Abstract
Multisubunit complexes containing molecular chaperones regulate protein production, stability, and degradation in virtually every cell type. We are beginning to recognize how generalized and tissue-specific chaperones regulate specialized aspects of erythropoiesis. For example, chaperones intersect with erythropoietin signaling pathways to protect erythroid precursors against apoptosis. Molecular chaperones also participate in hemoglobin synthesis, both directly and indirectly. Current knowledge in these areas only scratches the surface of what is to be learned. Improved understanding of how molecular chaperones regulate erythropoietic development and hemoglobin homeostasis should identify biochemical pathways amenable to pharmacologic manipulation in a variety of red blood cell disorders including thalassemia and other anemias associated with hemoglobin instability.
Introduction
Erythropoiesis epitomizes highly specialized cellular differentiation and gene expression. The major role of red blood cells is to deliver oxygen from pulmonary venous capillary beds to peripheral tissues. To streamline their functional capacity, erythrocyte precursors shed most organelles and produce prodigious amounts of hemoglobin, which eventually comprises approximately 95% of the total cellular protein. Erythropoiesis is regulated in part by the concerted actions of cytokine signaling pathways and transcription factors. However, many specialized aspects of mammalian erythroid development are regulated posttranscriptionally, especially at the final stages, which occur in the absence of a nucleus. Approximately 20 years ago, several groups noted that the molecular chaperone Hsp70 accumulates to high levels in erythroid precursors.1-5 Accordingly, investigators speculated that Hsp70 and related chaperones, proteins that regulate the folding, degradation, and activities of other proteins, might have specialized functions in streamlining erythroid maturation.2
Molecular chaperones are defined as a diverse group of proteins that guide the folding and assembly of other proteins, but are not associated with the functional end product.6 The general structure, biochemistry, and nomenclature of molecular chaperones are described in numerous reviews (see Frydman6 ; Hartl and Hayer-Hartl7 ; Liberek et al8 ; Saibil9 ; and Young et al10 for recent examples). Major classes of molecular chaperones are named according to how they were discovered. Thus, heat shock proteins (Hsps) are induced by increased temperature and other stresses. Examples include Hsp40, Hsp60, Hsp70, Hsp90, Hsp110, and small Hsps, each of which represent distinct protein families named according to their molecular mass. Most chaperones function within multiprotein complexes, termed “chaperone machinery,” which contain cochaperones and accessory proteins that modulate functional activities. The Hsps are also expressed at basal levels and exert important functions even in the absence of stress. In addition, Hsp homologues, termed heat shock cognate proteins (Hscs), are expressed constitutively at relatively high levels and have essential housekeeping roles. Molecular chaperones are conserved throughout evolution and there is some overlap of nomenclature based on different modes of discovery. For example, DnaK and DnaJ, orthologues of Hsp70 and Hsp40, respectively, were identified as proteins that are essential for bacteriophage DNA replication in Escherichia coli.
Multifunctional activities of molecular chaperones
Molecular chaperones bind substrate or “client” proteins to modulate their structural integrity and activities through several distinct mechanisms:
Molecular chaperones bind partially folded proteins to prevent their irreversible denaturation and aggregation. This role is exerted at 2 different stages in the lifetime of many proteins. First, chaperones help newly synthesized proteins to achieve their native functional state, either during or shortly after translation. This is particularly important for large multidomain proteins and for the assembly of multiprotein complexes. Second, chaperones help denatured proteins to refold as a protective mechanism against damage caused by various cellular stresses including heat and nutrient deprivation. In higher organisms, distinct networks of molecular chaperone families regulate these 2 major functions.11
In metazoans, chaperones maintain the solubility of denatured proteins, but aggregated proteins can be eliminated only through degradation. However, yeast, bacteria, and fungi express a protein-remodeling factor named Hsp104, which cooperates with other chaperones to dissolve protein aggregates and restore their functional activities.12
Molecular chaperones can facilitate degradation of denatured proteins. For example, the E3 ubiquitin ligase CHIP (carboxyl terminus of Hsc70-interacting protein), recognizes chaperone-bound unfolded substrates and targets them for degradation.13-15 The DnaJ-type chaperone HSJ1 contains ubiquitin-interacting motifs that bind ubiquitinated proteins and direct them to proteasomes.16 Through this mechanism, HSJ1 may protect neurons against toxic protein aggregation.
Protein misfolding, precipitation, and aggregation threaten all cells, particularly those under environmental or metabolic stress. The ability of chaperones to alleviate this problem by recognizing and either repairing or eliminating damaged polypeptides is termed protein quality control.16-18 Diseases of protein precipitation ensue when levels of unstable polypeptides exceed the protective capacity of resident chaperones in target tissues. Examples include neurodegenerative disorders, cataracts, the major form of cystic fibrosis, and myopathies.19-22 In the thalassemia syndromes, synthetic imbalance of globin chains leads to the formation of toxic protein precipitates in erythroid cells.23,24 In this way, the thalassemias are largely disorders of unstable protein accumulation and, therefore, potentially subject to modulation by protein quality control pathways.
In addition to interacting with denatured proteins, molecular chaperones bind many native folded proteins to modulate their cellular activities. For example, Hsp70- and Hsp90-containing chaperone machines regulate not only folding of nascent glucocorticoid receptor but also its subsequent translocation to the nucleus after ligand binding.25
Molecular chaperone machineries are ubiquitously expressed and influence virtually all aspects of normal cellular function including signaling, transcription, apoptosis, and cell division. In addition, chaperone-client protein interactions are involved in disorders of protein precipitation (discussed earlier), cancer,26 inflammation,27 and viral infections.28 Accordingly, chaperones are important drug discovery targets.29,30
Of particular interest to erythroid biology, molecular chaperones play important roles in the pathogenesis of malaria.31-33 The genomes of Plasmodia species encode a diverse complement of molecular chaperones, which are expressed abundantly and are believed to provide at least 2 essential functions. The first function is to protect the parasite against heat shock stresses that occur after transmission from the poikilothermic mosquito vector to humans and also during repeated febrile episodes in infected patients. The second function is to help remodel host erythrocyte cellular machinery for optimal parasite replication and evasion of immune clearance. For these reasons, drugs that target malarial chaperones represent promising therapeutic approaches.34-36
Molecular chaperones in erythroid development
Given the ubiquitous distribution and function of molecular chaperones, these proteins are likely to participate in red blood cell development. Several unique features of erythroid maturation also support this concept. For example, erythroid precursors are particularly susceptible to impaired protein synthesis, as evidenced by Diamond Blackfan anemia (DBA), a disorder in which haploinsufficiency of large or small ribosomal protein subunits leads to selective red cell aplasia.37 Molecular chaperone activity is likely to fine-tune and optimize protein synthesis in immature erythroblasts, the developmental stage at which DBA defects are manifested. Later in erythropoiesis, at the reticulocyte stage, chaperone complexes are present at high levels and equipped to facilitate 2 hallmarks of maturation: high-level hemoglobin synthesis and degradation of unnecessary proteins. Emerging evidence supports these predictions by indicating that molecular chaperones modulate numerous aspects of early and late erythropoiesis.
Hsp70 regulates erythroblast viability
Healthy maturing erythroblasts exhibit some features of apoptosis including mitochondrial depolarization and transient activation of caspases.38-41 In vitro, caspase inhibitors or depletion of caspase 3 by small interfering RNAs (siRNAs) inhibit erythroid maturation. However, these same precursors die upon further activation of apoptotic pathways, for example through stimulation of the Fas receptor or deprivation of erythropoietin (Epo).42-44 Indeed, these mechanisms are used physiologically to prevent excessive erythrocyte production. Several studies elucidate how erythroblast survival is regulated during caspase activation and other apoptotic stimuli. One pathway involves interplay between Epo, Hsp70, and the essential transcription factor GATA-1. In human erythroid cells, activated caspases can cleave GATA-1 leading to maturation arrest and/or apoptosis.43 Ribeil et al showed that in cultured erythroblasts Epo causes Hsp70 to enter the nucleus, bind GATA-1, and protect it from degradation by caspase 3.45 Conversely, during Epo deprivation, Hsp70 is excluded from the nucleus and GATA-1 is cleaved by caspase 3, causing apoptotic death. Thus, Hsp70 appears to play a critical role in erythroblast viability by interacting with an essential transcription factor. There are several outstanding questions. For example, how does Epo signaling regulate subcellular compartmentalization of Hsp70 and by what mechanisms does Hsp70 protect GATA1 from caspase cleavage?
Physiologic activation of caspases during erythropoiesis may be triggered by transient mitochondrial depolarization, which releases procaspases and the caspase activator, cytochrome c.38,40 This process also releases mitochondrial apoptosis-inducing factor (AIF), which can translocate to the nucleus and induce caspase-independent DNA fragmentation, triggering full-blown apoptotic death.46,47 Lui et al showed that in human TF-1 cells, which recapitulate some aspects of erythroid maturation, Epo induces mitochondrial depolarization with release of AIF.38 Concurrently, Hsp70 is up-regulated, binds AIF and sequesters it in the cytosol to limit apoptotic activity. Other groups have demonstrated that Hsp70 can inhibit apoptosis by inhibiting the nuclear localization of AIF in a variety of cells48-50 ; the study by Liu et al extends this finding to erythroblasts. Of note, in this experimental system, Hsp70 was not observed in the nucleus, which contrasts with observations of primary culture erythroblasts reported by Ribeil et al. This could reflect different stages of erythroid maturation examined in the 2 experimental systems or limitations in the extent to which TF-1 cells reflect normal erythropoiesis.
A mitochondrial chaperone promotes erythropoiesis
Forward genetic screens in zebrafish (Danio rerio) have identified numerous genes that participate in blood development. Craven et al used this approach to show that the hspa9b gene, which encodes a conserved membrane-bound Hsp70-like protein, is important for erythropoiesis.51 This ubiquitous chaperone (also called mortalin/mthsp70/Grp75/mot-2/PBP74) is expressed in mitochondria where it is believed to participate in the transport, folding, and assembly of matrix proteins.52
HSPA9B protein is required for viability in yeast.53 Its overexpression increases longevity in Caenorhabditis elegans and in human fibroblasts.54,55 In zebrafish, homozygosity for a missense mutation in the substrate binding domain of hsp9b (most likely a null allele) selectively impairs the development of erythrocytes, granulocytes, and hematopoietic progenitors.51 These deleterious effects probably result from mitochondrial dysfunction and increased oxidative stress. Of note, abnormalities observed in the hspa9b mutant fish resemble those of human myelodysplastic syndrome (MDS). These findings underscore the importance of mitochondrial function in hematopoiesis, including erythroid development and provide an animal model for human MDS. In erythroid cells, Epo stimulates hspa9b/mortalin mRNA and protein expression via the downstream effector phosphatidylinositol 3-kinase.56
The ability of Epo to stimulate nuclear translocation of Hsp70 and induce hspa9b expression illustrates 2 specialized, distinct mechanisms through which the cytokine may promote viability and/or proliferation of erythroid precursors via functional relationships with molecular chaperones. It is also worth noting that molecular chaperones, especially Hsp90-associated machinery, modulate the folding and functions of many protein kinases involved in signal transduction.57,58 These general activities likely promote erythropoiesis by supporting essential cytokine signaling pathways, including those involving Epo and stem cell factor (SCF, Kit ligand).
Molecular chaperones and hemoglobin production
During their terminal maturation, erythroblasts produce large amounts of hemoglobin and eventually expel their nucleus, degrade other organelles, and eliminate unnecessary proteins. Hemoglobin is a heterotetramer composed of 2 α globin and 2 β globin polypeptide subunits, each bound to a heme prosthetic group.
Heme is a tetrapyrrole that contains a single central iron molecule, which binds oxygen reversibly. Several challenges accompany the high-level production of hemoglobin, which reaches a concentration of approximately 35 g/dL (350 g/L; 350 mg/mL) in mature red blood cells. First, there is the general problem of macromolecular crowding. High protein concentrations and compartmentalization within cells can cause excluded volume effects that alter protein folding and promote aggregation.6 Second, hemoglobin synthesis and assembly must be exquisitely regulated to avoid accumulation of individual components, which are cytotoxic. This is evidenced by various genetic disorders in which unbalanced hemoglobin production results in accumulation of excessive free globin proteins, heme, or its synthetic intermediates, all of which damage erythroid cells. There is emerging evidence that molecular chaperones participate both directly and indirectly in protein folding and subunit balance during hemoglobin synthesis.
Balancing hemoglobin
How α and β globin synthesis are balanced is largely unknown. The complexity of this problem is illustrated by observations that the α and β globin gene clusters reside within very different chromatin environments and are likely to be regulated differently.59,60
However, several mechanisms are known to help coordinate the production of globins with heme. This is important because apo globin subunits (the heme-free form) are largely unstructured and must be folded either during or soon after synthesis to remain competent for hemoglobin assembly. Binding to heme increases the solubility of globin chains by stabilizing folding intermediates, particularly for the α subunit.61-68 Indeed, some studies indicate that heme binds nascent globin chains cotranslationally to fold and stabilize the emerging polypeptide.69,70 In the absence of heme, apo-globin subunits precipitate more readily.
Therefore, it is advantageous for erythroid cells to decrease globin production when heme is limiting and there are at least 2 mechanisms for this. Bach I is a nuclear repressor that inhibits both α and β globin gene transcription when heme concentration is low.71-73 Heme regulated inhibitor of translation (HRI) is a cytosolic heme-binding protein that represses globin translation in erythroid precursors during heme deficiency.74
HRI is a member of a small family of protein kinases that inhibit translation by phosphorylating the α subunit of eukaryotic translational initiation factor 2 (eIF2α). HRI kinase activity is inhibited by its binding to heme. In this way, abundant heme promotes translation of globin mRNA. Conversely, this provides an important adaptation mechanism to prevent the accumulation and precipitation of unstable apo globins when heme is limiting. Mice lacking HRI are generally healthy at baseline. However, loss of HRI exacerbates the anemias associated with iron deficiency, erythropoietic porphyria, and thalassemia by causing increased accumulation of precipitated apo globin proteins.75-77 Interestingly, heat shock, high osmolarity, and oxidant exposure all activate HRI to inhibit translation independent of heme status.78
Through all of these mechanisms, HRI participates in protein quality control during a variety of genetic and environmental erythropoietic stresses.
HRI engages in several important interactions with molecular chaperone machinery.79-85 For example, Hsp90- and Hsc70-containing complexes bind nascent HRI cotranslationally to facilitate its folding. These interactions are required for the production of a functionally competent HRI kinase. Moreover, Hsc70 can bind native-folded HRI to modulate its activity. Addition of purified Hsc70 to HRI reduces its kinase activity during heme deficiency or heat shock. In reticulocyte lysates exposed to similar stresses, pharmacologic disruption of the Hsc70-HRI interaction enhances HRI kinase activity and eIF2α phosphorylation. Thus, Hsc70 appears to attenuate the ability of mature kinase-competent HRI to inhibit translation.
Biologic consequences of unbalanced globin synthesis
The deleterious effects of unbalanced globin synthesis are evidenced by the thalassemias, a group of common inherited anemias in which mutations in α or β globin genes inhibit production of the corresponding protein, resulting in buildup of the unaffected subunit.23,86 In β thalassemia, β globin gene mutations cause holo α globin subunits to accumulate. This monomeric subunit tends to produce damaging reactive oxygen species (ROS) via chemical reactions catalyzed by the heme-bound iron.87 In addition, free holo α globin is inherently unstable and either precipitates within the plasma membrane or denatures, resulting in damage to cells. Alpha thalassemia produces similar effects whereby holo β globin accumulates and damages cells. However, free α globin is more unstable than free β globin, in part because the latter has a greater tendency to self-associate into homo tetramers, which impart some structural stability.88
Several lines of evidence suggest that erythroid cells have adaptive mechanisms to cope with free α globin. First, erythroid precursors contain a small pool of excess free α globin that is well tolerated.89,90 Second, individuals with β globin gene haploinsufficiency (β thalassemia trait) usually exhibit minimal erythroid pathology, despite approximately 50% reduced β globin gene output.91 Excess free α globin is degraded in erythroid precursors through poorly defined proteolytic mechanisms, some of which involve ubiquitinylation.92-94 Together, these observations indicate that protein quality control mechanisms using molecular chaperone machinery help to maintain globin chain balance and homeostasis.
Recent studies demonstrate that protein quality control occurs within at least 2 distinct compartments in eukaryotic cells.95 Thus, soluble ubiquitinated misfolded proteins colocalize with proteasome components in a juxtanuclear region. Terminally aggregated proteins, such as those associated with amyloid deposits in neuronal disorders, are sequestered in perivacuolar inclusions, perhaps to facilitate clearance through proteasome-independent mechanisms. Along these lines, it will be interesting to determine how erythroblasts partition unstable globin chains in thalassemias and other hemoglobinopathies.
An erythroid-specific molecular chaperone for α globin
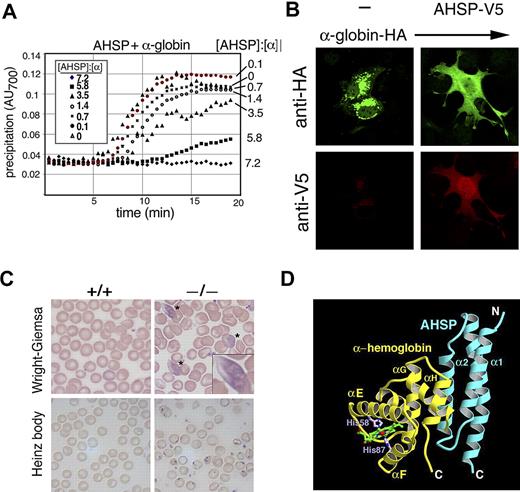
Our laboratory identified α hemoglobin–stabilizing protein (AHSP), a small erythroid protein that specifically binds free α globin, maintains its native folded structure, and inhibits its ability to catalyze the production of ROS. Recombinant AHSP stabilizes free α globin in E coli,96 in solution (Figure 1A) and in nonerythroid cells97 (Figure 1B). In mice, ablation of the Ahsp gene causes mild hemolytic anemia with hemoglobin precipitation97,98 (Figure 1C). In human erythroleukemia cells, reduction of AHSP siRNA causes α globin precipitation and apoptosis.99 Together, these data demonstrate that AHSP is important for hemoglobin stability during erythroid development.
Biologic and biochemical features of AHSP, a molecular chaperone for a globin. (A) AHSP prevents oxidant-induced α hemoglobin precipitation in solution. Recombinant human AHSP was preincubated with purified oxygenated (oxy) α globin at the indicated molar ratios for 60 minutes. Potassium ferricyanide (50 μM final concentration) was added at time 0 to induce heme oxidation. Protein precipitation was monitored by light scattering at 700 nm. Reproduced from Kihm et al.97 (B) AHSP-α hemoglobin interactions in mammalian cells. COS cells expressing HA-α hemoglobin and V5-AHSP, either separately or in combination, were stained with antibodies against V5 and HA and analyzed by indirect immunofluorescence. Original magnification ×400. Reproduced from Kihm et al.97 (C) Ahsp−/− erythrocytes exhibit abnormal morphology and hemoglobin precipitates (Heinz bodies). Wright-Giemsa stains of mutant erythrocytes (top panels) show morphologic abnormalities and inclusion bodies, designated by * and magnified in the inset. Heinz body stain, which detects denatured globin chains (bottom panels), is positive in Ahsp−/− cells. Photographs were taken using an Axioskop 2 microscope equipped with an Axiocam digital camera (Carl Zeiss). Images were processed using Axiovision Product Suite (Carl Zeiss) and Photoshop (Adobe, San Jose, CA). Original magnifications: panel B, 1000×; panel C, 400×. From Kong et al.98 (D) Structure of oxidized (FeIII) holo α globin bound to AHSP. α globin and AHSP are colored yellow and blue, respectively. The heme group is shown in green and His58 and His87 are in purple. The α globin heme iron (red sphere) is in a bis-histidyl conformation, bound by His58 and His87 (shown in purple). Adapted from Feng et al.102
Biologic and biochemical features of AHSP, a molecular chaperone for a globin. (A) AHSP prevents oxidant-induced α hemoglobin precipitation in solution. Recombinant human AHSP was preincubated with purified oxygenated (oxy) α globin at the indicated molar ratios for 60 minutes. Potassium ferricyanide (50 μM final concentration) was added at time 0 to induce heme oxidation. Protein precipitation was monitored by light scattering at 700 nm. Reproduced from Kihm et al.97 (B) AHSP-α hemoglobin interactions in mammalian cells. COS cells expressing HA-α hemoglobin and V5-AHSP, either separately or in combination, were stained with antibodies against V5 and HA and analyzed by indirect immunofluorescence. Original magnification ×400. Reproduced from Kihm et al.97 (C) Ahsp−/− erythrocytes exhibit abnormal morphology and hemoglobin precipitates (Heinz bodies). Wright-Giemsa stains of mutant erythrocytes (top panels) show morphologic abnormalities and inclusion bodies, designated by * and magnified in the inset. Heinz body stain, which detects denatured globin chains (bottom panels), is positive in Ahsp−/− cells. Photographs were taken using an Axioskop 2 microscope equipped with an Axiocam digital camera (Carl Zeiss). Images were processed using Axiovision Product Suite (Carl Zeiss) and Photoshop (Adobe, San Jose, CA). Original magnifications: panel B, 1000×; panel C, 400×. From Kong et al.98 (D) Structure of oxidized (FeIII) holo α globin bound to AHSP. α globin and AHSP are colored yellow and blue, respectively. The heme group is shown in green and His58 and His87 are in purple. The α globin heme iron (red sphere) is in a bis-histidyl conformation, bound by His58 and His87 (shown in purple). Adapted from Feng et al.102
AHSP forms an elongated 3 α helix bundle that binds α globin on the opposite side of the heme pocket to form a simple heterodimer (Figure 1D).100-103 The AHSP binding surface of α globin overlaps with the α1β1 interface for β globin binding. Biochemical studies using purified proteins show that AHSP competes with β globin for binding to α globin. However, β globin binds α globin much more tightly than AHSP. Thus, addition of equimolar β globin to oxygenated (oxy) α globin-AHSP dimer results in the formation of hemoglobin A tetramer and free AHSP.97,104,105 These findings indicate that α globin-AHSP complexes could exist as intermediates during hemoglobin A synthesis in vivo. Biochemical studies support this possibility, although it has been difficult to purify large amounts of α globin-AHSP complexes from erythroid cells, presumably due to their relatively low concentration and transient nature. AHSP binds multiple forms of α globin including apo and holo with the heme bound iron either oxidized (ferric, Fe[III]) or reduced (ferrous, Fe[II]). We hypothesize that the ability of AHSP to interact with multiple forms of α globin reflects different functions that predominate under different circumstances during normal or pathologic erythropoiesis.
Our studies indicate that AHSP has at least 2 related but biochemically distinct functions. First, AHSP acts as a molecular chaperone to facilitate the structural integrity of α globin en route to hemoglobin A synthesis.106 In murine reticulocytes, newly formed α globin is destabilized by loss of AHSP. In vitro, AHSP helps nascent apo α globin to fold and promotes the refolding of denatured apo α globin, 2 hallmark features of a molecular chaperone. Stabilization of native folded apo α globin by AHSP may be particularly important when heme is limiting, for example, during iron deficiency. Indeed, the presence of a functional iron response element (IRE) in the 3′ untranslated region of human AHSP mRNA stabilizes the transcript under low iron conditions.107
A second function of AHSP is to detoxify excess α globin. This is indicated by our observation that loss of AHSP increases the damaging effects of free α globin during β thalassemia in mice.98 In vitro studies also support this function and suggest a potential mechanism. In solution, AHSP can pass oxy α globin off to β globin to form functional hemoglobin A. However, in the absence of β globin, AHSP induces structural alterations to bound oxy α globin. Specifically, ·O2− anion (oxygen plus an extra electron) is lost in a process termed autoxidation, resulting in the formation of deoxygenated ferric (FeIII) heme. Rapidly thereafter, the heme iron becomes liganded on both sides of the porphyrin ring by histidines E7 and F8 of the α globin polypeptide.102,103,105
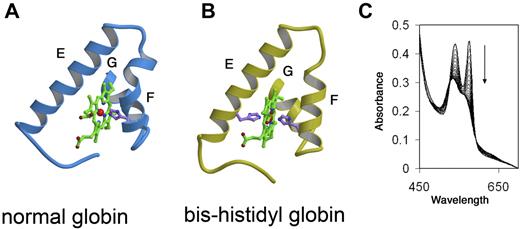
This contrasts with normal holo α globin where the heme iron is bound only by histidine F8 with the sixth coordinate position open for reversible oxygen binding (Figure 2). The hexacoordinate, or “bis-histidyl” structure conferred to α globin by bound AHSP is relatively nonreactive because heme iron is fully coordinated and therefore unable to catalyze redox reactions involved in ROS production. Formation of bis-histidyl α globin-AHSP is favored during low oxygen concentration, oxidant exposure, and β globin deficiency.102,103,105 This structure may represent a protective mechanism that is particularly important during various stresses including β thalassemia and pro-oxidant states. However, the bis-histidyl-α globin AHSP complex has not been shown to exist in vivo and further studies are required to determine its biologic significance. Recently, we created several missense AHSP mutations that specifically inhibit its ability generate bis-histidyl α globin-AHSP, which should facilitate future investigations (Joel Mackay, Yigong Shi, M.J.W., and Andrew Gow, unpublished, October 2008).
AHSP-induced alterations to the heme pocket and iron coordination of αHb. (A) The heme pocket of normal globin subunits, including oxy αHb. Helices E and F, which line the distal and proximal aspects of the planar heme structure, respectively, are indicated. Residues within helix G, located posteriorly in the diagram, participate in heme binding. Iron is indicated as a small sphere in the center of heme. The proximal HisF8 is shown bound to iron. The distal histidine E7 is not bound to iron and not shown. Oxygen, not shown, binds iron at the distal surface of heme, which is opposed by helix E. (B) The bis-histidyl globin, which is generated in holo α globin-AHSP complex. Helices E and F are distorted and both proximal His F8 and distal His E7 bind the heme iron. (C) Spectrophotometric changes reflecting conversion of AHSP-bound αHb from the oxygenated ferrous form to the ferric bis-histidyl form shown in panel B. The reaction was performed at 37°C. Tracings were recorded every 2 minutes with the direction of change over time indicated by. Adapted from Weiss et al.108
AHSP-induced alterations to the heme pocket and iron coordination of αHb. (A) The heme pocket of normal globin subunits, including oxy αHb. Helices E and F, which line the distal and proximal aspects of the planar heme structure, respectively, are indicated. Residues within helix G, located posteriorly in the diagram, participate in heme binding. Iron is indicated as a small sphere in the center of heme. The proximal HisF8 is shown bound to iron. The distal histidine E7 is not bound to iron and not shown. Oxygen, not shown, binds iron at the distal surface of heme, which is opposed by helix E. (B) The bis-histidyl globin, which is generated in holo α globin-AHSP complex. Helices E and F are distorted and both proximal His F8 and distal His E7 bind the heme iron. (C) Spectrophotometric changes reflecting conversion of AHSP-bound αHb from the oxygenated ferrous form to the ferric bis-histidyl form shown in panel B. The reaction was performed at 37°C. Tracings were recorded every 2 minutes with the direction of change over time indicated by. Adapted from Weiss et al.108
Many general molecular chaperones recognize exposed hydrophobic stretches of unfolded proteins that would be sequestered from solvent in the natively folded state. In contrast, AHSP binds specifically to a unique helix-loop-helix fold on a surface of α globin that also interacts with β globin. Indeed, α globin is the only known client protein for the chaperone activities of AHSP. In this regard, AHSP can be considered a “private” chaperone. Similarly, single protein-specific molecular chaperones function in the assembly of tubulin109,110 and collagen,111,112 other multisubunit proteins that are produced at relatively high levels in cells. The AHSP gene has been identified only in mammals, including eutherians and marsupials. No evidence for AHSP homologues has been found in other tetrapods such as chickens, lizards, or amphibians. Thus, AHSP has appeared relatively recently in evolution, most likely as a new mechanism to fine-tune hemoglobin production and homeostasis.
The role of AHSP in human disease remains an open question. Some naturally occurring missense mutations that render α globin unstable alter the binding surface for AHSP and inhibit the protein interaction.113-115 This suggests that failure to interact with AHSP may inhibit human α globin stability in vivo, supporting our model that AHSP acts as a molecular chaperone for hemoglobin synthesis. However, one caveat is that the AHSP binding surface on α globin overlaps with the α1β1 interface.103 Therefore, it will be important to determine whether α globin mutations reported to affect AHSP binding also inhibit β globin binding, which could represent an alternate mechanism for destabilization.
Naturally occurring mutations that ablate AHSP expression or alter the protein structure are rare,116,117 although several studies indicate that AHSP expression varies significantly between different individuals.118-120 Some of this variation may be caused by a variable length T homopolymer in the AHSP promoter region and/or by an intron 1 single nucleotide polymorphism (SNP) that inhibits binding of the transcriptional activator Oct-1.116,119,121
Quantitative differences in AHSP expression could influence susceptibility to erythropoietic stresses. Indeed, reduced AHSP expression has been linked to increased severity of several disorders including drug- and infection-induced oxidant stress,122 abnormal pregnancies,123 and β thalassemia.108,119,124-126 However, most of these studies are preliminary, and causal relationships between decreased AHSP expression and severity of these disorders have not been established unequivocally.
Summary and future outlook
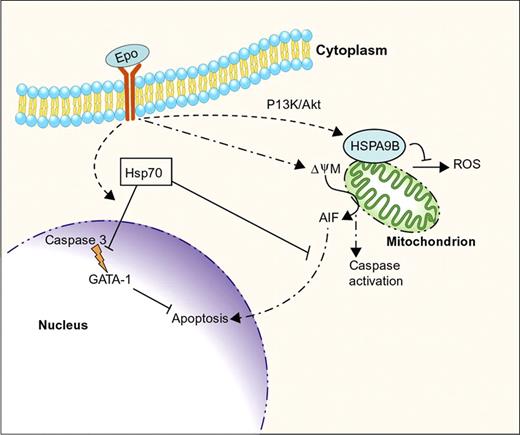
Current evidence suggests that molecular chaperones regulate at least 2 specialized areas of erythropoiesis. First, Hsp70 and the related protein HSPA9B/mortalin help to maintain cell survival in the face of apoptotic stimuli that are, somewhat paradoxically, required for normal erythropoiesis (Figure 3). Regulated apoptosis is an important mechanism for modulating red cell production according to physiologic needs. In this regard, it is interesting that Epo, the most important survival factor for erythropoietic development, positively influences Hsp70 and HSP9B activities and/or expression to enhance cell survival. These examples illustrate mechanisms through which Epo promotes red blood cell production. Second, molecular chaperones regulate hemoglobin homeostasis, a critical aspect of erythrocyte production and function (Figure 4). Hsp70 and Hsp90 are required to generate active HRI, which in turn, balances globin synthesis with heme availability. AHSP acts as a private chaperone for α globin.
Intersection of erythropoietin signaling, apoptotic pathways, and molecular chaperones during erythropoiesis. Erythropoietin (Epo) promotes erythroblast survival and maturation. Caspase activation, initiated in part through Epo-induced mitochondrial depolarization (ψΔΜ), is essential for erythroid maturation. Hsp70 inhibits apoptosis by binding cytoplasmic apoptosis initiating factor (AIF), inhibiting its accumulation in the nucleus. In addition, Epo signaling causes Hsp70 to translocate to the nucleus and bind the essential transcription factor GATA-1 to inhibit its cleavage by activated caspase 3. Erythropoietin signaling also induces expression of HSPA9B/mortalin, a mitochondrial Hsp70-like protein that inhibits apoptosis, in part by suppressing the production of reactive oxygen species (ROS). Professional illustration by Paulette Dennis.
Intersection of erythropoietin signaling, apoptotic pathways, and molecular chaperones during erythropoiesis. Erythropoietin (Epo) promotes erythroblast survival and maturation. Caspase activation, initiated in part through Epo-induced mitochondrial depolarization (ψΔΜ), is essential for erythroid maturation. Hsp70 inhibits apoptosis by binding cytoplasmic apoptosis initiating factor (AIF), inhibiting its accumulation in the nucleus. In addition, Epo signaling causes Hsp70 to translocate to the nucleus and bind the essential transcription factor GATA-1 to inhibit its cleavage by activated caspase 3. Erythropoietin signaling also induces expression of HSPA9B/mortalin, a mitochondrial Hsp70-like protein that inhibits apoptosis, in part by suppressing the production of reactive oxygen species (ROS). Professional illustration by Paulette Dennis.
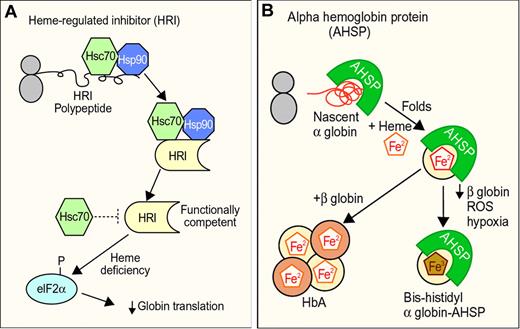
Molecular chaperones and hemoglobin synthesis. (A) Hsp90 and Hsp70 are required for the biogenesis of functional heme regulated inhibitor kinase (HRI), a protein that regulates globin synthesis according to heme availability. In the absence of heme or during other erythroid stresses, HRI phosphorylates translational initiation factor eIF2a, which blocks globin synthesis. Hsc70 may also negatively regulate HRI activity during heme deficiency. (B) Activities of alpha hemoglobin stabilizing protein (AHSP). AHSP binds nascent apo α globin, facilitates its folding, and promotes its incorporation into hemoglobin A (HbA). During β globin deficiency, exposure to ROS or hypoxia, AHSP generates a more stable “bis-histidyl” form of α globin in which the heme iron is oxidized and liganded by 2 histidines within the globin polypeptide (Figure 2). The biologic functions of the AHSP-bis-histidyl α globin complex are unknown. Professional illustration by Paulette Dennis.
Molecular chaperones and hemoglobin synthesis. (A) Hsp90 and Hsp70 are required for the biogenesis of functional heme regulated inhibitor kinase (HRI), a protein that regulates globin synthesis according to heme availability. In the absence of heme or during other erythroid stresses, HRI phosphorylates translational initiation factor eIF2a, which blocks globin synthesis. Hsc70 may also negatively regulate HRI activity during heme deficiency. (B) Activities of alpha hemoglobin stabilizing protein (AHSP). AHSP binds nascent apo α globin, facilitates its folding, and promotes its incorporation into hemoglobin A (HbA). During β globin deficiency, exposure to ROS or hypoxia, AHSP generates a more stable “bis-histidyl” form of α globin in which the heme iron is oxidized and liganded by 2 histidines within the globin polypeptide (Figure 2). The biologic functions of the AHSP-bis-histidyl α globin complex are unknown. Professional illustration by Paulette Dennis.
We are only beginning to understand how molecular chaperones and protein quality control regulate specialized aspects of erythropoiesis and there are many outstanding questions of relevance to basic biology and medicine. For example, the full extent to which molecular chaperones participate in normal hemoglobin production is not fully defined. It will be interesting to determine whether AHSP interacts with generalized cellular chaperones and/or proteolytic pathways to modulate α globin folding, degradation, or assembly with heme and β globin. Regulated proteolysis is an important part of normal erythroid development, particularly during the late stages as reticulocytes streamline their protein repertoire to consist mainly of hemoglobin. Molecular chaperones likely take part in this process, as predicted by Banerji et al more than 20 years ago.2 However, the specific molecules and pathways are unknown. Several candidate proteins are worthy of future investigation. For example CHIP is a ubiquitin ligase that helps guide selected chaperone-bound proteins to proteasomes.13-15 Erythroid-specific ubiquitin-conjugating enzymes have been identified, but nothing is known about their functions or interactions with chaperones as part of protein quality control or cellular maturation.127 It is also possible that erythroblasts eliminate unwanted soluble proteins by chaperone-mediated autophagy, a process in which molecular chaperones guide proteins into lysosomes for subsequent degradation.128 A different form of autophagy is essential for eliminating mitochondria during maturation of reticulocytes,129-131 underscoring the importance of lysosomal activity during late erythropoiesis.
Protein quality control is also likely to be an important adaptive mechanism in the thalassemia syndromes where toxic accumulation of the unaffected globin subunit is believed to be mitigated by proteolysis. For example, it is possible that AHSP-mediated conversion of excess holo α globin to the bis-histidyl heme form marks the protein for degradation. One candidate ubiquitin ligase is HOIL-1, which is reported to recognize oxidized heme in the context of iron regulatory protein 2 (IRP2), targeting it for degradation,132,133 although this finding is controversial.134
Alteration of protein homeostasis networks by pharmacologic manipulation of chaperone machinery and protein degradation pathways represents an exciting new therapeutic approach for numerous diseases.17,34-36,135,136 One extensively explored area relates to the treatment of neurodegenerative disorders caused by accumulation and/or aggregation of toxic proteins. Modulation of molecular chaperone activity in neurons by small molecules or gene transfer can suppress aggregation and stimulate degradation of the offending protein, enhancing neuronal survival in cultured cells and in whole organisms.22,137-145 It may even be possible to dissolve protein aggregates in mammalian cells by ectopic expression of the Hsp 104, which is normally expressed only in lower organisms.146 Similar approaches should be applicable to the treatment of erythroid disorders such as thalassemia where accumulation and precipitation of unstable globin proteins play a predominant role in disease pathophysiology.
Acknowledgments
We thank Yair Argon, Ron Rubenstein, Olivier Hermine, and Jim Shorter for comments on the paper.
Our work on AHSP is supported by the Cooley's Anemia Foundation (New York, NY), The National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (Bethesda, MD), and the American Heart Association (Dallas, TX). M.J.W. is a Leukemia & Lymphoma Society (White Plains, NY) Scholar. C.O.S. is an American Heart Association postdoctoral fellow.
National Institutes of Health
Authorship
Contribution: M.J.W. and C.O.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mitchell J. Weiss, Division of Hematology 3615 Civic Center Boulevard, Abramson Research Center, Room 316B, Philadelphia, PA 19104; e-mail: weissmi@email.chop.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal