Abstract
Plasmacytoid predendritic cells (pDCs) play a key role in antiviral immunity through their capacity to produce large amounts of type I interferons in response to Toll-like receptor triggering, and to differentiate into dendritic cells (DCs). However, their antigen processing and presentation pathways remain poorly characterized. In this study, we analyzed major histocompatibility complex class II (MHC II) synthesis and transport in primary human pDCs. We show that stimulation of pDCs with influenza virus leads to a sustained neosynthesis of MHC II molecules, which rapidly accumulate in antigen loading compartments organized around the microtubule organization center. MHC II endocytosis as well as antigen internalization remain active during the entire process of pDC differentiation into DCs, suggesting a capacity to constantly renew surface peptide–MHC II complexes. Formation of the intracellular pool of MHC II in activated pDCs is nuclear factor-κB–dependent and associated with acquisition of a dendritic phenotype, but independent of the IRF7-type I interferon-dependent pathway, suggesting that innate and adaptive functions of pDCs are differentially regulated. Our data demonstrate that the regulation of MHC II expression and transport is drastically different in pDCs compared with conventional DCs, indicating distinct and potentially complementary immunoregulatory functions.
Introduction
Human peripheral blood contains 2 main populations of dendritic cells (DCs): the “conventional” DCs (cDCs), and the plasmacytoid predendritic cells (pDCs).1 These 2 subsets express different sets of Toll-like receptors (TLR) and differentially respond to TLR ligands, indicating that they display nonredundant functions.2 pDCs play a central role in the immune system by linking the innate with the adaptive immune responses.3 First, pDCs rapidly produce large amounts of type I interferons (IFNs) after microbial stimulation.4,5 pDC activation occurs through the recognition of viral nucleic acids (single-stranded RNA) by TLR7, and viral and bacterial DNA by TLR9.6 Second, depending on the stimuli, pDCs can differentiate into cells with a dendritic phenotype.3,7 This differentiation is accompanied by a strong up-regulation of the surface expression of major histocompatibility complex class I (MHC I) and class II (MHC II), as well as T-cell costimulatory molecules, suggesting that they become potent antigen-presenting cells.3,7,8 However, the capacity of pDCs to act as antigen-presenting cells remains controversial. Depending on the stimuli,3,9,10 the source of pDCs,11,12 the way the antigen is internalized,13,14 and the dose of antigen,12 pDCs are able or not to activate naive CD4 T cells. It has also been proposed that pDCs are endowed with tolerogenic capacities by inducing regulatory T cells producing interleukin-10 (IL-10) both in vitro15-20 and in vivo.21,22
It is well established that antigen presentation by MHC II heavily depends on their intracellular traffic. The biosynthetic route followed by nascent MHC II has been well characterized in various antigen-presenting cells.23 MHC II are synthesized in the endoplasmic reticulum (ER) where they associate with the chaperone invariant chain (Ii) to form αβIi complexes. Such complexes exit from the ER and traffic through the Golgi apparatus to most probably reach the plasma membrane where they are rapidly internalized into endosomes. There, Ii is degraded, allowing MHC II to acquire their peptide load. MHC II peptide-loaded complexes are then exported to the cell surface.
MHC II traffic and presentation in cDCs are tightly controlled along their maturation.23 Immature cDCs acquire antigen through active endocytosis and possess low levels of surface MHC II that are constantly internalized and degraded in lysosomal compartments. Induction of cDC maturation is accompanied by a rapid but transient boost in neosynthesis of MHC II24 and in macropinocytosis,25 which are then shut down as well as the endocytosis of MHC II.26 cDCs appear therefore well suited for the presentation of antigen encountered before or at the onset of maturation.27,28 The proteolytic activity of the endosomal system of cDC is also subjected to a fine temporal regulation during cDC maturation.29 These different levels of regulation increase the formation of peptide-loaded MHC II and their half-life. Ultimately, in fully mature cDCs, MHC II are absent from intracellular compartments and essentially displayed at the plasma membrane.
In this study, we analyzed MHC II transport and regulation during pDC activation. We show that the activation of freshly isolated human pDCs by influenza virus (flu) is associated with an important and long-lasting MHC II neosynthesis, as well as a sustained MHC II–peptide endocytosis. Flu activation of pDC gives rise in a nuclear factor-κB (NF-κB)–dependent manner to a strong accumulation and clustering of the MHC II molecules in intracellular compartments. Moreover, pDC endocytic activity persists during the whole activation process, suggesting a capacity to constantly renew surface peptide–MHC II complexes. Therefore, considering MHC II localization and dynamics, activated pDCs are totally different from mature cDCs. Our data provide the first insight into the biology of MHC II in human pDCs and the molecular events underlying pDC differentiation into antigen-presenting cells.
Methods
Reagents and antibodies
Oligonucleotides CpG-A 264, CpG-B 118, and CpG-C C274 were kindly provided by F. Barrat (Dynavax Technologies, Berkeley, CA). Beta propiolactone inactivated and purified influenza virus (H1N1, strain A/PR/8/34) was from Charles River Breeding Laboratories (Portage, MI). Lipopolysaccharide (LPS) and cycloheximide (CHX) were from Sigma-Aldrich (St Louis, MO). The phosphatidylinositol 3 (PI 3)–kinase inhibitor, LY294002 and the IKKγ NEMO-binding domain (NBD) peptide were purchased from Imgenex (San Diego, CA) and Calbiochem (San Diego, CA), respectively. The recombinant bacterial superantigen TSST1 was from Toxin Technology (Sarasota, FL). Concentrations of IFN-α were determined in culture supernatants using an enzyme-linked immunosorbent assay (ELISA) from BioSource International (Camarillo, CA)/Invitrogen (Carlsbad, CA). This study was approved by the Institut Curie International Review Board and by the French National Blood Agency.
We used antibodies specific for BDCA-2 and BDCA-4 (Miltenyi Biotec, Auburn, CA), HLA-DR, LAMP-1, CD80, CD86, and HLA-DM (BD Biosciences, San Jose, CA), LAMP-1 (BD PharMingen, San Diego, CA), and rabbit anti–mouse IgG (Dako North America, Carpinteria, CA). The rabbit anti–MHC II was a kind gift from H. L. Ploegh (Massachusetts Institute of Technology, Cambridge, MA), whereas culture supernatants form the hybridomas L243 and BU45 were produced in the laboratory. The mAb H5C6 specific for CD63 developed by August and Hildreth was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biologic Sciences (Iowa City, IA). The fluorescein isothiocyanate (FITC)–dextran (40 kDa) and the secondary antibodies Alexa Fluor 488–goat anti–mouse IgG and Alexa Fluor 594–donkey anti–rabbit IgG were from Invitrogen, and phycoerythrin (PE)–donkey anti–mouse IgG from Jackson ImmunoResearch Laboratories (West Grove, PA).
Isolation and stimulation of DC subsets
Buffy coats were obtained from adult healthy donors (Saint-Antoine Crozatier Blood Bank, Paris, France) where all donors signed informed consent to allow the use of their blood for research purpose in accordance with the Declaration of Helsinki. pDCs were isolated by magnetic sorting using a negative selection kit (Miltenyi Biotec). pDCs were 97% to 99% BDCA-2+ BDCA-4+ as determined by flow cytometry.
Anti-CD14–conjugated magnetic microbeads (Miltenyi Biotec) were used to purify monocytes from peripheral blood mononuclear cells. DCs were generated, as described,24 in medium supplemented with 100 ng/mL granulocyte-macrophage colony-stimulating factor and 40 ng/mL IL-4 for 5 days. Populations of immature DCs obtained were 99% CD1a+CD14−.
For stimulation, 2 × 105 pDCs per well cultured in 96 flat–bottom plates in complete medium were incubated with 10 μg/mL CpG-A,-B, -C, or 20 μg/mL flu. We used a chemically inactivated flu strain, which can fuse but cannot replicate in target cells.
Monocyte-derived DCs were stimulated under the same conditions with 1 μg/mL LPS.
Flow cytometry
Cell surface stainings were performed in phosphate-buffered saline (PBS) supplemented with 3% fetal calf serum and 0.05% azide. For the experiments aimed at comparing surface and total HLA-DR levels, cells were first surface stained, fixed in 3% paraformaldehyde for 20 minutes, and permeabilized in PBS 0.125% saponin 1.25% bovine serum albumin for 20 minutes at 20°C. Cells were then either used to measure surface levels or further processed and incubated with appropriate antibodies to stain intracellular HLA-DR molecules as well. Samples were analyzed by flow cytometry on a FACSCalibur using CellQuest Pro software (BD Biosciences, San Jose, CA).
Immunofluorescence
pDCs were plated on poly-L-lysine–coated glass coverslips and processed for immunofluorescent staining. The cells were examined under a motorized upright wide-field microscope (Leica DMRA2; Leica, Wetzlar, Germany) equipped for image deconvolution. Acquisition was performed using an oil-immersion objective (100× PL APO HCX, 1.4 NA) and a high-sensitive cooled interlined CCD camera (Roper CoolSnap HQ; Roper, Ottobrunn, Germany). The Z-positionning was accomplished by a piezo-electric motor (LVDT; Physik Instrument, Karlsruhe/Palmbach, Germany). Z-series of images were taken at 0.2-μm increments and treated by the 3-dimensional deconvolution module from the Metamorph software (Molecular Devices, Sunnyvale, CA), using the fast Iterative Constrained PSF-based algorithm. When present, white crosses delimit the zone of the captured images, which was subjected to the deconvolution process.
Endocytosis assays
pDCs, activated or not, were incubated at 0°C for 45 minutes with the mAbs L243 or BU45, washed in cold PBS, then shifted or not at 37°C in complete medium supplemented with 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid for various periods of time and rapidly transferred to cold medium. Samples were then stained with PE-conjugated anti–mouse antibodies and analyzed by flow cytometry. For the uptake of FITC-dextran, pDCs and cDCs, activated or not, were incubated with 1 mg/mL FITC-dextran in PBS supplemented with 2% FBS for 1 hour at 4°C (as a control for background binding) or at 37°C. Cells were then extensively washed with ice-cold PBS and resuspended in 0.2 mg/mL Trypan Blue in 0.1 M citrate buffer, pH 4.0, to quench surface-bound FITC. Samples were analyzed by flow cytometry.
Immunoelectron microscopy
A total of 1 to 2 × 106 of purified pDCs were stimulated with flu for 0, 3, or 24 hours, then fixed by incubation for 2 hours at room temperature in a mixture of 2% paraformaldehyde in 0.2 M phosphate buffer (PB), pH 7.4, and 0.125% glutaraldehyde. Fixed cells were processed for ultrathin cryosectioning and immunogold labeling as described previously.30 Briefly, cells were washed with PB and 50 mM glycine in PB and were then embedded in 10% gelatin. Small blocks were infiltrated with 2.3 M sucrose at 4°C for 2 hours and then frozen in liquid nitrogen. Ultrathin sections were cut with a Leica ultracut fetal calf serum and retrieved in a mixture of 2% methylcellulose and 2.3 M sucrose (vol/vol). The sections were indirectly immunogold-labeled with the mouse anti–LAMP-1, followed by a rabbit anti–mouse antibody (Dako North America), or directly with the rabbit anti–MHC II. Bound antibodies were detected with protein A coupled to 10- and 15-nm gold particles. The sections were contrast stained, embedded in a mixture of methylcellulose and uranyl acetate, and viewed with a CM120 Twin Phillips electron microscope (Eindhoven, The Netherlands).
Results
Flu activation of pDC induces a long-lasting neosynthesis and intracellular accumulation of MHC II molecules
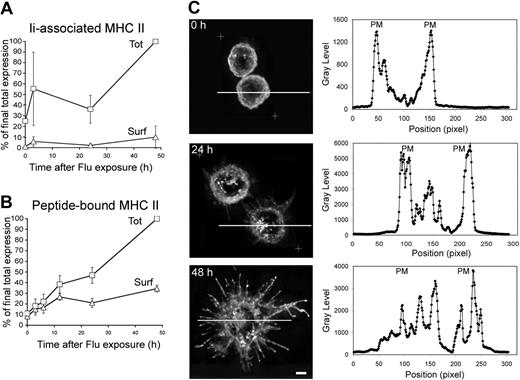
To gain insight into MHC II–restricted antigen presentation during pDC activation, we performed kinetics of activation with flu and followed MHC II expression. pDCs were freshly isolated from buffy coats by magnetic sorting. Their purity was routinely more than 98% as assessed by BDCA-2 and BDCA-4 staining (data not shown). Newly synthesized MHC II associate with their chaperone, the Ii chain, which can be followed using antibodies specific for the luminal side of the Ii chain (BU45). Cell-surface and “total” (ie surface + intracellular) Ii chain levels were quantified by flow cytometry in pDCs activated for different time periods (Figure 1A; Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). In fresh pDCs, total Ii levels were very low but on flu exposure increased substantially with time. Surface-displayed Ii chain represented only a minor part of the Ii chain pool at every time point up to 48 hours of flu exposure. These data indicated that flu stimulation of pDCs led to neosynthesis of Ii chain and probably formation of Ii-associated MHC II complexes, which were essentially detected intracellularly.
MHC II synthesis and redistribution in pDCs stimulated by flu. (A) Freshly purified (2 × 105) pDCs were stimulated or not with 20 μg/mL flu for different periods of time, as indicated. Levels of expression of αβIi complexes were analyzed by flow cytometry using an Ii chain-specific mAb for surface staining (Surf), or for surface and intracellular staining of permeabilized cells (Tot; “Methods”). (B) Similarly, total and surface levels of expression of peptide-loaded MHC II were estimated by flow cytometry using a mAb specific for peptide-bound MHC II. (A,B) Results shown were obtained from 3 independent donors and normalized taking the “Tot” MFI values obtained at 48 hours as 100%. See Figure S1 for raw data expressed in MFI from a typical experiment. Error bars represent SD. (C) Purified pDCs (105) were stimulated with 20 μg/mL flu for 0, 24, or 48 hours. Cells were fixed and stained for HLA-DR and analyzed by 3-dimensional deconvolution microscopy. Equatorial zones of 3-dimensional reconstructions are presented on the left panels. When present, white crosses delimit the zone of the captured images, which was subjected to the deconvolution process. Representative images of 6 individual donors are presented. On the right panels are displayed the quantification of the fluorescence means by a line scanning the corresponding cell on the left. Scale bar represents 3 μm. PM indicates plasma membrane.
MHC II synthesis and redistribution in pDCs stimulated by flu. (A) Freshly purified (2 × 105) pDCs were stimulated or not with 20 μg/mL flu for different periods of time, as indicated. Levels of expression of αβIi complexes were analyzed by flow cytometry using an Ii chain-specific mAb for surface staining (Surf), or for surface and intracellular staining of permeabilized cells (Tot; “Methods”). (B) Similarly, total and surface levels of expression of peptide-loaded MHC II were estimated by flow cytometry using a mAb specific for peptide-bound MHC II. (A,B) Results shown were obtained from 3 independent donors and normalized taking the “Tot” MFI values obtained at 48 hours as 100%. See Figure S1 for raw data expressed in MFI from a typical experiment. Error bars represent SD. (C) Purified pDCs (105) were stimulated with 20 μg/mL flu for 0, 24, or 48 hours. Cells were fixed and stained for HLA-DR and analyzed by 3-dimensional deconvolution microscopy. Equatorial zones of 3-dimensional reconstructions are presented on the left panels. When present, white crosses delimit the zone of the captured images, which was subjected to the deconvolution process. Representative images of 6 individual donors are presented. On the right panels are displayed the quantification of the fluorescence means by a line scanning the corresponding cell on the left. Scale bar represents 3 μm. PM indicates plasma membrane.
Cell-surface and total MHC II were also quantified by flow cytometry using a mAb specific for peptide-bound MHC II (L243). In fresh pDCs, the major part of MHC II molecules was displayed at their cell surface (Figures 1B, S1B). On flu exposure, total MHC II levels increased in an almost linear manner during the 48 hours of the experiments, reaching a 10-fold increase (Figure 1B). In contrast, surface MHC II levels increased at a slower rate during the same time period. After 48 hours of flu exposure, only 30% of the total MHC II molecules were displayed at the cell surface. Therefore, an increasing fraction of peptide-bound MHC II molecules accumulates intracellularly during the 48 hours of pDC activation (Figure S1C). These data indicate that acquisition of peptide cargo by MHC II continuously occurs during pDC activation.
pDC stimulation is associated with a profound redistribution of MHC II
Having shown an accumulation of intracellular MHC II after pDC activation, we investigated the subcellular localization of the MHC II by 3-dimensional deconvolution microscopy. At t = 0 hours, MHC II were mostly present at the cell surface, but also in vesicles just beneath the plasma membrane (Figure 1C). Activation of pDCs with flu was accompanied by important morphologic changes with large plasma membrane extensions leading to the acquisition of a dendritic shape (Figure 1C). At 24 and 48 hours of flu exposure, MHC II were still present at the cell surface but also accumulated in intracellular vesicles that formed clusters. Quantification of fluorescence intensity means by line scanning confirmed the appearance of an intracellular MHC II–enriched compartment (Figure 1C). Quantitative analysis revealed that approximately 75% of the pDCs displayed an intracellular MHC II clustering after 24 and 48 hours of activation with flu. In sharp contrast, during the maturation of monocyte-derived DCs (cDCs), MHC II shifted from an endosomal localization to the plasma membrane after 24 hours of LPS exposure (Figure S2).
Intracellular MHC II clusters are still present in fully matured pDCs
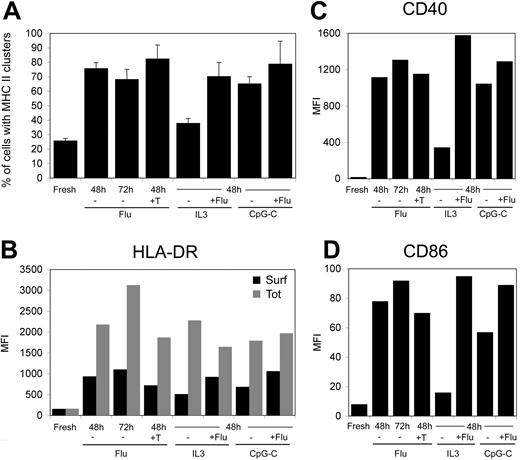
The presence of an intracellular pool of MHC II after flu stimulation of pDCs could be interpreted as an incomplete maturation of the cells. Because flu exposure is known to be a very potent stimulus of pDCs, we tested several conditions of activation with flu alone or in combinations with other stimuli to fully maturate pDCs. Extending the time of flu stimulation from 48 to 72 hours, as well as adding allogenic CD4 T cells together with the super-antigen TSST1 and flu, did not significantly change the phenotype of the pDC (Figure 2A). The same held true for combination of flu with IL-3 or with CpG-C. Levels of CD40, CD86, and surface and total HLA-DR were strongly increased compared with fresh pDC levels but remained similar in all conditions of stimulation (Figure 2B-D). We concluded that the accumulation of an intracellular pool of MHC II and its clustering occur in fully matured pDCs.
MHC II clustering and expression in pDCs exposed to various stimuli. pDCs were activated for the indicated periods of time with flu (20 μg/mL), IL-3 (10 ng/mL), CpG-C (10 μg/mL), or flu combined with IL-3 or CpG-C. In parallel, pDCs were activated with flu in presence of allogenic CD4+ T lymphocytes (ratio pDC/T 1:2) and of TSST1 at 10 ng/mL. (A) Cells were fixed and stained for HLA-DR and analyzed by 3-dimensional deconvolution microscopy. The percentage of cells exhibiting an intracellular pool of MHC II were estimated by 2 independent researchers (n = 75-150 cells per time point; 3 different donors). (B) Surface and total levels of HLA-DR expression were estimated by flow cytometry on fixed and permeabilized cells as in Figure 1B. (C,D) Surface expression of CD40 and CD86 were estimated by flow cytometry. MFI values presented are from a typical experiment representative of 3 independent experiments.
MHC II clustering and expression in pDCs exposed to various stimuli. pDCs were activated for the indicated periods of time with flu (20 μg/mL), IL-3 (10 ng/mL), CpG-C (10 μg/mL), or flu combined with IL-3 or CpG-C. In parallel, pDCs were activated with flu in presence of allogenic CD4+ T lymphocytes (ratio pDC/T 1:2) and of TSST1 at 10 ng/mL. (A) Cells were fixed and stained for HLA-DR and analyzed by 3-dimensional deconvolution microscopy. The percentage of cells exhibiting an intracellular pool of MHC II were estimated by 2 independent researchers (n = 75-150 cells per time point; 3 different donors). (B) Surface and total levels of HLA-DR expression were estimated by flow cytometry on fixed and permeabilized cells as in Figure 1B. (C,D) Surface expression of CD40 and CD86 were estimated by flow cytometry. MFI values presented are from a typical experiment representative of 3 independent experiments.
Polarized MHC II accumulation in peptide-loading compartments
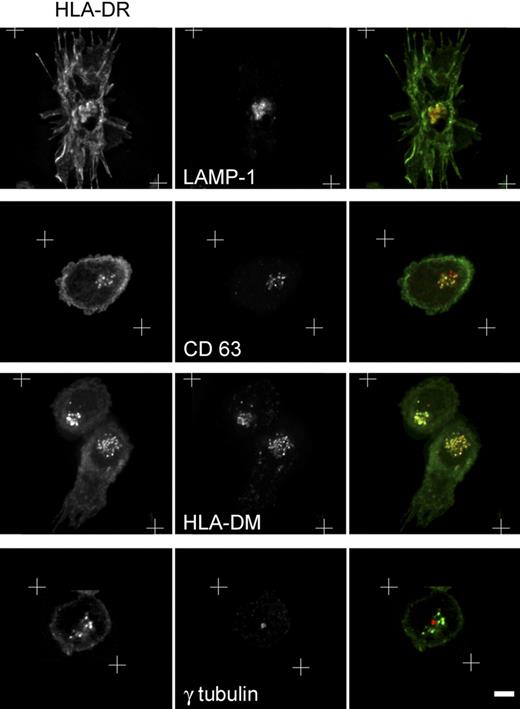
To further characterize the intracellular compartments in which MHC II molecules accumulated, 24-hour–stimulated pDCs were stained for different markers of the endocytic pathway and analyzed by 3-dimensional deconvolution microscopy. On pDC activation, MHC II molecules converge to compartments colocalized with LAMP-1, CD63, and HLA-DM markers (Figure 3), but not with transferrin receptor and EEA1 markers (data not shown). After the localization of the microtubule organization center (MTOC) with γ-tubulin–specific antibodies, we observed that the MHC II clusters were organized around the MTOC (Figure 3).
Characterization of the MHC II–containing compartment in activated pDCs. Twenty-four–hour flu-activated pDCs were costained for MHC II and the indicated markers of the endocytic pathway and then analyzed by 3-dimensional deconvolution microscopy. White crosses represent the zone of the captured images, which was subjected to the deconvolution process Equatorial zones of the 3-dimensional reconstruction are presented excepted for the bottom row, where a single plan containing the MTOC is shown. Scale bar represents 3 μm.
Characterization of the MHC II–containing compartment in activated pDCs. Twenty-four–hour flu-activated pDCs were costained for MHC II and the indicated markers of the endocytic pathway and then analyzed by 3-dimensional deconvolution microscopy. White crosses represent the zone of the captured images, which was subjected to the deconvolution process Equatorial zones of the 3-dimensional reconstruction are presented excepted for the bottom row, where a single plan containing the MTOC is shown. Scale bar represents 3 μm.
We next performed an ultrastructural analysis of pDCs stimulated for 0, 3, or 24 hours by the flu. Cells were fixed and processed for immunoelectron microscopy. Ultrathin cryosections were labeled for MHC II and LAMP-1 (Figure 4). Unstimulated pDCs displayed a round shape with a voluminous nucleus containing heterogeneous chromatin occupying most of the cell volume. A highly extended network of ER membranes was seen around the nucleus, a characteristic of pDCs related to their high capacity for type I IFN secretion.7 At 3 hours of flu exposure, pDCs exhibited important morphologic changes, which were more pronounced at 24 hours of flu exposure (Figure 4). The nucleus presented more compact chromatin. The cytoplasm contained numerous mitochondria and MHC II+ LAMP-1+ compartments often electron lucent, consistent with our immunofluorescence analysis (Figure 3). Some of these compartments were “fuzzy” and appeared to be rich in degraded material, whereas fewer compartments were rich in internal membrane sheets (Figure 4). pDCs developed dendrites and became polarized: most of the organelles tended to accumulate in the pole opposite to the nucleus.
Immunoelectron microscopy analysis of pDCs during activation by flu. Ultrathin cryosections from pDCs exposed for 0, 3, or 24 hours to flu were prepared. Sections were then labeled using 10-nm and 15-nm immunogold particles for MHC II and LAMP-1 molecules, respectively. The 3 large sections of pDC presented (time 0, 3, and 24 hours) are electron microscopy images, which have been obtained using the Multiple Image Alignment function of the iTEM software (Eloïse; Electron Optics Instrument Service, West Orange, NJ). The 2 images on the bottom right show enlargements of typical MHC II+ compartments after 24 hours of flu exposure.
Immunoelectron microscopy analysis of pDCs during activation by flu. Ultrathin cryosections from pDCs exposed for 0, 3, or 24 hours to flu were prepared. Sections were then labeled using 10-nm and 15-nm immunogold particles for MHC II and LAMP-1 molecules, respectively. The 3 large sections of pDC presented (time 0, 3, and 24 hours) are electron microscopy images, which have been obtained using the Multiple Image Alignment function of the iTEM software (Eloïse; Electron Optics Instrument Service, West Orange, NJ). The 2 images on the bottom right show enlargements of typical MHC II+ compartments after 24 hours of flu exposure.
We conclude that, on flu activation, pDCs rapidly undergo important morphologic changes related to their acquisition of a DC phenotype. In addition, MHC II accumulate in a polarized manner together with typical markers of compartments devoted to peptide-loading onto MHC II.
Constitution of the intracellular MHC II clusters occurs rapidly after pDC activation and depends on protein neosynthesis
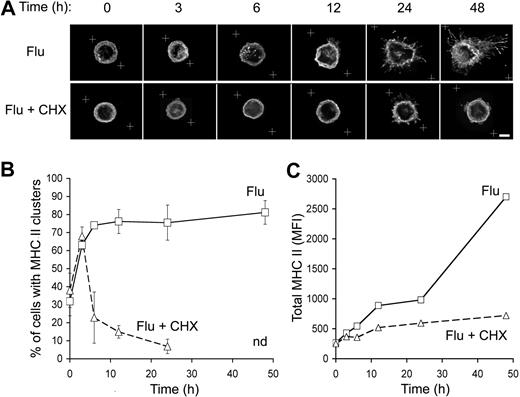
To investigate the kinetics of the appearance of the MHC II intracellular pool, peptide-bound MHC II subcellular distribution was analyzed at various time points after pDC exposure to flu by 3-dimensional deconvolution microscopy. MHC II started to cluster from 3 hours after flu exposure in more than 60% of the pDCs (Figure 5A). This proportion reached a plateau at 75% to 80% of the pDCs from 12-hour exposure and remained at this level until 48 hours of exposure (Figure 5B). The clustering of MHC II+ intracellular vesicles increased with time.
Kinetics of MHC II intracellular pool constitution in flu-stimulated pDCs. (A) Purified pDCs were activated by flu in the presence or the absence of CHX (10 μg/mL) for the indicated periods of time. Then, pDCs were stained for MHC II and analyzed by 3-dimensional deconvolution microscopy. White crosses represent the zone of the captured images, which was subjected to the deconvolution process. Equatorial zones of 3-dimensional reconstructions are presented. Scale bar represents 3 μm. (B) Percentage of pDCs exhibiting an intracellular pool of MHC II as seen in panel A were estimated as described in Figure 2. n.d. indicates not determined because long exposure to CHX affected cell viability. Error bars represent SD. (C) Levels of expression of peptide-bound MHC II by pDCs exposed to flu and treated or not with CHX were determined by flow cytometry as in Figure 1B. Results presented are representative of 3 independent experiments.
Kinetics of MHC II intracellular pool constitution in flu-stimulated pDCs. (A) Purified pDCs were activated by flu in the presence or the absence of CHX (10 μg/mL) for the indicated periods of time. Then, pDCs were stained for MHC II and analyzed by 3-dimensional deconvolution microscopy. White crosses represent the zone of the captured images, which was subjected to the deconvolution process. Equatorial zones of 3-dimensional reconstructions are presented. Scale bar represents 3 μm. (B) Percentage of pDCs exhibiting an intracellular pool of MHC II as seen in panel A were estimated as described in Figure 2. n.d. indicates not determined because long exposure to CHX affected cell viability. Error bars represent SD. (C) Levels of expression of peptide-bound MHC II by pDCs exposed to flu and treated or not with CHX were determined by flow cytometry as in Figure 1B. Results presented are representative of 3 independent experiments.
To determine whether the MHC II intracellular pool was constituted of newly synthesized MHC II, pDCs were activated in the presence of CHX, a protein synthesis inhibitor. CHX treatment strongly inhibited the formation of the intracellular pool of MHC II from 6 up to 48 hours of activation (Figure 5A,B). However, at 3 hours in the presence of CHX, 50% of the pDCs exhibited intracellular clusters of MHC II (Figure 5B). Efficiency of CHX treatment was verified in parallel by analyzing total MHC II expression by flow cytometry. In the absence of CHX, total MHC II levels increased with time reflecting intense neosynthesis, which was in most part abrogated by CHX treatment (Figure 5C). These data suggested that at least a part of the intracellular clusters observed after 3 hours of flu exposure originated from the recruitment of MHC II+ vesicles that were already present in fresh pDCs.
We conclude that pDC activation triggers a rapid coalescence of preexisting MHC II molecules and induces a sustained MHC II neosynthesis, both contributing to intracellular MHC II clustering.
MHC II internalization and FITC-dextran uptake persist during pDC activation
During maturation of cDCs, tight regulation of endocytosis of both Ii-associated and peptide-bound MHC II complexes directly impacts on their half-life.24,31 Similarly, endocytosis of MHC II could be regulated during pDC activation and contribute to the constitution of the intracellular pool of MHC II. To test this hypothesis, we measured endocytosis of MHC II in pDCs activated for 0, 3, or 24 hours by the flu. First, αβIi complexes were followed with the Ii chain specific mAb BU45. At t = 0 hours of activation, surface-bound BU45 mAb was quickly internalized (60% in 3 minutes) to rapidly reach a plateau at approximately 75% (Figure 6A). These data suggested that newly synthesized αβIi complexes trafficked very transiently to the plasma membrane before being internalized into the endocytic pathway. Importantly, this rapid rate of endocytosis was not modified after pDC activation with flu even after 24 hours (Figure 6A).
Kinetics of MHC II and dextran endocytosis in pDCs. Fresh pDCs were exposed to flu for 0, 3, or 24 hours. Cells were then collected and allowed to bind at 0°C with either (A) the Ii chain–specific mAb BU45 or (B) the peptide-bound MHC II specific mAb L243. Cells were then shifted to 37°C for the length of time indicated. Ligands remaining on the cell surface were detected using PE-labeled secondary antibodies. Estimation of the percentages of antibody present at the cell surface was made by flow cytometric analysis of the samples and using the following formula (MFI of each time point/MFI at t = 0) × 100. Data from 3 independent experiments were used to calculate means and SD. (C) Uptake of FITC-dextran. Flow cytometry profiles of the indicated populations of DCs are presented. Cells were activated for 0, 24, or 48 hours and then exposed to FITC-dextran at 37°C for 60 minutes (black lines). As a control, cells were incubated at 0°C during the same time (gray lines). Numbers indicate MFI values. Results presented are representative of 4 independent experiments.
Kinetics of MHC II and dextran endocytosis in pDCs. Fresh pDCs were exposed to flu for 0, 3, or 24 hours. Cells were then collected and allowed to bind at 0°C with either (A) the Ii chain–specific mAb BU45 or (B) the peptide-bound MHC II specific mAb L243. Cells were then shifted to 37°C for the length of time indicated. Ligands remaining on the cell surface were detected using PE-labeled secondary antibodies. Estimation of the percentages of antibody present at the cell surface was made by flow cytometric analysis of the samples and using the following formula (MFI of each time point/MFI at t = 0) × 100. Data from 3 independent experiments were used to calculate means and SD. (C) Uptake of FITC-dextran. Flow cytometry profiles of the indicated populations of DCs are presented. Cells were activated for 0, 24, or 48 hours and then exposed to FITC-dextran at 37°C for 60 minutes (black lines). As a control, cells were incubated at 0°C during the same time (gray lines). Numbers indicate MFI values. Results presented are representative of 4 independent experiments.
We then measured internalization of peptide-bound MHC II using the mAb L243. In fresh pDCs, approximately 25% of the surface-bound L243 disappeared from the cell surface in 20 minutes (Figure 6B) and was found intracellularly (data not shown). Subsequently, a slowdown of mAb internalization suggested that recycling of MHC II was taking place. After pDC stimulation with flu for 3 or 24 hours, the kinetics of MHC II remained similar. The only difference concerned late time points of internalization (120 minutes) where fresh pDCs exhibited a higher rate of endocytosis. To evaluate the access of potential antigens to the MHC II present in the endocytic pathway, we compared pDCs and monocyte-derived DCs, before and after their activation, for their ability to take up FITC-dextran. As expected,24 immature cDCs showed high levels of endocytic activity, which drastically decreased after 24 and 48 hours of exposure to LPS (Figure 6C). Fresh pDCs exhibited a slightly lower endocytic activity than immature cDCs, which, however, remained high after 24 and 48 hours of exposure to flu (Figure 6C).
We conclude that endocytosis of both Ii-associated and peptide-bound MHC II as well as dextran uptake are sustained in pDCs on activation.
The formation of the intracellular pool of MHC II in activated pDCs is NF-κB dependent
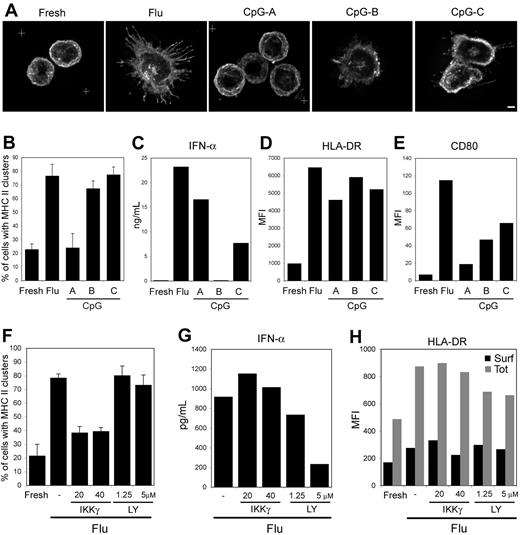
pDC activation by live as well as inactivated flu has been shown to be strictly TLR7-dependent.32,33 Stimulation of human pDC with flu virus or other TLR ligands can activate 2 pathways: the MyD88/IRF-7 pathway leads to type I IFN secretion, whereas the MyD88/NF-κB pathway results in the production of proinflammatory cytokines, such as TNF-α and IL-6, as well as the acquisition of a dendritic phenotype with up-regulation of costimulatory molecules.34-36 To pharmacologically dissociate these 2 pathways, we analyzed MHC II redistribution in response to a 24-hour exposure of pDCs to different types of CpG oligonucleotides: CpG-A and CpG-B, which induce type I IFN or dendritic cell differentiation, respectively, as well as CpG-C, which activates both pathways.36,37 As expected, CpG-A exposure led to IFN-α secretion and did not induce the up-regulation of the costimulatory molecules CD80 and CD86 (Figure 7C,E). However, CpG-A exposure increased surface levels of MHC II without affecting their intracellular distribution, which remained in peripheral vesicles and at the plasma membrane similar to fresh pDCs (Figure 7A,D). In contrast, CpG-B exposure did not induce IFN-α secretion but led to DC differentiation and MHC II clustering. Finally, CpG-C and flu exposures induced a similar phenotype regarding the different parameters followed (Figure 7A-E).
Specificity of the signaling required for acquisition of a dendritic phenotype. pDCs were exposed for 24 hours to the indicated stimulus (A-E) or were preincubated for 1 hour with the IKKγ NBD peptide (IKKγ) or LY294002 (LY), and exposed to flu for 12 hours (F-H). Cells were then stained for HLA-DR and analyzed by 3-dimensional deconvolution microscopy. (A) Typical images (equatorial zone of 3-dimensional reconstruction) for each condition are presented. Scale bar represents 2 μm. (B,F) Quantification of pDCs displaying an intracellular MHC II pool on stimulation. Percentages of pDCs exhibiting an intracellular pool of MHC II were blindly estimated by 2 independent researchers (n = 75-150 cells per time point; 3 different donors). Error bars represent SD. (C,G) Levels of IFN-α produced in the culture supernatants were determined by ELISA. (D,E,H) Surface expression of the indicated markers was estimated by flow cytometry. (H) Surface (■) and total (▩) levels of peptide-loaded MHC II were determined as in Figure 1B. CD86 surface expression in the various cell populations was very similar to the CD80 pattern and therefore is not shown. MFI values of a typical experiment representative of 3 to 4 independent experiments are presented.
Specificity of the signaling required for acquisition of a dendritic phenotype. pDCs were exposed for 24 hours to the indicated stimulus (A-E) or were preincubated for 1 hour with the IKKγ NBD peptide (IKKγ) or LY294002 (LY), and exposed to flu for 12 hours (F-H). Cells were then stained for HLA-DR and analyzed by 3-dimensional deconvolution microscopy. (A) Typical images (equatorial zone of 3-dimensional reconstruction) for each condition are presented. Scale bar represents 2 μm. (B,F) Quantification of pDCs displaying an intracellular MHC II pool on stimulation. Percentages of pDCs exhibiting an intracellular pool of MHC II were blindly estimated by 2 independent researchers (n = 75-150 cells per time point; 3 different donors). Error bars represent SD. (C,G) Levels of IFN-α produced in the culture supernatants were determined by ELISA. (D,E,H) Surface expression of the indicated markers was estimated by flow cytometry. (H) Surface (■) and total (▩) levels of peptide-loaded MHC II were determined as in Figure 1B. CD86 surface expression in the various cell populations was very similar to the CD80 pattern and therefore is not shown. MFI values of a typical experiment representative of 3 to 4 independent experiments are presented.
The involvement of the MyD88/NF-κB rather than the MyD88/IRF-7 pathway was directly tested with specific inhibitors. The PI 3-kinase inhibitor LY294002 has been recently shown to inhibit the IRF-7 pathway leading to IFN-α secretion.38 The IKKγ NBD peptide has been shown in different systems to be an efficient inhibitor of the NF-κB pathway.39 pDCs were preincubated for 1 hour with these inhibitors and then exposed to flu for 12 hours. As expected, LY294002 exposure, but not the NBD peptide, inhibited IFN-α secretion in a dose-dependent manner (Figure 7G). Both inhibitors did not affect surface and total levels of MHC II (Figure 7H), indicating that neosynthesis of MHC II remained unaffected by the treatments. However, we observed that exposure to the NBD peptide, but not LY294002, strongly reduced the appearance of MHC II intracellular clusters in response to flu (Figure 7F).
These data further establish that MHC II intracellular clustering induced by TLR triggering in pDCs depends on the NF-κB pathway and is IRF-7 independent.
Discussion
In this study, we provide molecular and ultrastructural analysis of MHC II trafficking in primary human pDCs. Despite the technical difficulties resulting from the scarcity and the weak viability of human pDCs, we have outlined the developmental regulation of MHC II antigen presentation machinery during the differentiation from the plasmacytoid state to the DC state. Our results establish that stimulation of primary human pDC induces a strong and long-lasting neosynthesis of MHC II and Ii chains. pDCs, like B lymphocytes, differ from cDCs regarding the expression of the transactivator CIITA that drives the expression of MHC II genes.40 cDC maturation is rapidly accompanied by a shutdown of de novo MHC II synthesis resulting from silencing of CIITA expression, both at the RNA and protein levels, which does not occur during pDC activation40,41 and thus may explain our results.
Persistent synthesis of MHC II molecules in activated pDCs is accompanied by sustained endocytosis of both Ii-associated and peptide-bound MHC II. Again, this is in sharp contrast to the situation of cDCs: on maturation, MHC II endocytosis and lysosomal degradation are rapidly turned off, resulting in increased stability and half-life of peptide-loaded MHC II at the plasma membrane.24 In human cDCs, oligoubiquitination of MHC II by the ubiquitin ligase MARCH I42 promotes their endocytosis and is down-regulated on cDC maturation, resulting in the accumulation of MHC II at the cell surface.43,44 Sustained endocytosis of MHC II along pDC activation suggests that such an ubiquitin-mediated regulation might not take place in pDCs.
Sustained MHC II endocytosis may explain the moderate up-regulation of MHC II displayed at the pDC surface compared with the large increase observed at the intracellular level and could be involved in the constitution of the intracellular clusters of MHC II during the activation of pDCs by flu. These MHC II vesicles contain markers of peptide-loading compartments including HLA-DM and are organized around the MTOC, a feature shared with B lymphocytes on activation through their antigen receptor.45
In activated pDCs, sustained MHC II synthesis is accompanied by acquisition of peptide cargo, as shown by the constant increase of the amounts of peptide-bound MHC II complexes detected. In addition, prolonged neosynthesis and endocytosis of MHC II could lead to constant renewal of the antigenic peptide cargos presented by MHC II. These peptides may originate from endogenous antigens or derive from internalized antigens having access to the MHC II clusters because the capacity of pDC to uptake FITC-dextran persists during their whole maturation process. Such constant peptide renewal in pDCs might contribute to reduce their stimulatory capacity compared with mature cDCs where the MHC II complexes remain at the plasma membrane for extended periods of time.24 Indeed, optimal half-life of the T-cell receptor/MHC II interaction is required for efficient T-cell activation.46 Whether this dynamic is involved in the proposed role of pDCs in the development of regulatory T cells and in the maintenance of tolerance15,16,22 remains to be determined.
On flu activation of pDCs, the appearance of the MHC II intracellular clusters is rapid and in large part dependent on newly synthesized MHC II molecules. The accumulation of an intracellular pool of MHC II does not represent an intermediate stage of pDC maturation because such pools are also observed in fully mature pDCs, activated by flu for 3 days or by various combinations of TLR stimuli. The extent of the changes we document and their rapidity suggest that this process is tightly regulated. Flu stimulation activates both the IRF-7 and the NF-κB–dependent pathways, whereas CpG-A activates only the first one and CpG-B only the second one. Both CpG-A and -B induced surface up-regulation of MHC II, but only CpG-B activation of pDC led to the formation of the MHC II intracellular clusters. A recent study shows that CpG-B, but not CpG-A-activated pDCs, are able to activate memory CD4+ T lymphocytes specific for a viral antigen.9 Finally, usage of specific inhibitors in the presence of flu further established the dependency of the NF-κB pathway in the formation of MHC II clusters in pDCs. These results provide another line of evidence for the dissociation of the type I IFN production from the T cell–stimulatory capacities of human pDC. This issue has been controversial, and some authors suggested that these 2 functions are tightly linked or triggered sequentially.47
Overall, our study provides definitive evidence for the presence of highly dynamic and regulated MHC II trafficking in pDC and suggests that pDCs and cDCs have complementary functions in eliciting immune responses. cDCs appear particularly well suited for providing an antigenic memory in the form of peptides derived from antigens captured at the onset of maturation.27,28 Rather, pDCs could be adapted to present (endogenous or exogenous) antigens that may have access to the MHC II intracellular clusters at anytime of the activation process because MHC II synthesis and antigen loading persist.
Our study also suggests that pDCs could play a central role in case of immunosuppression like the one commonly induced by septicemia.48 Indeed, systemic activation depletes the immune system of cDCs able to respond to a newly encountered antigen,28 whereas in these conditions, pDCs would be still able to capture, load, and present newly encountered antigen. Future work aiming at dissecting the cross-talks between these 2 populations of DCs in various physiopathologic conditions should shed light on the initiation of the immune responses and will be crucial for the development of DC-based immunotherapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Note added after review.
A study was recently published reaching similar conclusions on MHC II biology in mouse pDCs to ours in humans (Young L, et al. Differential MHC II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9:1244-1252).
Acknowledgments
We thank F.-X. Gobert for his help; S. Amigorena, F. Faure, G. Raposo, and A.-M. Lennon-Dumesnil for discussion; and J. A. Villadangos for sharing data before publication.
C.S. was supported by a fellowship, and P.B. was supported by grants from Agence Nationale de Recherche Contre le SIDA and from the Agence Nationale de Recherche.
Authorship
Contribution: C.S. performed research and analyzed data; M.-A.M.-P. contributed cell purifications and flow cytometry; V.S. contributed to analysis and writing of the paper; P.B. designed and supervised the research; and C.S. and P.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philippe Benaroch, Institut Curie, Inserm U653, 26 rue d'Ulm 75248 Paris Cedex 05, France; e-mail: benaroch@curie.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal