Chronic lymphocytic leukemia (CLL) is an indolent leukemia that is characterized by a relentless accumulation of mature lymphocytes with typical B-cell markers. Leukemic lymphocytes that are replicationally quiescent accumulate both in the bone marrow and the peripheral blood due to intrinsic defects in their apoptotic machinery and/or dysregulated production of survival signals from their Microenvironment. A balance between the anti- and proapoptotic proteins maintains the rheostat of B cells and all hematopoietic cells. While several members of antiapoptotic and proapoptotic Bcl-2 family have been identified, Mcl-1 surfaces as the most significant antiapoptotic protein associated with normal as well as malignant B lymphocytes.
Mcl-1 is essential during lymphoid development and maintenance of mature T and B lymphocytes1 , and the expression level of antiapoptotic proteins in normal and malignant lymphocytes is in concordance with its role in survival.2 High levels of Mcl-1 and Bcl-2 mRNA and protein have been found in CLL, which are inversely correlated with in vitro response to chemotherapeutic agents or with the failure of CLL patients to respond to fludarabine therapy.3 Conversely, down-regulation of Mcl-1 protein expression by antisense oligonucleotides or through indirect Mcl-1 transcription and translation inhibitors results in cell death during in vitro culture or in vivo therapy. In addition, overexpression of Mcl-1 prolongs the survival of CLL cells exposed to a variety of apoptosis-inducing stimuli.2 These key pieces of evidence establish Mcl-1 as a critical survival factor for CLL.
In this issue of Blood, Pepper et al use primary leukemia cells from a cohort of 185 patients with CLL to determine an association between antiapoptotic proteins and other prognostic parameters. To circumvent the qualitative nature of immunoblot analysis, flow cytometry was used to measure expression of antiapoptotic and proapoptotic proteins in a quantitative fashion. There is an expected heterogeneity among samples, yet the data beautifully demonstrate a relationship between Mcl-1 protein expression and other prognostic markers, such as stage of the disease, IgVH mutation status, ZAP-70 positivity, and CD38 expression. The study begs for analysis of other antiapoptotic and proapo-ptotic molecules, although they have included Bcl-2 and Bax. We also need to appreciate that quantitation of protein levels in 185 primary samples is a phenomenal effort. They also confirm association between these proteins and in vitro sensitivity to fludarabine, however, this was not a direct relationship; the correlation coefficients (r2 values) are not strong. Mcl-1 expression was also prognostic in overall survival of these patients and time to first treatment; the latter is under debate as there is not a consensus regarding the optimal time to treat CLL. Since the majority (70%) of the patients were of the Binet stage A, similar relationships were observed in this group also. Questions regarding a possible relationship between endogenous Mcl-1 levels and cytoreduction during therapy still remain unanswered. Nonetheless, the study provides new knowledge regarding the role of Mcl-1 in CLL patients, something that was previously assumed but now is substantiated by data and statistical analyses.
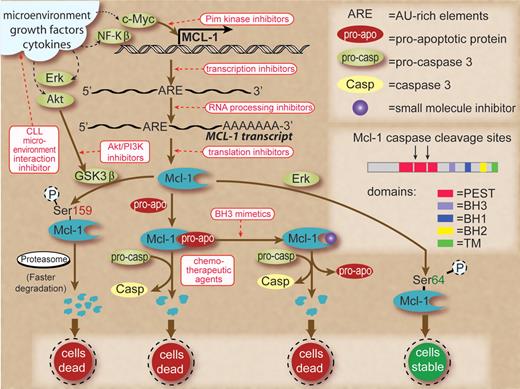
So how can we take advantage of this knowledge? Since specific genetic targets are not defined in CLL, Mcl-1 seems to be an appropriate biomolecule to therapeutically manipulate. As shown in the figure, Mcl-1 protein production and maintenance are dependent on several pathways. At the apical level, the microenvironment provides factors that dramatically increase this protein in CLL cells.4 Hence, a strategy that interferes with interaction of microenvironment and CLL cells is a logical approach. Production of Mcl-1 through these signals is carried via increased transcription of the MCL-1 gene. Transcription and polyadenylation inhibition, albeit not selective, is an approach that works because of AU-rich elements in the transcript of Mcl-1, which leads to its rapid turnover.5 The N-terminal region of Mcl-1 protein contains 2 PEST domains that are rich in proline, glutamic acid, serine, and threonine residues, resulting in a short half-life of the protein5 and making translation inhibition and rapid degradation of endogenous Mcl-1 via proteasome pathway a viable option to reduce the protein level.6 Mcl-1 is also essential during early lymphoid development1 and is abundantly expressed in the germinal center B-cell compartment. Pim kinase and Akt-PI3–kinase pathways and down-stream of BLyS have been identified to maintain the Mcl-1 levels in B cells.7 The roles of these pathways and consequence of their perturbations need to be investigated in malignant lymphocytes. Similarly, work is needed on posttranslational modification leading to increased or decreased half-life of Mcl-1 protein. Finally, and probably most intriguingly, small molecule antagonists of Mcl-1 protein that bind to the BH3 domain releasing proapoptotic proteins provide a new avenue of research and therapeutics.2,8
Schema to show transcription, translation, and posttranslation modifications that lead to production and maintenance of Mcl-1 proteins in normal and/or malignant B cells and therapeutic strategies (shown in red rectangles and red text) to interfere with these processes. Signals from microenvironment, growth factors, and cytokines are shown in black dashed lines as they result in increased expression of transcription factors and survival factors in CLL cells. Small molecule agents that interfere with microenvironment and CLL cell interactions reduce survival signals. Myc-driven transcription could be inhibited by Pim kinase inhibitors. The adenylate/uridylate-rich elements in the 3' untranslated region of Mcl-1 transcripts target them for rapid degradation. Global transcription inhibitors, such as flavopiridol or polyadenylation inhibitors, work to reduce Mcl-1 transcripts due to their fast turnover. Pharmacologic agents that shut down protein synthesis result in lower Mcl-1 protein levels due to the short half-life of this protein. Phosphorylation of Mcl-1 on Ser159 by activated GSK3β (unphosphorylated form) results in faster degradation of Mcl-1 through proteasomal pathway. However, GSK3β could be phosphorylated and inactivated by Akt. Hence, Akt inhibition is a therapeutic strategy. Mcl-1 sequesters proapoptotic proteins, and the latter could be released by use of BH3 mimetics, leading to cell death and Mcl-1 degradation. Similarly, chemotherapeutic agents activate caspases that could cleave Mcl-1. Mcl-1 is phosphorylated on Ser64 by Erk, resulting in stabilization of Mcl-1 protein and cell survival. Inset shows the structure of Mcl-1 protein with BH1, 2, and 3 domains, transmembrane domain, and PEST sequences where caspase cleavage sites are located. Some of these pathways are recognized only in normal B cells and are of unknown relevance in CLL B lymphocytes.
Schema to show transcription, translation, and posttranslation modifications that lead to production and maintenance of Mcl-1 proteins in normal and/or malignant B cells and therapeutic strategies (shown in red rectangles and red text) to interfere with these processes. Signals from microenvironment, growth factors, and cytokines are shown in black dashed lines as they result in increased expression of transcription factors and survival factors in CLL cells. Small molecule agents that interfere with microenvironment and CLL cell interactions reduce survival signals. Myc-driven transcription could be inhibited by Pim kinase inhibitors. The adenylate/uridylate-rich elements in the 3' untranslated region of Mcl-1 transcripts target them for rapid degradation. Global transcription inhibitors, such as flavopiridol or polyadenylation inhibitors, work to reduce Mcl-1 transcripts due to their fast turnover. Pharmacologic agents that shut down protein synthesis result in lower Mcl-1 protein levels due to the short half-life of this protein. Phosphorylation of Mcl-1 on Ser159 by activated GSK3β (unphosphorylated form) results in faster degradation of Mcl-1 through proteasomal pathway. However, GSK3β could be phosphorylated and inactivated by Akt. Hence, Akt inhibition is a therapeutic strategy. Mcl-1 sequesters proapoptotic proteins, and the latter could be released by use of BH3 mimetics, leading to cell death and Mcl-1 degradation. Similarly, chemotherapeutic agents activate caspases that could cleave Mcl-1. Mcl-1 is phosphorylated on Ser64 by Erk, resulting in stabilization of Mcl-1 protein and cell survival. Inset shows the structure of Mcl-1 protein with BH1, 2, and 3 domains, transmembrane domain, and PEST sequences where caspase cleavage sites are located. Some of these pathways are recognized only in normal B cells and are of unknown relevance in CLL B lymphocytes.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal