To the editor:
In this journal and others, Rüter1 and Raj2 have previously reported superior response to treatment with DNA-hypomethylating agents, such as decitabine or azacitidine (AC), in adults with myelodysplastic syndrome (MDS) and clonal monosomy 7. The use of AC has not yet been reported for childhood myelodysplastic or myeloproliferative disorders. In this letter we report a case of juvenile myelomonocytic leukemia (JMML) and clonal monosomy 7 with complete hematologic and molecular response to AC.
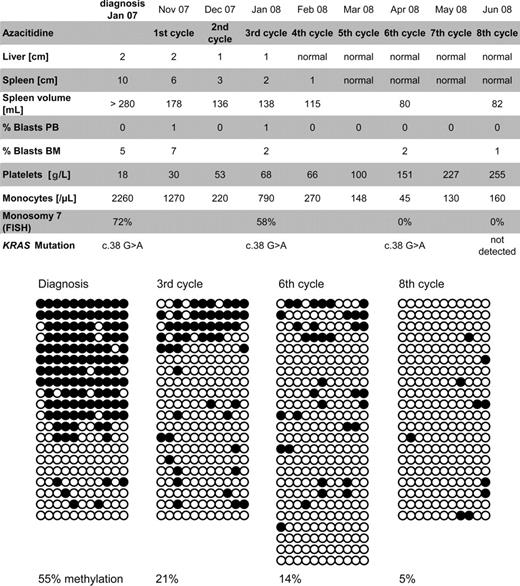
The boy presented at the age of 1.5 years with hepatosplenomegaly, myeloid precursors in the peripheral blood, monocytosis, anemia, thrombocytopenia (18 g/L), 6.8% hemoglobin F (HbF), and 5% blasts in the bone marrow (Figure 1). Metaphase cytogenetics revealed isolated monosomy 7, which was present in 72% of nucleated bone marrow cells as indicated by fluorescence in situ hybridization. In addition, a somatic KRAS c.38 G > A mutation was present. In accordance with current guidelines of the European Working Group of Myelodysplastic Syndromes in Childhood (EWOG-MDS), hematopoietic stem cell transplantation (HSCT) was offered but refused by the patient's guardians. Instead, azacitidine infusions were initiated as an experimental therapy (100 mg/m2 over 1 hour intravenously on 5 consecutive days, repeated every 4 weeks). AC was tolerated without major side effects. The clinical and hematologic response was impressive, including reduction of liver/spleen size and resolution of monocytosis, thrombocytopenia, and elevated blast count (Figure 1). Cytogenetically, monosomy 7 disappeared at initiation of the sixth cycle. Likewise, the molecular KRAS lesion was undetectable before the eighth cycle. At this time, the parents' consent to HSCT was obtained. Twelve weeks after the eighth cycle, HSCT was performed using peripheral blood stem cells from a 10/10 matched unrelated donor. There were no major toxicities or acute graft-versus-host disease; the child remains disease-free after a follow-up of 4 months from HSCT.
Summary of clinical course and methylation studies. The top panel shows clinical response parameters as liver and spleen size (in centimeters below costal margin), blast percentage, platelet count, monocyte count, and cytogenetic studies of bone marrow cells. Diagrams in the bottom panel depict the CALCA gene methylation status in bone marrow cells at diagnosis, before the third cycle and eighth cycle of azacitidine. The methylation of 11 CpG dinucleotides located between +172 bp and +325 bp relative to the CALCA transcription start site was mapped by sequencing multiple cloned alleles obtained from PCR on bisulfite-treated genomic DNA. Each horizontal line represents an individual allele (20-24 per sample). ● represents methylated CpG sites; ○, unmethylated CpG sites. FISH indicates fluorescence in situ hybridization.
Summary of clinical course and methylation studies. The top panel shows clinical response parameters as liver and spleen size (in centimeters below costal margin), blast percentage, platelet count, monocyte count, and cytogenetic studies of bone marrow cells. Diagrams in the bottom panel depict the CALCA gene methylation status in bone marrow cells at diagnosis, before the third cycle and eighth cycle of azacitidine. The methylation of 11 CpG dinucleotides located between +172 bp and +325 bp relative to the CALCA transcription start site was mapped by sequencing multiple cloned alleles obtained from PCR on bisulfite-treated genomic DNA. Each horizontal line represents an individual allele (20-24 per sample). ● represents methylated CpG sites; ○, unmethylated CpG sites. FISH indicates fluorescence in situ hybridization.
To test whether reduction of aberrant DNA methylation may have contributed to the efficacy of AC, we used bisulfite sequencing for high-resolution mapping of cytosine methylation at the CALCA gene promoter (Figure 1). CALCA was chosen as marker because of reported hypermethylation in childhood MDS.3 Our patient initially showed 55.4% CALCA methylation in the peripheral blood, which decreased to 4.5% before the eighth cycle of AC. Importantly, reduction in methylation was not only due to clearance of malignant cells, but was evident in the clone itself.4 Before the third cycle, 58% of cells still carried monosomy 7, but completely methylated alleles were no longer detected (Figure 1).
Here we report the first patient with JMML and monosomy 7 treated with AC. The favorable response fits well with previous studies1,2 but has additional remarkable aspects. Thrombocytopenia and HbF at diagnosis indicated that this was a rather aggressive case of JMML.5 Using conventional cytostatic therapy, remissions are rare in these cases6 and disappearance of the monosomy 7 clone in JMML without HSCT has never been reported before. In summary, our results suggest that AC therapy may be a viable option for JMML/monosomy 7 patients, either as a pretransplant window or in the absence of a suitable HSCT donor.
Authorship
Acknowledgment: This work was supported by Deutsche Krebshilfe 108220 (C.B., C.F.).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Dr C. Niemeyer, Division of Pediatric Hematology-Oncology, Department of Pediatric and Adolescent Medicine, University of Freiburg, Mathildenstrasse 1, 79106 Freiburg, Germany; e-mail: charlotte.niemeyer@uniklinik-freiburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal