To the editor:
We read the recent article by Wakkach et al1 regarding differentiation of murine dendritic cells (DCs) into osteoclasts (OCs) in bone marrow (BM) microenvironment in vivo, with different views to stimulate clarification before any conclusions may be drawn.
The authors report that CD11c+ dendritic cells (cDCs) with “100% purity,” carrying a “mature” phenotype of MHC-II+CD80+/CD86+, can differentiate into OCs in vitro and in vivo. Caution should be exercised as potential contaminating OC precursors (OCPs) may alter the observed results, whereas the exclusion of Ly-6C and F4/80 expressions on cDCs is not sufficient to completely eliminate monocyte/macrophage contaminants that may contribute to the rescued phenotype observed (ie, CD11c+ monocytes reported2 ). We and others3,4 showed that only “immature” cDCs (MHC-IIloCD80−CD86−) carry osteoclastogenic potential and that upon maturation this potential is lost even if receptor activator NFKB ligand (RANKL) was provided thereafter (Figure 1 below). In our studies, both splenic and BM-derived CD11c+ DCs act as OCPs based on phenotypic and functional analyses in vitro/in vivo.4,5 Wakkach et al report that cDC maturation via activation of Toll-like receptors does not alter their osteoclastogenic potential, where no evidence is provided nor discussed based on previous opposite reports.4-6 Splenic DCs were known to become apoptotic in less than 3 days upon maturation in vitro/in vivo,7,8 whereas the authors detected TRAP+ multinucleated OCs after 7 days' culture of mature cDCs, requiring explanation. Whether macrophage-colony stimulating factor (M-CSF) can rescue such osteoclastogenic potential of mature DCs as described becomes another issue, yet to be further addressed.
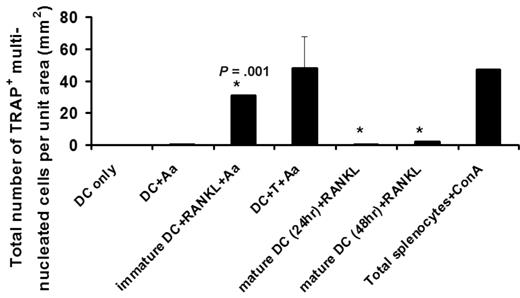
Immature but not mature CD11c+ DCs carry the osteoclastogenic potential. Freshly purified CD11c+ DCs (immature) or bacteria Aggregatibactor actinomycetemcomitans (Aa)–treated DCs for 24 or 48 hours (mature) were cultured with 30 to 100 ng/mL soluble receptor activator nb NFKB ligand (sRANKL) in the presence of 10 μg/mL Aa sonicated Ag (see Alnaeeli4 ). On day 5, the total number of TRAP+ multinucleated DC-derived OCs developed per unit area were quantified, using the protocol reported previously4 where the results are shown as mean plus or minus SE with *P = .001 (N = 5 experiments), and DC-only and DC + Aa as negative controls and Splenocytes + ConA as positive control.
Immature but not mature CD11c+ DCs carry the osteoclastogenic potential. Freshly purified CD11c+ DCs (immature) or bacteria Aggregatibactor actinomycetemcomitans (Aa)–treated DCs for 24 or 48 hours (mature) were cultured with 30 to 100 ng/mL soluble receptor activator nb NFKB ligand (sRANKL) in the presence of 10 μg/mL Aa sonicated Ag (see Alnaeeli4 ). On day 5, the total number of TRAP+ multinucleated DC-derived OCs developed per unit area were quantified, using the protocol reported previously4 where the results are shown as mean plus or minus SE with *P = .001 (N = 5 experiments), and DC-only and DC + Aa as negative controls and Splenocytes + ConA as positive control.
Wakkach et al report 100% detection of TRAP+ cells in cDC cocultures, 80% of which appear multinucleated osteoclast like cells (OCLs) without quantitative data for substantiation, particularly considering that such a high level of osteoclastogenesis hasn't been observed in DCs2-4 or classic OCPs. In Wakkach et al's Figure 2C, “160” TRAP+ multinucleated cells/cm2 are detected when 25 000 CD11c+ DCs are cultured per 48-well/plate (1 cm2-surface area/well). If so, how can this “80%” yield be accounted for? The proliferation of splenic cDCs cannot be the explanation because 100% pure cDCs are “mature” and can no longer proliferate. This raises concerns regarding the presence/contribution of contaminating CD11c+ and/or CD11c− OCPs.
To directly substantiate and support the findings that injected CFSE+-CD11c+ DCs and those of Diphtheria Toxin Receptor/Green flourescent protein mice are indeed the ones that develop into functional OCs in vivo, dual labeling via immunohistochemistry or immunofluorescence is required. The “singly stained images” presented in their Figure 4C and D suffer (1) low yield of positively stained cells for quantification, (2) insufficient explanation regarding the relatively low resorptive activity detected in the “DC-treated oc/oc” group (Figure 4D) and rescued phenotypes measured in their Figures 3A through D and 4C, yet the authors state that there is a spectacular recovery/restoration of bone resorption activity in 50% of the DC-treated mice in vivo (their Figure 3D,E), where in vivo control for “DC-treated oc/oc” with in vivo DC depletion in their Figure 4D is lacking. Therefore, the interpretation of Wakkach et al's Figure 4 that “functional OCL recovered from DC-treatedoc/oc mice have differentiated from injected splenic cDCs” is not fully substantiated, and other interpretations must be considered.
Wakkach and colleagues report that in oc/oc mice, BM stromal cells constitute the main RANKL source and are controlled by inflammatory CD4+ T cells, solely based on the correlation of relative cytokine gene expressions (their Figures 5C,D and 6B-D). Yet no direct evidence (ie, protein production/secretion) is provided to fully substantiate this in vivo conclusion. Particularly, it remains unclear whether oc/oc mice have more BM CD4+ T cells. Our studies support that as long as M-CSF and RANKL are provided, “immature” CD11c+ DCs can develop into OCs via an alternative pathway of osteoclastogensis proposed.4,5 Although CD4+ T cell–depletion analyses support an important role for CD4+ T cells in RANKL production (Figure 5C), the result presented in their Figure 6E is only relative with the use of oc/oc T cells without proper positive control to support the conclusion.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments: This work was supported by grants to Y.-T.A.T. from the Canadian Institute of Health Research, the University of Rochester, and the National Institutes of Health (DE015786, 018356).
Correspondence: Dr Yen-Tung Andy Teng, Eastman Dental Center and Department of Microbiology and Immunology, School of Medicine and Den-tistry, University of Rochester, Rochester, NY 14620; e-mail: andy_teng@urmc.rochester.edu.
References
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal