Abstract
Heat shock protein 70 (HSP70) has gained plenty of attention because of its adjuvant capability to induce CD8+ cytotoxic T lymphocyte and CD4+ T-helper cell responses. We investigated the behavior of T-cell subsets stimulated with endotoxin-free HSP70 with respect to proliferation, cytokine expression, cytotoxicity against allogeneic B-lymphoblastoid cell line and K562 cells, as well as target-independent cytotoxicity. CD4+ cells exhibited a strong increase in proliferation after stimulation with HSP70 (29%). In the presence of targets, a 35-fold up-regulation of granzyme B was observed after stimulation of CD4+ T cells with HSP70 in combination with interleukin-7 (IL-7)/IL-12/IL-15. The target cell-independent secretion of granzyme B by CD4+ cells was greatly augmented after stimulation with HSP70 plus IL-2 or IL-7/IL-12/IL-15. In this study, we showed that HSP70 is capable of inducing a cytotoxic response of T-helper cells in the absence of lipopolysaccharide. The granzyme B secretion and cytolytic activity of T-helper cells are induced in a target-independent way, whereas the cytotoxic activity of CD3+ and CD8+ T cells can be further enhanced in the presence of target cells. Our data provide novel insights into the role of extracellular HSP70 on T-cell immune response concerning the induction of target-independent T-helper cell cytotoxicity.
Introduction
Heat shock proteins (HSPs) are highly conserved proteins constitutively or stress-inducibly expressed and widely distributed in microorganisms and mammalian cells. They play an important primary role as intracellular molecular chaperones that prevent the aggregation and control the folding of proteins.1-3 Furthermore, HSPs can act at multiple points in apoptotic pathways to ensure that stress-induced damage does not inappropriately trigger cell death.4-6
The immunologic functions of HSPs emerged with the observation that certain HSPs (eg, HSP70, HSP90, glycoprotein 96 [gp96], calreticulin, HSP110, glucose-regulated protein 170 [GRP170]) isolated from tumor cells can initiate adoptive, tumor-specific T-cell responses and protective immunity, whereas HSP preparations from normal healthy cells did not.7-10 The immunogenicity of tumor-derived HSPs has been attributed to the noncovalent association of HSPs with peptides generated by the degradation of intracellular tumor-specific proteins expressed from the cells from which the HSPs were isolated.11 Peptides chaperoned by members of the HSP70 (HSP70, HSC70) and HSP90 (gp96) families can be taken up quite efficiently in a receptor-mediated manner (CD91, LOX-1)12,13 by antigen-presenting cells (APCs), such as dendritic cells (DCs),8,9 resulting in the peptide cross-presentation via human leukocyte antigen (HLA) class I molecules.14-17 HSPs, especially HSP70, have therefore gained much attention as enhancers of antigen-specific CD8+ cytotoxic as well as CD4+ T-helper cell (Th1) responses by representation of exogenous antigens.18
The interaction of HSPs with APCs, such as macrophages or DCs, also leads to several nonantigen-specific reactions that promote the stimulation of the innate immune system. HSP receptors identified as being involved in the innate immune response include CD14,19 CD91,12 CD40,20 LOX-1,13 CD36,21 SR-A,22 CCR5,23 and Toll-like receptors 2 and 4 (TLR2/4).24,25 Many of the HSP activities resulting in the activation of the innate immune response are strictly reminiscent of invariant molecular structures called pathogen-associated molecular patterns (PAMPs; eg, lipopolysaccharide [LPS], endotoxins, peptidoglycan, unmethylated CpG DNA), which are shared by numerous pathogens but are normally not expressed in host tissues.26
It has been reported that peptide-free HSP70 can enhance natural killer (NK)–cell activity against tumors in mice and in humans by stimulating the proliferation and the cytolytic activity of NK cells, contributing to increased cytotoxicity against HSP70 or NKG2D ligands expressing tumor cells.27-30 In this study about the immunomodulatory impact of extracellular HSP70 on T-cell activity, we demonstrated that HSP70 can enhance T-cell activation in the absence of LPS as the most important PAMP. Our findings highlight the role of extracellular HSP70 in the activation of the innate and adaptive immune response. In particular, we showed that HSP70 can induce cytolytic activity of T-helper cells and that the release of granzyme B is target-independent.
Methods
RNA isolation and RT-PCR
Peripheral blood mononuclear cells (PBMCs) were isolated from an HLA-A*0101–positive blood sample by discontinuous gradient centrifugation. Total RNA was isolated from PBMCs using the RNeasy Mini Kit (QIAGEN, Hilden, Germany). To facilitate the eukaryotic expression and isolation procedures, we developed a strategy for expression of soluble HSP70 (sHSP70) secreted into the cell culture supernatant. For this purpose, a fusion protein was constructed by connecting the signal peptide of HLA-A*0101 (exon 1) with the N terminus of HSP70. The fusion protein segments encoding the signal peptide of HLA-A*0101 and the complete sequence of HSP70 (gene HSPA1A) were generated by one-step reverse-transcribed polymerase chain reaction (RT-PCR) according to the manufacturer's instructions (OneStep RT-PCR Kit; QIAGEN). The following primers were used: HLA-A–TAS (5′-GAG ATG GCC GTC ATG GCG-3′ and HLA-E1 + HSP70-AS (5′-CTG ACC CAG ACC TGG GCG GCC AAA GCC GCG GCG ATC-3′) for amplification of exon 1 sequence, and HSP70-FS (5′-GCC AAA GCC GCG GCG ATC-3′) and HSP70-WAS (5′-CCC ACC ATT GAG GAG GTA GAT-3′) for amplification of the HSPA1A gene. HLA-E1 + HSP70-AS contained the sequence for the 3′ end of exon 1 and the first 18 bp of the 5′ end of the HSP70. The 2 PCR products were mixed at a ratio of 1:1 and fused in an overlap extension PCR using the primers HLA-A-TAS and HSP70-WAS. The resulting PCR product was cloned in the eukaryotic expression vector pcDNA3.1V5/His (Invitrogen, Carlsbad, CA). Because the antisense primer (HSP70-WAS) did not contain a stop codon, the cloned sequence was followed by the vector sequences for V5 and 6xHis tags. Vector purification was performed using the QIAGEN EndoFree Maxi Plasmid Kit.
Expression of sHSP70 in mammalian cells and verification of sHSP70 expression
The pcDNA3.1/sHSP70 construct was transfected by lipofection (Lipofectamine 2000; Invitrogen) into the human embryonic kidney cell line HEK293. Selection for geneticin (G418; Invitrogen)–resistant clones was done 48 hours after transfection using 1000 μg/mL. Geneticin-resistant clones were subcloned by limiting dilution to screen for high expression of sHSP70. Expression of sHSP70 from the supernatant of transfected HEK293 cells was quantified using a sandwich HSP70 enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). The HEK293 clone with the highest recombinant protein expression was used for large-scale production in the Cellspin cultivation system (Integra Biosciences, Chur, Switzerland). Harvests of the cell-culture supernatant were centrifuged for 10 minutes at 300g filtered through a 0.45-μm filter (Sartorius, Göttingen, Germany), and stored until further processing at −20°C.
Immobilized-metal affinity chromatography purification of sHSP70 from cell-culture supernatant
Purification of the recombinant V5/His-tagged sHSP70 protein from the cell-culture supernatant was performed using immobilized-metal affinity chromatography and HisTrapFF columns (GE Healthcare, Little Chalfont, United Kingdom) retaining the hexahistidine-tagged proteins. The supernatants were pooled, adjusted to pH 8.0, and loaded onto the column (BioLogic DuoFlow System; Bio-Rad, Hercules, CA). sHSP70 was eluted by an elution buffer consisting of 20 mM sodium phosphate, 0.5 M NaCl, 0.5 M imidazole, pH 7.4. The recombinant protein was quality controlled by Western blot and ELISA using detection antibodies against HSP70 (Assay Designs, Ann Arbor, MI), V5 epitope and 6xHis tag (both from Invitrogen). The HSP70 ELISA (R&D Systems) was used for quantification of the eluted protein. Limulus amebocyte lysate assay (LAL) was applied to exclude endotoxin contamination in the purified protein preparations (Rapid Endo-Test; Lonza Verviers, Verviers, Belgium; sensitivity = 0.005 endotoxin units [EU]/mL). To confirm the cleavage of the HLA-A*0101 signal peptide from the mature sHSP70, Edman sequencing was done to determine the first 6 N-terminal expressed amino acids of the protein (Toplab, Martinsried, Germany).
Immunofluorescence staining of T cells with sHSP70-FITC
A total of 3 mg purified sHSP70 was conjugated with fluorescein isothiocyanate (FITC) using the Fluoro Tag FITC Conjugation Kit (Sigma Chemie, Deisenhofen, Germany) according to the manufacturer's instructions. Concentration of the labeled protein sHSP70-FITC was determined in the sandwich HSP70 ELISA (R&D Systems).
PBMCs from healthy donors were isolated by discontinuous gradient centrifugation, washed twice in sterile phosphate-buffered saline (PBS, pH 7.2), and resuspended at a concentration of 107 cells/mL PBS containing 0.5% BSA and 2 mM ethylenediaminetetraacetic acid. CD3+ and CD4+ T cells were isolated from PBMCs by kits for negative selection (Miltenyi Biotec, Auburn, CA). Purity of the resulting CD3+ and CD4+ T cells was verified by flow cytometry analysis (mouse anti–human CD3 monoclonal antibody [mAb] and mouse anti–human CD4 mAb; BD Biosciences, San Jose, CA). The T cells were resuspended in Iscove medium (Biochrom, Berlin, Germany) supplemented with 4% heat-inactivated human AB serum (C.C.pro, Oberdorla, Germany). Peripheral blood of healthy blood donors was collected with informed consent in accordance with the Declaration of Helsinki as approved by the local ethics committee of Hannover Medical School.
A total of 2 × 105 T cells (purity > 99%) was incubated with 5 μg/mL sHSP70-FITC alone and in combination with 100 μg unlabeled sHSP70 in 200 μL medium (37°C, 5% CO2) for uptake. After 4 hours, cells were washed carefully and resuspended in PBS containing 0.2% gelatin, 20 mM sodium azide, and 10 μg/mL heat-aggregated human IgG (Sigma Chemie). Subsequently, the cells were incubated with phycoerythrin (PE)–conjugated anti–human CD3 mAb (Beckman Coulter, Fullerton, CA) on ice for 45 minutes. The stained cells were carefully washed and mounted on Starfrost slides (Menzel, Braunschweig, Germany) by Cytospin 3 (Shandon, Cheshire, United Kingdom). The slides were mounted in ProLong Gold Antifade Reagent mounting medium (Invitrogen). T cells were placed under constant illumination on the Zeiss Axiolab microscope (Carl Zeiss, Jena, Germany) with an FITC or PE filter set using a 100×, 1.25 N.A. oil immersion objective. Images were acquired using a Zeiss AxioCam camera (Carl Zeiss) and analyzed using Zeiss AxioVision image software (Carl Zeiss).
Stimulation of T cells with sHSP70
Twelve healthy blood donors with no prior history of blood transfusion were chosen for the stimulation of T cells. PBMCs were isolated by discontinuous gradient centrifugation, washed twice in sterile PBS, and resuspended at a concentration of 2.5 × 106 cells/mL in RPMI 1640 culture medium (Lonza Verviers) supplemented with 10% heat-inactivated human AB serum (C.C.pro). Negative isolation of CD3+ T cells and CD4+ or CD8+ T-cell subpopulations was performed by magnetic bead separation using the Pan T-Cell Isolation Kit, CD4+ T-Cell Isolation Kit II, and CD8+ T-Cell Isolation Kit II, respectively (all purchased by Miltenyi Biotec). Purity of the resulting T cells was verified by flow cytometry (FITC-conjugated anti-CD3, PE-conjugated anti-CD8, and peridinin chlorophyll protein (PerCP)–conjugated anti-CD4; all from BD Biosciences).
A total of 2.5 × 105 CD3+ T cells, CD4+ or CD8+ T-cell subpopulations (purity > 95%) were incubated on a 96-well plate for 7 days. Negative control cells were cultured in RPMI 1640 supplemented with 10% AB serum. Positive control cells were stimulated with 2 μg/mL staphylococcal enterotoxin B (Sigma Chemie), respectively. Cells were stimulated with sHSP70 in the following combinations: 10 μg/mL sHSP70; 100 U/mL interleukin-2 (IL-2) with and without 10 μg/mL sHSP70; 10 ng/mL IL-7/0.1 ng/mL IL-12/5 ng/mL IL-15 with and without 10 μg/mL sHSP70 (all cytokines from PeproTech, Rocky Hill, NJ). All stimulation assays were performed in triplicate.
To ensure that the T-cell responses were specific for recombinant sHSP70, a control experiment was performed using the recombinant Sema7A protein (sSema7A)31 and the recombinant viral sCMVpp65 protein, which were expressed and isolated under the same conditions as described here for the sHSP70. Experiments were performed using T-cell subsets from 5 CMV-seronegative donors and 10 μg/mL of the respective protein alone or in combination with 100 U/mL IL-2.
T-cell proliferation assay
Freshly isolated CD3+ T cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CSFE) [5- or 6-(N-succinimidyloxicarbonyl)-3′,6′-O,O′-diacetylfluorescein)] purchased from Invitrogen at a final concentration of 1 μM. After 1 week, T cells were stained with PerCP-conjugated anti-CD4 (BD Biosciences) and cell proliferation was evaluated by flow cytometry.
T-cell cytotoxicity assay
HLA-deficient K562 cells or B-lymphoblastoid cell line (B-LCL) cells, typed as homozygous for HLA-A*68, HLA-B*07, and HLA-Cw*02, *04, were labeled with CFSE as previously described and used as target cells.32,33 After 7 days of stimulation, T cells were incubated with target cells at an effector/target (E/T) ratio of 10:1 in the presence of 20 U/mL IL-2 (PeproTech) for 6 hours. Target cell lysis was assessed by 7-aminoactinomycin D (7-AAD) staining (BD Biosciences).
Evaluation of granzyme B mRNA levels by real-time RT-PCR
To assess the mRNA levels of granzyme B, total cellular RNA was isolated from T cells (RNeasy Mini Kit; QIAGEN) after 1-week stimulation with sHSP70 and the cytokines but without target cells (target-independent). To determine the target-dependent expression of granzyme B, RNA of the prestimulated T cells was analyzed after 6 hours incubation with K562 cells. Primers were designed to amplify transcripts of granzyme B mRNA (5′-TGC AAC CAA TCC TGC TTC TG-3′ and 5′-CCG ATG ATC TCC CCT GCA T-3′). One-step real-time RT-PCR (RT-PCR Master Mix; Applied Biosystems, Foster City, CA) was performed using a MGB-TaqMan probe for granzyme B (5′-TGG CCT TCC TCC TGC TGC CCA-3′) as previously described.34 A total of 50 ng RNA was used in each reaction. The thermal cycling conditions used on a ONEStep Plus (Applied Biosystems) were: 50°C for 15 minutes and 95°C for 10 minutes followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. The constitutively expressed β-actin gene was used as the reference standard for normalization of mRNA levels. β-Actin mRNA was detected using a primer pair combination (5′-ATG ATG ATA TCG CCG CGC-3′ and 5′-GCC TTG CAC ATG CCG G-3′) and a MGB-TaqMan probe (5′-CGT CGT CGA CAA CGG-3′).
Evaluation of granzyme B secretion by ELISA
Target-independent secretion of granzyme B was detected in the supernatant of the cultures on day 7 after stimulation of the T-cell populations with the different combinations of sHSP70 and cytokines. The protein was assayed by ELISA according to the manufacturer's instructions (Bender MedSystems, Vienna, Austria).
Multiple cytokine detection
A bead-based multiplexed assay (FlowCytomix Human Th1/Th2 11plex Sample Kit; Bender MedSystems) that quantifies multiple cytokines in single-sample supernatant was used for analysis of cytokine expression patterns of the cultured T cells. After 7 days of culture, cytokine detection was performed according to the manufacturer's instructions.
Statistics
Statistical analyses were performed using 2-tailed t tests run on GraphPad Prism version 4.03 software (GraphPad Software, San Diego, CA).
Results
Expression of sHSP70, protein purification, and LAL assay
For expression of sHSP70, the signal peptide of HLA A*0101 was fused to the N terminus of HSP70. The resulting chimeric gene consisted of 1992 nucleotides (HLA exon 1 72 bp, HSP70 1920 bp) and encoded for a 664-amino acid protein. A V5/His tag (45 amino acids length) was additionally fused to the sHSP70 for purification and detection purposes. Twelve to 32 μg/mL sHSP70 was accumulated in the supernatant of transfected HEK293 cells cultured in 500-mL spinner flasks. The final concentration of purified sHSP70 ranged between 0.8 and 1.2 mg/mL. Western blotting from sodium dodecyl sulfate–polyacrylamide gel electrophoresis demonstrated the purity of the isolated recombinant sHSP70. Potential contamination with endotoxin was detected in the sHSP70 preparations by LAL assay. Preparations containing less than 1 EU/mL, referred as endotoxin-free, were used in the stimulation assays. For positive control, we used prokaryotic expressed HSP70 containing approximately 470 EU/mL LPS. This protein was isolated from inclusion bodies and purified via the C-terminal 6xHis tag (data not shown). The cleavage of the HLA-A*0101 signal peptide of the sHSP70 was identified by sequencing the first 6 N-terminal amino acids. The determined sequence was coincident with the known HSP70 protein sequence (Ala-Lys-Ala-Ala-Ala-Ile).
Immunofluorescence staining of T cells with sHSP70-FITC
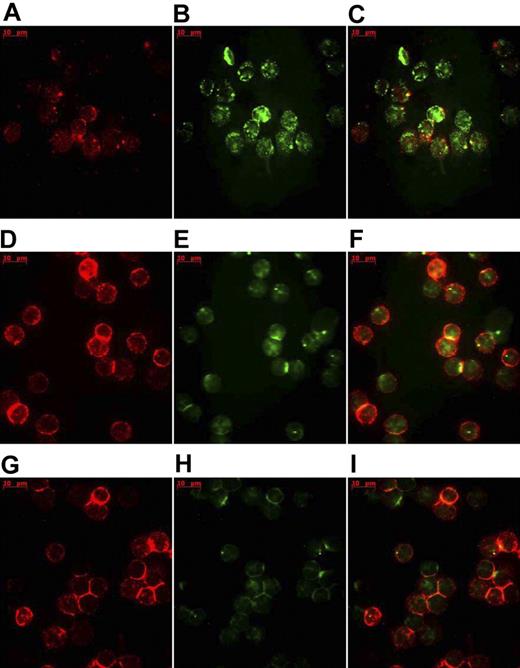
Figure 1 shows the cellular uptake of sHSP70-FITC by CD3+ (Figure 1A-C) and CD4+ T cells (Figure 1D-F). The uptake of sHSP70-FITC was greatly diminished by the incubation with unlabeled sHSP70 (Figure 1G-I). These results indicate that uptake of the protein most probably occurred by an endocytosis process mediated by HSP70 receptors.
Analysis of sHSP70 uptake by T-cell subsets using fluorescent microscopy. Representative results of fluorescent microscopic images of isolated CD3+ and CD4+ T cells, respectively, incubated with sHSP70-FITC. CD3-PE–stained isolated CD3+ T cells (A) and isolated CD4+ T cells (D) and internalized sHSP70-FITC (B,E) are depicted separately, as well as with the merged images (C,F). Incubation of CD4+ T cells (G) with unlabeled sHSP70 resulted in a decrease of the uptake of the sHSP70-FITC as shown separately (H) and in the overlay (I).
Analysis of sHSP70 uptake by T-cell subsets using fluorescent microscopy. Representative results of fluorescent microscopic images of isolated CD3+ and CD4+ T cells, respectively, incubated with sHSP70-FITC. CD3-PE–stained isolated CD3+ T cells (A) and isolated CD4+ T cells (D) and internalized sHSP70-FITC (B,E) are depicted separately, as well as with the merged images (C,F). Incubation of CD4+ T cells (G) with unlabeled sHSP70 resulted in a decrease of the uptake of the sHSP70-FITC as shown separately (H) and in the overlay (I).
Cytokine expression of T-cell populations in response to sHSP70
The activity of T-cell populations in the presence of sHSP70 or different cytokines was assayed by determining the secretion levels of Th1 cytokine interferon-gamma (IFN-γ) and inflammatory cytokines (Table 1). In all donors tested, cytokine secretion increased in response to sHSP70. The highest cytokine secretion levels were observed on stimulation with sHSP70 plus IL-2. The treatment with sHSP70 alone or sHSP70 plus IL-2 induced a significant increase of IFN-γ secretion, whereas coincubation of sHSP70 with IL-7/IL-12/IL-15 did not. The cytokine combination IL-7/IL-12/IL-15 alone had no effect on IFN-γ secretion by T cells. Therefore, especially with regard to the secretion of IFN-γ, an inhibitory effect of the combination IL-7/IL-12/IL-15 on the sHSP70-induced cytokine accumulation was detectable in each T-cell subset (Table 1; CD3+ T cells: sHSP70 462.9 pg/mL IFN-γ vs sHSP70 + IL-7/IL-12/IL-15 54.7 pg/mL IFN-γ; CD4+ T cells: sHSP70 74.9 pg/mL IFN-γ vs sHSP70 + IL-7/IL-12/IL-15 0 pg/mL IFN-γ; CD8+ T cells: sHSP70 91.9 pg/mL IFN-γ vs sHSP70 plus IL-7/IL-12/IL-15 0 pg/mL IFN-γ). Because there was no detectable secretion of IL-5, IL-6, and IL-8 in unstimulated CD3+ and CD4+ T cells, stimulation with sHSP70 induced de novo production of these inflammatory mediators in CD3+ and CD4+ T cells.
Secretion of cytokines by T-cell populations cultured in the presence of the different combinations of sHSP70 and/or cytokines
| Analytes, pg/mL . | ||||||
|---|---|---|---|---|---|---|
| Experiment . | IFN-γ . | IL-1β . | IL-5 . | IL-6 . | IL-8 . | TNF-α . |
| Multiple cytokine analysis for CD3+ T cells | ||||||
| SEB | 1163.9 ± 875.7 | 21.4 ± 42.7 | 63.0 ± 51.3 | 54.4 ± 92.6 | 3655.8 ± 4308.5 | 200.0 ± 225.6 |
| sHSP70 | 462.9 ± 853.7 | 17.4 ± 34.8 | 37.8 ± 0 | 5.7 ± 14.3 | 2162.3 ± 2207.7 | 64.3 ± 128.6 |
| IL-2 | 24.7 ± 34.7 | 0 | 0 | 0 | 27.1 ± 135.2 | 42.8 ± 41.0 |
| sHSP70 + IL-2 | 985.8 ± 648.4* | 69.7 ± 0 | 844.9 ± 138.6** | 129.5 ± 147.6** | 4034.8 ± 4522.2* | 82.9 ± 88.2* |
| IL-7/IL-12/IL-15 | 0 | 0 | 0 | 0 | 376.8 ± 834.5 | 8.6 ± 17.1 |
| sHSP70 + IL-7/IL-12/IL-15 | 54.7 ± 91.4* | 0 | 0 | 68.0 ± 114.7* | 3895.9 ± 4195.4** | 29.5 ± 27.4* |
| Multiple cytokine analysis for CD4+ T cells | ||||||
| SEB | 418.7 ± 725.2 | 65.6 ± 69.7 | 92.2 ± 144.4 | 123.0 ± 177.7 | 7928.4 ± 3400.1 | 205.9 ± 211.7 |
| sHSP70 | 74.9 ± 79.3 | 0 | 0 | 99.7 ± 126.3 | 3403.4 ± 2671.0 | 0 |
| IL-2 | 74.0 ± 90.6 | 0 | 0 | 0 | 61.5 ± 300.8 | 0 |
| sHSP70 + IL-2 | 199.8 ± 92.4 | 0 | 256.5 ± 444.3* | 107.6 ± 109.8* | 6685.1 ± 0** | 21.7 ± 0 |
| IL-7/IL-12/IL-15 | 0 | 0 | 0 | 0 | 742.6 ± 1788.1 | 0 |
| sHSP70 + IL-7/IL-12/IL-15 | 0 | 0 | 0 | 28.7 ± 40.5* | 6051.7 ± 5533.6* | 0 |
| Multiple cytokine analysis for CD8+ T cells | ||||||
| SEB | 1428.0 ± 326.5 | 0 | 720.5 ± 647.2 | 0.0 | 1367.1 ± 1782.0 | 88.1 ± 80.8 |
| sHSP70 | 91.9 ± 4.1 | 0 | 0 | 5.2 ± 9.0 | 278.9 ± 355.5 | 0 |
| IL-2 | 44.3 ± 39.4 | 0 | 0 | 0 | 42.1 ± 4.1 | 0 |
| sHSP70 + IL-2 | 198.6 ± 24.1* | 0 | 0 | 0 | 1077.5 ± 0* | 0 |
| IL-7/IL-12/IL-15 | 0 | 0 | 0 | 5.2 ± 9.0 | 19.9 ± 0 | 0 |
| sHSP70 + IL-7/IL-12/IL-15 | 0 | 0 | 0 | 22.3 ± 19.6 | 621.3 ± 604.3* | 0 |
| Analytes, pg/mL . | ||||||
|---|---|---|---|---|---|---|
| Experiment . | IFN-γ . | IL-1β . | IL-5 . | IL-6 . | IL-8 . | TNF-α . |
| Multiple cytokine analysis for CD3+ T cells | ||||||
| SEB | 1163.9 ± 875.7 | 21.4 ± 42.7 | 63.0 ± 51.3 | 54.4 ± 92.6 | 3655.8 ± 4308.5 | 200.0 ± 225.6 |
| sHSP70 | 462.9 ± 853.7 | 17.4 ± 34.8 | 37.8 ± 0 | 5.7 ± 14.3 | 2162.3 ± 2207.7 | 64.3 ± 128.6 |
| IL-2 | 24.7 ± 34.7 | 0 | 0 | 0 | 27.1 ± 135.2 | 42.8 ± 41.0 |
| sHSP70 + IL-2 | 985.8 ± 648.4* | 69.7 ± 0 | 844.9 ± 138.6** | 129.5 ± 147.6** | 4034.8 ± 4522.2* | 82.9 ± 88.2* |
| IL-7/IL-12/IL-15 | 0 | 0 | 0 | 0 | 376.8 ± 834.5 | 8.6 ± 17.1 |
| sHSP70 + IL-7/IL-12/IL-15 | 54.7 ± 91.4* | 0 | 0 | 68.0 ± 114.7* | 3895.9 ± 4195.4** | 29.5 ± 27.4* |
| Multiple cytokine analysis for CD4+ T cells | ||||||
| SEB | 418.7 ± 725.2 | 65.6 ± 69.7 | 92.2 ± 144.4 | 123.0 ± 177.7 | 7928.4 ± 3400.1 | 205.9 ± 211.7 |
| sHSP70 | 74.9 ± 79.3 | 0 | 0 | 99.7 ± 126.3 | 3403.4 ± 2671.0 | 0 |
| IL-2 | 74.0 ± 90.6 | 0 | 0 | 0 | 61.5 ± 300.8 | 0 |
| sHSP70 + IL-2 | 199.8 ± 92.4 | 0 | 256.5 ± 444.3* | 107.6 ± 109.8* | 6685.1 ± 0** | 21.7 ± 0 |
| IL-7/IL-12/IL-15 | 0 | 0 | 0 | 0 | 742.6 ± 1788.1 | 0 |
| sHSP70 + IL-7/IL-12/IL-15 | 0 | 0 | 0 | 28.7 ± 40.5* | 6051.7 ± 5533.6* | 0 |
| Multiple cytokine analysis for CD8+ T cells | ||||||
| SEB | 1428.0 ± 326.5 | 0 | 720.5 ± 647.2 | 0.0 | 1367.1 ± 1782.0 | 88.1 ± 80.8 |
| sHSP70 | 91.9 ± 4.1 | 0 | 0 | 5.2 ± 9.0 | 278.9 ± 355.5 | 0 |
| IL-2 | 44.3 ± 39.4 | 0 | 0 | 0 | 42.1 ± 4.1 | 0 |
| sHSP70 + IL-2 | 198.6 ± 24.1* | 0 | 0 | 0 | 1077.5 ± 0* | 0 |
| IL-7/IL-12/IL-15 | 0 | 0 | 0 | 5.2 ± 9.0 | 19.9 ± 0 | 0 |
| sHSP70 + IL-7/IL-12/IL-15 | 0 | 0 | 0 | 22.3 ± 19.6 | 621.3 ± 604.3* | 0 |
The capacity of T-cell subpopulations (CD3+ T cells, CD4+ T cells, CD8+ T cells) from 4 healthy donors to proliferate in the presence of sHSP70 and different cytokines was assessed by determining the secretion levels of Th-type 1 (Th1) cytokine IFN-γ and proinflammatory cytokines using a bead-based flow cytometric assay (picograms per milliliter). The basal cytokine expression by unstimulated cells was subtracted from the values measured after stimulation. There were no secretion of IL-5, IL-6, and IL-8 in both CD3+ and CD4+ T cells by unstimulated T cells detectable. Therefore stimulation with sHSP70 resulted in the de novo production of these inflammatory mediators. Cells stimulated with staphylococcal enterotoxin B (SEB) served as positive secretion controls. Statistically significant differences between the stimulation with sHSP70 plus IL-2 versus IL-2 and sHSP70 plus IL-7/IL-12/IL-15 versus IL-7/IL-12/IL-15 are indicated with asterisks (*P < 0.05 or **P < 0.01).
Proliferation assay
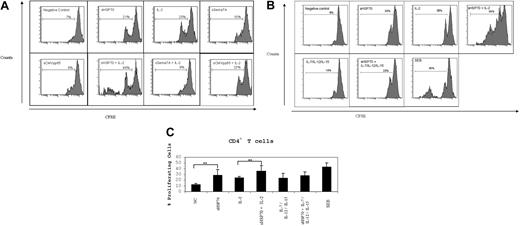
Isolated CD3+ T cells (constituted of 55.8% ± 9.8% CD4, 2% ± 0.5% CD4CD25, and 37.1% ± 9.2% CD8 T cells) were labeled with CSFE, and cell proliferation was analyzed 7 days after incubation under different stimulatory conditions. The control proteins sSema7A and sCMVpp65 did not induce proliferation of CD4+ T cells in the absence or presence of IL-2 (Figure 2A). One division cycle was observed on average for 12% of the unstimulated CD4+ T cells (Figures 2B,C). Stimulation with IL-2 or sHSP70 induced one division cycle of a mean of 24% to 28% of CD4+ T cells, respectively (Figure 2C). Dual stimulation with sHSP70 plus IL-2 led to a significantly higher proliferation rate (36%) of CD4+ T cells. One division cycle of CD4+ T cells was also observed on stimulation with IL-7/IL-12/IL-15 without (23%) or with (27%) sHSP70. No significant change in CD8+ T-cell proliferation was observed (data not shown).
sHSP70 induces T-cell proliferation. CD3+ T cells were labeled with CSFE and stimulated under different conditions for 1 week. Cell proliferation within the CD4+ T-cell populations was evaluated by CFSE dilution. (A) One representative experiment of CD4+ T-cell proliferation under different stimulation conditions and using the control protein sCMVpp65 or sSema7A in the absence or presence of IL-2. (B) One representative experiment of CD4+ T-cell proliferation under different stimulation conditions. (C) Results of 8 independent experiments are mean plus or minus SD. **P < .01.
sHSP70 induces T-cell proliferation. CD3+ T cells were labeled with CSFE and stimulated under different conditions for 1 week. Cell proliferation within the CD4+ T-cell populations was evaluated by CFSE dilution. (A) One representative experiment of CD4+ T-cell proliferation under different stimulation conditions and using the control protein sCMVpp65 or sSema7A in the absence or presence of IL-2. (B) One representative experiment of CD4+ T-cell proliferation under different stimulation conditions. (C) Results of 8 independent experiments are mean plus or minus SD. **P < .01.
Cytotoxicity assay
After stimulation, isolated CD3+, CD4+, or CD8+ T cells were used as effector cells in cytotoxic assays using K562 (Figure 3) or allogeneic B-LCL cells (Figure 4) as targets. The results are the mean percentage of target cell lysis plus or minus SD (Figures 3, 4).
Cytotoxic assays using K562 cells as target cells. (A) CD3+ T cells, (B) CD4+ T cells, and (C) CD8+ T cells were stimulated under the indicated conditions for 7 days. After stimulation, the cells were used as effector cells in cytotoxic assays performed in an allogeneic setting using K562 cells as targets. The cells were incubated to an E/T ratio of 10:1. Cell death of CFSE-stained target cells was evaluated by FACS after 7-AAD staining. The results are presented as a percentage of specific lysis. Data from 5 independent experiments are mean plus or minus SD. Comparison between groups was performed using t tests. *P < .05; **P < .01.
Cytotoxic assays using K562 cells as target cells. (A) CD3+ T cells, (B) CD4+ T cells, and (C) CD8+ T cells were stimulated under the indicated conditions for 7 days. After stimulation, the cells were used as effector cells in cytotoxic assays performed in an allogeneic setting using K562 cells as targets. The cells were incubated to an E/T ratio of 10:1. Cell death of CFSE-stained target cells was evaluated by FACS after 7-AAD staining. The results are presented as a percentage of specific lysis. Data from 5 independent experiments are mean plus or minus SD. Comparison between groups was performed using t tests. *P < .05; **P < .01.
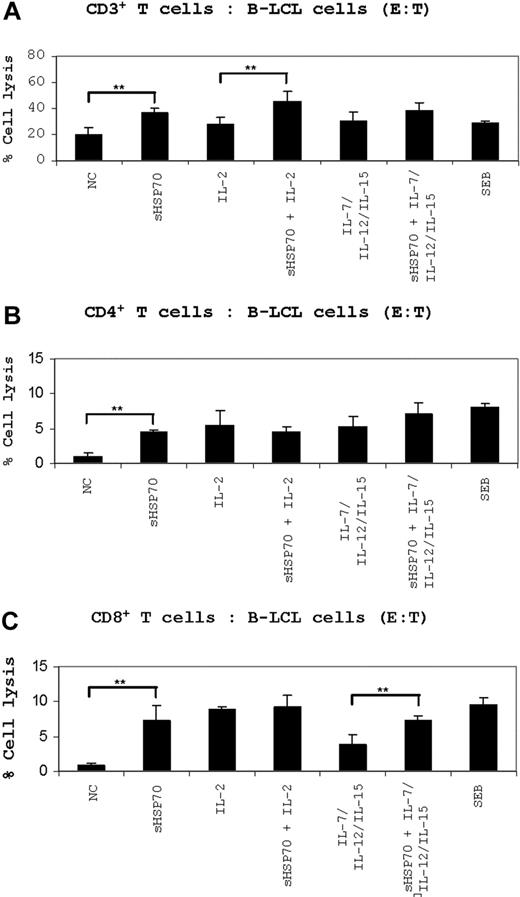
Cytotoxic assays using B-LCL cells as target cells. (A) CD3+ T cells, (B) CD4+ T cells, and (C) CD8+ T cells were stimulated under the indicated conditions for 7 days. After stimulation, the cells were used as effector cells in cytotoxic assays performed in an allogeneic setting using allogeneic B-LCL cells as targets. The cells were incubated to an E/T ratio of 10:1. Cell death of CFSE-stained target cells was evaluated by FACS after 7-AAD staining. The results are presented as a percentage of specific lysis. Results of 5 independent experiments are mean plus or minus SD. Comparison between groups was performed using t tests. **P < .01.
Cytotoxic assays using B-LCL cells as target cells. (A) CD3+ T cells, (B) CD4+ T cells, and (C) CD8+ T cells were stimulated under the indicated conditions for 7 days. After stimulation, the cells were used as effector cells in cytotoxic assays performed in an allogeneic setting using allogeneic B-LCL cells as targets. The cells were incubated to an E/T ratio of 10:1. Cell death of CFSE-stained target cells was evaluated by FACS after 7-AAD staining. The results are presented as a percentage of specific lysis. Results of 5 independent experiments are mean plus or minus SD. Comparison between groups was performed using t tests. **P < .01.
Lysis of K562 target cells
Unstimulated cultured CD3+ T cells lysed 8% of the K562 cells. IL-2-prestimulated T cells lysed 11% of the target cells. A significant increase in the lysis of K562 cells (19%) was observed after stimulation with sHSP70 alone. The level of K562 cell lysis could be further enhanced up to 20% in the presence of sHSP70 and IL-2. Stimulation with IL-7/IL-12/IL-15 resulted in only 13% target cell lysis compared with 22% after addition of sHSP70 to IL-7/IL-12/IL-15 (Figure 3A).
Unstimulated CD4+ T cells lysed only 1% of the K562 target cells. Stimulation with IL-2 resulted in 3%, whereas IL-7/IL-12/IL-15 had no effect on target cell lysis. Soluble HSP70 increased significantly the cytolytic potential of CD4+ T cells against K562 cells alone and in combination with IL-7/IL-12/IL-15 to a mean of 4% (Figure 3B).
The spontaneous CD8+ T-cell cytotoxicity (8%) was not augmented by sHSP70 (10%), by IL-2 (9%), or by a combination of IL-7/IL-12/IL-15 (10%). There was also no synergistic effect of sHSP70 plus IL-2 or sHSP70 plus IL-7/IL-12/IL-15 on the cytolytic potential of CD8+ T cells (Figure 3C).
Allogeneic B-LCL target cells
Untreated CD3+ T cells lysed 20% of allogeneic B-LCL cells, and stimulation with sHSP70 increased cytotoxicity significantly to 35% on average. T-cell prestimulation with IL-2 did lead to a target cell lysis of 28%, and preincubation with sHSP70 plus IL-2 resulted in a target cell lysis of 45% (Figure 4A). When IL-7/IL-12/IL-15 was used as stimulators, a target cell lysis of 29% was observed. This increased in combination with sHSP70 to 38%.
Unstimulated CD4+ T cells lysed 1% of the allogeneic B-LCL target cells, whereas stimulation with sHSP70 increased the cytolytic potential of CD4+ T cells significantly to a mean of 4%. Prestimulation of CD4+ T cells with a combination of sHSP70 and IL-2 or sHSP70 plus IL-7/IL-12/IL-15 increased target cell lysis to values of 4% and 7%, respectively (Figure 4B).
Unstimulated CD8+ T cells lysed on average 1% of the allogeneic cell targets. A strong increase in target cell lysis (9%) was observed when IL-2–prestimulated CD8+ T cells were used as the effector cells. Similarly, sHSP70 stimulation resulted in a significantly increased CD8+ cell-mediated target cell lysis of 7% in the mean. After prestimulation of the effector cells by IL-2 and sHSP70, the target cell lysis was on average 9%; thus, no synergistic effect between IL-2 and sHSP70 was observed. Stimulation of CD8+ cells with IL-7/IL-12/IL-15 resulted in only 4% target cell lysis compared with 7% lysis after the addition of sHSP70 to these 3 cytokines (Figure 4C).
Real-time PCR assessment of target-dependent granzyme B mRNA levels
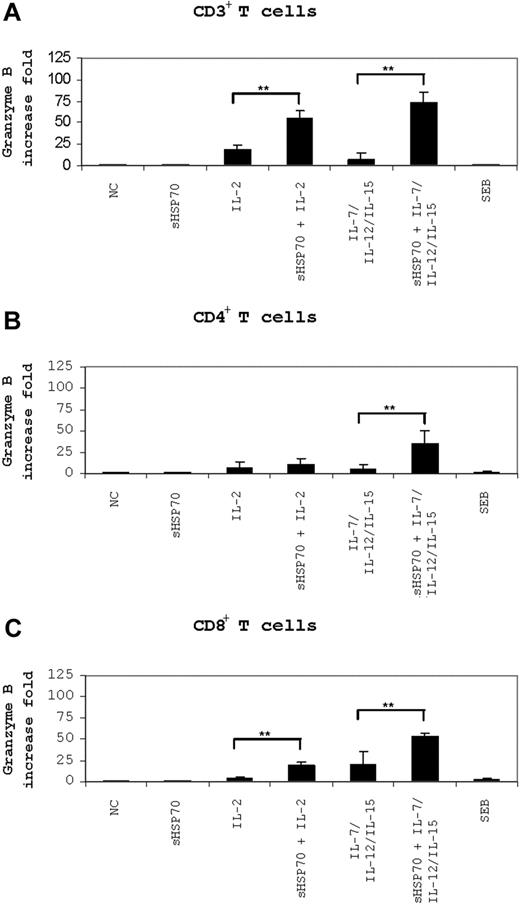
Granzyme B mRNA levels were measured in stimulated CD3+ (Figure 5A), CD4+, and CD8+ T-cell subsets (Figure 5B,C) after 6 hours of incubation with K562 target cells. Interestingly, stimulated CD3+ T cells had higher granzyme B mRNA levels than stimulated CD4+ and CD8+ T-cell isolates. Compared with the levels measured in unstimulated CD3+ T cells, stimulation of CD3+ T cells with IL-2 alone caused a 16-fold increase in granzyme B levels compared with 54- and 73-fold increases in cells stimulated with sHSP70 plus IL-2 or with sHSP70 plus IL-7/IL-12/IL-15 (Figure 5A). Thus, sHSP70 had together with these cytokines a synergistic effect on granzyme B mRNA expression in CD3+ T cells. In CD4+ T cells, granzyme B mRNA levels increased 10- or 6-fold on stimulation with IL-2 or IL-7/IL-12/IL-15, respectively (Figure 5B). Stimulation of CD4+ T cells with sHSP70 alone did not have an effect on granzyme B transcript levels, but stimulation with sHSP70 plus IL-7/IL-12/IL-15 caused a 35-fold increase in granzyme B mRNA levels; the synergistic effect was significant. Stimulation of CD8+ T cells with sHSP70 and IL-2 or IL-7/IL-12/IL-15 induced a 20-fold increase in granzyme B levels (Figure 5C), and stimulation with sHSP70 plus IL-7/IL-12/IL-15 resulted in a 53-fold increase. Soluble HSP70 had together with the cytokines a synergistic effect on granzyme B mRNA expression also in CD8+ T cells.
Granzyme B mRNA level is affected by sHSP70. Granzyme B mRNA levels in CD3+ T cells (A) or independently stimulated CD4+ (B) or CD8+ T cells (C) were assessed by real-time PCR after 6 hours of stimulation with the K562 target cells. Results of 8 independent experiments are expressed as mean plus or minus SD. Comparison between groups was performed using t tests. Statistically significant differences are indicated with asterisks (*P < .05 or **P < .01).
Granzyme B mRNA level is affected by sHSP70. Granzyme B mRNA levels in CD3+ T cells (A) or independently stimulated CD4+ (B) or CD8+ T cells (C) were assessed by real-time PCR after 6 hours of stimulation with the K562 target cells. Results of 8 independent experiments are expressed as mean plus or minus SD. Comparison between groups was performed using t tests. Statistically significant differences are indicated with asterisks (*P < .05 or **P < .01).
Detection of target-independent granzyme B expression and secretion by real-time RT-PCR and ELISA
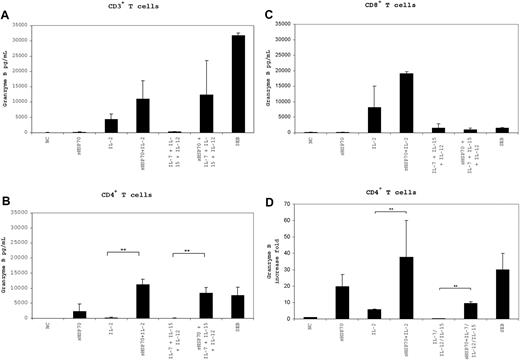
To determine whether T-cell cytotoxicity was induced in a target-independent manner, we measured granzyme B secretion levels in the supernatant of the T-cell populations and granzyme B mRNA levels after 7 days of culture under the different stimulation conditions (Figure 6). The control proteins sSema7A and sCMVpp65 did not cause an increase in the mRNA and protein level of granzyme B in the T-cell subsets (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The highest levels of granzyme B were secreted by CD3+ T cells (Figure 6A) cultured in the presence of sHSP70 plus IL-2 (11 040 pg/mL) and sHSP70 plus IL-7/IL-12/IL-15 (12 343 pg/mL), and by CD8+ T cells (Figure 6C) cultured in the presence of sHSP70 plus IL-2 (19 168 pg/mL). Granzyme B secretion by CD4+ T cells (Figure 6B) increased significantly in the presence of sHSP70 plus IL-2 (11 165 pg/mL) and sHSP70 plus IL-7/IL-12/IL-15 (8369 pg/mL) in comparison with IL-2 alone (285 pg/mL) and IL-7/IL-12/IL-15 (96 pg/mL). These results clearly demonstrate that the presence of sHSP70 can influence the effects of the cytokines, resulting in a target-independent increase in cytotoxic activity in all of the studied T-cell populations. This effect was significantly stronger in the CD4+ T-cell subset in terms of granzyme B mRNA levels, where a 38-fold increase of granzyme B mRNA was detectable after stimulation with sHSP70 plus IL-2 (Figure 6D). In addition, the combination of sHSP70 plus IL-7/IL-12/IL-15 led to a significant up-regulation of the mRNA level compared with stimulation with the cytokines alone. The increase of granzyme B was nearly identical in the presence or absence of target cells, indicating for the target-independent capacity of cell lysis by T-helper cells.
Expression and secretion of granzyme B by T cells is target-independent. Secretion of granzyme B by CD3+ (A), CD4+ (B), and CD8+ (C) T cells in the absence of target cells was assessed by ELISA after 7 days of stimulation with the different cocktails. mRNA expression of granzyme B by CD4+ T cells (D) was assessed by real-time PCR after 7 days of stimulation with the different stimulation combinations and in the absence of target cells. Results of 4 independent experiments are mean plus or minus SD. Comparison between groups was performed using t tests. **P < .01.
Expression and secretion of granzyme B by T cells is target-independent. Secretion of granzyme B by CD3+ (A), CD4+ (B), and CD8+ (C) T cells in the absence of target cells was assessed by ELISA after 7 days of stimulation with the different cocktails. mRNA expression of granzyme B by CD4+ T cells (D) was assessed by real-time PCR after 7 days of stimulation with the different stimulation combinations and in the absence of target cells. Results of 4 independent experiments are mean plus or minus SD. Comparison between groups was performed using t tests. **P < .01.
Discussion
In this study, we have established a strategy to express eukaryotic, soluble, LPS-free recombinant HSP70 and investigated the effects of this protein on T-cell response. Our data suggest that extracellular HSP70 can strongly activate CD4+ T cells and change their phenotype to a more cytotoxic one, whereas the functional properties of CD8+ cytotoxic T lymphocytes are less affected by the protein.
To circumvent major PAMP contaminations, we developed a eukaryotic strategy for expression of soluble HSP70 by connecting the signal peptide of HLA-A*0101 to the N terminus of HSP70. In the last few years, the use of HSPs expressed in Escherichia coli to stimulate immune responses has been controversially discussed because, as it is now known, many of the proinflammatory responses thought to be induced by HSP were the result of contamination of the recombinant HSP preparations with bacterial LPS, endotoxin, and other bacterial structures belonging to the group of PAMP molecules. A major concern was that these contaminants rather than the HSPs themselves were responsible for the observed immune responses.35,36 Therefore, special attention has been paid to LPS-free HSP70 preparation because LPS is the bacterial ligand of the TLR4/CD14 receptor complex, which is also involved in HSP signaling.37,38 LPS-free HSP preparation is a prerequisite in functional studies assessing the role of HSP in adoptive and innate immune responses. Purification of LPS-free HSP using polymyxin B columns was shown to be insufficient.39 In our studies, HSP70 expressed in eukaryotic cells and further processed by affinity chromatography proved to be highly pure, endotoxin-free, and functional.
The release of HSPs from cells is triggered by exposure to different kinds of environmental stress conditions (such as infection, inflammation, exposure of the cell to toxins, water deprivation) as well as by exposure to immunologic danger signals; release occurs both through physiologic secretion mechanisms and during cell death by necrosis. Several danger signals have been described, eg, mammalian DNA, RNA, ATP, IL-1α, ureic acid, HSPs released from necrotic cells or type I interferons secreted by virus-infected cells. HSPs behave as danger signal by activating DCs to express costimuli and proinflammatory cytokines and by regulating the surface expression of their own receptors.24,40,41
Many of the effects of extracellular stress proteins are mediated by cell surface receptors mainly found on APCs. Such receptors include TLR2/4,24,25 CD40,20 CD91,12 CD14,19 CCR5,23 and members of the scavenger receptor family (eg, LOX-1 and SREC-1).13,42 So far, little is known about HSP70 receptors on activated and resting T cells. It was previously found that TLR2 is expressed to a higher extent than TLR4 on the T-cell surface and that HSP60 interacts with these receptor molecules.43 Therefore, it is probable that HSP70 also interacts via TLR2 with CD3+ T-cell populations, resulting in the activation of innate and adoptive immune responses. The immunofluorescence data of our study showed that HSP70 is taken up during 4 hours of incubation and that it accumulates near the nuclei of the T cells. However, further studies are required to determine which receptors may be involved in the interaction of HP70 and T cells.
Previously, it was shown that extracellular HSP70 improves antigen-specific cell killing of cytotoxic CD8+ T cells.44 In addition, HSP70 molecule or a 14-mer peptide derived from the HSP70 protein sequence induces proliferation and enhances the cytotoxicity of NK cells. Similar to the effect observed in NK cells, we found that sHSP70 increased the proliferation rate and the cytolytic capacity of T-cell subsets. The functional properties of sHSP70 on T cells are similar to those of IL-2.45 The effect of sHSP70 on the innate response is stronger in CD3+ and CD4+ T cells than in the CD8+ cytotoxic T cells. This could be because cytotoxic T cells are mainly responsible for the adoptive antigen-dependent immune response.
Allez et al46 discovered that a unique subset of CD4+NKG2D+ T cells in inflammatory Crohn disease can have inflammatory and cytotoxic properties, as was measured based on IFN-γ secretion and perforin expression. Our study provides evidence that extracellular HSP70 molecules cause proliferation and cytotoxicity of the CD4+ T-cell population. It will be interesting to further identify which CD4+ T cells are affected by HSP70. Interestingly, HSP60 can act as costimulator of T-regulatory cells (Tregs, CD4+CD25+) via TLR2, and the HSP60-treated Tregs are significantly more effective than the untreated Tregs in inhibition of the target cell proliferation and secretion of IFN-γ and tumor necrosis factor-α (TNF-α).47,48 The effect of HSP70 on the functionality of Tregs regarding their cytotoxicity needs to be further addressed using purified CD4+CD25+ T-cell populations. Here, we demonstrated that stimulation of CD4+ T cells with sHSP70 alone or in combination with cytokines can lead to antigen-independent secretion of granzyme B. In the widely accepted model of granule-mediated killing by T lymphocytes and NK cells, exocytosis of granzyme B into the target cell is facilitated by the pore-forming molecule, perforin.49,50 On the other hand, it was shown that granzyme B can enter the target cells in a perforin-independent way.51,52 If granzyme B is released into the cytoplasm, it translocates rapidly to the nucleus and induces programmed cell death by promoting the fragmentation of the DNA and trigger apoptotic pathways. HSP70 might serve as an entry port for isolated granzyme B molecules into tumor cells expressing HSP70 on their cell surface, resulting in the induction of apoptosis selectively in these HSP70-positive tumor cells.51 Because perforin-expressing CD4+ T cells have been reported recently, it is probable that the release of granzyme B by T cells can trigger cell death also in a perforin-dependent way. Further studies are required to investigate whether sHSP70-induced cytotoxicity depends on perforin release.
It is known that the interaction of HSP70 with T cells leads to several peptide-independent activities, resulting in the translocation of nuclear factor-κB (NF-κB) into the nucleus of the cells.4 It is probable that NF-κB can interact with a downstream κB site (as an enhancer element) to up-regulate human granzyme B gene transcription in CD4+ cells, as already shown for NK and Jurkat T cells.53 The same mechanism might be responsible for the strong inflammatory immune response in T cells. HSP70 has been shown to specifically bind and change the cytokine profile of CD14+ cells favoring the secretion of IL-1β, IL-6, and TNF-α.19 The present study demonstrates that HSP70 induced de novo production of inflammatory mediators, such as IL-6 and IL-8 in CD3+ and CD4+ T cells.
The novel properties of HSP70 described here are highly indicative that this protein may play a role in the pathogenesis of diseases, such as lupus erythematosus. Intracellular up-regulation of HSP70 and positive staining patterns for HSP70 in lesional tissue have been described in lupus.54 In addition, the noninflammatory clearance of apoptotic cells seems to be impaired, and cytokines such as IL-6, IL-1β, and TNF-α are known to be up-regulated.55 Thus, access of (intra)cellular HSP70 to immunocompetent cells may further trigger the cytotoxic effector response in lupus erythematosus.
The release of HSPs from cells is triggered by cell stress as well as by exposure to immunologic danger signals. Once released into the extracellular fluid or bloodstream, HSPs can bind to the cell surface, initiate signal cascades, and stimulate the uptake of antigenic peptides. This study demonstrates, for the first time, that extracellular HSP70 can change the helper phenotype of CD4+ T cells to a more cytotoxic one. In contrast, the functional properties of CD8+ cytotoxic T lymphocytes are less affected by the protein. Our data suggest that activation of the innate immune mechanism can increase cytotoxicity and the de novo production of inflammatory cytokines by T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sarina Lukis, Doerthe Rokitta, and Jana Zenk for their excellent technical assistance.
This study was supported in part by Deutsche José Carreras Leukämie Stiftung and Deutsche Forschungsgemeinschaft (DFG Wi1822/5-1).
Authorship
Contribution: C.F. designed and performed the experiments for T-cell stimulation and functionality assays, analysis of the data, and writing the manuscript; M.W. and D.W. designed and performed the experiments for immunofluorescence staining; R.D. designed the experiments for target-independent killing and help in discussion; A.S. contributed with helpful discussion and wrote the manuscript; R.B. contributed with helpful discussion; and B.E.-V. designed and performed the experiments for protein cloning and expression, analysis of the data, T-cell stimulation, and functionality assays, and writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Britta Eiz-Vesper, Institute for Transfusion Medicine, Carl-Neuberg-Strasse 1, D-30625 Hannover, Germany; e-mail: eiz-vesper.britta@mh-hannover.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal