Abstract
We have previously shown that imatinib uptake into chronic myeloid leukemia (CML) cells is dependent on human organic cation transporter 1 (hOCT1; SLC22A1), and that low hOCT1 expression is an important determinant of clinical outcome to imatinib treatment. We hypothesized that dasatinib might be transported differently than imatinib, possibly accounting for its favorable effects in imatinib-resistant patients. 14C-dasatinib uptake was greater in KCL22-transfected cells with pcDNA3-hOCT1 plasmid (high hOCT1-expressing cells) than in control cells (P = .02). However, hOCT inhibitors did not decrease dasatinib uptake into either control or primary cells, in contrast to their block on imatinib uptake. Dasa-tinib decreased the level of phosphorylated CrkL to 49.9% in control and 40.3% in high hOCT1-expressing cells. Dasa-tinib efflux was investigated in confluent ABCB1-transfected MDCKII cell monolayers. Both dasatinib and imatinib were transported from the basal to the apical layer, indicating that they were transported by ABCB1, which was confirmed using the ABCB1 inhibitor PSC833 (P = .001 and P < .001, respectively). Compared with imatinib, dasatinib achieved superior intracellular levels and BCR-ABL suppression even in cells with low or blocked hOCT1. Efflux of dasatinib and imatinib appear similar via ABCB1. Dasatinib may therefore offer an advantage over imatinib in patients with low hOCT1 expression.
Introduction
The advent of the tyrosine kinase inhibitor (TKI) imatinib has transformed the treatment of chronic myeloid leukemia (CML). In comparison with earlier treatment options, it has an excellent safety profile, and the majority of patients will continue to respond well after 5 years of therapy.1 However, with increasing clinical experience it is becoming clear that some patients may develop resistance to imatinib. Many cases of acquired imatinib resistance are associated with the emergence of mutations in the BCR-ABL kinase domain (KD). However, some patients may develop resistance without KD mutations, whereas others develop KD mutations without developing imatinib resistance,2 suggesting that additional factors are required to produce a fully drug-resistant phenotype.
High expression of the efflux transporter P-glycoprotein, the product of the ABCB1 (MDR1) gene, may be associated with imatinib resistance in CML cell lines,3 and silencing of ABCB1 expression increases the intracellular concentration of imatinib.4 We have previously shown that imatinib uptake into CML cells is dependent on the uptake transporter hOCT1 (SLC22A1).5 In more recent work on clinical samples, we have shown that low hOCT1 expression may be an important mechanism of imatinib resistance.6 In contrast, pretreatment expression of the efflux transporters ABCB1, ABCC1 (MRP-1), and ABCG2 (breast cancer resistance protein) was unrelated to clinical outcome,6 suggesting that hOCT1 expression is the dominant transporter controlling intracellular imatinib concentration in CML cells.
Dasatinib is a second generation novel, oral, multitargeted inhibitor of BCR-ABL and SRC family kinases that has recently been licensed for the treatment of imatinib-resistant CML. In vitro, the drug has more than 300-fold greater potency than imatinib, and is effective against many KD mutations that confer imatinib resistance, with the notable exception of T315I.7 In a phase 1 study, hematologic and cytogenetic responses were observed in both chronic-phase and advanced-phase imatinib-resistant patients.8 In a phase 2 study of dasatinib at a dose of 70 mg twice daily in 186 patients with imatinib-resistant or -intolerant chronic-phase CML, 90% and 52% of patients achieved complete hematologic and major cytogenetic responses, respectively, at 8 months of follow up. Responses were seen in patients with KD mutations that confer resistance to imatinib.9 In a randomized phase 2 study in patients resistant to 400 mg imatinib, dasatinib induced better cytogenetic response rates and progression-free survival than dose escalation of imatinib to 800 mg daily.10 Dasatinib may induce major cytogenetic responses in up to 50% of patients in blast crisis that are resistant to imatinib and many of these responses are complete cytogenetic responses.11 Similarly, in a phase 2 study in 36 patients with Philadelphia chromosome (Ph)–positive acute lymphoblastic leukemia with a minimum follow up of 8 months, 140 mg daily dasatinib produced hematologic responses in 15 (42%), 10 of whom remained progression free. Complete cytogenetic responses were attained by 21 (58%), and again the presence of BCR-ABL mutations conferring imatinib resistance did not preclude a response to dasatinib.12
The activity of dasatinib in imatinib-resistant patients who lack KD mutations suggests that its uptake and efflux may differ from imatinib. Here we present the first data on the uptake and efflux of dasatinib, and compare the characteristics with those of imatinib. We report that dasatinib is less dependent than imatinib on hOCT1-mediated uptake into cells. Data on newly diagnosed CML patients showing different levels of hOCT1 expression support the in vitro findings.
Methods
Cell lines
For studies on TKI uptake, the CML cell line KCL22 was selected, since it expresses low basal levels of hOCT1.5 Cells were grown in RPMI 1640 (Biosera, East Sussex, United Kingdom) medium supplemented with 1% l-glutamine, penicillin/streptomycin, and 10% fetal calf serum (FCS). KCL22 cells were transfected by nucleofection (AMAXA Biosystems, Cologne, Germany) to introduce the pcDNA-hOCT1 plasmid (kind gift of D. Gründemann, Cologne, Germany). Two stable cell lines were generated, expressing high and low (but not absent) levels of hOCT1 by real-time polymerase chain reaction (PCR), as previously described.6 Cells were also transfected with the empty vector pcDNA3.1 to yield mock-transfected control lines.
The MDCKII canine kidney cell line was used to study ABCB1-mediated efflux, as previously described.5 The parental and ABCB1-transfected cells were kind gifts from Prof P. Borst (Amsterdam, The Netherlands) and were grown in Dulbecco modified Eagle medium (DMEM; Sigma-Aldrich, Poole, United Kingdom), supplemented with 1% l-glutamine, penicillin/streptomycin, and 10% FCS.
Analysis of cells from patients with newly diagnosed CML
Peripheral blood leukocytes were collected from 15 newly diagnosed CML chronic-phase patients prior to any treatment. Each patient gave informed consent to the study, which was approved by the Liverpool Local Research Ethics Committee, and this study was conducted in accordance with the Declaration of Helsinki. Expression levels of hOCT1 in these primary CML cells were assessed by real-time reverse-transcription (RT)–PCR, as previously described.5,6 Patients were stratified into low hOCT1 expressers (n = 8) and high hOCT1 expressers (n = 7), as previously defined.6
Evaluation of the uptake of TKIs
Studies of TKI transport used radiolabeled drug in transport medium (1% HEPES in Hanks buffered salt solution [HBSS]). For imatinib, 14C-imatinib (kind gift from Novartis, Basel, Switzerland) was used at 1.7 μM and nonradiolabeled imatinib (Novartis) was added to achieve a final concentration of 5 μM (2.95 μg/mL). For dasatinib, 14C-dasatinib (kind gift from Bristol Myers Squibb Pharmaceuticals, New York, NY) was used at 150 nM (75.9 ng/mL). These concentrations were selected as they are readily achievable in vivo at standard doses of imatinib and dasatinib. For each drug, 106 cells were added to TKI-containing transport medium, and uptake was measured by scintillation counting, as previously described.5,6
For the inhibition studies, the following inhibitors were used: verapamil (blocks hOCT1 and ABCB1), amantadine (blocks hOCT1 and hOCT2), prazosin (blocks hOCT1 and hOCT3), PSC833 (blocks ABCB1; kind gift from Novartis), and tariquidar (blocks ABCB1 and ABCG2). The optimal concentrations were as previously reported: 500 μM for verapamil and amantadine, 100 μM for prazosin, 10 μM for PSC833, and 500 nM for tariquidar.5 Unless stated, chemicals were obtained from Sigma-Aldrich. The assay was performed for up to 2 hours, whereas the inhibition studies were performed after 30-minute incubation.
Evaluation of the transport of tyrosine kinase inhibitors in monolayers
MDCKII and MDCKII-ABCB1 cells were seeded at a density of 1.5-2 × 106 cells per well onto presoaked, 24-mm diameter, 0.4-μm pore size, polycarbonate transwell membrane inserts (Appleton Woods, Birmingham, United Kingdom). Monolayer transepithelial electrical resistance (TEER) was measured using a Millicell-ERS volt-ohmeter (Millipore, Watford, United Kingdom) as before.5 Radiolabeled 3H-mannitol was also used as control, at a concentration of 1.85 Gβq/mL. TEER values of more than 200 were used.
14C-imatinib and 14C-dasatinib at 5 μM and 150 nM, respectively, were used as for the TKI uptake in transport buffer. The drugs were added to the donor compartment (either at the apical or the basal side), and the receiver compartment was filled with transport buffer only. Monolayers were incubated with the drugs for a total of 4 hours, and at various time intervals 50 μL solution was sampled from both compartments. Samples were mixed with 4 mL scintillation fluid and measured by scintillation counting. The measured radioactivity over the initial radioactivity at the donor chamber was calculated as percentage of radioactivity transported across the monolayers at different time points.13 For ABCB1 inhibition studies, the MDCKII-ABCB1 monolayer was preincubated with 10 μM PSC833 in transport buffer for 30 minutes and the experiment was then performed as described.
Partition coefficient of dasatinib and imatinib
The partition coefficient (logD) was used as a measure of the lipophilicity of dasatinib and imatinib. This was assessed using 14C-labeled drug at a concentration of 500 nM in a biphasic system of 1 mL 1-octanol mixed with 1 mL 1% HEPES in HBSS. LogD was defined as previously described14 : log (disintegrations per minute for 1-octanol)/log (disintegrations per minute for HEPES/HBSS buffer).
Measurement of CrkL phosphorylation by fluorescence-activated cell sorting
Phosphorylation of the CT10 regulator of kinaselike adaptor protein (CrkL) was used as a measure of BCR-ABL activity to assess drug uptake and physiological drug function.15 A total of 2 × 106 cells were cultured with 150 nM dasatinib or 5 μM imatinib for 1 hour. Cells (5 × 105) were resuspended in 500 μL 2% paraformaldehyde (VWR, Lutterworth, United Kingdom) and fixed for 10 minutes at 37°C. Cells were then chilled on ice for 1 minute and centrifuged at 768 g for 3 minutes. Added to the cell pellet was 500 μL 90% methanol (Fisher Scientific, Leicestershire, United Kingdom); the cells were vortexed and then incubated on ice for 30 minutes. Cells were then washed (throughout with 1 mL incubation buffer containing phosphate-buffered saline and 0.5% bovine serum albumin), and centrifuged at 768 g for 3 minutes. Cells were resuspended in 25 μL incubation buffer and left at room temperature for 10 minutes. pCrkL antibody (28μg/mL; Cell Signaling Technology, Beverly, MA) were added and 28 μg/mL anti–normal rabbit immunoglobulins (R&D Systems, Abingdon, United Kingdom) were used as control. Cells were vortexed and incubated at room temperature for 40 minutes. Cells were then washed twice and resuspended in 100 μL incubation buffer with the fluorescein-labeled goat antirabbit second antibody Alexa Fluor 488 (Invitrogen, Paisley, United Kingdom), incubated at room temperature in the dark for 30 minutes, then washed twice and analyzed using flow cytometry (FACScalibur; Becton Dickinson, Oxford, United Kingdom), with Cellquest Pro software (Becton Dickinson) for data analysis.
Statistical analysis
Time course experiments for drug uptake and transport of drugs across the monolayers were analyzed using the area under the curve (AUC) and unpaired t test within StatsDirect software (StatsDirect, Altrincham, United Kingdom.5,6 AUC is useful for determining the average concentration of a substance over a time interval. In this instance, it measured the mass (ng) of drug in a million cells over time (120 minutes). For uptake in the presence of inhibitors and effect of drugs on pCrkL expression, the unpaired t test was used. Data are all presented as mean plus or minus SEM (standard error of mean).
Results
Uptake of dasatinib and imatinib in KCL22 cell lines
Figure 1A shows the time course of the uptake of radiolabeled dasatinib into parental (KCL22), mock-transfected (KCL22pcDNA), and hOCT1-transfected KCL22 cells with low and high expression of hOCT1 (n = 3). Figure 1B shows similar data for the uptake of radiolabeled imatinib (n = 5). At 30 minutes, where maximum uptake is observed for both drugs, uptake was greater in cells with high expression of hOCT1 than in mock-transfected cells (P = .013 for dasatinib; P = .015 for imatinib). Moreover, uptake was greater in cells with high hOCT1 expression than into mock-transfected cells (P = .02 for dasatinib; P = .01 for imatinib) throughout the 120 minutes of testing, indicating that hOCT1 can transport both drugs. However, dasatinib uptake into parental, mock, and low hOCT1-expressing cells continued to increase up to 120 minutes. This effect was not seen with imatinib uptake. In addition, at baseline there was a difference in the level of dasatinib uptake compared with imatinib. These data suggest that although dasatinib is a substrate for hOCT1, its uptake is less dependent on hOCT1 than for imatinib, and in vivo, simple diffusion may be much more important than active uptake. This is consistent with the fact that at these pharmacologically achievable concentrations, the AUC for dasatinib uptake more than 120 minutes was 2-fold greater than that for imatinib both in the parental (67.6 vs 33.3; P = .001) and the high hOCT1-expressing (92.1 vs 47.7; P = .003) KCL22 cells.
Time course of dasatinib and imatinib uptake. (A) The uptake of 14C radiolabeled dasatinib into parental (◇), mock-transfected (■), and hOCT1-transfected KCL22 cells with low (▴) and high (×) expression of hOCT1. Data represent mean plus or minus SEM from 3 experiments. (B) The uptake of 14C radiolabeled imatinib for comparison. Data represent mean plus or minus SEM from 5 experiments. There is a greater uptake in high hOCT1-expressing than into mock-transfected cells, with P = .02 for dasatinib and P = .01 for imatinib between 0 to 120 minutes and P = .013 and P = .015, respectively, at 30 minutes. AUC for dasatinib versus imatinib uptake on KCL22 cells is 67.6 versus 33.3 (P = .001) and on high hOCT1-expressing cells is 92.1 versus 47.7 (P = .003).
Time course of dasatinib and imatinib uptake. (A) The uptake of 14C radiolabeled dasatinib into parental (◇), mock-transfected (■), and hOCT1-transfected KCL22 cells with low (▴) and high (×) expression of hOCT1. Data represent mean plus or minus SEM from 3 experiments. (B) The uptake of 14C radiolabeled imatinib for comparison. Data represent mean plus or minus SEM from 5 experiments. There is a greater uptake in high hOCT1-expressing than into mock-transfected cells, with P = .02 for dasatinib and P = .01 for imatinib between 0 to 120 minutes and P = .013 and P = .015, respectively, at 30 minutes. AUC for dasatinib versus imatinib uptake on KCL22 cells is 67.6 versus 33.3 (P = .001) and on high hOCT1-expressing cells is 92.1 versus 47.7 (P = .003).
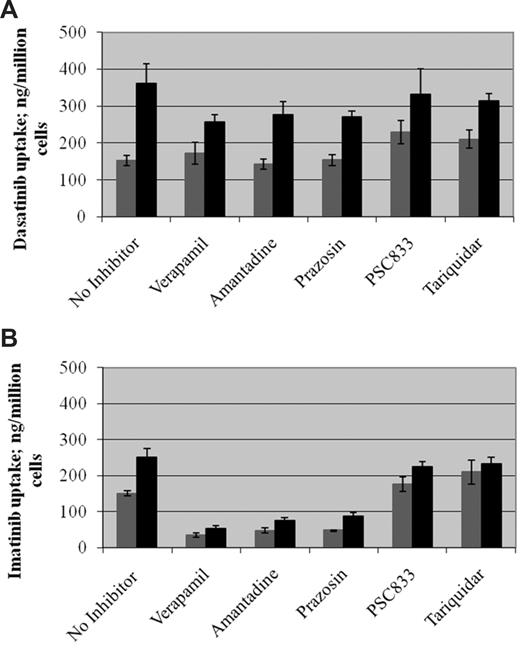
These data on hOCT1-mediated uptake of radiolabeled dasatinib (results of 4-6 replicate experiments at t = 30 minutes) were further confirmed using the hOCT1 inhibitors verapamil, amantadine, and prazosin (Figure 2A). Under these conditions, drug uptake was again greater in cells with high hOCT1 expression than in mock-transfected cells (P = .004 for dasatinib; P = .005 for imatinib). The hOCT1 inhibitors did not significantly decrease the uptake of dasatinib either into mock-transfected or into high hOCT1-expressing cells (P > .05 for all 3 inhibitors in both cell lines). The same information for the uptake of imatinib is given in Figure 2B. In contrast, these show that all 3 hOCT1 blockers decreased the uptake of imatinib into both mock-transfected and high hOCT1-expressing cells (P < .005 for all 3 inhibitors for both cell lines).
Effect of inhibitors of hOCT1 and ABCB1 on drug uptake by KCL22 cell lines. The effect of hOCT1 inhibitors (verapamil, amantadine, prazosin) and ABCB1 inhibitors (PSC833, tariquidar) on the uptake of radiolabeled dasatinib (A) and imatinib (B) into mock-transfected KCL22 cells ( ) and into high hOCT1-expressing cells (■) was examined (t = 30 minutes). No statistically significant effect was seen for inhibiting dasatinib uptake into either cell line (P = NS for all inhibitors in both cell lines). In contrast, all inhibitors significantly inhibited imatinib uptake (P < .005 for all 3 blockers for both cell lines). Under conditions with no inhibitor present, there was a significant difference on the uptake of drugs between hOCT1-expressing and mock-transfected cells with P = .004 for dasatinib and P = .005 for imatinib. Blockade of ABCB1 by PSC833 and tariquidar resulted in both cell lines showing similar levels of intracellular drugs. Data represent mean plus or minus SEM from 4 to 6 replicate experiments.
) and into high hOCT1-expressing cells (■) was examined (t = 30 minutes). No statistically significant effect was seen for inhibiting dasatinib uptake into either cell line (P = NS for all inhibitors in both cell lines). In contrast, all inhibitors significantly inhibited imatinib uptake (P < .005 for all 3 blockers for both cell lines). Under conditions with no inhibitor present, there was a significant difference on the uptake of drugs between hOCT1-expressing and mock-transfected cells with P = .004 for dasatinib and P = .005 for imatinib. Blockade of ABCB1 by PSC833 and tariquidar resulted in both cell lines showing similar levels of intracellular drugs. Data represent mean plus or minus SEM from 4 to 6 replicate experiments.
Effect of inhibitors of hOCT1 and ABCB1 on drug uptake by KCL22 cell lines. The effect of hOCT1 inhibitors (verapamil, amantadine, prazosin) and ABCB1 inhibitors (PSC833, tariquidar) on the uptake of radiolabeled dasatinib (A) and imatinib (B) into mock-transfected KCL22 cells ( ) and into high hOCT1-expressing cells (■) was examined (t = 30 minutes). No statistically significant effect was seen for inhibiting dasatinib uptake into either cell line (P = NS for all inhibitors in both cell lines). In contrast, all inhibitors significantly inhibited imatinib uptake (P < .005 for all 3 blockers for both cell lines). Under conditions with no inhibitor present, there was a significant difference on the uptake of drugs between hOCT1-expressing and mock-transfected cells with P = .004 for dasatinib and P = .005 for imatinib. Blockade of ABCB1 by PSC833 and tariquidar resulted in both cell lines showing similar levels of intracellular drugs. Data represent mean plus or minus SEM from 4 to 6 replicate experiments.
) and into high hOCT1-expressing cells (■) was examined (t = 30 minutes). No statistically significant effect was seen for inhibiting dasatinib uptake into either cell line (P = NS for all inhibitors in both cell lines). In contrast, all inhibitors significantly inhibited imatinib uptake (P < .005 for all 3 blockers for both cell lines). Under conditions with no inhibitor present, there was a significant difference on the uptake of drugs between hOCT1-expressing and mock-transfected cells with P = .004 for dasatinib and P = .005 for imatinib. Blockade of ABCB1 by PSC833 and tariquidar resulted in both cell lines showing similar levels of intracellular drugs. Data represent mean plus or minus SEM from 4 to 6 replicate experiments.
Taken together, these findings are consistent with the view that dasatinib uptake into cells with low hOCT1 expression may be independent of hOCT1 transport. This contrasts with the dependence of imatinib uptake on hOCT1, in line with previously reported data.5 These results are supported by the finding that dasatinib had greater cytotoxicity than imatinib after 72 hours in culture, in both parental and mock-transfected cell lines (data not shown).
Efflux of dasatinib and imatinib by ABCB1
Figure 2 also shows the effect of the ABCB1 inhibitors PSC833 and tariquidar on the intracellular concentration of dasatinib and imatinib. ABCB1 blockade increased the intracellular level of both dasatinib and imatinib, though this was not statistically significant. However, these cell lines express basal levels of ABCB1 and other transporters, and it is possible that their high level of drug uptake may have swamped more subtle effects on efflux.
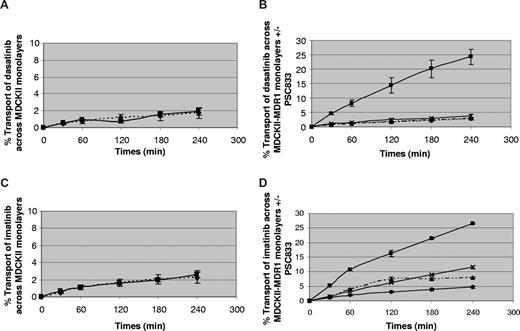
To investigate this more thoroughly, ABCB1-transfected MDCKII cells were used to study the role of ABCB1 in drug efflux—a model that has been widely used.5,16 When grown to a confluent monolayer on a semipermeable membrane, this cell line develops ABCB1 expression only on the apical aspect, and can therefore be used to study ABCB1 function, since this contributes to transport in the basal to apical but not in the apical to basal direction. Figure 3 shows the results on 3 or 4 separate monolayers. The left-hand panels show the transport of dasatinib and imatinib across parental MDCKII cells as a control. There was no significant difference between basal to apical (B-A) and apical to basal (A-B) transport, indicating minimal transport of the drugs across those cells. The right-hand panels show the transport across ABCB1-transfected cells that express ABCB1 on their apical surface, and demonstrate significantly increased transport of both drugs in the B-A direction in comparison with A-B (P = .001 for dasatinib and P < .001 for imatinib). This effect was abolished by the ABCB1 inhibitor PSC833 for each drug, indicating that they are both effluxed by ABCB1 (P = .001 for dasatinib and P < .001 for imatinib).
Efflux of dasatinib and imatinib by ABCB1. Efflux of dasatinib (A,B) and imatinib (C,D) in MDCKII-transfected (A,C) and MDCKII-ABCB1–transfected (B,D) cells. ▴ indicates A-B (apical to basal transport); ■, B-A (basal to apical transport); ♦, A-B + PSC833; and ×, B-A + PSC833. There was significantly increased transport of both drugs in the B-A direction in comparison with A-B (P = .001 for dasatinib and P < .001 for imatinib). This effect is abolished by PSC833 for each drug, indicating that they are both effluxed by ABCB1 (P = .001 for dasatinib and P < .001 for imatinib). Data represent mean plus or minus SEM from 3 to 4 separate monolayers.
Efflux of dasatinib and imatinib by ABCB1. Efflux of dasatinib (A,B) and imatinib (C,D) in MDCKII-transfected (A,C) and MDCKII-ABCB1–transfected (B,D) cells. ▴ indicates A-B (apical to basal transport); ■, B-A (basal to apical transport); ♦, A-B + PSC833; and ×, B-A + PSC833. There was significantly increased transport of both drugs in the B-A direction in comparison with A-B (P = .001 for dasatinib and P < .001 for imatinib). This effect is abolished by PSC833 for each drug, indicating that they are both effluxed by ABCB1 (P = .001 for dasatinib and P < .001 for imatinib). Data represent mean plus or minus SEM from 3 to 4 separate monolayers.
Transport of dasatinib and imatinib in primary CML cells
To investigate the effect of hOCT1 expression on primary cells, samples from 15 newly diagnosed patients were studied. Figure 4A shows the uptake of radiolabeled dasatinib into patients cells with low and high expression of hOCT1. Figure 4B shows similar data for the uptake of radiolabeled imatinib. The findings are similar to the cell lines shown in Figure 2. Prazosin decreases imatinib uptake in both low and high hOCT1-expressing samples (P = .001 and P = .04, respectively), but has no effect on dasatinib uptake in either cell type. As in the cell lines, dasatinib uptake is maintained in low hOCT1-expressing primary cells unlike the uptake of imatinib. Similar to the cell line data, cytotoxicity studies also showed that after 3 days in culture, dasatinib exerts greater cytotoxicity than imatinib (data not shown).
Transport of dasatinib and imatinib in primary CML cells. Differences on dasatinib (A) and imatinib (B) uptake on primary cells. The effect of hOCT1 inhibitor prazosin on the uptake of radiolabeled dasatinib (A) and imatinib (B) into patients expressing low ( , n = 8) and high (■, n = 7) hOCT1 levels was examined. The uptake of imatinib is inhibited in both low and high hOCT1 samples (P = .001 and P = .04, respectively), but there was no effect on the uptake of dasatinib. Data represent mean plus or minus SEM from 7 (■) or 8 (
, n = 8) and high (■, n = 7) hOCT1 levels was examined. The uptake of imatinib is inhibited in both low and high hOCT1 samples (P = .001 and P = .04, respectively), but there was no effect on the uptake of dasatinib. Data represent mean plus or minus SEM from 7 (■) or 8 ( ) hOCT1 expresses.
) hOCT1 expresses.
Transport of dasatinib and imatinib in primary CML cells. Differences on dasatinib (A) and imatinib (B) uptake on primary cells. The effect of hOCT1 inhibitor prazosin on the uptake of radiolabeled dasatinib (A) and imatinib (B) into patients expressing low ( , n = 8) and high (■, n = 7) hOCT1 levels was examined. The uptake of imatinib is inhibited in both low and high hOCT1 samples (P = .001 and P = .04, respectively), but there was no effect on the uptake of dasatinib. Data represent mean plus or minus SEM from 7 (■) or 8 (
, n = 8) and high (■, n = 7) hOCT1 levels was examined. The uptake of imatinib is inhibited in both low and high hOCT1 samples (P = .001 and P = .04, respectively), but there was no effect on the uptake of dasatinib. Data represent mean plus or minus SEM from 7 (■) or 8 ( ) hOCT1 expresses.
) hOCT1 expresses.
Effect of dasatinib and imatinib on BCR-ABL function
To investigate the relationship between the uptake and the efficacy of each drug, their effect on pCrkL expression was studied (Figure 5). Dasatinib produced a significant decrease in pCrkL expression for both cell lines, with reduction of pCrkL to 49.9% (P = .011) in mock-transfected KCL22 cells and 40.3% (P = .003) in high hOCT1-expressing cells, both in relation to untreated cells. In contrast, imatinib produced a significant decrease of pCrkL (to 39.7%, P = .001) only in high hOCT1-expressing cells, with no significant effect in mock-transfected cells, in relation again to untreated cells. Comparison of hOCT1 and mock-transfected cells showed that the degree of pCrkL suppression by imatinib was greater in high hOCT1-expressing cells than in mock-transfected cells (P = .03). These data are consistent with the view that hOCT1 transport is more important for imatinib than dasatinib.
Effect of dasatinib and imatinib on BCR-ABL function. Effect of dasatinib and imatinib on pCrkL expression (A) in mock-transfected KCL22 cells ( bars) and KCL22 cells with high hOCT1 expression (■ bars). Data are expressed as relative to untreated cells. Dasatinib produced a significant decrease in pCrkL expression for both cell lines, P = .011 and P = .003 in mock and high hOCT1-expressing cells, respectively. Imatinib produced a significant decrease, with P = .001 only in high hOCT1-expressing cells. * denotes a significant effect. pCrkL suppression by imatinib was greater in high hOCT1-expressing cells than in mock-transfected cells (P = .026). Data represent mean plus or minus SEM from 5 experiments. Examples of the corresponding p-CrkL flow histograms for mock (B) and hOCT1-expressing KCL22 cells (C) are also presented.
bars) and KCL22 cells with high hOCT1 expression (■ bars). Data are expressed as relative to untreated cells. Dasatinib produced a significant decrease in pCrkL expression for both cell lines, P = .011 and P = .003 in mock and high hOCT1-expressing cells, respectively. Imatinib produced a significant decrease, with P = .001 only in high hOCT1-expressing cells. * denotes a significant effect. pCrkL suppression by imatinib was greater in high hOCT1-expressing cells than in mock-transfected cells (P = .026). Data represent mean plus or minus SEM from 5 experiments. Examples of the corresponding p-CrkL flow histograms for mock (B) and hOCT1-expressing KCL22 cells (C) are also presented.
Effect of dasatinib and imatinib on BCR-ABL function. Effect of dasatinib and imatinib on pCrkL expression (A) in mock-transfected KCL22 cells ( bars) and KCL22 cells with high hOCT1 expression (■ bars). Data are expressed as relative to untreated cells. Dasatinib produced a significant decrease in pCrkL expression for both cell lines, P = .011 and P = .003 in mock and high hOCT1-expressing cells, respectively. Imatinib produced a significant decrease, with P = .001 only in high hOCT1-expressing cells. * denotes a significant effect. pCrkL suppression by imatinib was greater in high hOCT1-expressing cells than in mock-transfected cells (P = .026). Data represent mean plus or minus SEM from 5 experiments. Examples of the corresponding p-CrkL flow histograms for mock (B) and hOCT1-expressing KCL22 cells (C) are also presented.
bars) and KCL22 cells with high hOCT1 expression (■ bars). Data are expressed as relative to untreated cells. Dasatinib produced a significant decrease in pCrkL expression for both cell lines, P = .011 and P = .003 in mock and high hOCT1-expressing cells, respectively. Imatinib produced a significant decrease, with P = .001 only in high hOCT1-expressing cells. * denotes a significant effect. pCrkL suppression by imatinib was greater in high hOCT1-expressing cells than in mock-transfected cells (P = .026). Data represent mean plus or minus SEM from 5 experiments. Examples of the corresponding p-CrkL flow histograms for mock (B) and hOCT1-expressing KCL22 cells (C) are also presented.
Comparison of lipophilicity of dasatinib and imatinib
The relative lipophilic properties of dasatinib and imatinib were compared by measuring their logD partition coefficient between 1-octanol and HEPES/HBSS buffer. For dasatinib the logD was 2.05, whereas for imatinib it was 0.81. This demonstrates that dasatinib is more lipophilic than imatinib and, therefore, potentially able to get into cells more easily and independently of hOCT1.
Discussion
Dasatinib is currently the only licensed drug for the treatment of imatinib-resistant CML, and has been shown to be effective in several settings of imatinib resistance.9-12 Many cases of imatinib resistance arise because of the emergence of KD mutations, and dasatinib is effective against most KD mutations.7,9 However, it is also effective in patients with no demonstrable KD mutation, indicating that other mechanisms of imatinib resistance are clinically important. We have recently shown that the level of hOCT1 expression is a critical determinant of the outcome of imatinib treatment, and is an independent predictor of outcome in comparison with the components of the Sokal or Hasford prognostic scoring systems.6 Similar data for hOCT1 activity and imatinib uptake have also recently been demonstrated by White et al.17 These findings suggest that drug transporter expression and activity may be relevant to the clinical response to TKIs.
Here we present novel data on the transport of dasatinib at pharmacologically relevant concentrations, and compare it with that for imatinib. We show that the efflux of dasatinib is dependent on ABCB1, in a similar manner to imatinib. Overexpression of ABCB1, which reduces intracellular concentration of the drug, has been extensively implicated in treatment resistance in many forms of malignant diseases,18 and more recently, also in nonmalignant diseases such as epilepsy.19 Use of ABCB1 inhibitors has been suggested as a possible therapeutic maneuver to overcome drug resistance, but to date has not been shown to be clinically effective. Whether ABCB1 overexpression will also be important for dasatinib resistance will become clear only with further clinical experience.
Although imatinib and dasatinib both seem to be substrates for the efflux transporter ABCB1, in this study, we demonstrate important differences in the uptake of the 2 drugs. Dasatinib uptake into CML cell lines with particularly low hOCT1 expression (parental and mock-transfected KCL22 cells) was about 2-fold greater than that of imatinib. This was sufficient to cause suppression of pCrkL, unlike that with imatinib. CrkL has been widely used as a surrogate marker for physiological BCR-ABL tyrosine kinase activity15,20 and was adapted here as a quantifiable readout on BCR-ABL function over a short time period. Interestingly, use of transfected cells with high hOCT1 expression augmented dasatinib uptake, but did not lead to significant further suppression of pCrkL. One possibility is that suppression of pCrkL has reached its maximum level with respect to the sensitivity of the technique. Again, this pattern contrasts with that seen with imatinib, where high hOCT1 expression increases both uptake and pCrkL suppression. These findings were supported by the effect of inhibitors of hOCT1 function on dasatinib and imatinib uptake. Our data in both cell lines and primary cells expressing high and low levels of hOCT1 indicate that the uptake of dasatinib is less dependent on hOCT1 compared with imatinib. Indeed, our data suggest that effective uptake of dasatinib is likely to occur even at very low levels of expression of hOCT1, at which imatinib is ineffective. The uptake is likely to be due to passive diffusion, and is consistent with our finding that dasatinib is more lipophilic than imatinib in the octanol partition coefficient assay. Although we cannot completely exclude the role of other uptake transporters, taken together, our data demonstrate that effective BCR-ABL suppression by dasatinib is independent of hOCT1 expression, whereas suppression by imatinib is dependent on hOCT1.
The present findings may have clinical relevance. In our recent study of hOCT1 expression in imatinib-treated CML patients, 6 of 62 cases had an hOCT1 expression level below 3 arbitrary units (relative to a VBL cell line standard, as defined in Wang et al), which is the level of hOCT1 expression in mock-transfected KCL22 cells. Four of these 6 cases failed to achieve any cytogenetic response after 12 months of imatinib therapy, and 1 further case relapsed after an initial response with a BCR-ABL KD mutation.6 Our data suggest that dasatinib may still lead to effective BCR-ABL suppression, even at this low level of hOCT1 expression.
In summary, the present data suggest that dasatinib may be clinically effective in patients with low hOCT1 expression, who may be at risk of developing imatinib resistance. Similar suggestions have previously been made for nilotinib.21 The data give impetus to prospective phase 3 clinical trials comparing dasatinib and imatinib from original diagnosis. These trials are commencing, but will clearly take time to generate comparative data. Such studies will be essential in determining whether dasatinib is superior to imatinib in patients with a low baseline level of expression of hOCT1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all the authors for contributing to the paper.
This work was supported by a research project grant from the Leukemia Research Fund (LRF; London, United Kingdom).
Authorship
Contribution: A.G. and A.D. carried out all the main experimental work; C.M.L. and R.J.H. helped with the CrkL assay and fluorescence-activated cell sorting analysis; and M.P. and R.E.C. supervised the project and helped with the writing of this paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard E. Clark, Department of Haematology, Royal Liverpool University Hospital, Prescot Street, Liverpool L7 8XP, United Kingdom; e-mail: clarkre@liverpool.ac.uk.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal