Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that patrol tissues to sense danger signals and activate specific immune responses. In addition, they also play a role in inflammation and tissue repair. Here, we show that oxygen availability is necessary to promote full monocyte-derived DC differentiation and maturation. Low oxygen tension (hypoxia) inhibits expression of several differentiation and maturation markers (CD1a, CD40, CD80, CD83, CD86, and MHC class II molecules) in response to lipopolysaccharide (LPS), as well as their stimulatory capacity for T-cell functions. These events are paralleled by impaired up-regulation of the chemokine receptor CCR7, an otherwise necessary event for the homing of mature DCs to lymph nodes. In contrast, hypoxia strongly up-regulates production of proinflammatory cytokines, particularly TNFα and IL-1β, as well as the inflammatory chemokine receptor CCR5. Subcutaneous injection of hypoxic DCs into the footpads of mice results in defective DC homing to draining lymph nodes, but enhanced leukocyte recruitment at the site of injection. Thus, hypoxia uncouples the promotion of inflammatory and tissue repair from sentinel functions in DCs, which we suggest is a safeguard mechanism against immune reactivity to damaged tissues.
Introduction
Low oxygen tension (hypoxia) has been described at virtually every site of extensive inflammation, including necrotic foci and cutaneous sites of infection and wounding.1 Sites of inflammation are also characterized by extensive infiltration of inflammatory leukocytes, which need to move against oxygen gradients. As a consequence, immune effector cells in hypoxic sites, including dendritic cells (DCs), have an acute need to respond to these demanding conditions to maintain their viability and activity. DCs are powerful antigen-presenting cells (APCs) specialized for the activation of resting T cells and the initiation and regulation of many types of immune response.2,,–5 Because of this, we have investigated the functional changes that accompanying the metabolic adaptation of DCs to hypoxia, as these events are likely to affect the development of both inflammatory and immune functions
The capacity of DCs to activate and regulate T-cell responses is acquired during a complex differentiation and maturation program.2,,–5 DCs originate from bone marrow and, at an “immature” stage, they patrol peripheral tissues for the presence of pathogen-associated antigens. In order to perform this function, DCs express a rich repertoire of pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), which permit DCs to recognize distinct pathogen-associated molecules.6,7 The engagement of TLRs initiates a cascade of signaling events in DCs that leads, in the process of “maturation,” to the secretion of inflammatory and immunomodulatory factors, which mediate protective immunity.6,7 For instance, stimulation of DC by lipopolysaccharide (LPS), through the participation of TLR4, leads to up-regulation of MHC molecules; costimulatory molecules such as CD40, CD80, and CD86; the “maturation markers” CD83 and DC-lysosome–associated membrane protein (LAMP); chemokine receptors CXCR4 and CCR7; a diversity of cytokines and chemokines; and potent APC function.4,5 Such inflammatory signals also induce a chemokine receptor switch, with down-regulation of inflammatory receptors (such as CCR1, CCR2, and CCR5) associated with the induction of CCR7.8 This chemokine receptor switch facilitates the emigration of DCs out of peripheral tissues and their localization into the T-cell areas of secondary lymphoid organs, where they encounter naive T cells and initiate immune responses.8
Several studies have provided evidence that the maturation of DCs is a highly regulated process that can be influenced by several factors present in the tissue microenvironment. Critical factors include IL-109,–11 and VEGF,12,13 whose expression is modulated by hypoxia.1 These cytokines inhibit monocyte-derived DC differentiation as well as maturation, and also block the functional activities normally associated with a “mature” state.9,10,12 As DCs often localize at inflammatory sites characterized by low oxygen tension, such as wounds, tumors, and other sites of ischemia,1,14 we investigated whether low oxygen could modulate the differentiation and maturation of DCs. Our results indicate that hypoxia strongly enhances the innate immune functions of DCs by inhibiting their maturation, but increasing both their production of inflammatory cytokines and their chemotactic response toward chemokines selectively expressed at peripheral sites of inflammation. We propose that this modulation represents a safeguard against immune reactivity to damaged tissues.
Methods
Cells and culture conditions
Human monocytes were separated from the peripheral blood of healthy human donors (courtesy of the Ospedale di Desio, Milan, Italy) by Percoll gradient, as previously described.15 DCs were generated from monocytes by incubation for 6 days at 106/mL in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, antibiotics, 50 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF), and 20 ng/mL IL-13 under normoxic (20% O2, 5% CO2, and 75% N2) or hypoxic conditions (1% O2, 5% CO2, and 94% N2). After 6 days of culture, the population consisted of typical immature DCs that generally expressed CD1a (> 90% positive cells), low levels of CD80 and CD86, and little or no CD83 (< 10%) and CD14 (< 10%).16,–18 To induce terminal maturation, 10 ng/mL LPS was added at day 6 for 18 hours in normoxic or hypoxic conditions. Hypoxia treatment was performed by placing cells in the InVivo2 400 hypoxic workstation (Ruskinn-Biotrace, Bridgend, United Kingdom) maintained at 1% O2 and 37°C, or in a modular incubator chamber (Billups-Rothemberg, Del Mar, CA) flushed with a mixture of 1% O2, 5% CO2, and 94% N2 and placed at 37°C. Highly enriched CD1c+ DCs (> 90%) were obtained from peripheral blood mononuclear cells (PBMCs) of healthy donors by immunomagnetic depletion of contaminating CD19+CD1c+ B cells using CD19 mAb-conjugated microbeads (Miltenyi Biotec, Auburn, CA) followed by immunomagnetic enrichment of CD1c+ cells.
Fluorescence-activated cell sorter analysis
Monocytes were stained with FITC-conjugated mouse anti–human monoclonal CD1a (clone HI149; BD Biosciences, San Jose, CA), APC-conjugated mouse anti–human monoclonal CD14 (clone M5E2; BD Biosciences), R-PE–conjugated mouse anti–human CD16 (clone 3G8; AbD Serotec, Oxford, United Kingdom) and R-PE–conjugated mouse anti–human CD68 (clone Y1/82A; BD Biosciences). Human DC staining was performed using APC-conjugated mouse anti–human monoclonal CXCR4 (clone 12G5; BD Biosciences), PE-Cy7–conjugated rat anti–human monoclonal CCR7 (clone 3D12; BD biosciences), R-PE–conjugated mouse anti–human monoclonal CD80 (clone L307.4; BD Biosciences), R-PE–conjugated mouse anti–human monoclonal CD40 (clone 5C3; BD Biosciences), and APC-conjugated mouse anti–human monoclonal CD86 (clone 2331 [FUN-1]; BD Biosciences). For each antibody, the proper isotype controls were used. Furthermore, DCs were stained with mouse anti–human monoclonal CCR5 (clone CTC5; R&D Systems, Minneapolis, MN), mouse anti–human monoclonal CD83 (clone HB15e; BD Biosciences), and MHC class II (hybridoma L243; ATCC, Manassas, VA), followed by Alexa 488–conjugated, isotype-matched, affinity-purified goat anti–mouse antibody (Molecular Probes/Invitrogen, Carlsbad, CA). For each antibody, the proper mouse isotype-control antibody was used followed by Alexa 488–conjugated goat anti–mouse antibody.
Cytokines and reagents
Human recombinant CXCL12/stromal-derived factor 1 (SDF-1), CCL5/RANTES, and CCL19/MIP3β were from PeproTech (Rocky Hill, NJ); LPS Escherichia coli strain 055:B5 was from Sigma-Aldrich (St Louis, MO) and LPS Salmonella abortus equi S-form was from Alexis Biochemicals (San Diego, CA); PAM3CSK4 was from Alexis Biochemicals; poly (I:C) was from GE Healthcare Life Sciences (Little Chalfont, United Kingdom); resiquimod (R-848) was from Alexis Biochemicals; deferoxamine was from Sigma-Aldrich; human recombinant GM-CSF was a kind gift from Novartis (Basel, Switzerland); human recombinant IL-13 was from R&D Systems; and anti–human VEGF antibody was from R&D Systems.
Migration assay
DC migration was evaluated using a chemotaxis microchamber technique as described previously.15 Briefly, 30 μL of chemoattractant solution or control medium (RPMI 1640 with 1% FBS) was added to the lower wells of a chemotaxis chamber (Neuroprobe, Gaithersburg, MD) and a polycarbonate filter (5 μm pore size; Neuroprobe) was placed into the wells and covered with a silicon gasket. A total of 50 μL of cell suspension (106/mL) were seeded in the upper wells, and the chamber was incubated at 37°C for 90 minutes. At the end of this period, filters were removed and stained with Diff-Quik (Baxter, McGaw Park, IL), and 10 high-power oil-immersion fields were counted.
Enzyme-linked immunosorbent assay
The concentrations of hTNFα, hIL-1b, hIL-10, hCXCL10, hCCL22, mTNFα, and mIL-1β levels in DC supernatants were measured using specific Duo-Set kits purchased from R&D Systems, in accordance with the manufacturer's instructions.
Real-time PCR
Total RNA was obtained using Trizol (Invitrogen, Carlsbad, CA). Reverse transcriptase–polymerase chain reaction (RT-PCR) from a 1-μg RNA template was performed using an RT-PCR kit (cDNA Achieve kit; Applied Biosystems, Foster City, CA). Real-time PCR was performed using SyBr Green PCR Master Mix (Applied Biosystems) and detected with a 7900HT Sequence Detection System (Applied Biosystems). The primers used were designed using Beacon Designer5 software (Premier Biosoft International, Palo Alto, CA). Data were normalized to the expression of the housekeeping gene, β-ACTIN, in the PCR reactions and results were expressed as fold increase in mRNA expression with respect to the control cells.
Proliferation assay
After 18 hours of culture under normoxic or hypoxic conditions in the presence or absence of 10 ng/mL LPS, DCs were exposed to 20 Gy (2000 rad) in cell irradiator and then collected and redistributed in 96-well plates in ratios of 1:10 and 1:20 with CD4+ T cells; CD4+ T cells were isolated from peripheral blood of healthy donors by RosetteSep Human CD4+ T Cell Enrichment Cocktail (StemCell Technologies, Vancouver, BC) in accordance with manufacturer's instructions. After 3 days of coculture, the T-cell proliferation was assessed using the Cell Proliferation Biotrak ELISA System (Amersham Biosciences, Uppsala, Sweden). Briefly, BrdU was added to the cells and incubated overnight, after which the culture medium was removed, the cells were fixed, and peroxidase-labeled anti-BrdU was added. The immune complexes were detected by the subsequent substrate reaction and read at 450 nm.
Immunoblotting
DCs were washed with ice-cold phosphate-buffered saline (PBS) containing 1 mM Na3VO4, then lysed in 50 μL of lysis buffer (20 mM Tris-HCl [pH 8], 137 mM NaCl, 10% glycerol [vol/vol], 1% Triton X-100 [vol/vol], 1 mM Na3VO4, 2 mM EDTA, 1 mM PMSF, 20 μM leupeptin, and 0.15 U/mL aprotonin) for 20 minutes at 4°C. The lysates were centrifuged at 13 220g at 4°C for 15 minutes, and the supernatants (containing Triton X-100 soluble proteins) were run on a 10% (wt/vol) sodium dodecyl sulfate polyacrylamide gel electrophoresis (50 μg protein/lane). Separated proteins were transferred onto a nitrocellulose membrane (1 hour at 125 mA) and immunoblotted for specific rabbit anti–human HIF-1α antibody (Cell Signaling Technologies, Danvers, MA) or for specific mouse anti–mouse HIF-1α antibody (Novus Biologicals, Littleton, CO) according to the manufacturer's instructions. Blocking was done with 5% (wt/vol) bovine serum albumin (BSA) in TBS-0.1% Tween (TBST) for 1 hour at room temperature. Antibody dilutions were prepared in 5% (wt/vol) BSA-TBST. Primary antibodies were used at 1:1000 or 1:500 dilutions, respectively, overnight at 4°C. Horseradish peroxidase (HRP)–conjugated anti–rabbit or anti–mouse secondary antibodies (Amersham Biosciences) were used at 1:10 000 or 1:2000 dilution for 1 hour at room temperature. Blots were visualized using an electrochemiluminescence (ECL) kit (Amersham Biosciences). Immunoblotting for actin was performed using a goat polyclonal anti-actin (c-11; Santa Cruz Biotechnology, Santa Cruz, CA), diluted 1:2000 in 5% (wt/vol) BSA-TBST, and HRP-conjugated anti–goat secondary antibody (Santa Cruz Biotechnology) was used at 1:5000 dilution.
In vivo migration of murine DCs
CD34+-derived myeloid DCs were generated from femurs of C57BL/6 mice as previously described.19 After 9 days of culture, CD34+-derived myeloid DCs were collected (> 90% CD11c+) and exposed to 100 ng/mL LPS for 18 hours in normoxia, or in hypoxia, or in the presence of 400 μM deferoxamine (hypoxia mimicker). Afterward, DCs were labeled with 0.5 mM of the vital dye 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (mixed isomer (5-6-CFDA, SE [CFSE]; Molecular Probes). A total of 2 × 106 labeled cells was injected subcutaneously in the hind leg footpad. Popliteal lymph nodes were recovered 24 hours later, mechanically disaggregated and treated with collagenase A (1 mg/mL; Boehringer Mannheim, Indianapolis, IN) and DNase (0.4 mg/mL; Roche, Indianapolis, IN) for 30 minutes, after which the cell suspension was evaluated by FACScanto (Becton Dickinson, San Jose, CA).
Histology and immunohistochemistry
Consecutive frozen sections (8 μm) of footpads were cut, mounted on Superfrost slides (Bio-Optica, Milan, Italy), and used for histologic and immunohistochemical evaluation. Histologic examination was performed on hematoxylin-eosin–stained sections. Immunohistochemistry (IHC) was performed on acetone/chloroform-fixed slides by using the monoclonal antibody rat anti–mouse lymphocyte function–associated antigen-1/LFA1 (supernatant, dilution 1:10; raised in our laboratory) to detect lymphocytes, monocytes, granulocytes, and some plasma cells). The sections were rehydrated with PBS and then incubated with primary antibody for 2 hours in a humid chamber. The reactions were revealed by biotinylated anti–rat IgG (dilution 1:50, 1 hour incubation; Vector Laboratories, Burlingame, CA) followed by HRP-conjugated ZyMax Streptavidin (dilution 1:500, 30 minutes incubation; Zymed, San Francisco, CA). The chromogen was 3,3′-diaminobenzidine free base (DAB).
Annexin V staining
Murine tissues were collected and snap-frozen by liquid nitrogen; 8 μm sections were cut, mounted on Superfrost slides (Bio-Optica, Milan, Italy), and fixed with 4% paraformaldehyde for 15 minutes at room temperature. Sections were rehydrated with PBS (pH 7.00) twice for 10 minutes, incubated for 5 minutes with PBS–0.03% H2O2–1% BSA and with PBS–1% BSA for 10 minutes to block endogenous peroxidase and aspecific sites, respectively. A double immunofluorescence was performed with the following antibodies: FITC-conjugated anti–annexin V (Immunostep; Salamanca, Spain) and rat anti–mouse biotinylated CD11c followed by Alexa Fluor streptavidin 594 (Molecular Probes/Invitrogen). Nuclei were stained with DAPI (Molecular Probes/Invitrogen). The Fondazione Humanitas per la Salute adheres to the principles set out in the following laws, regulations, and policies governing the care and use of laboratory animals: Fondazione Humanitas Regulations and Policies providing internal authorization for persons conducting animal experiments; the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (1996 edition) Italian Governing Law Legislative decree 116 of January 27, 1992); European Union (EU) directives and guidlines (EEC Council Directive 86/609, OJ L 358, 12/12/1986); (Legislative Decree September 19, 1994, n. 626 (89/391/CEE, 89/654/CEE, 89/655/CEE, 89/656/CEE, 90/269/CEE, 90/270/CEE, 90/394/CEE, 90/679/CEE).
Statistical analysis
Data are presented as means with standard deviation. Statistical comparisons between groups were made using the Student t test, and P values less than .05 were considered to be statistically significant.
Results
Hypoxia-induced HIF-1α activation is paralleled by inhibition of monocyte-derived DC differentiation
Hypoxia is a common feature of several inflammatory diseases1,14 and strongly affects the expression of specific genes involved in leukocyte activation and recruitment.20,21 In hypoxia, cells undergo a metabolic adaptation, mainly mediated through the induction and stabilization of the HIF-1, a major regulator of cell adaptation to hypoxic stress.22 Because DCs are an important component of the inflammatory infiltrate characterizing inflammatory tissues, we evaluated the activation of HIF-1α in response to hypoxia during the differentiation and maturation phases of monocyte-derived DCs. Human monocytes were cultured for 6 days under normoxic or hypoxic conditions (1% O2) in the presence of GM-CSF and IL-13. On day 6, cells were treated for 24 hours with LPS to induce their maturation. HIF-1α expression was evaluated by Western blot at different times. As shown in Figure 1A, monocytes activate HIF-1α as part of their adaptation programs to low oxygen conditions (1%) after 3 days.
Effects of hypoxia on the differentiation of monocyte-derived DCs. (A) DCs express inducible HIF-1α in response to hypoxia. DCs were generated from monocytes cultured in the presence of IL-13 and GM-CSF under normoxic (Norm) and hypoxic (Hyp) conditions. Whole protein extracts were analyzed by Western blot at different time points as indicated. P indicates the protein extract from control Hela cells treated with the hypoxia-mimicking compound cobalt chloride (CoCl2).23 Vertical lines between day 3 and day 6 have been inserted to indicate a repositioned gel lane. (B) Expression profiles of CD14 and CD1a in DCs differentiated in normoxia or hypoxia, as indicated. Freshly isolated monocytes (day 0) and DCs differentiating on day 3 and day 6 of culture were stained with CD14-APC and CD1a-FITC. The results shown are representative of 4 independent experiments. Q1: single-positive CD14+ cells; Q2: double-positive CD14+/CD1a+ cells; and Q4: single-positive CD1a+ cells. (C) Expression profiles of CD16 and CD68 in the single-positive CD14+ population (Q1) in normoxia and hypoxia. Results are means plus or minus standard deviation (SD) of 3 independent experiments.
Effects of hypoxia on the differentiation of monocyte-derived DCs. (A) DCs express inducible HIF-1α in response to hypoxia. DCs were generated from monocytes cultured in the presence of IL-13 and GM-CSF under normoxic (Norm) and hypoxic (Hyp) conditions. Whole protein extracts were analyzed by Western blot at different time points as indicated. P indicates the protein extract from control Hela cells treated with the hypoxia-mimicking compound cobalt chloride (CoCl2).23 Vertical lines between day 3 and day 6 have been inserted to indicate a repositioned gel lane. (B) Expression profiles of CD14 and CD1a in DCs differentiated in normoxia or hypoxia, as indicated. Freshly isolated monocytes (day 0) and DCs differentiating on day 3 and day 6 of culture were stained with CD14-APC and CD1a-FITC. The results shown are representative of 4 independent experiments. Q1: single-positive CD14+ cells; Q2: double-positive CD14+/CD1a+ cells; and Q4: single-positive CD1a+ cells. (C) Expression profiles of CD16 and CD68 in the single-positive CD14+ population (Q1) in normoxia and hypoxia. Results are means plus or minus standard deviation (SD) of 3 independent experiments.
CD1a molecules are up-regulated during maturation of DCs, coincident with the functions of antigen capture and processing.24 In contrast, expression of the monocyte marker CD14 is lost during monocyte differentiation to DCs. Based on this, it was important to monitor the relative expression of these 2 markers during various stages of DC differentiation, in normoxia versus hypoxia. As shown in Figure 1B, monocytes cultured in the presence of the differentiation-inducing cytokines IL-13/GM-CSF showed differential and dynamic changes in the surface expression of CD14 and CD1a over time. As shown, at day 0, freshly isolated monocytes were 85.5% CD14+ and CD1a−. At day 3, 3 different cell populations were identified, both in normoxia and hypoxia. The first population (Q1) displayed a single positivity for CD14, and cytofluorimetric analysis identified 14.7% of total cells in Q1 in normoxia versus 29.7% in hypoxia. A CD14/CD1a double-positive population (Q2) was present at 43.3% in normoxia and 32.9% in hypoxia. A third population (Q4), CD1a single-positive, was 27.3% in normoxia and only 16.4% in hypoxia. This apparent delay of DC differentiation in hypoxia was further observed at day 6, when we observed 9.4% of the Q1 population in normoxia versus 14.7% in hypoxia; 17.6% of Q2 in normoxia versus 41.2% in hypoxia; and 61.4% of Q4 in normoxia versus 33.8% in hypoxia. The consistently higher expression of CD14 and lower expression of CD1a, in hypoxia versus normoxia, suggests that low oxygen availability restrains the differentiation process of monocyte-derived DCs. To investigate whether hypoxia could promote macrophage rather than DC differentiation, we measured the expression of the macrophage markers CD16 and CD68. As shown in Figure 1C, hypoxia promoted a selective up-regulation of CD16 in the CD14 single-positive monocyte population (Q1). Conversely, no changes were observed in CD68 surface expression, suggesting a lack of definite skewing toward macrophage differentiation.
Hypoxia inhibits the maturation of monocyte-derived DCs
DCs mature in response to various microbial compounds, including bacterial wall components such as LPS. To investigate the effect of hypoxia on DC maturation (as opposed to “differentiation”), human monocytes were first cultured for 6 days in normoxia in the presence of GM-CSF and IL-13. On day 6, immature DCs were subsequently incubated either in normoxia or hypoxia, and then treated with LPS for a further 18 hours. Hypoxia significantly reduced the expression of CD40, CD80, CD83, and CD86, both in terms of the percentage of positive cells and their mean channel of fluorescence intensity (Figure 2A). In addition, despite no differences being observed in the percentage of MHC class II–positive DCs, their mean fluorescence intensity was consistently reduced in hypoxia. As inhibition of DC maturation by hypoxia could affect their capability to promote adaptive immunity, we next determined the ability of hypoxic DCs to induce proliferation and activation of T cells. After exposure of DCs to LPS in normoxia or hypoxia, the cells were irradiated and subsequently cocultured in mixed leukocyte reactions with CD4+ T cells obtained from allogeneic donors (Figure 2B). Notably, in line with the observed decreased expression of costimulatory molecules by DCs in hypoxia, these cells induced lower proliferation of T cells, as well as lower secretion of IFN-γ, than DCs cultured in normoxia. These results indicate that oxygen availability is a limiting condition for the expression of costimulatory properties by DCs.
Effect of hypoxia on monocyte-derived DC maturation. (A) Human monocyte–derived DCs were cultured for 18 hours under normoxic or hypoxic conditions in the presence or absence of LPS and the surface expression of different maturation markers was analyzed by flow cytometry. Cells were stained with CD80–R-PE, CD83-Alexa488, CD86-APC, CD40–R-PE, and MHC II–Alexa488 antibodies, or with isotype controls. Left panels: dotted line indicates normoxia; dashed line, hypoxia; and solid line, isotype. Results are representative of 3 independent experiments. Right panels: the average of 3 independent experiments is shown. Data are means plus or minus SD. *P < .05 versus DCs matured in normoxia. (B) Effect of hypoxia on costimulatory functions of DCs. After 3 days of coculture with DCs, CD4+ T-cell proliferation (left) and IFN-γ production (right) were evaluated by enzyme-linked immunosorbent assay (ELISA). Data are means plus or minus SD of 3 different experiments. *P < .05 versus normoxic counterparts.
Effect of hypoxia on monocyte-derived DC maturation. (A) Human monocyte–derived DCs were cultured for 18 hours under normoxic or hypoxic conditions in the presence or absence of LPS and the surface expression of different maturation markers was analyzed by flow cytometry. Cells were stained with CD80–R-PE, CD83-Alexa488, CD86-APC, CD40–R-PE, and MHC II–Alexa488 antibodies, or with isotype controls. Left panels: dotted line indicates normoxia; dashed line, hypoxia; and solid line, isotype. Results are representative of 3 independent experiments. Right panels: the average of 3 independent experiments is shown. Data are means plus or minus SD. *P < .05 versus DCs matured in normoxia. (B) Effect of hypoxia on costimulatory functions of DCs. After 3 days of coculture with DCs, CD4+ T-cell proliferation (left) and IFN-γ production (right) were evaluated by enzyme-linked immunosorbent assay (ELISA). Data are means plus or minus SD of 3 different experiments. *P < .05 versus normoxic counterparts.
Hypoxia modulates cytokine and chemokine expression by DCs
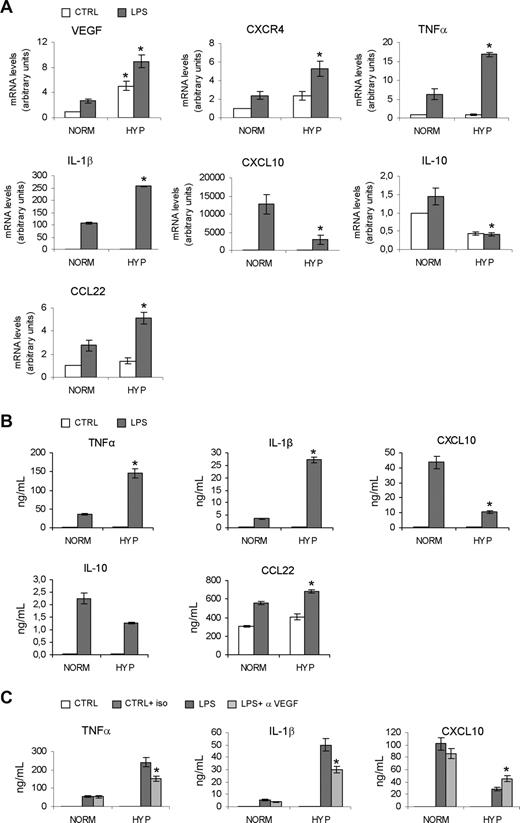
DCs orchestrate adaptive immunity and modulate the inflam-matory response by producing inflammatory cytokines and chemokines.2,,–5,8 Immature monocyte-derived DCs were treated for 18 hours with LPS in normoxia or hypoxia. Following this period, total RNA and supernatants were analyzed, respectively, for gene expression (Figure 3A) and secretion (Figure 3B) of selected cytokines and chemokines. As expected, in hypoxic conditions we observed increased mRNA levels of the prototypic hypoxia-inducible genes VEGF25 and CXCR426 (Figure 3A), as well as increased expression of TNFα, IL-1β, and CCL22 mRNAs. In contrast, CXCL10 and IL-10 mRNA expression was diminished. These results indicate that hypoxia can differentially modulate expression of selected cytokine and chemokine genes by DCs. In line with these results, we also observed higher secretion of TNFα, IL-1β, and CCL22 by hypoxic DCs, while CXCL10 and IL-10 secretion was diminished (Figure 3B). Similar results were obtained with hypoxic DCs exposed to CD40L rather than LPS (data not shown). Moreover, hypoxia induced enhanced VEGF protein secretion by DCs (data not shown) and, because DC maturation itself has been reported to be affected by VEGF,27 we also tested whether autocrine VEGF could have a role in the increased expression of proinflammatory cytokines observed in hypoxia. As shown in Figure 3C, an anti-VEGF antibody significantly decreased the secretion of TNFα and IL-1β in hypoxia, while significantly restoring CXCL10 production. These results indicate that autocrine hypoxia-induced VEGF plays a role in modulating the inflammatory phenotype of DCs. However, the anti-VEGF antibody did not elicit significant changes in the expression of DC maturation markers (eg, CD80, CD40, and MHC class II; data not shown).
Effects of hypoxia on cytokine expression by DCs. (A) Human monocyte–derived DCs were cultured under normoxic or hypoxic conditions in the presence or absence of LPS for 18 hours and analyzed for mRNA expression by real-time PCR. Results are means plus or minus SD of 3 different experiments. *P < .05 versus normoxic counterparts. (B) Supernatants were collected after 18 hours of LPS treatment and analyzed for cytokine production by ELISA. Results are means plus or minus SD of 4 experiments. *P < .05 versus DCs matured in normoxic conditions. (C) Effects of the inhibition of the biologic activity of VEGF on cytokine secretion by normoxic and hypoxic DCs. α-VEGF indicates anti-VEGF antibody (1 μg/mL); iso indicates isotype-matched antibody. Results are means plus or minus SD of 3 experiments. *P < .05 versus DCs matured in hypoxia.
Effects of hypoxia on cytokine expression by DCs. (A) Human monocyte–derived DCs were cultured under normoxic or hypoxic conditions in the presence or absence of LPS for 18 hours and analyzed for mRNA expression by real-time PCR. Results are means plus or minus SD of 3 different experiments. *P < .05 versus normoxic counterparts. (B) Supernatants were collected after 18 hours of LPS treatment and analyzed for cytokine production by ELISA. Results are means plus or minus SD of 4 experiments. *P < .05 versus DCs matured in normoxic conditions. (C) Effects of the inhibition of the biologic activity of VEGF on cytokine secretion by normoxic and hypoxic DCs. α-VEGF indicates anti-VEGF antibody (1 μg/mL); iso indicates isotype-matched antibody. Results are means plus or minus SD of 3 experiments. *P < .05 versus DCs matured in hypoxia.
Maturation of hypoxic DCs is refractory to different TLR ligands
To test whether maturation of DCs in hypoxia was refractory to TLR agonists other than LPS, we first analyzed the level of mRNA expression for different TLR members in immature DCs (day 6) cultured for an additional 18 hours under normoxic or hypoxic conditions (Figure 4A). As shown, no significant changes in TLR mRNA levels occurred in hypoxia. To evaluate the functional significance of these observations, immature DCs were exposed for 18 hours to several different TLR agonists and then analyzed for the surface expression of selected maturation markers (Figure 4B). In particular, we examined responses to an LPS preparation from Sigma-Aldrich, which binds both TLR2 and TLR4,28 and which was used in all our experiments, a TLR4-specific LPS (provided by Alexis Biochemicals), the TLR2-specific ligand PAM3CSK4, the TLR3-specific ligand poly (I:C), and the TLR7/8-specific ligand R-848. In all cases, hypoxia invariably inhibited the down-regulation of CCR5 and up-regulation of CCR7 that would otherwise accompany DC maturation, while, as expected, CXCR4 expression was induced by low oxygen conditions. Moreover, hypoxia inhibited the induction of CD83 surface expression (Figure 4B), as well as CD80 and MHC class II (data not shown). Furthermore, in most cases, hypoxia significantly enhanced TNFα and IL-1β secretion and had little effect on IL-10 secretion in response to the different TLR agonists (Figure 4C). These results suggest that DC “maturation” in hypoxia is impaired in response to a variety of different TLR ligands, thus highlighting the potential relevance of this event in various pathologic conditions.
Inhibition of DC maturation in response to different TLR ligands. (A) Human monocyte–derived DCs were cultured under normoxic or hypoxic conditions for 18 hours and analyzed for mRNA expression of TLR members by real-time PCR. Data are means plus or minus SD of 3 independent experiments. TLR mRNA levels in normoxic conditions ( ) were set to 1.0 arbitrary unit. (B) Surface expression (flow cytometric analysis) of selected chemokine receptors and the DC maturation marker CD83 on cells exposed to different TLR agonsists. DCs were cultured under normoxic or hypoxic conditions for 18 hours in the presence or absence of different TLRs agonists as indicated: 10 ng/mL LPS (TLR2/4; Sigma-Aldrich); 10 ng/mL LPS (TLR4; Alexis Biochemicals); 2 μg/mL PAM3CSK4 (TLR2); 10 μg/mL poly (I:C) (TLR3); or 3 μg/mL R-848 (TLR7/8). Data are means plus or minus SD of 3 independent experiments. *P < .05 versus normoxic counterparts. (C) Supernatants were collected 18 hours after treatments and analyzed for cytokine production by ELISA after exposure to different TLR agonists. Results are means plus or minus SD of 4 experiments. *P < .05 versus DCs matured in normoxia.
) were set to 1.0 arbitrary unit. (B) Surface expression (flow cytometric analysis) of selected chemokine receptors and the DC maturation marker CD83 on cells exposed to different TLR agonsists. DCs were cultured under normoxic or hypoxic conditions for 18 hours in the presence or absence of different TLRs agonists as indicated: 10 ng/mL LPS (TLR2/4; Sigma-Aldrich); 10 ng/mL LPS (TLR4; Alexis Biochemicals); 2 μg/mL PAM3CSK4 (TLR2); 10 μg/mL poly (I:C) (TLR3); or 3 μg/mL R-848 (TLR7/8). Data are means plus or minus SD of 3 independent experiments. *P < .05 versus normoxic counterparts. (C) Supernatants were collected 18 hours after treatments and analyzed for cytokine production by ELISA after exposure to different TLR agonists. Results are means plus or minus SD of 4 experiments. *P < .05 versus DCs matured in normoxia.
Inhibition of DC maturation in response to different TLR ligands. (A) Human monocyte–derived DCs were cultured under normoxic or hypoxic conditions for 18 hours and analyzed for mRNA expression of TLR members by real-time PCR. Data are means plus or minus SD of 3 independent experiments. TLR mRNA levels in normoxic conditions ( ) were set to 1.0 arbitrary unit. (B) Surface expression (flow cytometric analysis) of selected chemokine receptors and the DC maturation marker CD83 on cells exposed to different TLR agonsists. DCs were cultured under normoxic or hypoxic conditions for 18 hours in the presence or absence of different TLRs agonists as indicated: 10 ng/mL LPS (TLR2/4; Sigma-Aldrich); 10 ng/mL LPS (TLR4; Alexis Biochemicals); 2 μg/mL PAM3CSK4 (TLR2); 10 μg/mL poly (I:C) (TLR3); or 3 μg/mL R-848 (TLR7/8). Data are means plus or minus SD of 3 independent experiments. *P < .05 versus normoxic counterparts. (C) Supernatants were collected 18 hours after treatments and analyzed for cytokine production by ELISA after exposure to different TLR agonists. Results are means plus or minus SD of 4 experiments. *P < .05 versus DCs matured in normoxia.
) were set to 1.0 arbitrary unit. (B) Surface expression (flow cytometric analysis) of selected chemokine receptors and the DC maturation marker CD83 on cells exposed to different TLR agonsists. DCs were cultured under normoxic or hypoxic conditions for 18 hours in the presence or absence of different TLRs agonists as indicated: 10 ng/mL LPS (TLR2/4; Sigma-Aldrich); 10 ng/mL LPS (TLR4; Alexis Biochemicals); 2 μg/mL PAM3CSK4 (TLR2); 10 μg/mL poly (I:C) (TLR3); or 3 μg/mL R-848 (TLR7/8). Data are means plus or minus SD of 3 independent experiments. *P < .05 versus normoxic counterparts. (C) Supernatants were collected 18 hours after treatments and analyzed for cytokine production by ELISA after exposure to different TLR agonists. Results are means plus or minus SD of 4 experiments. *P < .05 versus DCs matured in normoxia.
Effects of hypoxia on DC chemotaxis in vitro
Maturing DCs migrate from inflamed tissues via the lymphatics into lymph nodes where CCR7 ligands are expressed; here, DCs initiate and orchestrate adaptive immunity. In contrast, maturing DCs are characterized by the loss of responsiveness toward inflammatory chemokines such as CCL5, which acts through CCR5 and CCR1.8,29 In addition, up-regulation of CXCR4 by DCs is also observed during maturation.30 To evaluate the effects of hypoxia on chemokine receptor functions, we measured the chemotactic responsiveness of DCs matured in hypoxia and normoxia. Hypoxia decreased the chemotactic responsiveness of mature DCs toward the CCR7 ligand CCL19, but enhanced DC migration to both the CCR5 and CXCR4 ligands, CCL5 and CXCL12, respectively (Figure 5A). Based on this observation, we next examined the surface expression of CCR7, CCR5, and CXCR4 (Figure 5B). In line with the modulation of chemotactic responsiveness, hypoxia significantly prevented the up-regulation of CCR7 and the down-regulation of CCR5 surface expression, and further enhanced the expression of CXCR4, suggesting that low oxygen tension may inhibit an otherwise efficient chemokine receptor switch that occurs during (normoxic) DC maturation. However, the entity of the decrease of CCR7 expression may suggest that hypoxia affects DC responsiveness to CCR7 ligands also at signaling levels.
Modulation of chemokine receptor expression and functions by hypoxia. Human monocyte–derived DCs were cultured for 18 hours under normoxic or hypoxic conditions in the presence or absence of LPS and analyzed for their chemotactic responsiveness and chemokine receptor expression. (A) Effects of hypoxia on the chemotactic responsiveness of monocyte-derived DCs toward CXCR4-, CCR5-, and CCR7-specific ligands, CXCL12/SDF-1, CCL5/RANTES, and CCL19/ MIP-3β, respectively. DCs were cultured for 18 hours in the indicated conditions, and the migration assay was performed using a chemotaxis microchamber. Chemokines were used at 100 ng/mL. Data are means plus or minus SD of 3 independent experiments done in triplicate. *P < .05 versus normoxic counterparts. (B) Flow cytometric analysis of chemokine receptor surface expression of monocyte-derived DCs. DCs were stained with CXCR4-APC, CCR7-PE-Cy7, CCR5 + Alexa488, and isotype-matched antibodies (isotypic control). Results are means plus or minus SD of 3 independent experiments. *P < .05 versus normoxic counterparts. (C,D) Effects of hypoxia on human myeloid DCs isolated from peripheral blood. CD1c+ myeloid DCs were cultured for 18 hours under normoxic or hypoxic conditions in the presence or absence of LPS. (C) In CD1c+ myeloid DCs isolated from blood, LPS-induced TNFα secretion was enhanced in hypoxia, while low oxygen tension reduced IL-10 and CXCL10 secretion. Furthermore (D), hypoxia promoted a strong decrease of CCR7 surface expression, partial reduction of CD40, CD83, MHC class II, and significant up-regulation of CXCR4. Numbers on top of bars show mean fluorescence intensity.
Modulation of chemokine receptor expression and functions by hypoxia. Human monocyte–derived DCs were cultured for 18 hours under normoxic or hypoxic conditions in the presence or absence of LPS and analyzed for their chemotactic responsiveness and chemokine receptor expression. (A) Effects of hypoxia on the chemotactic responsiveness of monocyte-derived DCs toward CXCR4-, CCR5-, and CCR7-specific ligands, CXCL12/SDF-1, CCL5/RANTES, and CCL19/ MIP-3β, respectively. DCs were cultured for 18 hours in the indicated conditions, and the migration assay was performed using a chemotaxis microchamber. Chemokines were used at 100 ng/mL. Data are means plus or minus SD of 3 independent experiments done in triplicate. *P < .05 versus normoxic counterparts. (B) Flow cytometric analysis of chemokine receptor surface expression of monocyte-derived DCs. DCs were stained with CXCR4-APC, CCR7-PE-Cy7, CCR5 + Alexa488, and isotype-matched antibodies (isotypic control). Results are means plus or minus SD of 3 independent experiments. *P < .05 versus normoxic counterparts. (C,D) Effects of hypoxia on human myeloid DCs isolated from peripheral blood. CD1c+ myeloid DCs were cultured for 18 hours under normoxic or hypoxic conditions in the presence or absence of LPS. (C) In CD1c+ myeloid DCs isolated from blood, LPS-induced TNFα secretion was enhanced in hypoxia, while low oxygen tension reduced IL-10 and CXCL10 secretion. Furthermore (D), hypoxia promoted a strong decrease of CCR7 surface expression, partial reduction of CD40, CD83, MHC class II, and significant up-regulation of CXCR4. Numbers on top of bars show mean fluorescence intensity.
The effects of hypoxia were also evaluated using human myeloid DCs from peripheral blood. After isolation, myeloid DCs were exposed to LPS and cultured in normoxia or hypoxia, as indicated (Figure 5C). Similarly to monocyte-derived DCs, culture in hypoxia enhanced the secretion of TNFα, reduced IL-10 expression, and partially inhibited CXCL10 secretion. Furthermore, hypoxia promoted a strong decrease in CCR7 surface expression, a partial reduction in levels of expression of CD40, CD83, and MHC class II (in terms of mean fluorescent intensities), along with a significant up-regulation of CXCR4 (Figure 5D). Taken together, these results from in vitro studies of both monocyte-derived DCs and primary myeloid DCs suggested that low oxygen conditions may alter the tissue distribution of maturing DCs in vivo (see next paragraph).
Hypoxia enhances inflammatory functions of DCs at peripheral sites
Cell reoxygenation strongly affects various biologic functions, including cell migration. Indeed, we observed that LPS-matured hypoxic DCs, reexposed to normoxic conditions for a further 24 hours, regain levels of expression of both chemokine receptors (CCR5, CCR7, and CXCR4) and maturation markers (CD83, CD80, and MHC class II) equivalent to those expressed by DCs matured in normoxia (data not shown). Therefore, to prevent reoxygenation and assess the in vivo relevance of the effects of hypoxia on DC maturation, mouse CD34+-derived myeloid DCs were treated with LPS (100 ng/mL) for 18 hours in the presence or absence of 400 μM of the hypoxia mimicker deferoxamine (DFX).26 Similarly to hypoxia, DFX treatment of DCs resulted in HIF-1α activation (Figure 6A), up-regulation of TNFα and IL-1β secretion (Figure 6B), decreased Ccr7 mRNA expression (Figure 6C), decreased chemotaxis toward CCL19 (Figure 6D), and increased migration toward the CXCR4 ligand CXCL12 (Figure 6D). These results indicate that, at the concentration used in our experiments, DFX treatment recapitulates the effects elicited by hypoxia on DCs.
Selective induction of DC inflammatory functions by hypoxia. Effects of DFX on HIF-1α activation (A), production of TNFα and IL-1β (B), Ccr7 mRNA expression (C), and chemotactic responsiveness to CCL19 and CXCL12 (D) of LPS-exposed murine DCs. (E) Effects of hypoxia on DC functions in vivo. CD34+-derived myeloid DCs were exposed to LPS for 18 hours in normoxia or in the presence of DFX (400 μM). Afterward, 2 × 106 of CFSE-labeled DCs were injected subcutaneously in the hind-leg footpad. The number of DCs that had migrated to the popliteal lymph nodes was evaluated by cytofluorimetry. Results are representative or average of 3 independent expreiments. *P < .05 versus normoxic counterparts. (F) Histologic (left panels) and immunohistochemical (right panels) evaluation of subcutaneous inflammatory infiltrates induced by injection of DCs cultured in normoxia (NORM), hypoxia (HYP), or in the presence of DFX. EE indicates hematoxylin-eosin; LFA1, anti-LFA1 monoclonal antibody; Ep, epidermis; De, dermis; and Sm, skeletal muscle. **Inflammatory infiltrate. (G) Viability of normoxic and DFX-treated DCs after injection. Footpad sections of mice injected with normoxic or DFX-treated DCs were stained for the DC marker CD11c (red), the apoptotic marker annexin (green), and the nuclei marker DAPI (blue), as indicated. Original magnification ×100. Bars represent 20 μm. Immunohistochemical and fluorescence analyses were performed using an Olympus BX-51 microscope (Olympus, Hamburg, Germany) with Plan N 10× objective for immunohistochemical and Plan N 20× objective for fluorescence using a Colour view III camera. Images were captured and processed using Cell ^F application software.
Selective induction of DC inflammatory functions by hypoxia. Effects of DFX on HIF-1α activation (A), production of TNFα and IL-1β (B), Ccr7 mRNA expression (C), and chemotactic responsiveness to CCL19 and CXCL12 (D) of LPS-exposed murine DCs. (E) Effects of hypoxia on DC functions in vivo. CD34+-derived myeloid DCs were exposed to LPS for 18 hours in normoxia or in the presence of DFX (400 μM). Afterward, 2 × 106 of CFSE-labeled DCs were injected subcutaneously in the hind-leg footpad. The number of DCs that had migrated to the popliteal lymph nodes was evaluated by cytofluorimetry. Results are representative or average of 3 independent expreiments. *P < .05 versus normoxic counterparts. (F) Histologic (left panels) and immunohistochemical (right panels) evaluation of subcutaneous inflammatory infiltrates induced by injection of DCs cultured in normoxia (NORM), hypoxia (HYP), or in the presence of DFX. EE indicates hematoxylin-eosin; LFA1, anti-LFA1 monoclonal antibody; Ep, epidermis; De, dermis; and Sm, skeletal muscle. **Inflammatory infiltrate. (G) Viability of normoxic and DFX-treated DCs after injection. Footpad sections of mice injected with normoxic or DFX-treated DCs were stained for the DC marker CD11c (red), the apoptotic marker annexin (green), and the nuclei marker DAPI (blue), as indicated. Original magnification ×100. Bars represent 20 μm. Immunohistochemical and fluorescence analyses were performed using an Olympus BX-51 microscope (Olympus, Hamburg, Germany) with Plan N 10× objective for immunohistochemical and Plan N 20× objective for fluorescence using a Colour view III camera. Images were captured and processed using Cell ^F application software.
These results enabled us to evaluate the potential in vivo significance of our in vitro findings in a mouse model. DCs were labeled with 0.5 mM of the vital dye CFSE,31 and a total of 2 × 106 labeled cells were injected subcutaneously in the hind-leg footpad; 24 hours later, the number of DCs recovered from the draining lymph nodes was evaluated by fluorescence-activated cell sorter (FACS) analysis. As shown in Figure 6E, we observed a drastic reduction in the number of DFX-treated DCs that had migrated to lymph nodes, as compared with their normoxic counterparts, supporting the in vivo significance of our observations. Next, we evaluated the inflammatory infiltrate induced by subcutaneous injection of hypoxic, DFX-treated, or normoxic DCs. In agreement with the enhanced expression of TNFα, IL-1β, and the inflammatory chemokine receptor CCR5, we observed that hypoxic and DFX-treated DCs promoted enhanced subcutaneous recruitment of leukocytes. As shown in Figure 6F, the inflammation was less intense in footpads of mice injected with normoxic DCs and, in this group, was mainly localized in the deeper zone of the dermis and in the hypodermis. In contrast, in the hypoxic and DFX-treated groups, a higher number of inflammatory cells were present, which also infiltrated the superficial papillary dermis. To rule out possible in vivo toxic effects of DFX on DCs, footpad sections of mice injected with normoxic or DFX-treated DCs were stained for the DC marker CD11c (red) and the apoptotic marker annexin (green; Figure 6G). As shown, only a few CD11c/annexin V double-positive cells were present in both groups, and with a similar density. In addition, the viability of normoxic and DFX-treated DCs was also tested in vitro, and no differences were found at the concentrations used in our experiments (data not shown).
Discussion
Our results indicate that hypoxia prevents full maturation of DCs by interfering with specialized functions linked to activation of adaptive immunity. In contrast, hypoxia enhances the capacity of DCs to express specialized inflammatory functions at peripheral sites. During the differentiation of monocytes to DCs, we observed that low oxygen levels impaired the induction of CD1a surface expression although promoting higher expression of the monocytic marker CD14. Only a minor reduction (20%) of the endocytic activity, a specialized function of immature DCs,32 was observed in hypoxia (data not shown). Further, after stimulation with LPS, hypoxia-matured DCs displayed lower expression of the maturation markers CD40, CD80, CD83, CD86, and MHC class II molecules, accompanied by their reduced ability to prime T-cell functions. These observations indicate that oxygen availability is a critical parameter for DC differentiation and maturation. In contrast to the selective impairment of specialized DC functions involved in T-cell activation described here, hypoxia induced higher expression of proinflammatory cytokines, TNFα and IL-1β in particular, by LPS-exposed DCs. This phenotypic adaptation of DCs appears instrumental for the early host response against danger signals, such as infections or trauma, during which establishment of strong inflammatory responses may prevent disease progression. As an example, wounds are characterized by local hypoxia due to blood clotting, an event preventing pathogen spread. In these conditions, hypoxia may reinforce local inflammation and antibacterial activities by acting on different cell populations, DCs in particular. In support of this contention, we also observed increased expression of proinflammatory cytokines (eg, TNFα and IL-1β) in LPS-exposed hypoxic monocytes and macrophages (L. Rubino, unpublished results, September 2006). Interestingly, the proinflammatory action of hypoxia was further strengthen by the increased differentiation of CD14/CD16 double-positive proinflammatory monocytes, which are known to play a relevant role in inflammation and infectious disease in humans.33
As compared with normoxia, the maturation of DCs in hypoxia was paralleled by both inhibition of CCR7 up-regulation and sustained expression of CCR5 and CXCR4. Our results partially disagree with a previous study by Zhao et al,34 who reported a slight up-regulation of CCR7 by DCs in hypoxia. This discrepancy may be due to different experimental settings, as we used a hypoxia workstation to avoid cell reoxygenation during the assay. Despite this, we confirmed the decreased migration of hypoxic DCs in response to CCR7 ligands, as previously reported.34,35 In agreement with our observation on the enhanced inflammatory functions acquired by DCs in hypoxia, Ricciardi et al recently demonstrated that hypoxic DCs express functional CCR3, CCR2, CX3CR1, and CXCR4.36 In contrast with DCs, it was reported in breast cancer cells that the hypoxia-mimicking compounds DFX and cobalt chloride induce functional CCR7, mainly through a posttranscriptional mechanism.37 Our data suggest that, in response to various TLR agonists, hypoxia promotes a “premature DC phenotype” characterized by enhanced inflammatory functions (high TNFα and IL-1β production), selective homing capacity to peripheral tissues (through sustained CCR5 expression), and impaired migration in response to lymphoid-specific chemokines (CCL19). In support of this, hypoxia also elicited similar effects in human myeloid DCs isolated from peripheral blood. In line with this scenario, subcutaneous injection of DFX-treated DCs in the hind-leg footpad of mice resulted in their impaired migration to draining lymph nodes and enhanced an inflammatory infiltrate at the site of injection. The enhanced capability of hypoxic myeloid-derived DCs to perform inflammatory activities is in agreement with the observation that metabolic adaptation to hypoxia, through HIF-1α activation, is instrumental in promoting the inflammatory functions of myeloid cells, such as migration and bacterial killing.38 In addition, hypoxia-induced CXCR4 expression may play a role in the recruitment and positioning of DCs in hypoxic sites, similar to what has been reported for macrophages.26 Of note, we found that inhibition of the biologic activity of VEGF in hypoxic DCs resulted in decreased IL-1β and TNFα secretion, 2 cytokines controlled by NF-κB activity.39 This result is in line with previous reports showing NF-κB induction by VEGF40 and suggests that, along with its inhibitory activity on DC maturation,27 hypoxia-induced VEGF may contribute to promote a “premature DC phenotype” with enhanced inflammatory properties.
The role of the VEGF as key regulator of inflammatory functions is further strengthened by previous observations on VEGF-induced up-regulation of functional CXCR4, both in endothelial cells and glioblastoma, where it supports angiogenesis41 and cancer cell invasion,42 respectively. Thus, the hypoxia/HIF-1/VEGF pathway appears to extend its activity to different components of hypoxic microenvironments, underlying its potential relevance in disease. In this context, inhibition of DC maturation by hypoxia may occur in solid tumors, where the presence of necrotic areas and immature DCs is often found, and tumor-derived VEGF has been proposed to impair DC maturation.43 The signal transducer and activator of transcription STAT3 was shown to enhance HIF-1 activity and to contribute to tumor angiogenesis and growth.44 However, we did not observe phospho-STAT3 up-regulation in hypoxic DCs, thus ruling out its possible involvement in hypoxia-driven DC differentiation and maturation (data not shown).
In addition to acting as antigen-presenting cells, DCs have inflammatory activities and promote angiogenesis and tissue repair.45 Here, we show that hypoxia likely dissociates the inflammatory and tissue repair functions of DCs from their capacity to act as sentinels for adaptive immunity. It is conceivable that, through this functional uncoupling under hypoxic conditions, DCs may contribute to tissue homeostasis. At the same time, inhibition of accessory functions and trafficking to lymph nodes is likely to act as a safeguard mechanism against autoimmunity elicited by tissue damage.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Associazione Italiana Ricerca sul Cancro (AIRC), Italy; European Community (DC-THERA); Ministero Istruzione Università Ricerca (MIUR), Italy; and Istituto Superiore Sanità (ISS). We acknowledge support of the EC FP6 Network of Excellence DC-THERA (project no. LSHB-CT-2004-512074) for collaborative studies.
Authorship
Contribution: A.M. performed the majority of the experiments and contributed to the manuscript writing; T.S. provided technical and scientific support; P.L. contributed to DC purification, differentiation, and analysis; F.P. performed the immunohistochemical analysis; M.N. is a pathologist who supervised the immunohistochemical analysis; I.-H.C. performed independent, parallel experiments on the effects of hypoxia on DC differentiation and provided data for Figure 1A; S.S. provided critical discussions and contributed to the analysis of myeloid DCs isolated from peripheral blood; J.A. provided critical discussions, contributed to the analysis of HIF-1 and DC differentiation, and assisted with manuscript preparation; A.M. provided critical discussions; and A.S. supervised the entire work and the manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonio Sica, Fondazione Humanitas per la Ricerca, via Manzoni, 56, 20089 Rozzano, Milan, Italy; e-mail: antonio.sica@humanitas.it.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal