Abstract
Homing of endothelial progenitor cells (EPCs) to the neovascular zone is now considered to be an essential step in the formation of vascular networks during embryonic development and also for neovascularization in postnatal life. We report here the prominent role of the insulin-like growth factor 2 (IGF2)/IGF2 receptor (IGF2R) system in promoting EPC homing. With high-level expression of IGF2R in EPCs, IGF2-induced hypoxic conditions stimulated multiple steps of EPC homing in vitro and promoted both EPC recruitment and incorporation into the neovascular area, resulting in enhanced angiogenesis in vivo. Remarkably, all IGF2 actions were exerted predominantly through IGF2R-linked G(i) protein signaling and required intracellular Ca2+ mobilization induced by the β2 isoform of phospholipase C. Together, these findings indicate that locally generated IGF2 at either ischemic or tumor sites may contribute to postnatal vasculogenesis by augmenting the recruitment of EPCs. The utilization of the IGF2/IGF2R system may therefore be useful for the development of novel means to treat angiogenesis-dependent diseases.
Introduction
Postnatal tissue neovascularization is an important adaptation for rescue from critical ischemia and is required for sufficient blood supply to growing tumors. This process was formerly attributed to the migration and proliferation of pre-existing, fully differentiated endothelial cells (ECs) in a process called angiogenesis. Recent studies have shown that circulating bone marrow (BM)–derived endothelial progenitor cells (EPCs) are recruited to the site of tissue regeneration and substantially contribute to neovascularization and re-endothelialization after acute vascular injury.1,2 Intravenous infusion of EPCs after ischemia was shown to improve neovascularization and cardiac function in both animal models and pilot clinical studies. Conversely, BM-derived EPCs participate in the pathogenesis of various diseases, such as cancer, retinopathy, and atherosclerosis.2,3 Impaired recruitment of BM-derived endothelial and hematopoietic precursor cells is known to block both tumor angiogenesis and growth.4 Injection of BM cells promotes injury-associated retinal angiogenesis.5 BM-derived progenitors also contribute to the formation of the microvasculature in allograft arteriosclerotic lesions.6 Therefore, EPCs have both physiologic and pathologic roles; thus, they have been widely considered for establishing new therapeutic strategies for the treatment of angiogenesis-related diseases
The number of circulating EPCs may limit the ultimate magnitude of angiogenesis therapies and, therefore, strategies that are based on the administration of ex vivo–expanded populations of EPCs harvested from the patient's circulating blood appear promising.7 In addition, increased recruitment and specific lodging of EPCs in ischemic tissues may constitute another therapeutic target. Likewise, mechanisms to prevent EPC homing show promise as therapeutic strategies for the treatment of excessive neovascularization. Recently, stromal cell-derived factor-1 (SDF-1), a member of the chemokine CXC subfamily, was identified as an important factor for the trafficking of EPCs to ischemic tissues; it has also been recognized as a prominent target for increasing the engraftment of progenitor cells.8,9 SDF-1 secreted from carcinoma-associated fibroblasts promotes angiogenesis by recruiting EPCs to tumor tissue.3 Furthermore, β2-integrin expressed in EPCs was shown to mediate augmentation of the homing and neovascularization capacity of EPCs in vitro and in vivo.10 CXCR2 also increased the homing of circulating EPCs to the sites of artery injury, possibly by enhancing adhesion of EPCs to extracellular matrix coimmobilized with chemokines.11,12 Therefore, the identification of novel, endogenous factors that lead to both the mobilization and homing of EPCs in response to tissue hypoxia is important for the understanding of the functional contribution of EPCs to postnatal neovascularization and to the establishment of new strategies for the treatment of angiogenesis-related diseases. Here, we report a novel role and underlying signaling mechanism for the insulin-like growth factor 2 (IGF2)/IGF2 receptor (IGF2R) system in both recruiting and incorporating EPCs to ischemic sites.
Methods
Micorarray data as described in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) has been deposited with Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE12891.
Isolation and cultivation of EPCs
We isolated EPCs from human umbilical cord blood (HUCB). HUCB samples (∼50 mL each) were collected from fresh placentas with attached umbilical cords by gravity flow. The protocol of the study was approved by Yonsei University Severance Hospital, and informed consent was obtained in accordance with the Declaration of Helsinki. EPCs were isolated by density gradient centrifugation over Biocoll (Biochrom, Berlin, Germany) for 30 minutes at 400g and washed 3 times in PBS (Biochrom). One million cells were seeded onto 6-well plates coated with human fibronectin (Sigma-Aldrich, St Louis, MO) in endothelial basal medium-2 (EBM-2; Clonetics, Cell Systems, St Katharinen, Germany). The medium was supplemented with endothelial growth medium-2 (EGM-2; Clonetics, Cell Systems) containing fetal bovine serum, human VEGF-A, human fibroblast growth factor-B, human epidermal growth factor, insulin-like growth factor 1 (IGF1), and ascorbic acid in appropriate amounts. After 3 days, nonadherent cells were removed, and fresh culture medium was applied. Cultures were maintained for 7 days. Phenotypic analyses of the cells were performed on days 3, 5, and 7. EPC identification and estimation of culture purity (90%-95%) were determined by staining cells with FITC-labeled Ulex europaeus lectin I (UEA-1; Vector Laboratories, Burlingame, CA) and uptake of Dil-conjugated acetylated low-density lipoprotein (acLDL; Molecular Probes, Leiden, The Netherlands).
Real-time polymerase chain reaction analysis
Total RNA (5 μg) isolated from EPCs with Trizol reagent (Invitrogen, Carlsbad, CA) was used for cDNA synthesis by SuperScript II reverse transcriptase (RT; Invitrogen). The abundance of transcripts in the cDNA samples was measured by real-time polymerase chain reaction (PCR) with specific primers according to the manufacturer's instructions. The reactions contained 1× SYBR Green mix (Invitrogen), 10 pmol of both forward and reverse primers, and cDNA corresponding to 0.1 μg total RNA. The reactions were subjected to 40 cycles of PCR amplification (95°C for 15 seconds and 55°C for 1 minute) in a Bio-Rad (Hercules, CA) iCycler and detection system. All results were normalized to GAPDH mRNA. The primers used are described in Table S2.
Hypoxia experiments
For normoxia experiments, the cancer cells (MDA-MB-435) and human umbilical vein endothelial cells (HUVECs) were cultured at 37°C in humidified 5% CO2/95% air, and, for hypoxia experiments, the cancer cells (MDA-MB-435) and HUVECs were cultured at 37°C with 5% CO2, 94% N2, and 1% O2 in a multigas incubator (Juji Field, Tokyo, Japan). Culture in hypoxic conditions was performed for 24 hours. To evaluate hypoxic effects in culture of cancer cells (MDA-MB-435) and HUVECs, vascular endothelial growth factor (VEGF) expressions were detected by RT-PCR. The primers of VEGF used in hypoxic culture were as follows: VEGF 5′-GTGGACATCTTCCAGGAGTA-3′ (sense) and 5′-GCGAGTCTGTGTTTTTGC-3′ (antisense). Each experimental condition was performed in quadruplicate.
Cell migration assay
Cell migration was assayed using the Transwell system (Corning Costar, Acton, MA) with 6.5-mm diameter polycarbonate filters (5-μm pore size). Briefly, EPCs (105) were seeded onto chemotaxis filters in EBM plus 0.5% FBS. Recombinant human IGF2 (Upstate Biotechnology, Lake Placid, NY), VEGF (Upstate Biotechnology), SDF-1 (R&D Systems, Minneapolis, MN), and conditioned media (EBM plus 0.5% FBS) from HUVECs or the breast cancer cell line MDA-MB-435 cultured under different oxygen tensions for 24 hours were then added to the lower chamber. After the 24-hour migration period, nonmigrating cells were completely removed from the top surface of the membrane. Migrating cells adhering to the undersurface of the filters were measured by hematoxylin and eosin staining and quantified using Kodak 1D software (Eastman Kodak, Rochester, NY). Results are representative of 4 independent experiments.
Cell-matrix adhesion
Cell-matrix adhesion assays were performed as described previously.13 The 96-well plates were coated overnight (4°C) with 0.1 mg/mL human fibronectin. EPCs in adhesion buffer were seeded at 105 cells/well in 100 μL volume in either the absence or presence of IGF2 and were incubated for 30 minutes at 37°C. After the removal of nonadherent cells after 2 washes, adhesion was quantified in triplicate by counting adherent cells in 5 randomly selected fields per well (Axiovert 100; Carl Zeiss Micro-Imaging, Thornwood, NY).
Vertical collagen gel invasion assay
To measure the invasive migration capacity of EPCs, a vertical collagen gel chamber assay was performed as described previously,14 except that 50 μL collagen and IGF2 in the first layer and 200 μL collagen in the second layer were used as an IGF2 gradient. After 48 hours, the capacity of downward invasion into the collagen gel was measured as the maximum distance from the upper margin of the collagen gel.
Murine bone marrow mononuclear cell homing into the Matrigel plug
Seven-week-old male C57/BL6 mice (Orient Company, Seoul, Korea) were injected subcutaneously with 0.6 mL Matrigel containing the indicated amount of vehicle or 200 ng IGF2 and 10 U heparin. The injected Matrigel rapidly formed a single, solid gel plug. After 1 day, CD34+ murine bone marrow mononuclear cells (mBMMNCs) isolated from enhanced green fluorescent protein (EGFP) transgenic mice were then infused into the tail vein. Six days after infusion, the skin of the mouse was easily pulled back to expose the Matrigel plug, which remained intact. Hemoglobin was measured using the Drabkin method and the Drabkin reagent kit 525 (Sigma-Aldrich) for the quantification of blood vessel formation. The concentration of hemoglobin was calculated from a known amount of hemoglobin assayed in parallel. Endothelial cells in the Matrigel plugs were identified by staining with anti-CD31 or anti–von Willebrand factor (VWF) antibody and infiltrated mBMMNCs were viewed using fluorescence microscopy. The total number of mBMMNCs (GFP+) cells per field for each Matrigel sample was counted.
Mouse hind limb ischemia model and transplantation of mBMMNCs
Seven-week-old male C57/BL6 mice (Orient Company) underwent surgery to induce unilateral hind limb ischemia as previously described.15 In brief, after the ligature was performed on the proximal origin of the right femoral artery, 5 × 105/CD34+ mBMMNCs (GFP+) were infused into the tail vein. To block ischemia-induced IGF2 signaling, 20 μg mouse IGF2-neutralizing antibody (IGF2N; R&D Systems) was administered to the ischemic area 30 minutes before cell transplantation. Mouse IGF2 (250 ng; R&D Systems) was also administered to the ischemic area 30 minutes before cell injection. Isotype antibody (rat IgG2A; R&D Systems) was used as a control with the same dose and concentration. To quantify the homing of mBMMNCs to the ischemic organ, direct counting of GFP+ cells in the histologic section of each organ, harvested 15 hours after transplantation, was performed. For the direct counting of homed cells in the hind limb, the medial section of the thigh muscles were frozen sectioned (10 μm) at 200-μm intervals. The mouse vasculature was stained with anti–mouse VWF antibody (Sigma-Aldrich). GFP+ cells were counted in each section under fluorescent microscopy (Olympus IX81-ZDC microscope [Olympus, Tokyo, Japan]; LUCPL FLN 20×/0.45 NA lens). Total cell number was divided by total muscle area, which was measured using Image-Pro Plus (Media Cybernetics, Bethesda, MD).
Measurement of intracellular calcium mobilization
The measurement of intracellular-free Ca2+ was performed as described previously with some modifications.16 Briefly, EPCs were incubated in Hanks balanced salt solution (HBSS; Sigma-Aldrich) containing 10 μM Fluo-4 (Molecular Probes) for 30 minutes at 37°C. Cells were diluted 1:5 in HBSS containing 1% FBS followed by incubation for 40 minutes. The cells were washed 3 times, resuspended in HEPES-buffered saline, and incubated for at least 10 minutes at 37°C. After stimulation with IGF2 (final concentration 50 ng/mL; Upstate Biotechnology), FL-1 fluorescence was analyzed using a FACSCalibur (BD PharMingen, San Diego, CA) in 5-second acquisition intervals.
siRNA transfections
Phospholipase C β2 (PLCβ2) siRNA against the target sequence 5′-AGAGGAACGCUUGGCACCA-3′ in a deprotected, duplexed, desalted, and purified form (Dharmacon RNA Technologies, Lafayette, CO) was used for PLCβ2 knockdown studies. Control siRNA consisted of the sequence 5′-AAUGGAAGACCACUCCCACUC-3′. EPCs were transfected with either PLCβ2 or control siRNAs at concentrations ranging from 200 to 800 nM using electroporation (Amaxa Biosystems, Gaithersburg, MD). PLCβ2 levels were analyzed by RT-PCR and Western blot at 48 hours after transfection.
Animal studies
Animals were maintained in a laminar airflow cabinet under specific pathogen-free conditions with a 12-hour light-dark cycle. All animal experiments were performed in accordance with Yonsei University Institutional Animal Care and Use Committee guidelines.
Statistical analyses
Data are presented as the mean plus or minus SE, and statistical comparisons between groups were performed by one-way analysis of variance followed by the Student t test.
Results
IGF2 increases EPC chemotaxis through its cognate receptor, IGF2R
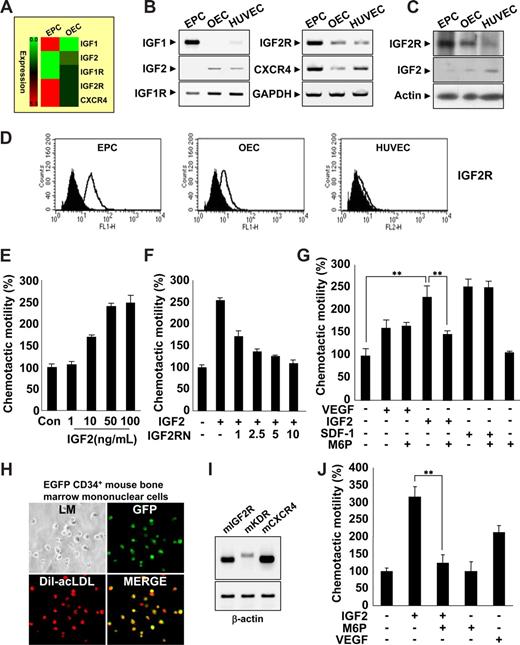
EPCs were isolated from human cord blood by Biocoll density gradient and cultured on fibronectin-coated plates. We confirmed the identity of EPCs (termed early EPCs) by the expression of multiple markers,17-19 including CD133 c-Kit, CXCR4, CD34, CD31, KDR, and UEA-1, as well as the incorporation of acLDL (Figure S1A-E). Furthermore, EPCs lacked expression of myeloid-specific markers CD33 and CD41 (Figure S1F). After culture for 7 to 14 days, the cells were differentiated into outgrowth endothelial cells (OECs, sometimes called late EPCs),14,20 which are characterized by an endothelial phenotype and expression of endothelial cell markers,17-19 such as CD146, CD31, endothelial nitric oxide synthase (eNOS), CDH5, vWF, Flt1, and CD105 (Figure S1B-E).
Gene expression profiles during the differentiation of EPCs into OECs were analyzed, and novel factors engaged in EPC homing were identified. Interestingly, we found that IGF2R was highly expressed in EPCs; however, its ligand, IGF2, which is a known hypoxia-inducible gene,21,22 was absent (Figure 1A-D). The expression patterns of both IGF2 and IGF2R were reminiscent of SDF-1 and CXCR4, which are have well-defined roles in EPC homing. Therefore, we first tested the ability of IGF2 to stimulate EPC chemotaxis, an initial step of EPC homing, using an in vitro transwell assay. IGF2 increased EPC chemotactic motility in a dose-dependent manner (Figure 1E), whereas IGF1, which has a significantly lower affinity to IGF2R than IGF2, had only a minor effect (Figure S2A). OECs derived from EPCs expressed a low level of IGF2R and responded weakly to IGF2 (Figure S2B). The specificity of IGF2 to IGF2R was confirmed by data showing that neutralizing antibody to IGF2R (ie, IGF2RN) inhibits IGF2-induced EPC migration (Figure 1F), whereas neutralizing antibody to IGF1R (ie, IGF1RN) had no effect (Figure S2C). The magnitude of EPC chemotaxis induced by IGF2 was much stronger than that induced by VEGF and was similar to that induced by SDF-1 (Figure 1G). Moreover, IGF2-induced EPC chemotaxis was inhibited by mannose-6-phosphate (M6P); M6P antagonizes IGF2 binding to IGF2R23 without affecting responses to either VEGF or SDF-1 (Figure 1G). The chemotactic effect of IGF2 was observed in CD34+ and CD34− EPCs, both of which were shown to have potential to differentiate to OECs (Figure S3).24 This effect was abolished in both by M6P treatment, indicating the specificity of IGF2-IGF2R binding in inducing chemotaxis of these cells.
IGF2 increases EPC chemotaxis. (A) Total mRNA was isolated from EPCs and OECs, and gene expression profiles were assessed using an Affymetrix gene chip. Red indicates high expression. IGF2R mRNA and protein levels were measured by RT-PCR (B),Western blotting (C), and flow cytometric analysis (D). (E) EPCs were treated with IGF2 (1, 10, 50, and 100 ng/mL). (F,G) EPCs were preincubated for 30 minutes with or without IGF2RN (1, 2.5, 5, and 10 μg/mL) or M6P (5 mM) and stimulated with IGF2 (50 ng/mL), VEGF (50 ng/mL), or SDF-1 (50 ng/mL). After 24 hours, chemotaxis was quantified by counting the cells that migrated to the lower side of the filter with optical microscopy at 200× magnification. (H,I) EGFP CD34+ mouse BMMNCs were isolated using the magnetic-activated cell sorting (MACS) system and then cultured for 7 days. Immunofluorescent staining of Dil-acLDL was performed (H) using an Olympus IX81-ZDC microscope with a UPL SAPO 10×/0.40 NA lens. Mouse IGF2R, KDR, and CXCR4 mRNA expression was measured by RT-PCR (I). (J) EGFP CD34+ mBMMNCs were preincubated for 30 minutes with or without 5 mM M6P and stimulated with 50 ng/mL mIGF2 or 50 ng/mL mVEGF. All data are presented as the mean plus or minus SE from 3 different experiments in duplicate. **P < .01 versus IGF2 alone.
IGF2 increases EPC chemotaxis. (A) Total mRNA was isolated from EPCs and OECs, and gene expression profiles were assessed using an Affymetrix gene chip. Red indicates high expression. IGF2R mRNA and protein levels were measured by RT-PCR (B),Western blotting (C), and flow cytometric analysis (D). (E) EPCs were treated with IGF2 (1, 10, 50, and 100 ng/mL). (F,G) EPCs were preincubated for 30 minutes with or without IGF2RN (1, 2.5, 5, and 10 μg/mL) or M6P (5 mM) and stimulated with IGF2 (50 ng/mL), VEGF (50 ng/mL), or SDF-1 (50 ng/mL). After 24 hours, chemotaxis was quantified by counting the cells that migrated to the lower side of the filter with optical microscopy at 200× magnification. (H,I) EGFP CD34+ mouse BMMNCs were isolated using the magnetic-activated cell sorting (MACS) system and then cultured for 7 days. Immunofluorescent staining of Dil-acLDL was performed (H) using an Olympus IX81-ZDC microscope with a UPL SAPO 10×/0.40 NA lens. Mouse IGF2R, KDR, and CXCR4 mRNA expression was measured by RT-PCR (I). (J) EGFP CD34+ mBMMNCs were preincubated for 30 minutes with or without 5 mM M6P and stimulated with 50 ng/mL mIGF2 or 50 ng/mL mVEGF. All data are presented as the mean plus or minus SE from 3 different experiments in duplicate. **P < .01 versus IGF2 alone.
Several lines of evidence have shown that mononuclear cells derived from bone marrow (BM) contribute to new vessel formation in ischemia and tumor models.25,26 To further investigate whether IGF2 induces chemotaxis of BM-derived EPCs, we isolated CD34+ mBMMNCs from EGFP transgenic mice and confirmed the potentiality of CD34+ mBMMNCs to differentiate into EPCs in vitro (Figure 1H). IGF2R was highly expressed in CD34+ mBMMNCs, along with both KDR and CXCR4 (Figure 1I). IGF2 increased the chemotaxis of CD34+ mBMMNCs more strongly than did VEGF, and the response to IGF2 was markedly inhibited by the addition of M6P (Figure 1J and Figure S4). Taken together, these results suggest that IGF2 may induce chemotactic migration in various types of circulating EPCs specifically through the stimulation of the IGF2R.
Hypoxia-induced IGF2 contributes to EPC chemotaxis
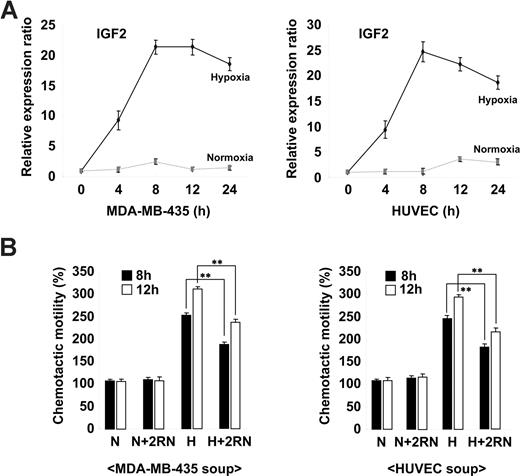
Ischemia is known to induce the mobilization of BM progenitor cells in both animal models and humans through the increased expression of genes such as VEGF and SDF-1.27-29 To assess whether IGF2 is regulated in a similar fashion, we analyzed its expression in the breast cancer cell line MDA-MB-435 and in HUVECs under both normoxic and hypoxic conditions. As shown in Figure 2A, the up-regulation of IGF2 mRNA was noted in cells under hypoxic conditions. The expression of VEGF was also increased in both types of cells under hypoxic conditions (Figure S5A). Compared with normoxic-conditioned media, hypoxic-conditioned media significantly increased EPC chemotaxis; this effect was partially but significantly blocked by IGF2RN treatment (Figure 2B and Figure S5B), suggesting that cells under hypoxic stress secrete IGF2 and subsequently induce EPC chemotaxis through IGF2R stimulation.
Hypoxia-induced IGF2 increases EPC chemotaxis. (A) MDA-MB-435 breast cancer cells and HUVECs were incubated for the indicated times under either normoxic (N) or hypoxic (H) conditions. (A) IGF2 mRNA was measured by real-time PCR. (B) EPCs were preincubated for 30 minutes with or without 10 μg/mL IGF2RN and stimulated with the conditioned media of either MDA-MB-435 cells or HUVECs. After 24 hours, chemotaxis was quantified by counting the cells that migrated to the lower side of the filter. Data are the mean plus or minus SE. **P < .01 versus hypoxic conditioned media alone.
Hypoxia-induced IGF2 increases EPC chemotaxis. (A) MDA-MB-435 breast cancer cells and HUVECs were incubated for the indicated times under either normoxic (N) or hypoxic (H) conditions. (A) IGF2 mRNA was measured by real-time PCR. (B) EPCs were preincubated for 30 minutes with or without 10 μg/mL IGF2RN and stimulated with the conditioned media of either MDA-MB-435 cells or HUVECs. After 24 hours, chemotaxis was quantified by counting the cells that migrated to the lower side of the filter. Data are the mean plus or minus SE. **P < .01 versus hypoxic conditioned media alone.
Role of IGF2 in EPC adhesion
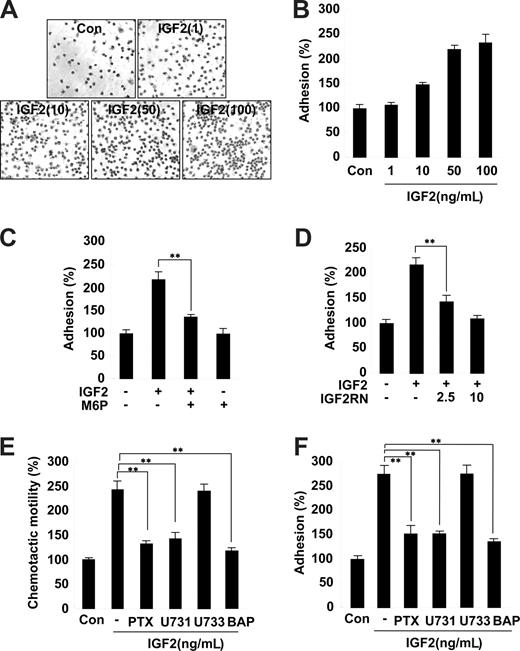
The second step of progenitor cell homing to ischemic tissues involves the adhesion of progenitor cells to the extracellular matrix.30 We thus examined the effects of IGF2 on EPC adhesion to the fibronectin matrix. Stimulation of EPCs with IGF2 significantly increased adhesion; this IGF2 effect was inhibited by pretreatment with both M6P and IGF2R neutralizing antibody (Figure 3A-D). In contrast, IGF1 had a minor effect on EPC adhesion (Figure S6). Similar to the results observed in the chemotaxis assays, the adhesion of both CD34+ and CD34− EPCs was increased by IGF2, and the effect was abrogated by M6P treatment (Figure S7).
Role and signaling of IGF2 in EPC chemotaxis and adhesion. (A,B) EPCs were treated with IGF2 (1, 10, 50, and 10 ng/mL). (C,D) EPCs were preincubated for 30 minutes with or without 5 mM M6P or IGF2RN (2.5, 5 μg/mL) and stimulated with 50 ng/mL IGF2. After 30 minutes, adhesion was quantified by counting the cells that attached to the fibronectin-coated matrix with optical microscopy at 200× magnification. (E,F) EPCs were preincubated for 6 hours with or without 50 ng/mL PTX or preincubated for 30 minutes with or without 5 μM U73122, 5 μM U73343, or 5 μM BAPTA-AM and stimulated with 50 ng/mL IGF2. After 24 hours, chemotaxis was quantified by counting the cells that migrated to the lower side of the filter with optical microscopy at 200× magnification (E). After 30 minutes, adhesion was quantified by counting the cells (F). Data are the mean plus or minus SE. **P < .01 versus IGF2 alone.
Role and signaling of IGF2 in EPC chemotaxis and adhesion. (A,B) EPCs were treated with IGF2 (1, 10, 50, and 10 ng/mL). (C,D) EPCs were preincubated for 30 minutes with or without 5 mM M6P or IGF2RN (2.5, 5 μg/mL) and stimulated with 50 ng/mL IGF2. After 30 minutes, adhesion was quantified by counting the cells that attached to the fibronectin-coated matrix with optical microscopy at 200× magnification. (E,F) EPCs were preincubated for 6 hours with or without 50 ng/mL PTX or preincubated for 30 minutes with or without 5 μM U73122, 5 μM U73343, or 5 μM BAPTA-AM and stimulated with 50 ng/mL IGF2. After 24 hours, chemotaxis was quantified by counting the cells that migrated to the lower side of the filter with optical microscopy at 200× magnification (E). After 30 minutes, adhesion was quantified by counting the cells (F). Data are the mean plus or minus SE. **P < .01 versus IGF2 alone.
Inhibition of IGF2-induced EPC chemotaxis and adhesion by antagonists of G(i) protein, PLC, and intracellular Ca2+ mobilization
Both IGF1R and the insulin receptor are homologous in that they both activate tyrosine kinases. In contrast, IGF2R is a type I transmembrane glycoprotein that lacks an intracellular tyrosine kinase domain.23 It has been suggested that the G(i) protein linked to IGF2R activates intracellular signaling pathways, such as PLC, mitogen-activated protein (MAP) kinase, and the small GTPase.31 To further elucidate the role of the IGF2/IGF2R system in EPC chemotaxis, downstream signaling targets of IGF2R were investigated using various pharmacologic inhibitors. Notably, pretreatment of EPCs with the G(i) inactivator PTX markedly inhibited IGF2-induced EPC chemotaxis (Figure 3E and Figure S8). Moreover, the PLC inhibitor U73122 as well as BAPTA-AM, an inhibitor of intracellular calcium mobilization, showed strong inhibitory effects (Figure 3E), whereas the phosphatidylinositol 3-kinase (PI-3K) inhibitor wortmannin, the MAP kinase kinase (MEK) inhibitor U0126, the PKC inhibitor chelerythrine chloride, and the inactive analog of the PLC inhibitor U73343 had no effect (Figure 3E and data not shown). The activities of the working concentrations of inhibitors used in this study were validated; the effective inhibitors showed no cytotoxicity to EPCs under the experimental conditions (data not shown). Furthermore, IGF2-induced EPC adhesion was specifically blocked by inhibitors of G(i) protein, PLC, and intracellular Ca2+ mobilization (Figure 3F). Together, these results suggest that IGF2 stimulates EPC migration and adhesion by a signaling pathway that involves G(i) protein, PLC, and intracellular Ca2+.
IGF2 enhances both invasion and MMP9 expression in EPCs
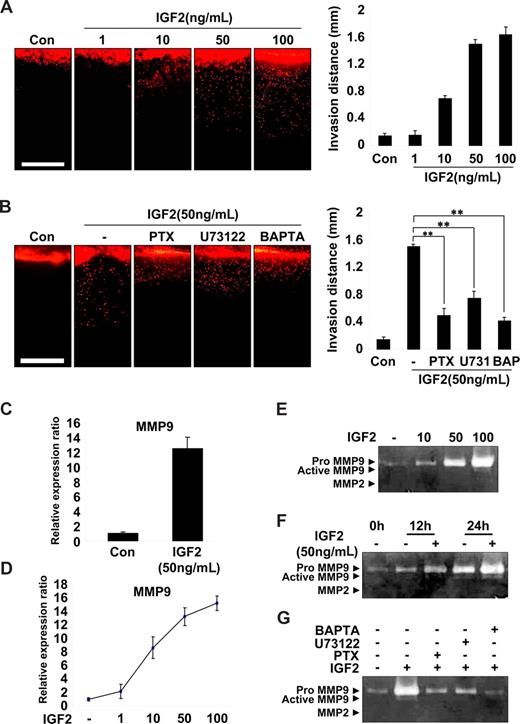
EPC invasion is an important step in the recruitment and incorporation of EPCs into ischemic or tumor sites.14,30 Therefore, a vertical collagen gel invasion assay was performed to assess the possible role of IGF2 in EPC invasion. As shown in Figure 4A, IGF2 significantly increased invasion depth in both a dose- and IGF2R-dependent manner. Moreover, the effect of IGF2 on EPC invasion was significantly attenuated by treatment with inhibitors of G(i), PLC, and calcium mobilization (Figure 4B). IGF2 stimulation of EPC invasion was also confirmed using the Transwell assay (Figure S9A).
IGF2-enhanced invasion of EPCs is mediated by an increase in MMP9 expression. (A) Invasion assay using vertical collagen gel chamber with IGF2 at the bottom for the chemotactic gradient. Dil-acLDL–labeled EPCs were stimulated with IGF2 (1, 10, 50, and 100 ng/mL). (B) Dil-acLDL–labeled EPCs were preincubated for 6 hours with or without 50 ng/mL PTX or preincubated for 30 minutes with or without 5 μM U73122 or 5 μM BAPTA-AM, and then stimulated with 50 ng/mL IGF2. After 48 hours, invasion was quantified by counting the cells. Scale bar represents 1 mm. (C,D) Total mRNA was isolated from EPCs treated with IGF2 (1, 10, 50, and 100 ng/mL) or untreated, and MMP9 gene expression was assessed by real-time PCR analysis. EPCs were incubated in 0.5% FBS-conditioned medium for 24 hours in the absence or presence of IGF2 (10, 50, and 100 ng/mL) (E) or for the indicated times in the absence or presence of 50 ng/mL IGF2 (F). (G) EPCs were preincubated for 6 hours with or without 50 ng/mL PTX or preincubated for 30 minutes with or without 5 μM U73122 or 5 μM BAPTA-AM, and then incubated in 0.5% FBS-conditioned medium for 24 hours in the absence or presence of 50 ng/mL IGF2. The culture medium was collected and subjected to gelatin zymography. Data are the mean plus or minus SE. **P < .01 versus IGF2 alone.
IGF2-enhanced invasion of EPCs is mediated by an increase in MMP9 expression. (A) Invasion assay using vertical collagen gel chamber with IGF2 at the bottom for the chemotactic gradient. Dil-acLDL–labeled EPCs were stimulated with IGF2 (1, 10, 50, and 100 ng/mL). (B) Dil-acLDL–labeled EPCs were preincubated for 6 hours with or without 50 ng/mL PTX or preincubated for 30 minutes with or without 5 μM U73122 or 5 μM BAPTA-AM, and then stimulated with 50 ng/mL IGF2. After 48 hours, invasion was quantified by counting the cells. Scale bar represents 1 mm. (C,D) Total mRNA was isolated from EPCs treated with IGF2 (1, 10, 50, and 100 ng/mL) or untreated, and MMP9 gene expression was assessed by real-time PCR analysis. EPCs were incubated in 0.5% FBS-conditioned medium for 24 hours in the absence or presence of IGF2 (10, 50, and 100 ng/mL) (E) or for the indicated times in the absence or presence of 50 ng/mL IGF2 (F). (G) EPCs were preincubated for 6 hours with or without 50 ng/mL PTX or preincubated for 30 minutes with or without 5 μM U73122 or 5 μM BAPTA-AM, and then incubated in 0.5% FBS-conditioned medium for 24 hours in the absence or presence of 50 ng/mL IGF2. The culture medium was collected and subjected to gelatin zymography. Data are the mean plus or minus SE. **P < .01 versus IGF2 alone.
Matrix metalloproteases (MMPs) are known to mediate the invasion of various cells32,33 ; thus, we examined the potential role of IGF2 in MMP expression in EPCs. Among the many MMPs evaluated, the expression of MMP9 mRNA was markedly increased by IGF2 in EPCs (Figure 4C) in a dose-dependent manner (Figure 4D), whereas other MMPs we examined were not significantly affected by IGF2 (Figure S9B). In addition, gelatin zymography of the EPC culture medium showed that IGF2 increased the production of proMMP9 (Mr 88 000) in both a dose- and time-dependent manner (Figure 4E,F). In agreement with IGF2 signaling in EPC invasion, the induction of MMP9 by IGF2 was blocked by treatment with inhibitors of G(i), PLC, and intracellular Ca2+ (Figure 4G). Collectively, these results suggest that IGF2 increases matrix degradation and invasion of EPCs through the IGF2R-dependent generation of MMP9.
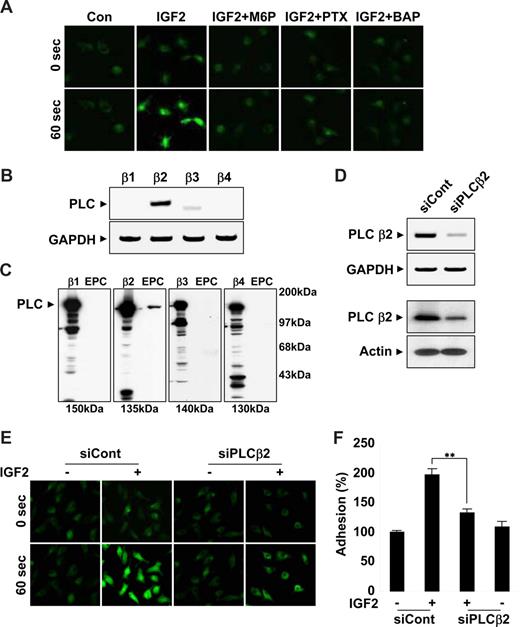
The β2 isoform of PLC is critical for IGF2-induced intracellular Ca2+ mobilization
Results from the use of pharmacologic inhibitors suggested that IGF2R downstream molecules, such as G(i) protein, PLC, and intracellular calcium, were commonly involved in migration, adhesion, and invasion of EPCs in response to IGF2. In addition, a strong increase in intracellular calcium levels was observed within seconds after IGF2 treatment; this increase was inhibited by both M6P and PTX as well as by a control calcium mobilization inhibitor (Figure 5A and Figure S10A), suggesting upstream roles of both IGF2R and G(i) in calcium mobilization. The βγ subunits of the G(i) protein induce the mobilization of intracellular calcium through PLCβ activation. Four PLCβ isoforms, β1, β2, β3, and β4, exist in mammals. To better elucidate the role of PLC in the IGF2-mediated calcium increase, we analyzed the expression of the PLC isozymes in EPCs by both RT-PCR (Figure 5B) and Western blot analysis (Figure 5C). Among the 4 isozymes, only PLCβ2 was prominently expressed in EPCs (Figure 5B,C). PLCβ2-specific siRNA successfully reduced both PLCβ2 mRNA (Figure 5D top) and protein levels in EPCs (Figure 5D bottom). In EPCs transfected with PLCβ2 siRNA, IGF2-induced intracellular calcium mobilization was significantly reduced (Figure 5E and Figure S10B). Moreover, PLCβ2-specific siRNA, but not control siRNA, reversed IGF2-induced EPC adhesion incrementally compared with control levels (Figure 5F). Taken together, these results suggest that an IGF2/IGF2R pathway uses PLCβ2 for the stimulation of intracellular calcium mobilization, which is involved in both recruitment and incorporation of EPCs into sites of ischemia.
IGF2 increases intracellular Ca2+ mobilization by PLCβ2. (A) EPCs were preincubated 6 hours with or without 50 ng/mL PTX or preincubated for 30 minutes with or without 5 mM M6P or 5 μM BAPTA-AM. Cells were stimulated with 50 ng/mL IGF2, and intracellular Ca2+ levels were measured. Both mRNA and protein levels of the PLC isozymes β1, β2, β3, and β4 were measured by RT-PCR (B) and Western blotting (C). (D) EPCs were transfected with nonsilencing (siCont) or PLCβ2 siRNA (siPLCβ2). After 48 hours, PLCβ2 mRNA and protein levels were measured by RT-PCR (top) and Western blotting (bottom). (E,F) EPCs were transfected as in panel D and stimulated with 50 ng/mL IGF2, and intracellular Ca2+ levels and adhesion were measured. (A,E) Images were viewed using an Olympus IX81-ZDC microscope with a LUCPL FLN 20×/0.45 NA lens, and processed using MetaMorph v.7.0 software. Data are the mean plus or minus SE. **P < .01 versus siCont + IGF2.
IGF2 increases intracellular Ca2+ mobilization by PLCβ2. (A) EPCs were preincubated 6 hours with or without 50 ng/mL PTX or preincubated for 30 minutes with or without 5 mM M6P or 5 μM BAPTA-AM. Cells were stimulated with 50 ng/mL IGF2, and intracellular Ca2+ levels were measured. Both mRNA and protein levels of the PLC isozymes β1, β2, β3, and β4 were measured by RT-PCR (B) and Western blotting (C). (D) EPCs were transfected with nonsilencing (siCont) or PLCβ2 siRNA (siPLCβ2). After 48 hours, PLCβ2 mRNA and protein levels were measured by RT-PCR (top) and Western blotting (bottom). (E,F) EPCs were transfected as in panel D and stimulated with 50 ng/mL IGF2, and intracellular Ca2+ levels and adhesion were measured. (A,E) Images were viewed using an Olympus IX81-ZDC microscope with a LUCPL FLN 20×/0.45 NA lens, and processed using MetaMorph v.7.0 software. Data are the mean plus or minus SE. **P < .01 versus siCont + IGF2.
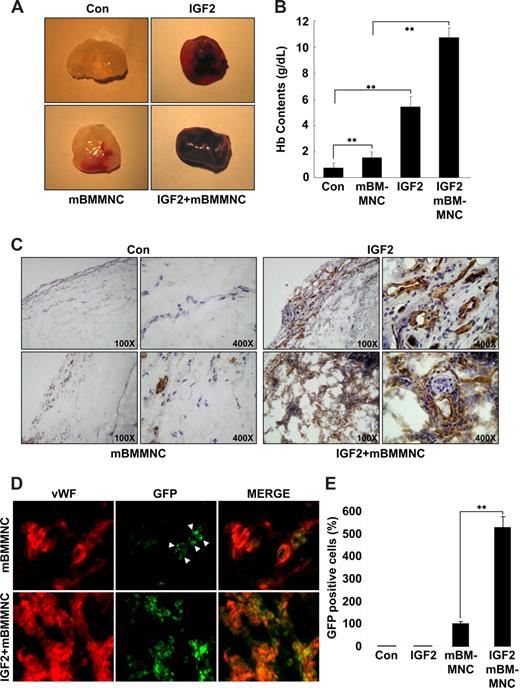
IGF2 increases mBMMNC homing and neovascularization in vivo
To assess the relevance of IGF2 for progenitor cell homing and neovascularization in vivo, the mouse Matrigel plug assay was performed. A hemoglobin assay showed that the amount of functional blood vessels formed in the Matrigel plug is significantly increased in response to IGF2 and further enhanced by cotreatment with mBMMNCs and IGF2, compared with controls or treatment with mBMMNCs alone (Figure 6A,B). In addition, a histologic analysis using anti-CD31 antibody further showed that the vascular density and the number of mature vascular structures in the IGF2-containing plugs after mBMMNC injection were much higher than those in plugs treated with IGF2 alone (Figure 6C). In addition, the number of GFP+ mBMMNCs recruited into the implanted Matrigel plug was significantly increased in response to IGF2; most of the GFP+ cells surrounding the blood vessels were positively stained with VWF, VE-Cadherin, and CD31, markers for endothelial cells (Figure 6D,E and Figure S11A). Furthermore, differentiation of GFP+ cells into ECs was confirmed by in vitro culture (Figure S11B,C).
IGF2 increases mBMMNC homing and neovascularization in the mouse Matrigel plug assay. C57BL/6 mice were injected with 0.6 mL Matrigel with or without IGF2 (200 ng) (n = 7 per group). After 1 day, CD34+ mBMMNCs from EGFP transgenic mice (5 × 105/mouse) were infused into the tail vein. Six days after infusion, mice were killed, and Matrigel plugs were excised and serially sectioned. (A) Representative Matrigel plugs. (B) Quantification of neovasculature formation by measuring hemoglobin levels in the Matrigel. (C) Plug sections were stained for infiltrating endothelial cells using anti-CD31antibody (Olympus CX31 RBSF microscope; 10×/0.25 NA and 40×/0.65 NA plan objectives). (D) Plug sections were used to identify ECs with anti-VWF antibody (red) and mBMMNCs with green fluorescence (Olympus IX81-ZDC microscope, LUCPL FLN 40×/0.60 NA lens). (E) Quantitative assessment of GFP+ mBMMNCs per field for each Matrigel sample. Data are the mean plus or minus SE. **P < .01 versus each mouse group.
IGF2 increases mBMMNC homing and neovascularization in the mouse Matrigel plug assay. C57BL/6 mice were injected with 0.6 mL Matrigel with or without IGF2 (200 ng) (n = 7 per group). After 1 day, CD34+ mBMMNCs from EGFP transgenic mice (5 × 105/mouse) were infused into the tail vein. Six days after infusion, mice were killed, and Matrigel plugs were excised and serially sectioned. (A) Representative Matrigel plugs. (B) Quantification of neovasculature formation by measuring hemoglobin levels in the Matrigel. (C) Plug sections were stained for infiltrating endothelial cells using anti-CD31antibody (Olympus CX31 RBSF microscope; 10×/0.25 NA and 40×/0.65 NA plan objectives). (D) Plug sections were used to identify ECs with anti-VWF antibody (red) and mBMMNCs with green fluorescence (Olympus IX81-ZDC microscope, LUCPL FLN 40×/0.60 NA lens). (E) Quantitative assessment of GFP+ mBMMNCs per field for each Matrigel sample. Data are the mean plus or minus SE. **P < .01 versus each mouse group.
The capacity of IGF2 for EPC chemoattraction in vivo was further confirmed by both mouse ear and retina models. In the ear model, real-time imaging showed that IGF2 significantly increased the chemoattraction of mBMMNCs to IGF2-treated ears (Figure S12A,B). The mouse retina model also showed that the recruitment of CD34+ mBMMNCs was significantly increased in the retina blood vessels of the eye injected with IGF2 compared with controls (Figure S12C,D). These results suggest that IGF2 may induce the formation of new blood vessels by augmenting the recruitment of endothelial progenitor cells to the neovascular zone.
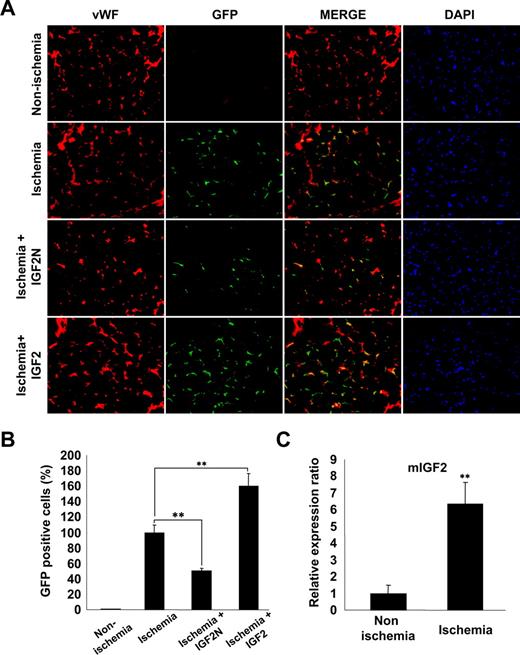
IGF2 increases the in vivo homing of mBMMNCs to sites of hind limb ischemia
We finally examined whether the IGF2/IGF2R axis is involved in ischemia-induced recruitment and incorporation of mBMMNCs into ischemic tissues. For this purpose, mice underwent femoral artery ligature and either IGF2N or IGF2 was injected into the ligature area. Subsequently, CD34+ mBMMNCs, isolated from EGFP transgenic mice, were intravenously injected into C57/BL6 mice. After 15 hours, mBMMNCs recruited to the ischemic limbs and incorporated into capillaries were examined histologically. GFP+ cells were prominently detected in the capillary spaces of ischemic muscle but nearly absent in nonischemic muscle (Figure 7A,B). The number of trafficked mBMMNCs in the ischemic limb was significantly decreased by treatment with IGF2N but increased after IGF2 treatment (Figure 7A,B). Real-time PCR analysis confirmed that the expression of IGF2 mRNA was significantly increased in the adductor muscles of ischemic mice compared with nonischemic controls (Figure 7C). These results suggest that IGF2, generated under ischemic conditions, may play an important role in the recruitment of EPCs to ischemic sites.
IGF2 increases in vivo homing of mBMMNCs in sites of hind limb ischemia. After the ligature was performed on the proximal origin of the right femoral artery (n = 6 per group), CD34+ mBMMNCs isolated from EGFP transgenic mice (5 × 105/mouse) were infused into the tail vein. Mouse IGF2 neutralizing antibody (IGF2N, 20 μg) or mouse IGF2 (250 ng) was administered to the ischemic area 30 minutes before cell transplantation. One day after infusion, the medial section of the thigh muscle was frozen and sectioned (10 μm) at 200-μm intervals. (A) Mouse vasculature was stained with anti–mouse VWF antibody (red fluorescence). Nuclei were stained with DAPI (blue fluorescence). Images were viewed using an Olympus IX81-ZDC microscope with a LUCPL FLN 20×/0.45 NA lens. (B) GFP+ mBMMNCs were counted in each section under fluorescent microscopy. Total cell number was divided by total muscle area, which was measured using Image-Pro Plus. (C) Real-time analysis of IGF2 transcript abundance in ischemic muscle. Data are the mean plus or minus SE. **P < .01 versus each mouse group.
IGF2 increases in vivo homing of mBMMNCs in sites of hind limb ischemia. After the ligature was performed on the proximal origin of the right femoral artery (n = 6 per group), CD34+ mBMMNCs isolated from EGFP transgenic mice (5 × 105/mouse) were infused into the tail vein. Mouse IGF2 neutralizing antibody (IGF2N, 20 μg) or mouse IGF2 (250 ng) was administered to the ischemic area 30 minutes before cell transplantation. One day after infusion, the medial section of the thigh muscle was frozen and sectioned (10 μm) at 200-μm intervals. (A) Mouse vasculature was stained with anti–mouse VWF antibody (red fluorescence). Nuclei were stained with DAPI (blue fluorescence). Images were viewed using an Olympus IX81-ZDC microscope with a LUCPL FLN 20×/0.45 NA lens. (B) GFP+ mBMMNCs were counted in each section under fluorescent microscopy. Total cell number was divided by total muscle area, which was measured using Image-Pro Plus. (C) Real-time analysis of IGF2 transcript abundance in ischemic muscle. Data are the mean plus or minus SE. **P < .01 versus each mouse group.
Discussion
Numerous studies have reported isolation and characterization of EPC which has the potential to become a mature, fully differentiated EC in a variety of systems. Furthermore, although there were some discrepancies in direct incorporation of ECs differentiated from BMMNCs into newly forming endothelium, the contribution of EPCs to neovascularization in adult pathologic states such as ischemia and tumor has recently become generally accepted.25,26 The homing of EPCs to neovascular area requires a coordinated sequence of multistep events, including chemoattraction, adhesion, and invasion, before differentiation into ECs.30,34 Importantly, our data showed that IGF2 strongly stimulates the events required for EPC homing. IGF2R is highly expressed on human cord blood EPCs as well as CD34+ mononuclear cells isolated from mouse BM. Consistent with the notion that homing of EPCs may be remotely controlled by factors generated from ischemic or tumor environments with low oxygen, IGF2 expressed under hypoxic conditions promoted both the recruitment and incorporation of EPCs specifically through an IGF2R-dependent signaling pathway (Figure S13). However, IGF2 had no significant effects on the expression of known molecules related to EPC recruitment such as CXCR4/CXCR2, VEGFR2, integrins, and their ligands (Figure S14) tested in this study, except significant induction of MMP9.
The IGF system consists of 2 ligands, IGF1 and IGF2, and 2 transmembrane receptors, IGF1R and IGF2R. IGF1 and IGF2 share greater than 60% sequence identity, and yet the IGF2R binds only IGF2 with high affinity.23 IGF1 is known to induce angiogenesis both in vitro and in vivo through its binding to IGF1Rs expressed on ECs.35,36 There is increasing evidence to suggest that IGF2 is also involved in the promotion of angiogenesis as well as lymphangiogenesis in several systems.37,38 Hypoxia-induced IGF2 contributes to angiogenesis in human hepatocellular carcinomas21 and growth of hemangiomas,39 with localized expression primarily at tumor vessel or vascular channel sites. However, the expression level of IGF2R and the proangiogenic activity of IGF2 in ECs is relatively weak compared with other angiogenic factors such as IGF1 and VEGF.40 Notably, IGF2R was highly expressed in human cord blood–derived EPCs and CD34+ BMMNCs, and IGF2 strongly stimulated homing of these EPCs in vitro and in vivo through its cognate receptor IGF2R, independently of IGF1R. Interestingly, the expression pattern and role of the IGF2/IGF2R signaling pathway in EPCs and ECs are reminiscent of SDF-1, which currently is the best characterized factor involved in EPC homing. SDF-1 is induced by hypoxia, and its receptor CXCR4 is highly expressed in EPCs, decreasing after differentiation into OECs.8,9 SDF-1 activates EPCs by ligation to CXCR4 and contributes to neovascularization by augmentation of EPC homing.41 Comparison of the expression patterns and activities of IGF2 and SDF-1 further suggest that locally generated IGF2 at ischemic or certain tumor sites may contribute to enhancements of postnatal neovascularization by the stimulation of EPCs in addition to its action on ECs.
Alternatively, recent study has shown that IGF1 ligation with IGF1R augments the number and the angiogenic capacity of circulating EPCs declined in elderly persons.42 Humpert et al43 also suggested the involvement of IGF1R in insulin-induced outgrowth and tube formation of EPCs. There are heterogeneous types of EPCs that are derived from different sources and culture conditions and show different phenotypes and gene expression patterns.44 The expression level of IGF2R in early or primitive EPCs such as cord blood EPCs and CD34+ BMMNCs was high but decreased with differentiation into late EPCs. Conversely, IGF1R expression showed the opposite pattern during EPC differentiation. In line with this notion, the weak action of IGF1 on cord blood–derived EPCs or CD34+ BMMNCs reflects that these EPCs devoid of EC characteristics may be more primitive or earlier staged than EPCs from circulating peripheral blood mononuclear cells (PBMCs), suggesting that the action of IGF1 by IGF1R may be increased with gradual gain of EC phenotypes. Therefore, the relative significance of IGF1 and IGF2 in EPC function appears to depend on the EPC context, and both IGFs may act synergistically during neovascularization. Notably, 2 recent studies have suggested that insulin-like growth factor–binding protein 3 (IGFBP3) may improve vessel repair by promoting the incorporation of CD34+ BMMNCs into the retina, acting independently of IGF1.45,46 IGFBP3 is known to carry serum IGF1 and IGF2 in heterotrimeric complexes and enhance the effect of IGFs.47 Given that IGFBP3 and IGF2 are induced on hypoxia, the possibility is raised that the vascular effects of IGFBP3 on the ischemic injury may be mediated at least in part through enhancing action of IGF2 to IGF2R in EPCs.
Our present data provide novel insights into the intracellular signaling pathways engaged in the homing of EPCs or BM progenitor cells. Although IGF2R is a single transmembrane receptor devoid of a kinase domain, several studies have shown that the ligation of IGF2 to the IGF2R promotes cellular responses through inhibitory G(i) protein-mediated signaling pathways.23,31 In our system, the G(i) protein antagonist PTX blocked EPC responses to IGF2, such as intracellular calcium mobilization and associated chemotaxis, adhesion, and invasion, indicating a prominent role for G(i) as an IGF2R downstream mediator. Interestingly, the importance of G(i) proteins in CXCR4/SDF-1 signaling has been shown in hematopoietic stem cells (HSCs)48 but has not been directly shown in EPCs. SDF-1–induced calcium flux and chemotaxis of human CD34+ progenitor cells and the subsequent responses were blocked by PTX treatment,49 suggesting the convergent and significant role of G(i)–mediated calcium signaling in the mobilization and homing of EPCs and BM progenitor cells in response to different stimuli. Our data further define the specific isoform of PLC involved in G(i)–mediated calcium mobilization. G(i) triggers intracellular calcium fluxes through PLCβ activation linked to the βγ subunits of the G proteins. Indeed, EPCs selectively expressed the β2 isoform of PLC; its knockdown by siRNA resulted in the marked reduction in the responses of EPCs to IGF2. On the basis of these data, we propose an important role for the G(i)/PLCβ2/calcium signaling cascade in the mobilization and recruitment of EPCs and BM CD34+ progenitor cells to the sites of vascular remodeling or neovascularization in response to IGF2 and other stimuli. Note that certain non–hematopoietic stem cells (HSCs) and stromal BM cells express high levels of IGF2; HSCs from both mouse fetal liver and adult BM cells express receptors for IGF2.50 Furthermore, IGF2 is thought to be functionally important in the adult BM to maintain homeostasis of adult stem cells. Therefore, similar to SDF-1, which acts as a potent chemoattractant for HSCs,49 IGF2 may have the potential to promote either BM homing of HSCs or the mobilization of a broad spectrum of stem cells in addition to the activation and recruitment of EPCs in vivo.
In summary, our data suggest, for the first time, a prominent role for the IGF2/IGF2R system in the activation and homing of EPCs and BM CD34+ progenitor cells both in vitro and in vivo. Regardless of the potentially additive mechanisms involved in EPC homing, our data substantially raise the possibility that IGF2 contributes to vascular remodeling and neovascularization by enhancing the recruitment and incorporation of progenitor cells into areas of vascular injury. Recently, EPCs isolated from human cord or peripheral blood are being used in clinical trials to improve neovascularization in patients with ischemic heart disease.48,50 Thus, our results could have important clinical implications because they disclose a mechanism for the enhanced homing of EPCs and, thereby, improve the neovascularization capacity of infused EPCs. Alternatively, because IGF2 generated locally at the hypoxic tumor sites could contribute to tumor growth by increasing both tumor vasculogenesis and angiogenesis, neutralization of IGF2/IGF2R may lead to the development of new therapeutic strategies for treating tumors by blocking tumor vascularization.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Research Laboratory (grant ROA-2007-000-20099-0[2008]), the Korea Biotech R&D Group (grant F104AC010003-08A0301-00310), the Vascular System Research Center (grant R11-2001-090-01001-0[2008]), and the Stem Cell Research Program (grant M10641450002-07N4145-00210) from the Korea Science and Engineering Foundation (KOSEF) funded by the Korean government (MEST).
Authorship
Contribution: Y.-S.M., H.-J.C., and Y.-G.K. planned the experimental design, analyzed data, and wrote the paper; J.-Y.K. and Y.-W.P. provided the human cord blood samples; K.-S.C. and K.-S.K. performed the in vivo homing experiment; J.-K.M. did the FACS work; Y.-H.K. and P.-G.S. performed the siRNA experiment; and M.-H.W. and Y.-M.K. conducted the microarray analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Young-Guen Kwon, Department of Biochemistry, College of Life Science and Biotechnology, Yonsei University, Seoul, 120-749, Republic of Korea; e-mail: ygkwon@yonsei.ac.kr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal