Abstract
We previously reported interim results of a phase 1 trial in patients with chronic lymphocytic leukemia (CLL) whereby flavopiridol was administered intravenously as a 30-minute bolus followed by 4-hour infusion. We now report full pharmacokinetic (PK) data, correlations of PK with clinical outcomes, and final response and progression-free survival (PFS). Twenty-one (40%) of 52 patients with relapsed CLL achieved a partial response (PR) with a median PFS of 12 months. Responders included 17 (40%) of 43 fludarabine refractory patients, 7 (39%) of 18 patients with del(17p13), and 14 (74%) of 19 patients with del(11q22). Six responders received repeat therapy at relapse, and 5 responded again with a second median PFS of 10 months. Noncompartmental analysis and nonlinear mixed effects modeling was used to estimate PK parameters and evaluate covariates. Two-compartment population parameter estimates were 31.4 L/h, 65.8 L, 8.49 L/h, and 157 L for CL, V1, Q, and V2, respectively. Flavopiridol area under the plasma concentration-time curve (AUC) correlated with clinical response and cytokine release syndrome, and glucuronide metabolite AUC correlated with tumor lysis syndrome. These composite results confirm high activity of this pharmacokinetically derived schedule in relapsed, genetically high-risk CLL. Furthermore, PK describes some, but not all, variability in response and toxicity.
Introduction
Flavopiridol (Alvocidib, NSC 649890) is a serine/threonine kinase inhibitor that broadly targets cyclin-dependent kinases (CDKs), including the CDK9/cyclin T complex (pTEF-b), preventing activation of RNA polymerase II.1-3 Flavopiridol initiates cell cycle arrest4,5 and apoptosis6-8 by down-regulating the antiapoptotic proteins Mcl-1 and X-linked inactivator of apoptosis (XIAP)7,9,10 via a p53-independent mechanism.8,11 This latter characteristic suggested that flavopiridol might be active in chronic lymphocytic leukemia (CLL), as advanced CLL commonly exhibits elevated Mcl-1 and dysfunctional or deleted p53, thereby rendering standard treatments such as alkylating agents, fludarabine, and rituximab ineffective.12-14 Clinical studies corroborating these pharmacodynamic responses have yet to be reported.
Based on promising preclinical activity, initial clinical trials of flavopiridol used a 72-hour intravenous infusion schedule in patients with refractory neoplasms. However, phase 1 and 2 trials using 24- to 72-hour intravenous infusion schedules in solid and hematologic malignancies produced few responses.15-21 These schedules failed to produce clinical responses in relapsed CLL, although a phase 2 study evaluating a 1-hour intravenous bolus observed a modest response rate with additional evidence of peripheral lymphocyte reduction in nonresponders.15 Our group subsequently implemented a phase 1 study (NCI-5746) using a pharmacokinetically (PK)–based dosing schedule with a 30-minute intravenous bolus loading dose (IVB) followed by a 4-hour continuous intravenous infusion (CIVI), in patients with refractory CLL. We previously reported interim results from 42 patients in the first 3 cohorts, which indicated a 45% overall response rate22 and showed marked improvement in clinical activity over previous 1-hour or 24- to 72-hour infusion schedules. Tumor lysis syndrome (TLS), indicative of rapid tumor cell death, was the most serious of observed toxicity. Compared with previous schedules, our dosing schedule generally achieved higher plasma drug concentrations that were maintained for the duration of the infusion, but significant relationships between PK and clinical outcomes were not yet apparent.
In this report, we present final clinical response data for the entire phase 1 study, and we present progression-free survival (PFS) data for the first time. We also present for the first time a population PK model for all cohorts of patients treated with this active schedule. These data indicate parent drug and glucuronide metabolite PK are significantly correlated with clinical outcomes, including response, cytokine release syndrome (CRS), and TLS. Although these relationships are significant, high intersubject variability in PK and clinical outcomes suggests further evaluation of covariates is warranted to potentially identify patients who are at increased risk of TLS and require more stringent observation when given flavopiridol.
Methods
Patients and study design
Eligibility criteria.
Patients with relapsed, symptomatic CLL or small lymphocytic lymphoma (SLL) were enrolled and received treatment between May 2003 and February 2006. This study (NCI-5746, OSU-0055) was approved by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI, Bethesda, MD) and the Institutional Review Board (IRB) of The Ohio State University. Informed consent was obtained in accordance with the Declaration of Helsinki. Eligibility criteria included relapsed CLL or SLL, age 17 years or older, Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less, serum creatinine and total bilirubin less than 2 times the upper limit of normal, platelet count of at least 50 × 109/L (cohorts 1-2 only), and no active infection or inflammatory bowel disease. To reduce the risk of severe tumor lysis, patients in cohort 4 were required to have a white blood cell (WBC) count less than 200 × 109/L.
Treatment plan.
Flavopiridol was administered through a central catheter by IVB plus CIVI weekly for 4 consecutive weeks, followed by 2 weeks without therapy (total of 6 weeks per cycle). Patients were treated until occurrence of dose-limiting or other unacceptable toxicity, disease progression, or completion of 6 cycles of therapy. Patients who responded to their initial treatment and subsequently progressed were eligible for retreatment.
Interpatient and intrapatient dose escalation.
As previously reported, intrapatient dose escalation was allowed beginning in cohort 3.22 Patients in cohorts 3 and 4 received initial doses at the cohort 1 level, and in the absence of severe toxicity, escalated doses on the first dose of cycle 2 (cohort 3) or the second dose of cycle 1 (cohort 4). Once a patient underwent dose escalation, all subsequent treatments were given at this escalated dose. The dose schema is depicted in Table 1.
Dosing scheme by cohort
| Cohort . | IVB, mg/m2 . | CIVI, mg/m2 . | Intrapatient dose escalation . | n . |
|---|---|---|---|---|
| 1 | 30 | 30 | 20 | |
| 2 | 40 | 40 | 3 | |
| 3 | 30 | 30 | 19 | |
| 30 | 50 | Dose 5 (cycle 2, day 1) | (14 of 19) | |
| 4 | 30 | 30 | 16 | |
| 30 | 50 | Dose 2 (cycle 1, day 8) | (14 of 16) |
| Cohort . | IVB, mg/m2 . | CIVI, mg/m2 . | Intrapatient dose escalation . | n . |
|---|---|---|---|---|
| 1 | 30 | 30 | 20 | |
| 2 | 40 | 40 | 3 | |
| 3 | 30 | 30 | 19 | |
| 30 | 50 | Dose 5 (cycle 2, day 1) | (14 of 19) | |
| 4 | 30 | 30 | 16 | |
| 30 | 50 | Dose 2 (cycle 1, day 8) | (14 of 16) |
IVB indicates 30-minute intravenous bolus dose; CIVI, 4-hour continuous intravenous infusion; and n, number of patients receiving the specified dose.
Supportive care.
Allopurinol 300 mg daily was started 1 day prior to the first dose of flavopiridol and continued throughout therapy. Prophylactic therapy for pneumocystis pneumoniae (PCP) and varicella zoster virus (VZV) was provided during and for 6 months following flavopiridol treatment. Rasburicase 0.15 mg/kg was given 2 hours prior to both the initial dose and the first escalated dose (when applicable). Patients received intravenous hydration and urine alkalization, phosphate binder, and hourly monitoring of serum potassium during flavopiridol administration, as previously described.22 Patients with elevated potassium levels during treatment received kayexalate, furosemide, albuterol, insulin, glucose, and calcium according to an established institutional protocol.
Assessment of response.
Pharmacokinetic study
Initial blood sampling occurred between 0.5 and 36 hours, but adjustments were made after cohort 2 to better characterize the elimination phase by extending sampling up to 48 hours. Sampling occurred immediately prior to, during, and up to 48 hours after the initial dose and again following dose escalation (ie, PK determined on 2 occasions in each dose-escalated patient). Venous blood was drawn from a peripheral catheter into sodium heparin tubes and centrifuged, and plasma was stored at −70°C until analysis. A validated liquid chromatography/mass spectrometry/mass spectrometry (LC-MS/MS) assay with 3 nM lower limit of quantitation (LLOQ) was used to determine parent drug plasma concentrations.25
Pharmacokinetic modeling
WinNonlin Professional, V5.2 (Pharsight, Mountain View, CA) was used to estimate noncompartmental PK parameters following initial and escalated doses in each patient. Subsequently, compartmental modeling in NONMEM V (Globomax, Ellicott City, MD) was carried out to generate a structural PK model for the population data with volume (V) and clearance (CL) parameters. Models included parameters for interindividual (IIV) and interoccasion (IOV) variability as well as proportional and additive residual error.
Each model was simultaneously fitted to the data for all patients with first order conditional estimation. Model selection was guided by the likelihood ratio test using a significance level of P value less than .05, which corresponded to an absolute decrease in the objective function value (OFV, −2 × log-likelihood function, which is distributed as a 1 degree of freedom χ2, χ12, under the null hypothesis) of 3.84 or greater when an additional parameter was added to the model. Coefficient of variation expressed as a percentage (CV%, ratio of the standard error of the estimate divided by the estimate) was evaluated as a standardized measure of uncertainty in the parameter estimates. Once a final model was obtained, single-factor ANOVA was used for parameter comparisons among categories unless otherwise noted.
Results
Patient characteristics
Forty-three patients with relapsed CLL and 9 patients with relapsed SLL (total: 52 unique patients) were treated in the 4 cohorts of this trial, including 6 patients who were retreated after disease progression following response to their initial course of flavopiridol therapy. Two patients received repeat flavopiridol therapy in cohort 3 and were included in our initial report of this trial. Four patients received repeat therapy in cohort 4. Of the 52 unique patients, 38 were male (72%), and the median age was 60 years (range, 38-84). Forty-two patients (81%) were Rai stage III or IV at the time of study enrollment. Patients had received a median of 4 prior therapies (range, 1-14). Fifty-one patients (98%) had received prior fludarabine therapy, and 43 patients (83%) were refractory to their most recent fludarabine regimen. High-risk cytogenetic features were identified in 40 patients (77%). A complex karyotype was observed in 25 patients (48%); deletion of 17p13, in 18 patients (35%); and deletion of 11q22, in 19 patients (37%). Thirty-eight patients (73%) had bulky lymphadenopathy, defined as having at least one lymph node of at least 5 cm in dimension. A summary of pretreatment characteristics is provided in Table 2.
Demographics and pretreatment characteristics of patients receiving initial flavopiridol therapy, N = 52
| . | No. . | Range . |
|---|---|---|
| Demographics | ||
| Sex, no. | ||
| Male | 38 | |
| Female | 14 | |
| Mean age, y (median) | 60 (60) | 38-84 |
| Mean weight, kg (median) | 82 (83) | 52-121 |
| Body surface area, m2, mean (median) | 2 (1.95) | 1.54-2.41 |
| Disease characteristics (%) | ||
| Lymphocyte count | ||
| More than 5000 (CLL) | 43 | |
| Less than 5000 (SLL) | 9 | |
| Rai stage III/IV | 42 (81) | |
| Bulky lymph nodes larger than 5 cm | 38 (73) | |
| Median prior therapies | 4 | 1-14 |
| Prior fludarabine therapy | 51 (98) | |
| Fludarabine refractory | 43 (83) | |
| Cytogenetics (%) | ||
| del(11q22) | 19 (37) | |
| del(17p13) | 18 (35) | |
| Complex karyotype | 25 (48) | |
| Baseline CBC | ||
| Platelets, ×109/L | 105 (106) | 6-284 |
| White blood cells, ×109/L | 59 (16.6) | 1.9-314.5 |
| Hemoglobin, mM | 10.5 (10.2) | 5.8-15.4 |
| Albumin, g/dL | 3.5 (3.4) | 2.6-4.7 |
| Protein, g/dL | 5.7 (5.6) | 4.4-8.5 |
| . | No. . | Range . |
|---|---|---|
| Demographics | ||
| Sex, no. | ||
| Male | 38 | |
| Female | 14 | |
| Mean age, y (median) | 60 (60) | 38-84 |
| Mean weight, kg (median) | 82 (83) | 52-121 |
| Body surface area, m2, mean (median) | 2 (1.95) | 1.54-2.41 |
| Disease characteristics (%) | ||
| Lymphocyte count | ||
| More than 5000 (CLL) | 43 | |
| Less than 5000 (SLL) | 9 | |
| Rai stage III/IV | 42 (81) | |
| Bulky lymph nodes larger than 5 cm | 38 (73) | |
| Median prior therapies | 4 | 1-14 |
| Prior fludarabine therapy | 51 (98) | |
| Fludarabine refractory | 43 (83) | |
| Cytogenetics (%) | ||
| del(11q22) | 19 (37) | |
| del(17p13) | 18 (35) | |
| Complex karyotype | 25 (48) | |
| Baseline CBC | ||
| Platelets, ×109/L | 105 (106) | 6-284 |
| White blood cells, ×109/L | 59 (16.6) | 1.9-314.5 |
| Hemoglobin, mM | 10.5 (10.2) | 5.8-15.4 |
| Albumin, g/dL | 3.5 (3.4) | 2.6-4.7 |
| Protein, g/dL | 5.7 (5.6) | 4.4-8.5 |
Response and toxicity
Cohort 4.
Response and toxicity for cohorts 1 to 3 were previously described.22 Sixteen patients were treated in cohort 4, including 12 flavopiridol-naive patients and patients who had responded to previous flavopiridol therapy in cohort 1 (n = 3) or cohort 3 (n = 1) and had subsequently relapsed. Median age of the 16 patients in cohort 4 was 60 years (range, 42-75), 75% were male, 69% had fludarabine refractory disease, 63% were Rai stage III or IV, and 50% had del(17p13); patients had received a median of 4 prior therapies (range, 1-10). Thus, their baseline characteristics were similar to those of patients in cohorts 1 to 3.
Fourteen of 16 patients in cohort 4 underwent dose escalation at dose 2 of cycle 1. One patient in this cohort required transient hemodialysis due to severe TLS. Because this occurred during the first treatment, this patient received the 30 plus 30 mg/m2 dose for the remainder of therapy (3 complete cycles). The other nonescalated patient tolerated the initial flavopiridol infusion without significant toxicity but developed abdominal pain and hypotension 48 hours following dose administration. This patient developed an acute abdomen and underwent exploratory laparotomy and resection of a portion of bowel, which demonstrated CLL infiltration. This patient later died of gastrointestinal bleeding, and postmortem study failed to demonstrate a clear relationship of these events to flavopiridol. Other toxicities observed in cohort 4 were similar to those previously reported for cohorts 1 to 3, including neutropenia, diarrhea, fatigue, and CRS.22 A summary of severe toxicities (grades 3 and 4) is presented in Table 3. Six (38%) of 16 patients in cohort 4 achieved a partial response (PR) with a median PFS of 9.7 months (range, 6.5-17.2 months), and 2 additional patients experienced more than 50% reduction of nodal disease but did not achieve sufficient improvement in blood counts to be classified as a PR.
Severe toxicities (grades 3 and 4) experienced by patients in cohort 4 (n = 16)
| . | No. of patients with toxicities . | |
|---|---|---|
| Grade 3 . | Grade 4 . | |
| Hematologic | ||
| ANC | 16 | |
| WBC | 7 | 4 |
| Platelets | 4 | 3 |
| Hemorrhage* | 1 | |
| Cardiac | ||
| Hypotension | 1 | |
| Ventricular arrhythmia | 1 | |
| Cardiac-ischemia/infraction | 1 | |
| Respiratory | ||
| Hypoxia | 2 | 1 |
| GI/Hepatic | ||
| Diarrhea | 12 | |
| Abdominal pain/cramping | 1 | |
| AST or ALT | 3 | |
| Bilirubin | 1 | |
| Renal† | ||
| Renal failure | 1 | |
| CNS | ||
| Dysphagia | 1 | |
| Myalgia | 1 | |
| Pain | 1 | |
| Pruritus | 1 | |
| Possible infection | ||
| Febrile neutropenia | 4 | |
| Infection w/neutropenia | 6 | |
| Other | ||
| Fatigue | 2 | |
| Biochemical tumor lysis | 7 | |
| Erectile dysfunction | 1 | |
| Nausea | 3 | |
| . | No. of patients with toxicities . | |
|---|---|---|
| Grade 3 . | Grade 4 . | |
| Hematologic | ||
| ANC | 16 | |
| WBC | 7 | 4 |
| Platelets | 4 | 3 |
| Hemorrhage* | 1 | |
| Cardiac | ||
| Hypotension | 1 | |
| Ventricular arrhythmia | 1 | |
| Cardiac-ischemia/infraction | 1 | |
| Respiratory | ||
| Hypoxia | 2 | 1 |
| GI/Hepatic | ||
| Diarrhea | 12 | |
| Abdominal pain/cramping | 1 | |
| AST or ALT | 3 | |
| Bilirubin | 1 | |
| Renal† | ||
| Renal failure | 1 | |
| CNS | ||
| Dysphagia | 1 | |
| Myalgia | 1 | |
| Pain | 1 | |
| Pruritus | 1 | |
| Possible infection | ||
| Febrile neutropenia | 4 | |
| Infection w/neutropenia | 6 | |
| Other | ||
| Fatigue | 2 | |
| Biochemical tumor lysis | 7 | |
| Erectile dysfunction | 1 | |
| Nausea | 3 | |
The table lists the number of patients who experienced severe (grades 3,4) toxicities at any point during their flavopiridol treatment.
One patient died from gastrointestinal bleeding.
Excludes transient electrolyte abnormalities.
Final response data for entire phase 1 study.
Of 52 patients receiving their first course of flavopiridol therapy in cohorts 1 to 4, 7 (13%) completed all 6 cycles of planned therapy, including 2 (13%) of 16 patients in cohort 4. The median number of completed cycles of therapy was 2, and this was also true of cohort 4. Overall, 21 (40%) of 52 patients achieved a PR. Two additional patients had marked reduction of their white blood cell count from 235.4 × 109/L and 213.5 × 109/L to 5.2 × 109/L and 23.5 × 109/L, respectively, but received only one dose due to severe TLS and were therefore not responders. The response rate was not affected by the number of prior treatments. Seventeen (40%) of 43 fludarabine refractory patients responded with a median PFS of 12.4 months, whereas 4 (44%) of 9 nonrefractory patients responded with a median PFS of 9.3 months. Responses were seen in 7 (39%) of 18 patients with del(17p13), 14 (74%) of 19 patients with del(11q22), and 9 (36%) of 25 patients with a complex karyotype. Furthermore, 17 (45%) of 38 patients with bulky lymphadenopathy achieved a PR. One responder was in remission but was lost to follow-up at 7.5 months. The other 20 responding patients all relapsed, and median PFS was 12 months. Median PFS duration for responders was 14.5 months for those with del(17p13), 11.7 months for those with del(11p22), 9.4 months for those with a complex karyotype, and 11.4 months for patients with bulky lymph nodes (Table 4).
Clinical response by cytogenetic risk group
| . | All patients, n = 52 . | Flu refractory, n = 43 . | Not flu refractory, n = 9 . | Complex karyotype, n = 25 . | Deletion of 17p13, n = 18 . | Deletion of 11q22, n = 21 . |
|---|---|---|---|---|---|---|
| Partial response | 21 | 17 | 4 | 9 | 7 | 15 |
| % PR | 40 | 40 | 44 | 36 | 39 | 74 |
| Median PFS, mo | 12.0 | 12.4 | 9.3 | 9.4 | 14.5 | 11.7 |
| . | All patients, n = 52 . | Flu refractory, n = 43 . | Not flu refractory, n = 9 . | Complex karyotype, n = 25 . | Deletion of 17p13, n = 18 . | Deletion of 11q22, n = 21 . |
|---|---|---|---|---|---|---|
| Partial response | 21 | 17 | 4 | 9 | 7 | 15 |
| % PR | 40 | 40 | 44 | 36 | 39 | 74 |
| Median PFS, mo | 12.0 | 12.4 | 9.3 | 9.4 | 14.5 | 11.7 |
Partial responses (PR), the PR rate, and median progression-free survival (PFS) are shown for all patients as well as for individual cytogenetic risk groups.
Six previous responders (median PFS, 12.7 months; range, 7.8-15.5 months) received repeat flavopiridol therapy after relapse. They received a median of 3.5 cycles of repeat therapy, and 2 completed all 6 planned cycles of retreatment. Five of 6 patients achieved a second PR with a median PFS of 10 months (range, 6.5-19.1 months). Thus, median PFS and response duration with repeat flavopiridol therapy were comparable with that achieved with initial flavopiridol therapy.
Pharmacokinetic analysis
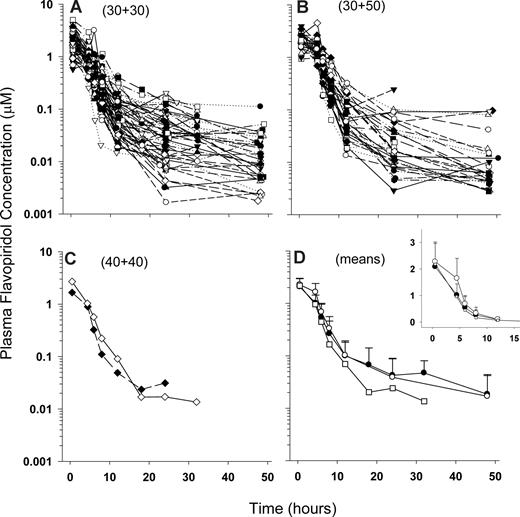
A total of 585 plasma concentration observations representing 85 concentration-versus-time profiles from 51 patients was available for PK analysis, including profiles for initial and escalated doses. PK parameters were determined for the 6 patients who were re-enrolled after relapse as if they were unique individual patients (ie, a total of 57 patients for which PK parameters were estimated) since retreatments occurred several months after initial therapy, and for each retreated patient a new set of baseline demographics (eg, age and BSA) and laboratory data as well as new toxicity and response data were available for correlation. Figure 1 displays concentration-versus-time profiles for all patients and among cohorts.
PK profiles. Semilogarithmic flavopiridol plasma concentration versus time plots for doses 30 + 30 (A), 40 + 40 (B), and 30 + 50 mg/m2 (C), and mean plus SD values for each dose level (D) (filled circles represent 30 + 30 mg/m2; squares, 40 + 40 mg/m2; and open circles, 30 + 50 mg/m2). The curve for the 40 + 40 mg/m2 dose level does not include SD because data from only 2 patients were available for mean calculation. The inset in panel D displays a linear y-axis to better illustrate the increased AUC achieved with the escalated dose (30 + 50 mg/m2).
PK profiles. Semilogarithmic flavopiridol plasma concentration versus time plots for doses 30 + 30 (A), 40 + 40 (B), and 30 + 50 mg/m2 (C), and mean plus SD values for each dose level (D) (filled circles represent 30 + 30 mg/m2; squares, 40 + 40 mg/m2; and open circles, 30 + 50 mg/m2). The curve for the 40 + 40 mg/m2 dose level does not include SD because data from only 2 patients were available for mean calculation. The inset in panel D displays a linear y-axis to better illustrate the increased AUC achieved with the escalated dose (30 + 50 mg/m2).
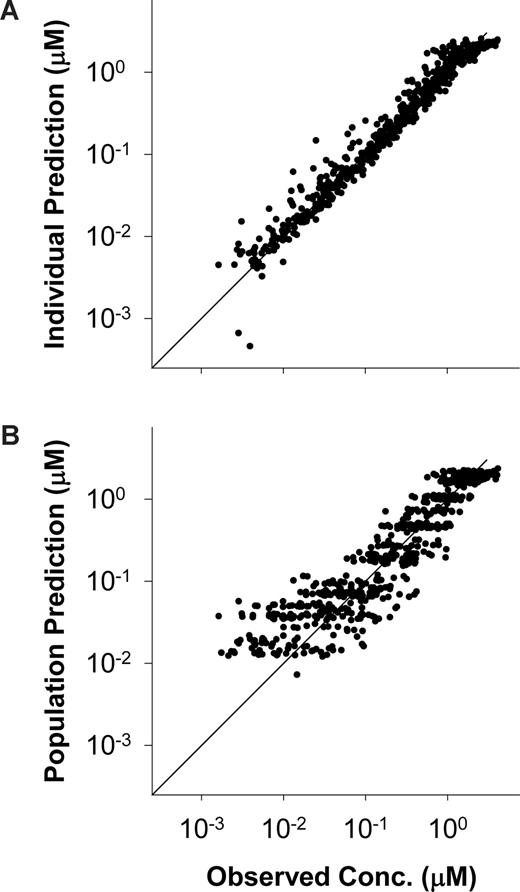
For structural model development, open 2- and 3-compartment PK models were evaluated. One outlier profile (patient 6, cohort 1) caused significant interpatient variability and was therefore removed from the data set. Similar to most other compartmental modeling reports with flavopiridol, including a population modeling study,26 a 2-compartment model with first-order elimination provided the best fit to the data. The final structural model included proportional and additive residual error, IIV on all PK parameters, and IOV on CL, Q, and V1. Patient demographics, complete blood count, and electrolytes were evaluated as covariates. Only body surface area (BSA) met the criteria for inclusion with a modest 5-unit reduction in OFV (χ12 = 5) when included as a covariate on CL. Proportional and additive residual error components were 32.4% and 1.2 ng/mL (3 nM) for the final model. Table 5 lists model parameters and error values and Figure 2 displays goodness- of-fit plots for individual and population predictions.
Two-compartment population parameter and variability estimates with body surface area (BSA) as a covariate on CL
| . | Estimate . | IIV . | IOV . |
|---|---|---|---|
| CL, L/h | 31.4 (5.4) | 28.7 (25.3) | 26.0 (43.7) |
| θ | 0.84 (52.3) | ||
| V1, L | 65.8 (3.7) | 13.7 (69.0) | 9.4 (241) |
| Q, L/h | 8.49 (10.7) | 66.9 (24.2) | 73.1 (63.3) |
| V2, L | 157 (6.0) | 87.4 (32.7) | - |
| . | Estimate . | IIV . | IOV . |
|---|---|---|---|
| CL, L/h | 31.4 (5.4) | 28.7 (25.3) | 26.0 (43.7) |
| θ | 0.84 (52.3) | ||
| V1, L | 65.8 (3.7) | 13.7 (69.0) | 9.4 (241) |
| Q, L/h | 8.49 (10.7) | 66.9 (24.2) | 73.1 (63.3) |
| V2, L | 157 (6.0) | 87.4 (32.7) | - |
θ is an estimated population parameter (exponent on the normalized BSA value, BSAθ). Interindividual variability (IIV) and interoccasion variability (IOV) estimates are represented as % CV. Relative standard errors (RSE as % CV) for estimated parameters are shown in parentheses.
Individual and population predicted versus observed concentrations. (A) Individual and (B) population predicted versus observed concentrations of plasma flavopiridol. Diagonals are lines of identity.
Individual and population predicted versus observed concentrations. (A) Individual and (B) population predicted versus observed concentrations of plasma flavopiridol. Diagonals are lines of identity.
Area under the concentration-versus-time curve (AUClast) ranged from 4.51 to 31.4 μM/h, although the 31.4 value (patient 6) was an outlier with the next highest exposure at 21.4 μM/h. The AUClast covered 95% plus or minus 8% of AUC extrapolated to infinity (AUC0-∞). The escalated 30 plus 50 doses resulted in a significant 26% increase in AUC0-∞ (mean ± SD, 12.9 ± 3.7 μM/h) compared with the 30 plus 30 doses in the same patients (10.2 ± 3.9 μM/h; paired 2-tailed t test, P = .006). Mean AUC values by cohort and dose level are presented in Table 6.
Mean AUC0-∞ (log-linear estimations) by cohort and dose level
| Cohort . | AUC0-∞, mean ± SD (μM × h) . | Patients . | Dose level, mg/m2 . | AUC0-∞, mean ± SD (μM × h) . | Profiles . |
|---|---|---|---|---|---|
| 1 | 10.0 ± 5.6 | 20 | 30 + 30 | 10.4 ± 4.6 | 55 |
| (9.0 ± 2.9)* | (19) | (10.0 ± 3.7)* | (54) | ||
| 2 | 9.0 | 2 | 40 + 40 | 9.0 | 2 |
| 3 | 11.4 ± 3.9 | 19 | 30 + 50 | 12.9 ± 3.7† | 28 |
| 4 | 12.1 ± 2.4 | 16 |
| Cohort . | AUC0-∞, mean ± SD (μM × h) . | Patients . | Dose level, mg/m2 . | AUC0-∞, mean ± SD (μM × h) . | Profiles . |
|---|---|---|---|---|---|
| 1 | 10.0 ± 5.6 | 20 | 30 + 30 | 10.4 ± 4.6 | 55 |
| (9.0 ± 2.9)* | (19) | (10.0 ± 3.7)* | (54) | ||
| 2 | 9.0 | 2 | 40 + 40 | 9.0 | 2 |
| 3 | 11.4 ± 3.9 | 19 | 30 + 50 | 12.9 ± 3.7† | 28 |
| 4 | 12.1 ± 2.4 | 16 |
Data for cohorts includes initial and escalated doses for all 58 patients (including re-enrolled patients).
Means without the outlier patient 6.
Comparison of AUC0-∞ for all 30+30 and 30+50 doses, P = .002.
Correlations of composite pharmacokinetics, response, and toxicity
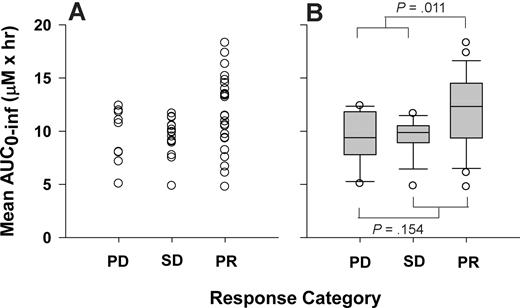
The basis for this trial was to attain and sustain a plasma flavopiridol concentration above 1.5 μM for 4 hours. Forty-five patients achieved plasma concentrations above 1.5 μM. Among these, 20 patients maintained this concentration for 4 or more hours during either their initial, escalated, or both doses. These included 11 partial responders (55%), 4 with stable disease (20%), 4 with progressive disease (20%), and 1 who was nonevaluable for response. Although this and other flavopiridol PK parameters indicate correlation with response in this study (including Cmax, C4.5 hour, and CL), only AUC met the .05 level of significance when comparing means across response groups. When the outlier point is removed (patient 6), significant differences in mean AUC0-∞ are observed between PR (11.9 ± 3.5 μM/h) and combined progressive (PD) and stable disease (SD) groups (9.4 ± 2.0 μM/h, P = .004). Table 7 lists response by cohort and dose level. Mean AUC0-∞ (ie, mean AUC0-∞ from initial and escalated dose profiles) versus response category is plotted in Figure 3.
Summary of responses for 52 patients by cohort and maximum dose received (data including 58 unique and re-enrolled patients are included in parentheses)
| Response . | Cohort . | Total . | Max dose level, mg/m2 . | |||||
|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 30+30 . | 40+40 . | 30+50 . | ||
| NE | 1 | 1 | 3 | 1 | 6 | 5 | 1 | 0 |
| PD | 6 | 0 | 1 | 4 | 11 | 6 | 0 | 5 |
| SD | 5 | 1 | 4 | 4 (5) | 14 (15) | 6 | 1 | 7 (8) |
| PR | 8 | 1 | 9 (11) | 3 (6) | 21 (26) | 9 (10) | 1 | 11 (15) |
| Total | 20 | 3 | 17 (19) | 12 (16) | 52 (58) | 26 (27) | 3 | 23 (28) |
| PR% | 40 | 33 | 53 (58) | 25 (38) | 40 (45) | 35 (37) | 33 | 48 (54) |
| Response . | Cohort . | Total . | Max dose level, mg/m2 . | |||||
|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 30+30 . | 40+40 . | 30+50 . | ||
| NE | 1 | 1 | 3 | 1 | 6 | 5 | 1 | 0 |
| PD | 6 | 0 | 1 | 4 | 11 | 6 | 0 | 5 |
| SD | 5 | 1 | 4 | 4 (5) | 14 (15) | 6 | 1 | 7 (8) |
| PR | 8 | 1 | 9 (11) | 3 (6) | 21 (26) | 9 (10) | 1 | 11 (15) |
| Total | 20 | 3 | 17 (19) | 12 (16) | 52 (58) | 26 (27) | 3 | 23 (28) |
| PR% | 40 | 33 | 53 (58) | 25 (38) | 40 (45) | 35 (37) | 33 | 48 (54) |
NE indicates nonevaluable; PD, progressive disease; SD, stable disease; and PR, partial response.
Flavopiridol exposure and response. Relationship between response category and AUC0-∞ or mean AUC0-∞ (ie, average of AUC0-∞ from initial and escalated doses) represented as a (A) dot plot or (B) box plot. ANOVA P values are displayed for comparison of PD/SD versus PR (.011) and PD versus SD/PR (.154). PD indicates progressive disease; SD, stable disease; and PR, partial response.
Flavopiridol exposure and response. Relationship between response category and AUC0-∞ or mean AUC0-∞ (ie, average of AUC0-∞ from initial and escalated doses) represented as a (A) dot plot or (B) box plot. ANOVA P values are displayed for comparison of PD/SD versus PR (.011) and PD versus SD/PR (.154). PD indicates progressive disease; SD, stable disease; and PR, partial response.
Because of the high response rate in retreated patients (5/6 achieved PR and 1/6 achieved SD), we repeated our analysis after removing the second set of PK data from the retreated patients in cohorts 3 and 4 (n = 6). Among partial responders (n = 5) in this group, average mean AUC0-∞ was 12.4 plus or minus 4.0 μM/h. The sixth patient achieved stable disease and had a mean AUC0-∞ of 10.5 μM/h. When repeat patients were removed from analysis, mean AUC0-∞ for the PD/SD (9.4 ± 3.1 μM/h) and PR groups (11.7 ± 3.4 μM/h, P = .008) remained statistically different.
Seventeen patients experienced grades 1 (1), 2 (13), or 3 (3) CRS. Mean AUCs were statistically lower in patients who did not experience CRS (10.5 ± 3.2 μM/h) versus those who experienced grades 1 to 3 CRS (13.4 ± 5.0 μM/h; P = .005). A similar observation was made with CL for the 2 groups (34.6 ± 9.1 and 27.1 ± 8.7 L/h, respectively, P = .003). Removing repeat patients in cohorts 3 and 4 again reduced the strength of the relationships slightly, but both maintained significance with P values of .020 and .016 for the AUC and CL comparisons, respectively.
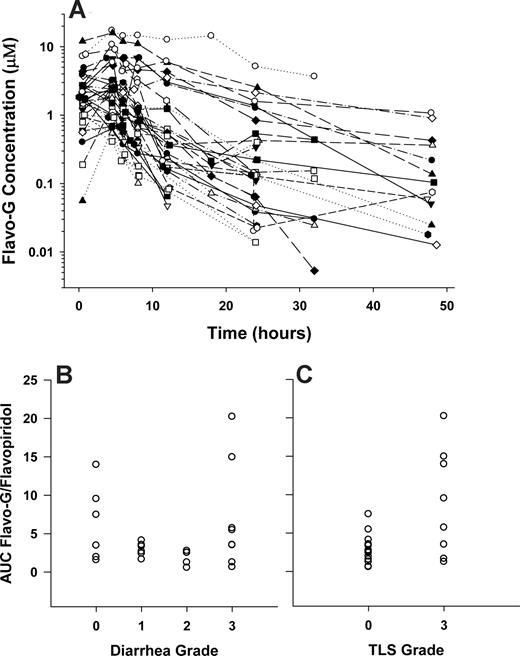
Flavopiridol PK parameters were not significantly correlated with TLS, diarrhea, or neutropenia. To evaluate a previously identified relationship between steady-state concentrations of flavopiridol glucuronide metabolite (flavo-G) and diarrhea using a 72-hour infusion,27 plasma samples collected after initial dosing in a subset of 25 patients (initial treatment only, no dose escalations, or re-enrolled patients were included) were remeasured to quantify flavo-G using β-glucuronidase as previously described.28 Figure 4A displays concentration versus time profiles for flavo-G, which illustrate the high and variable metabolite exposure with this IVB plus CIVI schedule (AUC0-∞, μM/h; range, 4.7-205; median, 26; mean, 57; SD, 61). No significant correlations were apparent between diarrhea and flavo-G or flavo-G/flavopiridol ratios. Figure 4B displays an example plot comparing diarrhea grade and flavo-G/flavopiridol AUC0-∞ ratios. Interestingly, a significant relationship was observed between metabolite exposure and TLS grade in our study. Patients experiencing no TLS had lower flavo-G/flavopiridol AUC0-∞ ratios (3.0 ± 1.8) compared with patients experiencing grade 3 TLS (8.9 ± 7.0, P = .005). Figure 4C displays this relationship via a dot plot.
Flavopiridol glucuronide (Flavo-G) concentration versus time profile and associations with TLS and diarrhea. (A) Flavo-G concentration-versus-time profiles for a subset of patients (n = 25); AUC flavo-G/flavopiridol ratio plotted against diarrhea (B) or TLS grade (C).
Flavopiridol glucuronide (Flavo-G) concentration versus time profile and associations with TLS and diarrhea. (A) Flavo-G concentration-versus-time profiles for a subset of patients (n = 25); AUC flavo-G/flavopiridol ratio plotted against diarrhea (B) or TLS grade (C).
Discussion
The interim analysis of this phase 1 trial demonstrated that flavopiridol is highly active in patients with refractory, genetically high-risk CLL when administered using a pharmacokinetically based dosing regimen.22 However, enthusiasm for this drug was initially dampened by severe TLS, which resulted in the death of one patient, required the transient use of hemodialysis in several other patients, and necessitated in-hospital admissions for the first 5 administrations of the drug. Furthermore, although we identified a WBC of 200 × 109/L or higher as a risk factor for severe TLS requiring hemodialysis, we were unable to correlate severe TLS with parent drug exposure. Thus, although our initial phase 1 report indicated promising clinical activity, several unanswered questions remained.
In this report, we confirm the clinical response rate initially reported in our interim analysis, and we present median PFS data that demonstrate that flavopiridol is able to achieve durable responses in genetically high-risk patients with limited treatment options. Furthermore, our experiences with cohort 4 (n = 16 patients) demonstrate that flavopiridol can be safely dose escalated at the second dose in patients who do not experience severe TLS with their first treatment, and we show that patients can safely transition from the in-hospital setting to outpatient treatment as early as the third dose. The safety data in cohort 4 are important, as they support an eventual role for flavopiridol in the community oncology practice. Finally, we establish correlations between flavopiridol AUC and both clinical response and cytokine release syndrome, and between flavopiridol glucuronide AUC and TLS. These findings suggest a potential for identifying patients with higher probability of response and those who may be at increased risk of severe toxicity.
The final clinical response data of this study confirm the previous observation that flavopiridol is active in CLL patients with poor-risk cytogenetic features such as del(17p13) and del(11q22). The only FDA-approved agent for relapsed CLL that has such activity in the high-risk cytogenetic groups is alemtuzumab. However, alemtuzumab therapy is ineffective as a single agent29 in patients with bulky adenopathy and is associated with significant hematologic and infectious toxicities. In contrast, flavopiridol induced responses in 45% of patients with bulky lymph nodes and was associated with only transient hematologic toxicity and few infectious complications.30 Whereas agents such as fludarabine and alemtuzumab cause profound T-cell suppression that often lasts several months or 1 to 2 years, we have not observed significant T-cell lymphopenia with flavopiridol.
We observed a median PFS of 12 months in responding patients, which ranged from 9.4 months in responders with a complex karyotype to 14.5 months in those with del(17p13). Furthermore, the response rate and median PFS were not affected by the number of prior treatments or refractoriness to fludarabine. The median PFS of 12.4 months in fludarabine refractory patients responding to flavopiridol is particularly encouraging, given that more than 80% of patients in this study were refractory to their last fludarabine regimen and the expected median overall survival of this population is only 9 months. Although we observed no complete responses in our phase 1 study, the lack of T-cell lymphopenia and severe infectious toxicity along with clinical responses in patients with bulky nodal disease indicates a potential role for flavopiridol in cytoreducing relapsed CLL patients to enable nonmyeloablative allogeneic stem cell transplantations. Furthermore, its lack of T-cell suppression or infectious toxicity suggests that it may be feasible to combine flavopiridol with other drugs in CLL and other lymphoid malignancies. We and others are actively investigating such combination regimens.
The results of cohort 4 indicate that the flavopiridol dose can be safely escalated as early as the second treatment in patients who do not experience severe TLS with the first dose. With this approach, the number of hospitalizations for flavopiridol treatment is reduced to 2, thus allowing early transition to outpatient treatment. This is an important finding, since early safe transition to the outpatient setting will enhance the ability of this drug to be administered in the community setting, should the drug gain eventual approval. Overall, dose escalation resulted in a higher response rate in this study (54% PR for dose-escalated versus 37% PR for non–dose-escalated patients). Despite escalating the dose and maintaining higher plasma drug levels beginning at dose 2 of cycle 1, we observed a relatively low response rate among patients in cohort 4 (38%) compared with cohorts 1 and 3 (40% and 58%, respectively). This discrepancy may be due to the small number of patients (n = 16) in cohort 4 and the high number of patients with del(17p13) and other high-risk cytogenetic features. Analysis of results of an ongoing phase 2 study (NCI-7000), where 64 patients with relapsed CLL have been treated using the day 8, cycle 1 dose-escalation schema, will provide additional data for assessing the safety and activity of earlier dose escalation.31
We also show that repeat flavopiridol therapy for patients who respond but subsequently relapse may be a viable treatment strategy. Six such patients who were not eligible for other clinical trials or did not want alternative therapies were retreated with flavopiridol. Five of these patients responded, and the median PFS after second treatment was comparable with the median PFS attained by this group of patients with their initial flavopiridol treatment. Although this is a small subset of patients, this relatively high response rate (83%) may be further evidence of interindividual differences in sensitivity to flavopiridol (ie, patients who respond to initial therapy may have a high likelihood of responding to later therapy after relapse). Thus, repeat flavopiridol therapy for patients with del(17p13) who may be poor candidates for alemtuzumab or allogeneic stem cell transplantation may be a reasonable therapeutic option. Identifying the source of interindividual variability in PK and response will be an important task in further clinical development of flavopiridol in CLL.
Flavopiridol population pharmacokinetics were described for the first time using this novel dosing schedule. BSA was the sole covariate included in the 2-compartment population model. Interindividual and intraindividual variability was less than 30% for V1 and CL but substantially higher for V2 and Q (up to 87%). Interestingly, IOV was similar to IIV, suggesting intrapatient parameters that were not evaluated significantly contribute to PK variability. Parameter and IIV estimates were similar to those reported in other studies, including a 34-patient, 2-compartment population model reported by Karp et al who used a 1-hour × 3-day dosing schema in adult relapsed and refractory acute leukemias with coadministration of 1-beta-d-arabinofuranosylcytosine and mitoxantrone.26 Primary differences in parameter estimates include higher V2 and Q (392 L and 25.3 L/h, respectively) and higher intersubject variability in CL and V1 (64% and 44.3%, respectively) in the acute leukemia study compared with this CLL study (Table 5). Although the differences in disease populations and dosing schedules may contribute to differences in parameter estimates, other possible contributors include a larger sample size (57 vs 34 patients) and improved sensitivity of the analytic assay (LLOQ of 3 nM vs 11 nM) in the present single-agent study.
Only 2 dose levels were evaluated in this study. It is therefore difficult to draw conclusions regarding PK linearity. We did observe a 26% increase in AUC within patients who received the 33% dose increase from 60 to 80 mg/m2 (n = 28). Although this data set needs to be expanded for future confirmation, this less-than-proportional increase in AUC may indicate nonlinear pharmacokinetics. In fact, other parameters support this possibility, including a decrease in T1/2 from 16.2 plus or minus 13.1 to 13.2 plus or minus 8.8 hours when patients receive the 30 plus 30 and 30 plus 50 mg/m2 doses, respectively (P = .068, paired t test, n = 28). Nonlinearity has previously been reported when administering flavopiridol as a 72-hour continuous infusion.32 In this study, Rudek et al observed saturation near their 50 mg/m2 per day dose level (range, 4-122.5 mg/m2 per day in this study). Beyond this dose level, clearance increased to yield less-than-proportional AUC increases.
Clinical outcome was associated with flavopiridol and metabolite PK. A significant, direct relationship was identified between flavopiridol AUC and response. Higher AUCs were also associated with occurrence of CRS. In a subset of 25 patients where PK samples were re-evaluated to measure flavo-G concentrations, a significant relationship was identified between flavo-G/flavopiridol AUC ratio and TLS. No relationship was evident between flavo-G levels and diarrhea. This was inconsistent with a relationship reported by Innocenti et al,27 which may be a function of the different dosing schedules, although neither study provides definitive sets of data for determining such a relationship since significant overlap of low flavo-G/flavopiridol ratios is observed among all diarrhea grades (0-3). Similar statements may be made about the response, CRS, and TLS relationships, as significant overlap in AUC or AUC ratios can be observed among the various response or toxicity categories (Figures 3,4). More data must be generated with this dosing schedule to determine the validity and clinical relevance of these associations. In addition, other contributing factors, such as single nucleotide polymorphisms in flavopiridol metabolizing enzymes or transporters should be factored into the models that have been developed thus far.
In conclusion, the findings of this phase 1 study demonstrated that flavopiridol is highly active in patients with high-risk, refractory CLL; that these responses are durable with a median PFS of 11.7 months across all cytogenetic risk groups; and that dose escalation at the second treatment, with transition to the outpatient setting with the third dose, is safe and effective. Correlative studies indicated that the AUC of flavopiridol correlated with CRS and clinical response, and the AUC ratio of glucuronide metabolites–parent drug was associated with TLS. These results suggest PK may be associated with risk for severe toxicity in the broader population. However, application of PK as a predictive measure may be unachievable. Therefore, yet unevaluated factors that contribute to the variability in PK, response, and severe toxicity (such as protein binding or pharmacogenomic variation) must be identified before these associations can be applied in the general clinical setting. There may also be yet undefined predictors of TLS that relate to the selective sensitivity of the leukemic cells. Identification of all of these potential predictors will be important in patient management for the future. The activity observed in this trial illustrates that rational PK-derived dosing schedules of antineoplastic agents can reveal clinical activity when it may otherwise be unapparent using empirically derived dosing regimens, thus underscoring the importance of proper design and conduct of PK studies in phase 1 drug development. This report provides a framework for future study of flavopiridol to further characterize genetic and other individual features that may contribute to overall outcomes in CLL and other malignancies.
Portions of the data in this paper were presented at the 48th Annual Meeting of the American Society for Hematology, Orlando, FL, December 8, 2006. A previous publication presented interim clinical data from this trial.22
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Leukemia & Lymphoma Society (White Plains, NY) SCOR grant (J.C.B.), the D. Warren Brown Foundation (Marion, OH; J.C.B.), and NCI grants 5U01 CA76576 (M.R.G.), P01 CA081534 (to Principal Investigator Thomas J. Kipps at the University of California, San Diego and to Coinvestigators M.R.G. and J.C.B. at OSU), P30 CA016058 (to Principal Investigator Michael A. Caligiuri and Coinvestigators M.R.G., J.C.B., and M.A.P.), K23 CA102276 (T.S.L.), and R21 CA112947-02 (T.S.L.).
National Institutes of Health
Authorship
Contribution: M.A.P. validated the PK assay, performed PK sample analysis and PK modeling, and drafted the paper; T.S.L. was the principal investigator on the clinical trial, supervised patients enrolled in the study, and supported paper writing; A.J.J. supervised and performed laboratory correlative work and assisted with paper writing; E.H. assisted with PK modeling; D.M.R. supported sample analysis and PK modeling; K.L.F. assisted with sample analysis and quality assurance of PK data; D.W. supported initial PK assay development, sample analysis, and paper writing; K.A.B. was coinvestigator on the study, evaluated patients, and assisted with paper writing; B.F. led the patient care effort, assisted in development of TLS management procedures, and assisted with paper writing; S.M.M. performed data management for the trial; M.E.M. was coinvestigator on the study and, with the principal investigator, oversaw patient management; M.B.-M. was key in patient care and management of TLS; N.A.H. directed interphase and metaphase cytogenetic analysis on all patients and assisted with paper writing; D.J. supported statistical analysis and paper writing; L.J.S. was director of the patient clinical treatment unit, sample processing laboratory, where he assisted in developing the outpatient algorithm and assisted with paper writing; J.C.B. and M.R.G. authored and coinvestigated the study, identified the initial schedule of administration, directed modifications of the study, and supported paper writing and revision; J.T.D. directed the bioanalytical and pharmacokinetic analyses and assisted with paper writing.
Conflict-of-interest disclosure: A patent has been filed on this method of administering flavopiridol. Inventors on this patent include J.C.B., M.R.G., T.S.L., and J.T.D. It has no financial value now. The remaining authors declare no competing financial interests.
Correspondence: Mitch A. Phelps, Comprehensive Cancer Center and College of Pharmacy, Division of Pharmaceutics, The Ohio State University, 500 W 12th Ave, Columbus, OH 43210; e-mail: mitch.phelps@osumc.edu; or Michael R. Grever, The Ohio State University, 215 Means Hall, 1654 Upham Dr, Columbus, OH 43210; e-mail: michael.grever@osumc.edu.
References
Author notes
*M.A.P. and T.S.L. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal