Abstract
Kaposi sarcoma (KS) is associated with human herpesvirus (HHV)-8 and is dependent on the induction of vascular endothelial growth factors (VEGFs). VEGF regulates genes that provide arterial or venous identity to endothelial cells, such as the induction of EphrinB2, which phenotypically defines arterial endothelial cells and pericytes, and represses EphB4, which defines venous endothelial cells. We conducted a comprehensive analysis of the Eph receptor tyrosine kinases to determine which members are expressed and therefore contribute to KS pathogenesis. We demonstrated limited Eph/Ephrin expression; notably, the only ligand highly expressed is EphrinB2. We next studied the biologic effects of blocking EphrinB2 using the extracellular domain of EphB4 fused with human serum albumin (sEphB4-HSA). sEphB4-HSA inhibited migration and invasion of the KS cells in vitro in response to various growth factors. Finally, we determined the biologic effects of combining sEphB4-HSA and an antibody to VEGF. sEphB4-HSA was more active than the VEGF antibody, and combination of the 2 had at least additive activity. sEphB4-HSA reduced blood vessel density, pericyte recruitment, vessel perfusion, and increased hypoxia, with an associated increase in VEGF and DLL4 expression. The combination of sEphB4-HSA and VEGF antibody is a rational treatment combination for further investigation.
Introduction
Kaposi sarcoma (KS) is a highly vascularized tumor that is associated with human herpesvirus (HHV)–8. KS manifests most frequently as an angioproliferative disease in the skin; in advanced cases, KS involves visceral organs such as the liver, lungs, or gastrointestinal (GI) tract, which can be fatal. KS lesions exhibit an extensive vascular network of slit-like spaces with abnormal spindle-shaped endothelial cells lining the tumor vessels, which lack basement membranes. Defective vasculature results in an accumulation of the blood components, including albumin and red and mononuclear cells, in the lesions.1
Vascular endothelial growth factors (VEGFs), including VEGF, VEGF-C, and their cognate receptors VEGFR1, -2, and -3, are highly expressed in KS cells and induced by HHV-8.2 VEGFs function as autocrine growth factors.2 Furthermore, VEGFs induce tumor vessels in a paracrine manner and regulate endothelial cell proliferation and migration. VEGFs regulate genes that provide arterial or venous identity to endothelial cells, such as the induction of EphrinB2, which phenotypically defines arterial endothelial cells and pericytes, and represses EphB4, which defines venous endothelial cells.2-4
The EphB4/EphrinB2 interaction plays a critical role in vessel maturation because the knockout of either protein is embryonically lethal in mice as a result of vascular arrest at the primitive capillary plexus stage.5,6 We previously noted the disorganized KS vasculature was caused by unbalanced expression of EphB4 and its ligand EphrinB2 and that the HHV-8 virus associated with KS regulates EphB4 and EphrinB2. Infection of venous endothelial cells with HHV-8 results in a switch from expression of EphB4 to EphrinB2, similar to that observed with VEGF.2 We also observed that EphrinB2 expression is required for KS cell viability by knock down with small interfering RNA (siRNA).2
EphrinB2 also may regulate biologic functions of cell migration, adhesion, and invasion in KS. EphrinB2 can interact with other members of Eph receptors, including EphB1, EphB2, EphB3, EphB4, and EphA4.7-9 Second, EphrinB2 can modulate vascular response by binding to EphB4 in adjacent tumor vessels.3,10-12 In the current work, we conducted a comprehensive analysis of the Eph receptor tyrosine kinases to determine which of the other members may be expressed and contribute to KS pathogenesis. Next, we studied the biologic effects of blocking EphrinB2 in vitro and in vivo using the soluble form of EphB4 (sEphB4) consisting of the extracellular domains of the receptor.13 Finally, we determined the biologic effects of combining sEphB4 fused to human serum albumin (sEphB4-HSA) and an antibody to VEGF.
Methods
Antibodies and other reagents
Antibodies to Eph receptors and ligands were obtained from R&D Systems (Minneapolis, MN); EphB2, EphB4, and EphrinB2 were generated at VasGene Therapeutics (Los Angeles, CA), anti-CD31 (M20) was from Santa Cruz Biotechnology (Santa Cruz, CA); anti–Ki-67 and anti-SMA were from (Dako, Carpinteria, CA); IgG-Fc fragment and anti–human Fc were from The Jackson Laboratory (Bar Harbor, ME); hypoxyprobe-1 from Chemicon International (Temecula, CA); rhodamine-labeled ricinus communis agglutinin I (RCA) from Vector Laboratories (Burlingame, CA); and alkaline phosphatase substrate para-Nitrophenylphosphate (pNPP) from Sigma-Aldrich (St Louis, MO).
Expression and purification of sEphB4 fused to HSA
Complementary (cDNA) encoding amino acids 1 to 539 of sEphB4 representing the entire extracellular domain was cloned upstream of the mature human serum albumin pCRscript/sEphB4-HSA and placed into the mammalian expression vector under control of the cytomegalovirus (CMV) promoter stably expressed in the Chinese hamster ovary (CHO) cell line. Supernatants were concentrated by tangential flow filtration and diafiltered into 20 mmol/L Tris, pH 8.0, 50 mmol/L NaCl. Concentrated sample was applied to a Q-FF column equilibrated in the same buffer. The sEphB4-HSA fusion protein was eluted with 25 mmol/L NaCl steps in 20 mmol/L Tris, pH 8.0. The elution was followed by NuPAGE gel (Invitrogen, Carlsbad, CA). Fractions containing sEphB4-HSA were diafiltered into 10 mmol/L sodium phosphate buffer, pH 7.0, 50 mmol/L NaCl, and applied to a hydroxyapatite column equilibrated in the same buffer. The sEphB4-HSA was eluted from the column using 10 mmol/L sodium phosphate steps at pH 7.0 in 50 mmol/L NaCl. The elution was followed by NuPAGE Gel.
Characterization of sEphB4-HSA by saturation binding
To characterize the functionality of sEphB4-HSA and sEphB4, a solid-phase enzyme-linked immunosorbent assay (ELISA) was developed in which sEphB4-HSA is trapped on 96-well ELISA plates (Nunc-Maxisorb; Roskilde, Denmark) with the use of EphB4 monoclonal antibody. Samples were diluted serially in phosphate-buffered saline (PBS) and run in triplicate. After incubation at 4°C and washing with PBS, sEphrinB2-alkaline phosphatase, generated at VasGene Therapeutics, Inc., was added to the wells and incubated at 4°C for 1 hour. After washing with PBS, 100 μL of pNPP substrate (Calbiochem, San Diego, CA) in 50 mmol/L carbonate buffer, pH 9.0, was added to each well. The colorimetric reaction was developed at 37°C for 4 hours and read on a Victor Wallac 1420 96-well multilabel plate reader. Absorbance at 405 nm was read, data transferred into GraphPad Prism (GraphPad, San Diego, CA), and the dissociation constant was calculated with the use of nonlinear regression.
Intraperitoneal pharmacokinetic analysis
Mice (n = 6) were dosed with a single intraperitoneal bolus injection of 10 mg/kg dose of either sEphB4 (545 pmol) or sEphB4-HSA (250 pmol). Plasma samples were obtained at 0.25, 1, 2, 4, 8, 16, 24, 48, and 72 hours. ELISAs were performed on serially diluted plasma samples, and concentrations were obtained from linear regression of standard curves on each ELISA plate. Area under the time-concentration curve was calculated with GraphPad Prism. According to first-order kinetics, the elimination half-life was determined from 0.693/Ke. The elimination constants were determined using the linear slope of the Log concentration–time curves during the elimination phase. All animal studies were performed with the approval of the University of Southern California Keck School of Medicine.
Phosphorylation analysis of EphB4
Endothelial cells were grown in 60-mm dishes until 100% confluence, serum-starved, and treated with recombinant mouse EphrinB2/Fc fusion protein or Fc alone at increasing concentrations for 10 minutes as previously described.14 Lysates were prepared with buffer containing 20 mmol/L Tris-HCl, pH 8.0; 150 mmol/L NaCl; 1% (vol/vol) Triton-X100; 1 mmol/L ethylene diamine tetraacetic acid (EDTA); 1 mmol/L phenylmethylsulphonyl fluoride (PMSF); and 1 mM sodium vanadate and centrifuged at 50 000g for 60 minutes at 4°C. The immunoprecipitated complexes were probed with anti-p-Tyr–specific antibody 4G10. EphB4 precipitation efficiency was tested by probing with EphB4 antibody.
Cell culture
KS-SLK and KS-IMM were cultured as previously described.2 Normal human umbilical vein endothelial cells (HUVECs) and human umbilical arterial ECs (HUAECs) were obtained from Cambrex (Walkersville, MD) and maintained in EGM2-supplemented medium (Invitrogen, Carlsbad, CA). For all experiments, HUVECs were used at passages 4 or below and collected from a confluent dish. HT29 colon cancer cell lines were obtained from ATCC (Manassas, VA) and cultured under recommended conditions.
RT-PCR
First-strand cDNA was synthesized from total RNA with the ImProm-IITM reverse transcription system (Promega, Madison, WI) and used for polymerase chain reaction (PCR) with specific primers (primer pairs used for this study are available on request).15 Equal amounts of cDNA from each cell line were used in each reaction (normalized to actin). Gene-specific amplification consisted of 35 cycles of denaturation at 94°C for 30 seconds, annealing at 60°C for 45 seconds, and extension at 72°C for 1 minute. PCR products were separated on 1.5% agarose gels and stained with ethidium bromide. HT-29 cell line was used as a control. Quantitative PCR analysis was performed as previously described.14
Western blot
Cell lysates were prepared with the use of cell lysis buffer (GeneHunter, Basgvukke, TN) supplemented with protease inhibitor cocktail (Pierce, Rockford, IL). Total protein was determined with the DC reagent system (Bio-Rad, Hercules, CA). Typically, 20 μg of whole-cell lysate was run on 4% to 20% Tris-glycine gradient gel, as previously described.14
Migration assay
SLK cells were seeded onto 6-well plates and cultured in RPMI 1640 until confluent. At time 0, the monolayer of cells was scraped with the use of a sterile pipette tip. The migration process was examined dynamically after treatment with various concentrations of sEphB4 and recorded with a Nikon Coolpix 5000 digital camera (Nikon, Melville, NY) with microscope adapter, as previously described.16,17 Filled area was quantitated in 5 independent fields with the use of ImageJ software from the National Institutes of Health (Bethesda, MD).
Invasion assay
SLK cells (5 × 104) were seeded into 8-μm Matrigel-precoated inserts (BD Bioscience, Palo Alto, CA). The inserts were placed in companion wells containing RPMI supplemented with 5% fetal bovine serum (FBS) and VEGF, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), or fibroblast growth factor (FGF) as chemoattractants. After 22 hours of incubation, the inserts were removed, and the noninvading cells on the upper surface were removed with a cotton swab. The cells on the lower surface of the membrane were fixed in 100% methanol for 15 minutes, air-dried, and stained with Diff-Quik stain for 2 minutes.14 The cells were counted in 5 individual high-powered fields for each membrane under a light microscope.2 Assays were performed in triplicate for each treatment group.
Cell viability assay
KS cells were seeded in 48-well plates at a density of 104 cells/well in a total volume of 500 μL. The medium was changed after cells were attached, and triplicate samples were treated with sEphB4-HSA. Cell viability was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) as described previously.14 Cell apoptosis was also studied by plating cells as described.14 Cells were serum starved overnight and apoptosis was quantified with the use of annexin-V FITC.
Immunohistochemistry and immunofluorescence
Fresh-frozen tissue embedded in OCT was sectioned at 5 μm and fixed in phosphate-buffered 4% paraformaldehyde and washed in PBS. For studies on formalin-fixed paraffin-embedded tissues, 5-μm sections were deparafinized and hydrated, and antigen epitope retrieval was performed by boiling slides in 10 mmol/L sodium citrate buffer (pH 8.5) at 80°C for 20 minutes. Endogenous peroxidase activity was blocked by incubation in 3% H2O2, followed by blocking of nonspecific sites with SuperBlock blocking buffer (Pierce). Sections were then incubated with primary antibody overnight at 4°C. After washing in PBS, antibody binding was localized with appropriate secondary antibodies. Nuclei were counterstained with 6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI). The number of cells staining positive was counted by a blinded observer in 5 random 40× fields and compared by the use of Student t test. Images were obtained with an Olympus AX70 fluorescence microscope and Spot v2.2.2 (Diagnostic Instruments, Sterling Heights, MI) digital imaging system. Apoptosis was detected in deparaffinized sections of tumor samples by TdT-mediated dUTP nick-end labeling (TUNEL) assay using the in situ cell death detection kit (Roche, Piscataway, NJ). Distribution and intensity of hypoxia were studied using the recommend protocol for hypoxyprobe-1 (HP1-100; Chemicon International).
Murine tumor xenograft and metastatic models
KS-SLK and KS-IMM cells were propagated, collected by trypsin digestion, and resuspended in serum-free medium. Cells (2 × 106) were injected in the flank of 10- to 12-week-old male Balb/C athymic mice. Tumor growth was measured 2 times a week and volume estimated as 0.52 × a × b2, where a and b are the largest and smallest lengths of the palpable tumor. On day 4 after cell implantation, tumor volumes were calculated to ensure uniformity in size, and animals were divided randomly into 4 groups (n = 4 mice per group, repeated twice). Each group was administered 3 times per week by intraperitoneal injection of 200 μL of PBS: PBS alone, 10 mg/kg VEGF monoclonal antibody (VEGF moAb; moAb A.4.6.1), 20 mg/kg sEphB4-HSA, or combination of VEGF moAb and sEphB4-HSA at the same dose levels. Animals were killed and tumors and normal organs harvested either after 5 weeks or when tumor volume was considered inhumane. Distribution and intensity of hypoxia were studied with the use of HP1-100 infused intraperitoneally at a dose of 60 mg/kg 1 hour before the tumor harvest. Vessel perfusion was studied using rhodamine-labeled ricinus communis agglutinin 1 (Vector Laboratories) infused 10 to 15 minutes before the tumor harvest and analyzed using the manufacturer's recommended protocol.
For the murine metastatic model, after appropriate anesthesia and sterile alcohol preparation, a left lateral flank incision was made with a no. 15 blade knife in the 8- to 10-week-old male Balb/C athymic mouse. The spleen was exposed through the incision and 106 SLK cells were injected. After 5 minutes, the splenic hilum was ligated, splenectomy was performed, the incision was closed with 4-O silk sutures, and the mouse was recovered. Starting on postoperative day 3, each group was administered 3 times per week by intraperitoneal injection of 200 μL of PBS: PBS alone (11 mice), 10 mg/kg VEGF monoclonal antibody (5 mice), 20 mg/kg sEphB4-HSA (5 mice), or a combination of VEGF moAb and sEphB4-HSA at the same dose levels (5 mice). After 5 weeks of treatment, mice were killed and livers evaluated for gross metastatic deposits. All procedures were approved by our Institutional Animal Care and Use Committees and performed in accordance with the Animal Welfare Act regulations.
Statistical analysis
Data are presented as mean plus or minus SE. Differences in tumor volume in vivo and number of cells staining positively were analyzed with the Student t test, and significance was set at P less than .05.
Results
Expression of the Eph receptors and Ephrin ligands in KS
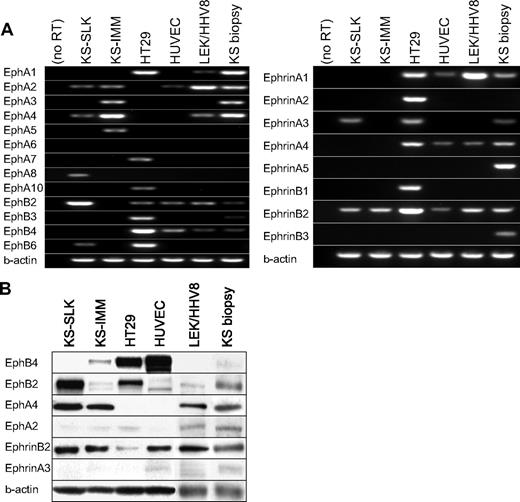
The Eph and Ephrin expression profiles of the KS cell lines KS-SLK and KS-IMM, HHV-8-transformed lymphatic endothelial cells (LEC/HHV8), and primary KS tissue were determined by reverse transcription (RT)–PCR (Figure 1A,B). HT29, a colon cancer cell line, expresses many members of Eph/Ephrin family and, thus, was included as a control. Of the 13 Eph receptors studied, the KS-SLK and KS-IMM cell lines expressed only EphA2, EphA3, EphA4, EphA5, and EphB2. Only 2 Ephrin ligands, EphrinB2 and EphrinA3, were expressed in the KS cell lines. LEC/HHV-8 and primary KS tissue showed similar expression patterns. In addition, EphA1 expression also was noted in LEC/HHV-8 and KS tumor biopsy. EphrinB2 was the only consistently expressed Ephrin ligand in KS cell lines, LEC/HHV-8, and KS biopsy. Furthermore, in protein analysis, EphrinB2 was highly expressed in both KS cell lines and KS tissue, whereas there was no appreciable level of EphrinA3. Thus, of the 8 different ligands, only EphrinB2 is expressed on Western blot. Protein analysis for Eph receptors shows expression of EphB2 and EphA4, both of which are known receptors for EphrinB2. EphA2 was not observed at the protein level. Thus, EphrinB2 and its receptors are the pertinent Eph/Ephrin members of importance in KS.
The Eph and Ephrin expression in KS cell lines KS-SLK and KS-IMM, lymphatic endothelial cells transformed with HHV-8 (LEC/HHV-8), and KS tumor biopsy. (A) The mRNA expression of 13 Eph receptors and 8 Ephrin ligands was determined with RT-PCR. HT29 colon cancer cell line and HUVEC were included as controls. (B) Western blot analysis to for protein expression of selected expressed Eph-Ephrins. β-actin expression was done to show comparable protein loading.
The Eph and Ephrin expression in KS cell lines KS-SLK and KS-IMM, lymphatic endothelial cells transformed with HHV-8 (LEC/HHV-8), and KS tumor biopsy. (A) The mRNA expression of 13 Eph receptors and 8 Ephrin ligands was determined with RT-PCR. HT29 colon cancer cell line and HUVEC were included as controls. (B) Western blot analysis to for protein expression of selected expressed Eph-Ephrins. β-actin expression was done to show comparable protein loading.
HUVEC expression analysis demonstrated abundant expression of EphB4 by RT-PCR and immunoblotting. In addition, EphB2, EphA2 and low levels of EphrinB2 were expressed in endothelial cells, with very little expression of other Eph/Ephrin members (Figure 1A). EphrinB2 from KS cells or adjacent endothelial cells can activate Eph receptors in KS. Furthermore, EphrinB2 from endothelial cells may activate EphB4 on adjacent vascular endothelial cells and regulate a vascular response.13 Inhibition of the EphrinB2 interaction with its cognate receptors with the use of the soluble form of EphB4 with improved pharmacokinetics was thus studied further. sEphB4-HSA was chosen for its specificity for EphrinB2 and lack of binding to any other Ephrin ligand.13
Expression and purification of the soluble form of EphB4 fused to HSA
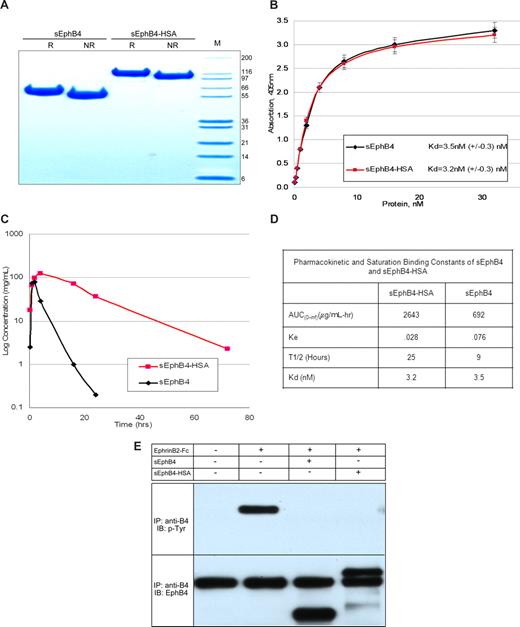
To develop a long-acting form of sEphB4, we choose to fuse the coding region of the extracellular domain of EphB4 and human serum albumin. This fusion protein (sEphB4-HSA) has the potential to provide long retention time in the circulation while retaining the antagonistic function of monomeric sEphB4. The fusion protein was thus expressed in CHO cells and purified from the supernatant to purity greater than 95% (Figure 2A). When the sEphB4-HSA binding kinetics were compared directly with sEphB4 in saturation binding studies, the affinity of the fusion protein to sEphrinB2-AP was not adversely affected, as indicated by the comparable dissociation constants (Figure 2B). The sEphB4-HSA fusion protein also retained activity to inhibit EphB4 phosphorylation upon treatment with dimeric EphrinB2. Recombinant sEphB4-HSA was tested for its ability to block EphrinB2-induced EphB4 phosphorylation in HUVECs13 and MCF7 cells (Figure 2B). Pharmacokinetic studies of the purified protein were conducted in mice and compared with sEphB4 lacking albumin. Circulation half-life for sEphB4-HSA was 25 hours compared with 9 hours for sEphB4; sEphB4-HSA area under the curve was 3.8-fold greater than sEphB4 (Figure 2C,D). From here on, all studies were completed with the albumin fusion derivative of sEphB4.
Expression and characterization of sEphB4 and sEphB4-HSA. (A) sEphB4-HSA was expressed in CHO cells and purified to near homogeneity and separated on SDS-PAGE (Coomassie staining) under reducing and nonreducing conditions. (B) Saturation binding kinetics of sEphB4 and sEphB4-HSA in a solid-phase ELISA. Interaction of increasing concentrations of sEphB4 or sEphB4-HSA with sEphrinB2-AP was determined in a solid-phase ELISA. Each point was determined in triplicate. Dissociation constants were calculated with the use of nonlinear regression and Graphpad Prism. (C) Systemic pharmacokinetics of sEphB4 and sEphB4-HSA administered intraperitoneally. Mice were injected with a 10 mg/kg dose of either sEphB4 or sEphB4-HSA administered intraperitoneally. Each point represents the average of 2 separate experiments. Error bars represent the SEM. (D) Pharmacokinetic and saturation binding constants of sEphB4 and sEphB4-HSA. (E) Tyrosine phosphorylation of EphB4 receptor in MCF7 cells in response to stimulation with EphrinB2-Fc (15 minutes) in the absence or presence of EphB4-derived soluble proteins.
Expression and characterization of sEphB4 and sEphB4-HSA. (A) sEphB4-HSA was expressed in CHO cells and purified to near homogeneity and separated on SDS-PAGE (Coomassie staining) under reducing and nonreducing conditions. (B) Saturation binding kinetics of sEphB4 and sEphB4-HSA in a solid-phase ELISA. Interaction of increasing concentrations of sEphB4 or sEphB4-HSA with sEphrinB2-AP was determined in a solid-phase ELISA. Each point was determined in triplicate. Dissociation constants were calculated with the use of nonlinear regression and Graphpad Prism. (C) Systemic pharmacokinetics of sEphB4 and sEphB4-HSA administered intraperitoneally. Mice were injected with a 10 mg/kg dose of either sEphB4 or sEphB4-HSA administered intraperitoneally. Each point represents the average of 2 separate experiments. Error bars represent the SEM. (D) Pharmacokinetic and saturation binding constants of sEphB4 and sEphB4-HSA. (E) Tyrosine phosphorylation of EphB4 receptor in MCF7 cells in response to stimulation with EphrinB2-Fc (15 minutes) in the absence or presence of EphB4-derived soluble proteins.
Effects of sEphB4-HSA on KS cell migration and invasion
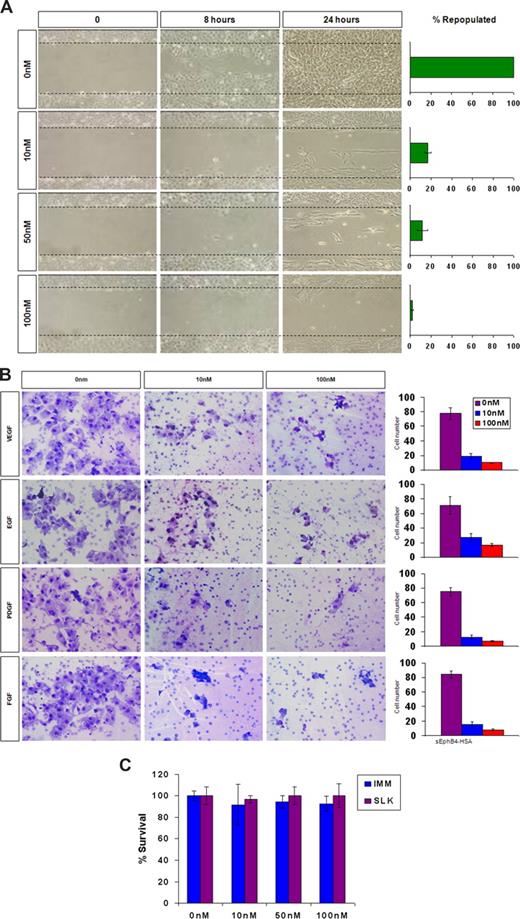
Given that EphrinB2 is the only major Eph ligand expressed in KS cells, we studied the effect of blocking EphrinB2 binding to various receptors on adhesion, migration, and invasion. Specifically, we determined whether sEphB4-HSA affects KS cell migration, invasion, and proliferation. KS cells grown to confluence were wounded and treated with sEphB4-HSA or diluents, and the occupancy of the cell free zone was examined over time with photography. sEphB4-HSA markedly inhibited the migration of KS cells to occupy the cell free zone, decreasing from 100% confluence at 24 hours with no treatment to 17% confluence with 10 nm of sEphB4-HSA, to 2% confluence with 100 nm of sEphB4-HSA (Figure 3A).
sEphB4-HSA activity in KS migration, invasion, and survival. (A) KS-SLK cells were grown to confluence, scraped, and treated with varied concentrations of sEphB4-HSA. Cell migration in the clear zone was documented by photographs at various time points at 20× fields. (B) KS cell invasion in response to growth factors. Modified Boyden chamber assay was used to determine KS cell invasion across Matrigel-precoated inserts. Data are presented as number of invading cells plus or minus SE from duplicate wells in 2 experiments. (C) Cell viability assay. KS cells were grown in triplicate in the presence of increasing concentrations of sEphB4-HSA for 72 hours. Cell viability was assessed by MTT assay. The experiment was repeated twice with similar results. Photomicrographs in panels A and B were taken with a Nikon Coolpix 5000 camera (Nikon, Tokyo, Japan) and a Carl Zeiss Invertoskop microscope (Zeiss, Goettingen, Germany) with a 4×/0.12 NA objective and 10× eyepiece.
sEphB4-HSA activity in KS migration, invasion, and survival. (A) KS-SLK cells were grown to confluence, scraped, and treated with varied concentrations of sEphB4-HSA. Cell migration in the clear zone was documented by photographs at various time points at 20× fields. (B) KS cell invasion in response to growth factors. Modified Boyden chamber assay was used to determine KS cell invasion across Matrigel-precoated inserts. Data are presented as number of invading cells plus or minus SE from duplicate wells in 2 experiments. (C) Cell viability assay. KS cells were grown in triplicate in the presence of increasing concentrations of sEphB4-HSA for 72 hours. Cell viability was assessed by MTT assay. The experiment was repeated twice with similar results. Photomicrographs in panels A and B were taken with a Nikon Coolpix 5000 camera (Nikon, Tokyo, Japan) and a Carl Zeiss Invertoskop microscope (Zeiss, Goettingen, Germany) with a 4×/0.12 NA objective and 10× eyepiece.
We then performed an in vitro invasion assay to measure KS cell ability of to degrade basement membrane and migrate toward a growth factor stimulus. Various growth factors, including FGF, VEGF, EGF, and PDGF, were used as chemoattractants. sEphB4-HSA markedly inhibited the invasion of KS cells in response to each of the growth factors; at 100 nm of sEphB4-HSA, a 5- to 10-fold reduction was demonstrated. Thus, sEphB4-HSA is active regardless of the growth factor used (Figure 3B). Growth factors increased the attachment of KS cells; however, sEphB4-HSA treated cells markedly reduced binding (data not shown). Finally, sEphB4-HSA was found to have no appreciable cytotoxic effect on KS cells as assessed by MTT (Figure 3C).
sEphB4-HSA inhibits KS tumor growth in a murine tumor xenograft model
sEphB4-HSA inhibits the EphrinB2 interaction with Eph receptors on both other KS tumor cells, as well as the Eph receptors expressed on newly formed vessels in vivo. We next studied the in vivo effect of sEphB4-HSA on KS cells as well as the new host vessels growing into the tumor using a KS tumor xenograft model (Figure 4A).
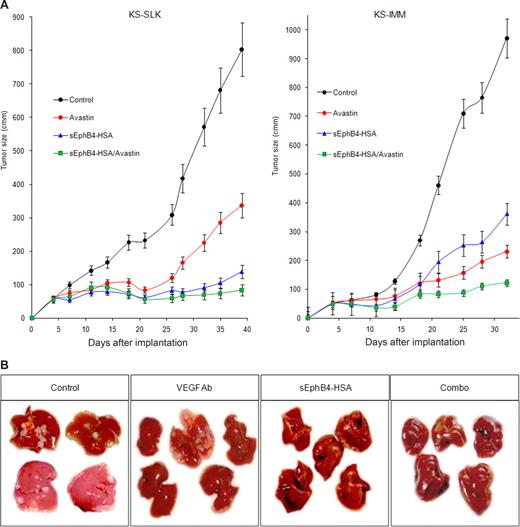
sEphB4-HSA inhibits KS tumor growth in a murine tumor xenograft model. (A) Mice (n = 8/group) were implanted with 2 × 106 KS-SLK or KS-IMM cells and treated with sEphB4-HSA, VEGF moAb, or combination sEphB4-HSA/VEGF moAb; tumor volumes were measured 2 times a week; and the data are presented as tumor volume. After 5 weeks, tumor volumes were as follows; sEphB4-HSA (KS-SLK = 15.9% of control, P < .001; KS-IMM = 37.2% of control, P < .001) or combination sEphB4-HSA plus VEGF moAb (KS-SLK = 12.1% of control, P < .001; KS-IMM = 12.6% reduction, P < .001). (B) Mice spleens were injected with 106 SLK cells and treated with sEphB4-HSA, VEGF moAb, or combination sEphB4 plus VEGF moAb. After 5 weeks, livers were harvested and examined for the number of tumor metastases (tumors/livers) and are as follows; control (8/11), VEGF moAb (2/5), sEphB4-HSA (2/5), and combination of sEphB4-HSA and VEGF moAb (0/5).
sEphB4-HSA inhibits KS tumor growth in a murine tumor xenograft model. (A) Mice (n = 8/group) were implanted with 2 × 106 KS-SLK or KS-IMM cells and treated with sEphB4-HSA, VEGF moAb, or combination sEphB4-HSA/VEGF moAb; tumor volumes were measured 2 times a week; and the data are presented as tumor volume. After 5 weeks, tumor volumes were as follows; sEphB4-HSA (KS-SLK = 15.9% of control, P < .001; KS-IMM = 37.2% of control, P < .001) or combination sEphB4-HSA plus VEGF moAb (KS-SLK = 12.1% of control, P < .001; KS-IMM = 12.6% reduction, P < .001). (B) Mice spleens were injected with 106 SLK cells and treated with sEphB4-HSA, VEGF moAb, or combination sEphB4 plus VEGF moAb. After 5 weeks, livers were harvested and examined for the number of tumor metastases (tumors/livers) and are as follows; control (8/11), VEGF moAb (2/5), sEphB4-HSA (2/5), and combination of sEphB4-HSA and VEGF moAb (0/5).
KS-SLK and KS-IMM cells (2 × 106 cells) were injected into the flank of 8- to 10-week old male Balb/C athymic mice. On day 4 after cell implantation, mice were randomly placed into 4 groups. Each group was then randomly chosen for systemic treatment 3 times a week with PBS (negative control), VEGF moAb (positive control), sEphB4-HSA, or combined sEphB4-HSA and VEGF moAb. Relative to PBS, sEphB-HSA inhibited tumor growth in both SLK and IMM tumors, with tumor volume reduced to 15.9% and 37.2% of control, respectively. sEphB4-HSA was more active in SLK tumors compared with VEGF moAb (P < .01) and it was equally as active as VEGF moAb in IMM tumors (P > .05). The combination of sEphB4-HSA and VEGF moAb was superior to each alone in both tumor types, with tumor volumes of 12.1% for KS-SLK (P < .001) and 12.6% for KS-IMM (P < .001).
KS is known to involve the GI tract, liver, and lungs. We thus studied metastasis of KS to the liver as a representative metastatic model. SLK cells (106) were injected into the spleens of 8- to 10-week-old male Balb/C athymic mice exposed by a flank incision. Splenectomy was performed after 5 minutes and mice were recovered. sEphB4-HSA was administered 3 times a week for a period of 5 weeks, and liver metastasis was quantified by visual examination. Metastatic deposits were noted in the livers of 8 of 11 mice treated with PBS, 2 of 5 mice treated with VEGF moAb (P = NS), 2 of 5 mice treated with sEphB4-HSA (P = NS), and 0 of 5 mice treated with sEphB4-HSA/VEGF moAb (P = .004, Figure 4B). Liver metastases were smaller in size in the mice receiving sEphB4-HSA or VEGF moAb individually. These results demonstrate the activity of each agent alone and the additive activity from combination therapy.
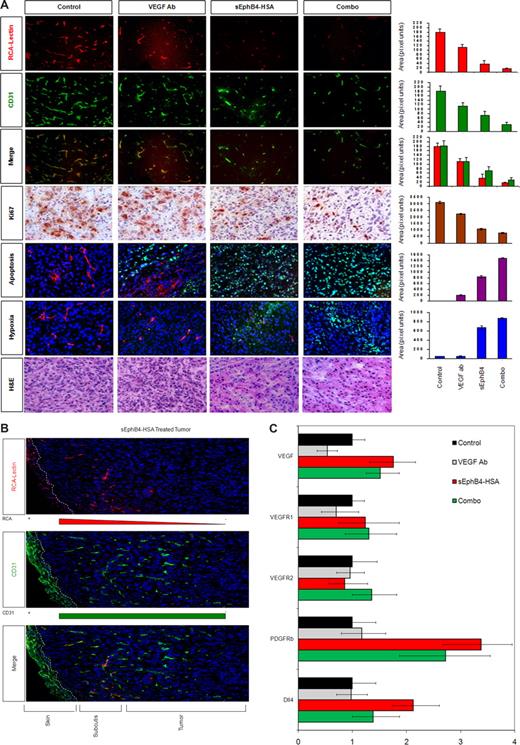
sEphB4-HSA affects tumor vessel density and perfusion
Tumors were harvested and examined for tumor vessel perfusion and tumor vessel growth. Vascular perfusion was determined by injecting fluorescent-labeled lectin 10 to 15 minutes before sacrificing the mice and harvesting KS tumors and lectin was localized to the perfused areas. Blood vessel density was delineated with platelet/endothelial cell adhesion molecule (PECAM) staining. Lectin and PECAM colocalized in the control group, whereas the treatment groups showed marked deficiency of perfusion and vessel density (Figure 5A). Adjacent normal tissue vessel density and perfusion were unaffected by sEphB4-HSA. Deeper tumor tissue shows progressive decrease in vessel density and even greater decrease in vessel perfusion (Figure 5B). Vessel density decreased with sEphB4-HSA (38.6% of control), VEGF moAb (62.5% of control), and even more with combination treatment (16.9% of control). Perfusion also decreased with sEphB4-HSA (20.6% of control) and VEGF moAb (61.7% of control; Figure 5A). Notably, after VEGF moAb treatment, the remaining vessels perfused well. In sharp contrast among the sEphB4-HSA treatment group, only a small fraction of the vessels showed perfusion, indicating a lack of maturation or collapse of the poorly formed vessels. In both instances, combination treatment with sEphB4-HSA and VEGF moAb decreased both vessel perfusion and vessel density more than sEphB4-HSA or VEGF moAb alone.
Analysis of vascular perfusion, vessel density, tumor cell proliferation, apoptosis and hypoxia. (A) Tumors were harvested at completion of the study and examined by hematoxylin and eosin staining. Just before harvest, mice were infused with RCA-Lectin and hypoxia probe. Nuclei were counterstained with DAPI. RCA-Lectin localized the perfused vessels, CD31 localized microvascular endothelial cells, and the merged picture shows perfusion of total vessels in the field. Quantitation was performed with the use of Bioquant Image Analysis (Bioquant, Nashville, TN). Proliferating cells within the tumor were assessed by immunohistochemical detection of Ki-67 protein and quantified as described. All values are expressed as mean plus or minus SEM. *P < .01 by Student t test. Ki-67 pictures were taken with Carl Zeiss Invertoskop microscope with a 4×/0.12 NA objective and 10× eyepiece. Photomicrographs were taken using a Nikon Coolpix 5000 camera and a Nikon Eclipse E400 microscope with a 10× eyepiece. Magnification was as 40×/0.75 NA objectives. (B) sEphB4-HSA–treated tumor and adjacent normal tissue vessel density and perfusion. Dotted line demarcates the skin showing autofluorescence. Vessels in the subcutaneous tissue (subcutis) and the margin of the tumor show perfusion. Deeper tumor tissue shows progressive decrease in the vessel density and even greater decrease in vessel perfusion. (C) Gene expression analysis of tumor tissues was performed by quantitative PCR for VEGF, VEGFR1, VEGFR2, PDGF-β, and Dll4. Gene expression levels were corrected for β-actin levels.
Analysis of vascular perfusion, vessel density, tumor cell proliferation, apoptosis and hypoxia. (A) Tumors were harvested at completion of the study and examined by hematoxylin and eosin staining. Just before harvest, mice were infused with RCA-Lectin and hypoxia probe. Nuclei were counterstained with DAPI. RCA-Lectin localized the perfused vessels, CD31 localized microvascular endothelial cells, and the merged picture shows perfusion of total vessels in the field. Quantitation was performed with the use of Bioquant Image Analysis (Bioquant, Nashville, TN). Proliferating cells within the tumor were assessed by immunohistochemical detection of Ki-67 protein and quantified as described. All values are expressed as mean plus or minus SEM. *P < .01 by Student t test. Ki-67 pictures were taken with Carl Zeiss Invertoskop microscope with a 4×/0.12 NA objective and 10× eyepiece. Photomicrographs were taken using a Nikon Coolpix 5000 camera and a Nikon Eclipse E400 microscope with a 10× eyepiece. Magnification was as 40×/0.75 NA objectives. (B) sEphB4-HSA–treated tumor and adjacent normal tissue vessel density and perfusion. Dotted line demarcates the skin showing autofluorescence. Vessels in the subcutaneous tissue (subcutis) and the margin of the tumor show perfusion. Deeper tumor tissue shows progressive decrease in the vessel density and even greater decrease in vessel perfusion. (C) Gene expression analysis of tumor tissues was performed by quantitative PCR for VEGF, VEGFR1, VEGFR2, PDGF-β, and Dll4. Gene expression levels were corrected for β-actin levels.
sEphB4-HSA decreases proliferation and increases apoptosis and hypoxia in KS murine tumor xenograft model
Hypoxia in situ (HP1-100) analysis showed that sEphB4-HSA treatment resulted in increased areas of tumor hypoxia. Both sEphB4-HSA and VEGF moAb decreased tumor cell proliferation as evaluated by Ki-67 staining (a 2- to 3-fold reduction in proliferative cells). Apoptosis was measured by TUNEL assay and combination therapy demonstrated a marked increase in TUNEL-positive cells consistent with induction of apoptosis.
sEphB4-HSA treatment affects regulators of blood vessel formation
We next examined the differential regulation of the VEGF family of receptors as well as the VEGF signaling proteins in response to sEphB4-HSA treatment. The levels of the VEGF, VEGFR1, and VEGFR2 levels in KS tumors were assessed by RT-PCR after treatment with VEGF moAb, sEphB4-HSA, and combination sEphB4-HSA/VEGF moAb. Treatment with VEGF moAb minimally decreased the levels of VEGF, whereas treatment with sEphB4-HSA increased VEGF levels, most likely from the marked increase in hypoxia. However, combination treatment with sEphB4-HSA and VEGF moAb still resulted in high VEGF levels (Figure 5C). Treatment with VEGF MoAb sEphB4-HSA alone or in combination did not affect VEGFR1 and VEGFR2 levels. EphB4-HSA also showed increase in murine Dll4 (mDll4), which is induced by VEGF (Figure 5C). Although VEGF MoAB had no effect, sEphB4-HSA also showed marked increase in PDGFR-β levels, an increase that was sustained even in the combination group (Figure 5C). PDGFR-β is expressed on pericytes and regulates pericyte recruitment to endothelial cells. sEphB4-HSA–treated tumors have poorly formed blood vessels. An increase in PDGFR-β may be caused by feedback from defective vessel maturation
Discussion
Eph is the largest family of receptor tyrosine kinases, with 14 members and 8 ligands. The Eph-Ephrin interaction plays important functions in morphogenesis, affecting nervous system, pancreas, GI, and genitourinary tract development.6,18-20 In vasculogenesis and angiogenesis, EphB4 and EphrinB2 play critical roles and notably lack redundancy in their important functions. A comprehensive review of the Eph–Ephrin family in KS demonstrated expression of limited members. Notably, only EphrinB2 is expressed at substantial levels in all KS cell lines, HHV-8–transformed lymphatic endothelial cells (LEC/HHV8), and KS tissue. In addition, EphrinB2 was previously reported in lymphatic endothelial cells, which does not contradict the lineage studies in KS.21 EphrinB2 is also induced by HHV-8 and a number of growth factors for KS, including VEGF, VEGF-C, and interleukin-8.1,2,4 EphrinB2 can bind several Eph receptors, of which EphB2 and EphA4 are expressed on KS cells, whereas EphB4 is expressed in the vessels. These findings suggested that EphrinB2 plays an important role in KS biology. To address this question, we used the soluble extracellular domain of EphB4 as an antagonist. sEphB4 only binds EphrinB2 and, thus, its function is highly predictable. However, this binding characteristic prevents EphrinB2 from binding to any other receptor. We have shown previously that sEphB4 is a highly active antagonist of the EphB4/EphrinB2 interaction.13 sEphB4 has suboptimal pharmacokinetics in mice. We thus developed an optimal version of sEphB4 by fusing it with the human albumin coding region placed on its C-terminus. This protein retains binding and functions as an antagonist to EphrinB2. Furthermore, the kinetics in the mouse are markedly improved such that sEphB4-HSA can be considered suitable for human clinical development.
To test the consequences of blocking EphrinB2 function in KS, we first conducted studies in vitro. sEphB4-HSA demonstrated a dramatic effect on the migration of KS cells in vitro. Furthermore, sEphB4-HSA inhibited the invasion of KS cells across matrix proteins, especially when stimulated with growth factors that promote KS growth and migration, including VEGF, bFGF, EGF, and PDGF-B. We have shown previously that sEphB4 inhibits angiogenesis in response to various growth factors. Given that KS cells express EphrinB2, which is rare among cancers, its interaction with EphB4 will further enhance angiogenesis. Thus, in vivo studies of sEphB4-HSA were expected to be highly effective. This finding indeed was the case; sEphB4-HSA is very active in vivo.
Given that VEGF inhibitors are active in many cancers and that KS is among the highest VEGF producers, we compared a VEGF antibody to sEphB4-HSA in vivo. sEphB4-HSA was substantially more active than VEGF moAb in KS-SLK and equally active in KS-IMM. A combination of the 2 has at least additive activity. This finding is not surprising, because sEphB4-HSA induced severe hypoxia in the tumor tissue and increased VEGF expression. The combination of VEGF inhibitor and sEphB4-HSA is thus a potent combination. sEphB4-HSA also increased mDll4 levels, which is consistent with the induction of VEGF and hypoxia both which induce Dll4. mDll4 expression in tumor vessels may inhibit VEGFR expression and thus inhibit VEGF activity and promote vessel maturation. As expected, the combination of sEphB4-HSA with VEGF antibody reduced mDll4 levels.
KS is lethal when spread to vital organs, including the lungs, GI tract, and liver; because sEphB4-HSA had profound effects on KS cell migration and invasion, we envisioned sEphB4-HSA would reduce the ability of KS to spread to distant sites. To test this function, we used a model of tumor cell migration, invasion, and growth by injecting KS-SLK cells into the spleen and monitoring for metastasis in the liver. KS metastasis indeed grew in the liver and sEphB4-HSA and VEGF moAb markedly reduced these metastasis to the liver. The combination of sEphB4-HSA and VEGF moAb was remarkable for complete blockade of liver metastasis.
In summary, the monomeric form of sEphB4-HSA ectodomain displays the following: (1) it functions as an EphrinB2 antagonist; (2) it has markedly improved pharmacokinetics compared with sEphB4; (3) it inhibits KS adhesion, migration, and invasion; (4) it inhibits the activity of several growth factors, including VEGF, bFGF, EGF, and PDGF; (5) it inhibits KS tumor growth with marked reduction in vessel density, vessel maturity, and vessel perfusion; and (6) it is comparable to or better than VEGF moAb in KS treatment with additive effect in combination. sEphB4-HSA is a candidate for investigation of how it prevents vessel maturation and for consideration for clinical investigation in KS and possibly other cancers.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Cancer Institute (RO1 CA 079218-07 to P.S.G.).
National Institutes of Health
Authorship
Contribution: E.J.L., J.S.S., and P.S.G. designed the studies,; E.J.L. and P.S.G. wrote the paper; and J.S.S., E.J.L., V.K., R.L., P.K.M., E.S., A.K., S.G., S.R.K., and P.S.G. performed research and analyzed data.
Conflict-of-interest disclosure: V.K. and E.S. are employees of Vasgene Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Parkash S. Gill, University of Southern California, Norris Hospital, NOR 6332, 1441 Eastlake Avenue, Los Angeles, CA 90033; e-mail: parkashg@usc.edu.
References
Author notes
*J.S.S. and E.J.L. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal