Response:
Our group published a microarray study of primary central nervous system lymphoma (PCNSL) in Blood in March 2008.1 We reported a comprehensive CNS signature of PCNSL, identifying single-gene differential expression as well as a pathway signature. Most notably, our pathway signature for PCNSL is characterized by differential expression of extracellular matrix (ECM)- and adhesion-related pathways. The most up-regulated gene is the ECM- and adhesion-related osteopontin (SPP1). Our study is unique in that we compared PCNSL to a broad spectrum of non-CNS diffuse large B-cell lymphoma (DLBCL), consisting of nodal and extranodal samples; and in-depth bioinformatics analysis was performed. This is in contrast to 2 other microarray studies in PCNSL2,3 in which the comparisons were made to nodal DLBCL and no pathway analysis was performed.
We are pleased that Rubenstein et al have confirmed our single-gene expression findings by retrospective analysis of their data and come up with concordant genes. We are also surprised, because their original findings2 published in 2006 were very different from ours.1 We would recommend that they also consider performing pathway analysis on their data. In our opinion, the pathway analysis makes it possible to obtain meaningful biologic insights with gene expression data. They mentioned lack of an independent validation set in both studies. We would point out that in our immunohistochemical validation, 10 of 15 PCNSL samples were not used in the microarray study.1 As such, we did validation on a largely independent sample set.
Their proposal on 2 major types of PCNSL based on cellular density of the tumor will need more validation. Autopsy studies have shown the presence of lymphoma cells throughout the brain, even in areas that look normal on imaging scans4,5 ; hence the term whole brain disease.5 One has to assume that high-density and low-density lesions do coexist and that tumors are scattered with intervening normal brain.
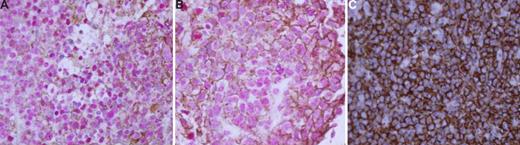
We would like to draw particular attention to SPP1. It is striking that SPP1 is uniquely expressed by DLBCL in the CNS, in which it is highly expressed under normal circumstances.6 Figure 1 shows dual IHC for CD-20 and SPP1 in PCNSL and non-CNS DLBCL samples; the findings confirm that SPP1 is highly expressed in PCNSL B cells. Montesinos-Rongen et al3 have shown that SPP1 is more significantly expressed in PCNSL than in normal brain or systemic DLBCL (please refer to their Figure S6b). We conclude that normal B-cell counterparts3 and non-CNS DLBCL1-3 do not express SPP1, but malignant B cells in PCNSL express it significantly.1-3
CD20 and osteopontin are coexpressed in PCNSL. Dual immunohistochemistry was performed using antibodies specific to CD20 (Dako, Carpenteria, CA) and osteopontin (R&D Systems, Minneapolis, MN). Diaminobenzidine (brown) dye (Dako) was used for CD20 and Vulcan fast red (red) dye (Biocare Medical, Concord, CA) was used for osteopontin. (A) PCNSL. Original magnification ×400. Both CD20 (membranous pattern) and osteopontin (predominantly nuclear) are clearly positive. (B) Second case of PCNSL. Original magnification ×1000. Again, both CD20 and osteopontin are positive. (C) Nodal DLBCL. Original magnification ×400. Only CD20 is positive with no expression of osteopontin. A Leica DMLB optical microscope (Leica Microsystems, Wetzlar, Germany) and Cytoseal-60 mounting media (Richard Allen, Kalamazoo, MI) were used. Images were acquired using a SPOT RT Color Camera (Diagnostic Instruments, Sterling Heights, MI), and were processed with SPOT Advanced program version 2.0 (Diagnostic Instruments) and Adobe Photoshop version 6.0 software (Adobe Systems, San Jose, CA).
CD20 and osteopontin are coexpressed in PCNSL. Dual immunohistochemistry was performed using antibodies specific to CD20 (Dako, Carpenteria, CA) and osteopontin (R&D Systems, Minneapolis, MN). Diaminobenzidine (brown) dye (Dako) was used for CD20 and Vulcan fast red (red) dye (Biocare Medical, Concord, CA) was used for osteopontin. (A) PCNSL. Original magnification ×400. Both CD20 (membranous pattern) and osteopontin (predominantly nuclear) are clearly positive. (B) Second case of PCNSL. Original magnification ×1000. Again, both CD20 and osteopontin are positive. (C) Nodal DLBCL. Original magnification ×400. Only CD20 is positive with no expression of osteopontin. A Leica DMLB optical microscope (Leica Microsystems, Wetzlar, Germany) and Cytoseal-60 mounting media (Richard Allen, Kalamazoo, MI) were used. Images were acquired using a SPOT RT Color Camera (Diagnostic Instruments, Sterling Heights, MI), and were processed with SPOT Advanced program version 2.0 (Diagnostic Instruments) and Adobe Photoshop version 6.0 software (Adobe Systems, San Jose, CA).
The role of SPP1 and ECM/adhesion pathway changes in the biology of PCNSL is not known. Our hypothesis is that they are likely linked to a transitional process B cells undergo to invade the CNS microenvironment. This B-cell lymphocytic-neurologic transition process may be akin to epithelial-mesenchymal transition (EMT), in which epithelial cancer cells become invasive by acquiring mesenchymal changes and losing some adhesion molecules.7 The malignant B cells in PCNSL apparently acquire expression of SPP1, which is highly expressed in CNS microenvironment. Our pathway analysis has shown down-regulation of multiple ECM and adhesion-related pathways in PCNSL compared with non-CNS DLBCL. These transitional changes may explain the invasiveness of PCNSL B cells in CNS microenvironment.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Han Win Tun, Department of Hematology/Oncology, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; e-mail: tun.han@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal