculture on OP9-DL1 stromal cells in the presence of various concentrations of the γ-secretase inhibitor DAPT (GSI) and with increased (50 ng/mL) or reduced (0.5 ng/mL) IL-7 concentrations. Data show TCR-γδ and TCR-αβ cellularity in comparison to normal IL-7 levels (5 ng/mL) as also shown in Figure 3. Data represent mean plus or minus SEM (n = 3).
IL-7 cannot rescue Notch-dependent TCR-γδ T-cell development. Flow cytometric analysis of human CD34+CD1− (A) and CD34+CD1+ (B) postnatal thymocytes after 3 weeks of coculture on OP9-DL1 stromal cells in the presence of various concentrations of the γ-secretase inhibitor DAPT (GSI) and with increased (50 ng/mL) or reduced (0.5 ng/mL) IL-7 concentrations. Data show TCR-γδ and TCR-αβ cellularity in comparison to normal IL-7 levels (5 ng/mL) as also shown in Figure 3. Data represent mean plus or minus SEM (n = 3).
Notch-signaling intensities influence the TCR-αβ potential
The striking difference in the Notch-dependent generation of TCR-αβ and TCR-γδ cells urged us to investigate whether Notch-signaling intensities influence this developmental lineage choice.
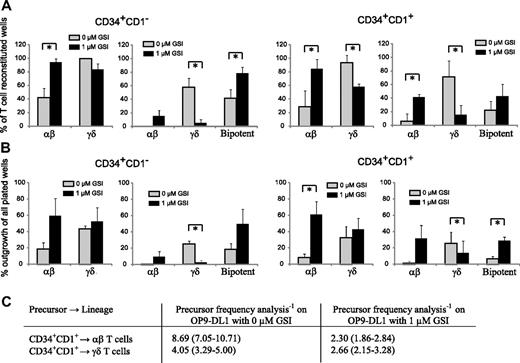
Figure 6 shows the result of clonal OP9-DL1 cultures with uncommitted CD34+CD1− and T-committed CD34+CD1+ thymocytes in the presence of maximal (0 μM GSI) or reduced (1 μM GSI) Notch stimulation. Figure 6A shows the relative frequency of TCR-αβ and TCR-γδ T cells, derived from unipotent or bipotent clones within the wells that showed T-cell reconstitution. Because CD34+CD1− thymocytes are the earliest T-cell precursors, we expected these cells to be mainly bipotent. However, in the absence of GSI, only 42% of the outgrowing cells were bipotent, whereas 58% generated exclusively γδ cells and less than 1% exclusively αβ T cells (Figure 6A). When Notch activity was reduced with 1 μM GSI, the bipotent potential of these precursors increased to 79%, but also more αβ-lineage committed precursor developed (15%), whereas very few precursors only had γδ-lineage potential (5%) (Figure 6A). Overall, the change in Notch-signaling intensity slightly reduced the TCR-γδ potential (99% and 84% for 0 and 1 μM GSI, respectively) but significantly increased the αβ-lineage potential (42% and 95% for 0 and 1 μM GSI, respectively). In contrast to CD34+CD1− thymocytes, the majority of T lineage–committed CD34+CD1+ precursors had lost bipotent T-lineage potential, independent of the amount of GSI (0 or 1 μM), and yielded either αβ or γδ cells, not both (Figure 6A), corresponding with the onset of TCR-β, TCR-γ, and TCR-δ rearrangements at this developmental stage.24 In the absence of GSI, only 22% of all CD34+CD1+ precursors were bipotent, whereas 71% generated γδ-lineage cells and 6% αβ-lineage cells (Figure 6A). With reduced Notch activation, the total αβ lineage potential again increased dramatically (29% and 84% for 0 and 1 μM GSI, respectively); but in contrast to CD34+CD1− precursors, this was not the result of an increase in bipotent precursors, but especially the result of an increase in committed αβ lineage precursors (from 6% to 42% for 0 and 1 μM GSI, respectively) (Figure 6A). Strikingly, the total γδ-lineage potential was significantly reduced when Notch activity was reduced (94% and 58% for 0 and 1 μM GSI, respectively), a result of a severe decline in single cells that exclusively yield γδ-lineage progeny (71% and 16% for 0 and 1 μM GSI, respectively). Thus, Notch-signaling intensities influence the outgrowth of human αβ- and γδ-lineage precursors.
High levels of Notch signaling reduces the frequency of TCR-αβ T-cell precursors. (A) Relative frequency of αβ or γδ lineage containing wells (left graph) or frequency of αβ-only, γδ-only, or bipotent αβ and γδ containing wells (right graph) within single-plated CD34+CD1− (left) and CD34+CD1+ (right) thymocytes on OP-DL1 with 0 or 1 μM GSI that showed T-cell reconstitution. Data represent mean plus or minus SEM (n = 3), encompassing 101 and 45 positive wells, of 216 and 72 wells plated for 0 and 1 μM GSI cultures, respectively, for CD34+CD1− thymocytes and 46 and 54 positive wells, of 168 and 72 wells plated for 0 and 1 μM GSI cultures, respectively, for CD34+CD1+ thymocytes. (B) Absolute frequency of αβ or γδ lineage containing wells (left graph) or frequency of αβ-only, γδ-only, or bipotent αβ and γδ containing wells (right graph) within all wells plated, derived from data as described in panel A. *Significant difference (P < .05). (C) Limiting dilution analysis to determine the TCR-αβ and TCR-γδ precursor frequency in CD34+CD1+ thymocytes after OP9-DL1 coculture in the absence or presence of 1 μM GSI. Data are presented as precursor frequency−1 (95% confidence limits) and is the result of 3 independent experiments with a total of 60, 60, 60, and 96 replicate wells for 30, 10, 3, and 1 CD34+CD1+ thymocytes plated.
High levels of Notch signaling reduces the frequency of TCR-αβ T-cell precursors. (A) Relative frequency of αβ or γδ lineage containing wells (left graph) or frequency of αβ-only, γδ-only, or bipotent αβ and γδ containing wells (right graph) within single-plated CD34+CD1− (left) and CD34+CD1+ (right) thymocytes on OP-DL1 with 0 or 1 μM GSI that showed T-cell reconstitution. Data represent mean plus or minus SEM (n = 3), encompassing 101 and 45 positive wells, of 216 and 72 wells plated for 0 and 1 μM GSI cultures, respectively, for CD34+CD1− thymocytes and 46 and 54 positive wells, of 168 and 72 wells plated for 0 and 1 μM GSI cultures, respectively, for CD34+CD1+ thymocytes. (B) Absolute frequency of αβ or γδ lineage containing wells (left graph) or frequency of αβ-only, γδ-only, or bipotent αβ and γδ containing wells (right graph) within all wells plated, derived from data as described in panel A. *Significant difference (P < .05). (C) Limiting dilution analysis to determine the TCR-αβ and TCR-γδ precursor frequency in CD34+CD1+ thymocytes after OP9-DL1 coculture in the absence or presence of 1 μM GSI. Data are presented as precursor frequency−1 (95% confidence limits) and is the result of 3 independent experiments with a total of 60, 60, 60, and 96 replicate wells for 30, 10, 3, and 1 CD34+CD1+ thymocytes plated.
To further investigate the direct impact of different Notch-signaling levels on the αβ- and γδ-lineage differentiation, we calculated the absolute outgrowth of unipotent or bipotent clones of all single-plated wells (including wells without cellular growth), thus expressing the frequency of lineage outgrowth within all wells plated (Figure 6B). We observed a net gain in wells that contain αβ-lineage cells when Notch signaling was reduced with 1 μM GSI, for both CD34+CD1− and CD34+CD1+ thymocytes, but only a very small increase in γδ-lineage outgrowth (Figure 6B). These findings were also confirmed in limiting dilution analysis experiments with CD34+CD1+ thymocytes on OP9-DL1 with 0 or 1 μM GSI (Figure 6C). CD34+CD1+ thymocytes were used as the αβ/γδ bifurcation point starts to occur at this stage of human T-cell development (Figure 6A,B). The addition of 1 μM GSI severely increased the frequency of αβ lineage precursors by almost 4-fold but only slightly altered the frequency of γδ lineage precursors within CD34+CD1+ cells (Figure 6C). Results for individual experiments are provided in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Thus, Notch signaling clearly needs to be reduced to allow the further differentiation of human CD34+ precursors into αβ T cells, in agreement with the reduction in Notch target gene expression that is observed as the cells differentiate along the TCR-αβ pathway (Figure 1).
Notch dose-dependent regulation of gene expression
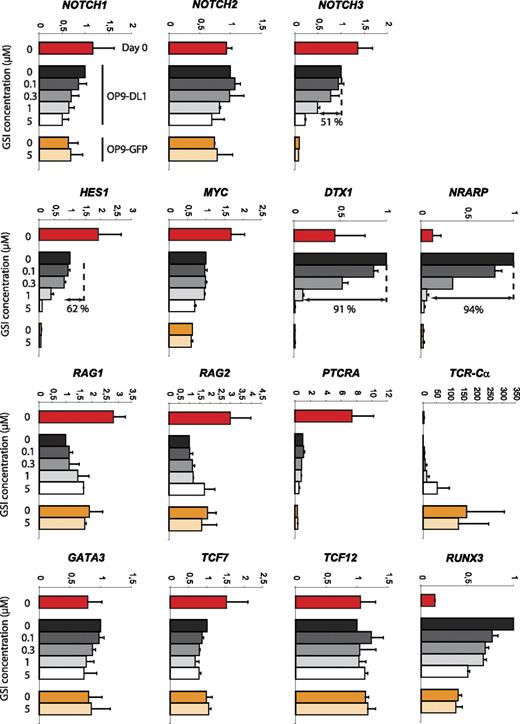
Developmental progression along the αβ-lineage pathway seems complex with respect to Notch signaling. Different changes in Notch target gene expression can be observed at the transition from uncommitted CD34+CD1− to T lineage–committed CD34+CD1+ thymocytes as characterized by a severe reduction in expression of the Notch target genes DTX1 and NRARP but not HES1 and MYC, suggesting a partial reduction in Notch signaling and differential gene regulation. Because both CD34+ thymocyte precursor subsets also showed a differential T-cell lineage outcome depending on the Notch signal strength, we analyzed GSI concentration effects on the expression of Notch-related genes or genes involved in T-cell development. CD34+ thymocytes (day 0) were cocultured for 40 hours on OP9-DL1 cells in the presence of 0, 0.1, 0.3, 1, or 5 μM GSI or on OP9-GFP cells in the presence of 0 or 5 μM GSI (Figure 7). This short 40-hour period was chosen to minimize differential cell death and/or differentiation effects. Absence of Notch signaling, through high concentration of GSI on OP9-DL1 (5 μM) or through absence of DL1 (OP9-GFP, 0 and 5 μM GSI), caused a severe reduction in NOTCH3, HES1, DTX1, and NRARP expression. The reduction was similar in these 3 conditions, indicating efficient Notch inhibition on OP9-DL1 with 5 μM GSI and that OP9-GFP cocultures cannot further be inhibited by additional GSI. These target genes showed different sensitivities to changes in Notch intensities as NOTCH3 and HES1 were only reduced by 51% and 64%, respectively, with 1 μM GSI, whereas DTX1 and NRARP were already reduced by more than 90% (differential sensitivity also visible at 0.3 μM GSI, Figure 7). We could not detect clear Notch dose-dependent effects on MYC and PTCRA expression. Both genes were expressed at lower levels in OP9 cocultures compared with freshly isolated CD34+ thymocytes, suggesting that the proper regulatory factors are missing in the in vitro cultures. The expression of GATA-3, TCF7, and TCF12 (coding for HEB) was hardly influenced by absence or presence of Notch signaling, but remarkably the expression TCR-Cα transcripts was drastically up-regulated in the absence of Notch/DL1 signaling. Both RAG genes were up-regulated 2-fold by removing Notch signaling (Figure 7). Surprisingly, RUNX3 expression was strongly up-regulated in OP9-DL1 cocultured cells, in a Notch dose-dependent fashion. In conclusion, these results show that Notch target genes display differential sensitivities toward changes in Notch activation and reveal potentially some novel targets that are regulated by the Notch pathway.
Differential sensitivity of Notch target genes in response to changes in Notch-signaling intensity. Quantitative real-time RT-PCR gene expression analysis of Notch and T cell–related genes in human postnatal CD34+ thymocytes, either freshly isolated (day 0) or after 40 hours of culture on OP9-DL1 and OP9-GFP stromal cells in the presence of various concentrations of GSI. Data shown are the mean plus or minus SEM from 2 sets of independent samples and expression levels for all genes are presented in units relative to those from CD34+ cells from the same donor cultured on OP9-DL1 cells in the absence of GSI and normalized to the geometric mean of β-actin, YWHAZ, and GAPDH.50
Differential sensitivity of Notch target genes in response to changes in Notch-signaling intensity. Quantitative real-time RT-PCR gene expression analysis of Notch and T cell–related genes in human postnatal CD34+ thymocytes, either freshly isolated (day 0) or after 40 hours of culture on OP9-DL1 and OP9-GFP stromal cells in the presence of various concentrations of GSI. Data shown are the mean plus or minus SEM from 2 sets of independent samples and expression levels for all genes are presented in units relative to those from CD34+ cells from the same donor cultured on OP9-DL1 cells in the absence of GSI and normalized to the geometric mean of β-actin, YWHAZ, and GAPDH.50
Discussion
Developmental processes are driven by stage-specific and complex molecular pathways. The Notch pathway is a major driver of T-cell development. Whereas Notch requirements have been well established during mouse T-cell development, its role during human T-cell development is less clear. Here, we demonstrate that different levels of Notch activation control major developmental checkpoints during human T-cell development, which are regulated at the molecular level by differential sensitivities of Notch target genes. We show that, in human, these Notch-driven events induce an opposing TCR-αβ versus TCR-γδ cell fate decision compared with during mouse T-cell development.
The first step of T-cell development comprises the migration of precursor cells into the thymus. On entry, it is thought that Delta-like Notch ligands induce T-cell differentiation. We show that human CB progenitors require high Notch activation to generate CD34+CD7high T-cell progenitors, consistent with previous results.19 Accordingly, the highest induction of Notch target genes, such as HES1, MYC, and DTX1, occurs at this stage. Although we and others34,35 can show a Notch dose-dependent expression pattern for NRARP, this gene was already highly expressed in CB progenitors and maintained in the earliest thymocytes. Because overexpression of this gene inhibits T-cell development,36 Nrarp may prevent early Notch activation,35 similar to LRF.37
Upon T-cell commitment, significant changes in Notch activation occur. DTX1 and NRARP expression is sharply down-regulated, whereas HES1 and MYC levels remain constant. Although all considered Notch target genes, our Notch dose-dependent gene expression analysis suggests that these differences result from differential sensitivities of these genes in response to different Notch activation levels. It is clear, however, that this slight reduction in Notch activity is required at these early CD34+CD1+ and ISP4+ stages because high Notch activation inhibits the development of TCR-αβ precursors, as also observed after intracellular Notch (ICN) transduction in early human thymocytes.38 Down-regulation of NRARP and DTX1 seems pivotal at this stage because overexpression of these genes inhibits T-cell development and commitment in the mouse.36,39 Expression of these genes is high in OP9-DL1–cultured human CD34+ thymocytes and significantly reduced with low GSI concentrations, conditions that promote TCR-αβ development. However, further work in human will be required to determine whether increased NRARP and/or DTX1 expression is indeed responsible for inhibiting human TCR-αβ T-cell development. A reduction in Notch activation also seems critical to induce accessibility of the TCR-Cα locus as shown by the increase in TCR-Cα expression after addition of GSI. This is important to allow TCR-α gene rearrangements, required for TCR-αβ T-cell development. Furthermore, clonal cultures and limiting dilution analysis (LDA) show that the amount of αβ-lineage precursors increases with lower Notch activity. This is in sharp contrast with early stages of mouse T-cell development where a gradual increase in Notch signaling is observed until β-selection,8,36,40,41 and several studies have shown that high Notch activation is required for αβ T-cell development.4-7
In contrast, TCR-γδ T cells preferentially grow in the presence of high Notch activation. Previous human studies were limited to ICN1 overexpression, resulting in increased TCR-γδ and reduced TCR-αβ cells,13,14 yielding different results compared with mouse.4,6,7 Our results now confirm, using identical tools as used in mouse,6 that high Notch activity enhances TCR-γδ development. Because increased IL-7, a critical growth factor for γδ cells in mouse,33 cannot compensate for reduced Notch activity and because the amount of γδ precursors was not reduced with lower Notch activation, these results suggest that, in human, Notch signaling is critical for expanding γδ T cells. Furthermore, because we have recently shown that even CD4+CD3−CD8α−CD28− pre-β-selection thymocytes, a population very similar to mouse DN3a cells,8 fail to efficiently generate TCR-γδ T cells in the absence of Notch signaling,42 Notch seems also critical for the final maturation of human γδ T cells.
There are multiple lines of evidence suggesting that the strength of signals from Notch4-6,8 and the TCR43-45 have a significant impact on the αβ/γδ lineage decision. As for other developmental systems,46 it seems probable that the integration of these various signals ultimately determines the lineage outcome.7 Therefore, in the absence of TCR signaling in early human CD34+ thymocytes,24 high Notch activation might be interpreted as a strong signal, thereby promoting γδ-lineage differentiation as proposed by the TCR signal strength concept.43-45 Why this phenomenon is then not observed in mouse6 remains unclear but could be attributed to inherited species differences. To clarify this further, future work will be required to investigate the impact of both TCR and Notch signaling on the outcome of human T-lineage differentiation. Our data suggest that the development of αβ-lineage cells, which proceeds through a stage of pre-TCR signaling, occurs more efficiently in conditions of lower Notch activation, consistent with mouse observations from Garbe et al.7
Gene expression analysis reveals regulatory differences between human TCR-αβ and TCR-γδ T cells. DTX1, NRARP, and RUNX3 are expressed at higher levels in γδ-lineage cells compared with αβ cells. Because these genes also show the most profound up-regulation on OP9-DL1 coculture of human CD34+ thymocytes, DTX1, NRARP, and RUNX3 must be considered as potential contributors to the preferential outgrowth of γδ T cells in these conditions of high Notch activity. However, further work will be required to investigate whether one or all of these factors are involved in these lineage decisions. γδ T-cell development certainly seems more permissive for high Notch activation compared with αβ-lineage differentiation. During mouse T-cell development, a similar role for Runx3 has previously been implicated at the earliest divergence point of αβ- and γδ-lineage cells.8
Surprisingly, some genes that are Notch targets, such as MYC and PTCRA47-49 , were not influenced by GSI inhibition in human thymocytes. Both genes, but especially PTCRA, were expressed at lower levels after OP9 coculture compared with their expression in freshly isolated CD34+ thymocytes, suggesting that some positive regulatory factors are lacking in the OP9 system that could cover Notch-dependent enhancer activities. Alternatively, in OP9-DL1 cocultures, the threshold for Notch-dependent activation may not have been reached for these genes. This is particularly relevant for MYC, given its crucial role in cellular transformation.47-49 Thus, activation of Notch genes may further depend on the Notch activation level, possibly in collaboration with other factors. This is also apparent from the differential Notch dose-dependent sensitivities of the target genes. Given the role of Notch in T-ALL,18 these sensitivities seem critical in balancing between normal and malignant differentiation and suggest that a certain threshold of Notch activation needs to occur before specific genes are expressed that result in oncogenic transformation.
Human TCR-αβ T-cell development thus highly depends on continuous alterations in Notch activity. Although some of these correlate with corresponding stages in mouse T-cell development, important differences were observed, indicating that the molecular events that control human and mouse T-cell development can be quite distinct. In light of T-cell malignancies, understanding these pathways in the human system is therefore critical. Our results also highlight the importance of the thymic microenvironment for providing appropriate levels of the different signaling events that control human T-cell development, which are critical for efficiently generating relevant and functional T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Zúñiga-Pflücker (University of Toronto, Toronto, ON) for the OP9-DL1 cells, Dr Parreira for the LZRS-hDL1 construct (Instituto de Histologia e Embriologia, Lisboa, Portugal), Dr Harris (University of Oxford, Oxford, United Kingdom), and Dr Tosato (National Institutes of Health, Bethesda, MD) for the LZRS-hDL4 construct, and Ellen Rothenberg and Tessa Kerre for critical reading of the manuscript.
This work was supported by the Odysseus program of the Fund for Scientific Research Flanders (FWO) and grants from the FWO, the Flemish Institute for the Advancement of Scientific-Technological Research in the Industry, and the Concerted Research Action of Ghent University. T.T. was supported by the action Return Grants of the Belgian Science Policy (Belspo) and is currently a postdoctoral researcher of the FWO. I.V.d.W. is a PhD student supported by a grant from the Institute for the Promotion of Innovation by Science and Technology in Flanders.
Authorship
Contribution: I.V.d.W. performed research and analyzed and interpreted data; G.D.S. performed research; M.D.S. performed research and analyzed and interpreted data; B.V. analyzed and interpreted data; G.L. analyzed and interpreted data; J.P. designed research and analyzed and interpreted data; and T.T. designed research, performed research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tom Taghon, Department of Clinical Chemistry, Microbiology and Immunology, Ghent University, University Hospital Ghent, 4BlokA, De Pintelaan 185, B-9000 Ghent, Belgium; e-mail: tom.taghon@ugent.be.