Abstract
The malignant Hodgkin/Reed-Sternberg (HRS) cells of classical Hodgkin lymphoma (HL) are derived from mature B cells, but have lost a considerable part of the B cell–specific gene expression pattern. Consequences of such a lineage infidelity for lymphoma pathogenesis are currently not defined. Here, we report that HRS cells aberrantly express the common cytokine-receptor γ-chain (γc) cytokine IL-21, which is usually restricted to a subset of CD4+ T cells, and the corresponding IL-21 receptor. We demonstrate that IL-21 activates STAT3 in HRS cells, up-regulates STAT3 target genes, and protects HRS cells from CD95 death receptor–induced apoptosis. Furthermore, IL-21 is involved in up-regulation of the CC chemokine macrophage-inflammatory protein-3α (MIP-3α) in HRS cells. MIP-3α in turn attracts CCR6+CD4+CD25+FoxP3+CD127lo regulatory T cells toward HRS cells, which might favor their immune escape. Together, these data support the concept that aberrant expression of B lineage–inappropriate genes plays an important role for the biology of HL tumor cells.
Introduction
Classical Hodgkin lymphoma (HL) is a common lymphoid malignancy supposed to be derived from mature B cells. As a unique characteristic among human lymphomas, the malignant Hodgkin/Reed-Sternberg (HRS) cells represent only a small proportion of the cellular mass in affected lymph nodes.1 Regarding proliferation and apoptosis resistance of HRS cells, the transcription factors nuclear factor κB (NF-κB) and activator protein 1 (AP-1) have been recognized as key regulatory genes.1 In addition, a major role in these processes can be attributed to the STAT gene family. The importance of these factors for HRS cell biology is supported not only by their identified target genes, but also by the plethora of genomic defects affecting key regulators of these pathways.1 Although originating from B cells, HRS cells have lost a considerable part of the B cell–specific gene expression pattern.2 As a key mechanism involved in this process, a functional disruption of the B cell–specific transcription factor program has been recognized.2,3 As a result, the expression of B cell–specific genes is blocked, and B lineage–inappropriate genes are up-regulated.1,3 These data offer an explanation for the unique phenotype of HRS cells and demonstrate an unexpected plasticity of human lymphoid cells. However, due to the lack of suitable model systems, consequences of such lineage infidelity for human lymphoma pathogenesis are unclear.
To clarify this issue, we searched for factors that are usually not expressed in B lineage cells, but might be expressed in a lineage-inappropriate fashion in B cell–derived HRS cells. We identified in HRS cells an aberrant expression of the common cytokine-receptor γ-chain (γc) cytokine IL-21, the expression of which is usually restricted to subsets of CD4+ T cells and natural killer (NK)–T cells.4 IL-21 acts on a broader range of effector cell types, including B, T, NK, and myeloid cells. Accordingly, expression of the IL-21 receptor (IL-21R) was found on these cell types.4,5 Since IL-21 stimulates the immune system, whereas, in contrast to other common γc cytokines like IL-2, it does not support proliferation of immunosuppressive regulatory T (Treg) cells, it has been introduced into tumor immunotherapy protocols.4,5 IL-21–mediated effects on normal B lymphoid cells and cells derived from lymphoproliferative disorders differ depending on the cell type and the cellular context.4-6 For example, whereas IL-21 enhances anti-CD40–induced proliferation of primary human B-cell subsets,7 human B-cell chronic lymphocytic leukemia (B-CLL) cells undergo apoptotic cell death in response to anti-CD40 and IL-21 treatment.8 On human multiple myeloma and acute T-cell leukemia cells, IL-21 has growth stimulatory effects, which are mediated by activation of various signaling pathways.5,9,10 Taken together, the role of IL-21 has to be defined for each lymphoid cell type.
In addition, IL-21 is highly expressed in inflamed tissues and in those involved in autoimmune diseases.5,11,12 The pathogenic role of IL-21 in these processes is underlined by the amelioration of disease symptoms following IL-21 inhibition.13,14 The mechanism of IL-21–mediated promotion of tissue inflammation is not fully understood. IL-21R is expressed not only on hematopoietic cells, but also on nonimmune cells like fibroblasts, keratinocytes, or endothelial cells.15,16 This finding points to an IL-21–mediated cross-talk between immune and nonimmune cells at the site of inflammation. In support of this model, IL-21 stimulates gut epithelial cells to produce the CC chemokine macrophage-inflammatory protein MIP-3 α (MIP-3α/CCL20/LARC/Exodus-1), a potent chemoattractant for immunmodulatory cells.17 MIP-3α is expressed in lymphoid organs, fetal liver, and epithelial cells, and is up-regulated by inflammatory stimuli.18,19 It attracts immunmodulatory cells following binding to its receptor CCR6, which is expressed on immature dendritic cells (DCs) as well as subsets of B cells and memory T cells.18 Importantly, CCR6 is highly expressed on Treg cells, which suppress immune responses and represent the predominant cellular population surrounding HRS cells of HL.20-23 We therefore investigated expression and function of IL-21 and MIP-3α in HRS cell lines and primary HL samples.
Methods
Cell lines and culture conditions
HRS (L428, L1236, KM-H2, L591 [EBV+], HDLM-2, L540, and L540Cy), pre-B (Reh), Burkitt lymphoma (Namalwa), and diffuse large B-cell lymphoma (DLBCL; SU-DHL-4) cell lines were cultured as described.24 Where indicated, cells were cultured in the presence of recombinant human (rh) IL-21 (PHC0214; Biosource, Karlsruhe, Germany), the agonistic anti-CD95 antibody CH-11 (Coulter-Immunotech, Hamburg, Germany) or the respective IgM isotype control (Qbiogene, Heidelberg, Germany), rhIL-21 receptor/Fc chimera (IL-21R:Fc; 991-R2; R&D Systems, Wiesbaden, Germany) or the respective control (recombinant human IgG1:Fc, 110-HG; R&D Systems), neutralizing anti–human MIP-3α antibody (AF360; R&D Systems), or the respective goat isotype control (AB-108-C; R&D Systems). To block the function of rhIL-21, rhIL-21 was preincubated for 90 minutes at room temperature with the IL-21R:Fc or the IgG1:Fc control construct. All antibodies used for cell culture experiments were free of sodium azide. Where indicated, cells were maintained prior to experiments in fetal calf serum (FCS)–reduced medium. CD19+ tonsillar B cells were purified by use of CD19 MicroBeads (no. 130–050-301; Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer′s recommendation. Purity of B cells was greater than 97%, as determined by staining of purified cells with a PE-conjugated anti-CD20 antibody (no. 130-091-109; Miltenyi Biotec) and subsequent fluorescence-activated cell sorter (FACS) analysis using a FACSCalibur flow cytometer and CELLQuest software (Becton Dickinson; Heidelberg, Germany). The use of the human material was approved by the Local Ethical Committee of the Charité, Medical University Berlin, and performed in accordance with the Declaration of Helsinki.
Immunofluorescence and flow cytometry
For analysis of IL-21R expression, cells were stained with a monoclonal anti–IL-21R antibody (MAB9911; R&D Systems), the respective mouse IgG1 isotype control (MAB002; R&D Systems), a polyclonal anti–IL-21R antibody (sc-32902), or the respective isotype control (sc-2027; both from Santa Cruz Biotechnology, Heidelberg, Germany); for analysis of CCR6 expression, cells were stained by use of a monoclonal anti-CCR6 antibody (MAB195; R&D Systems) or the respective isotype control, followed by incubation of cells with a PE-conjugated F(ab′)2 fragment goat anti–mouse IgG or goat anti–rabbit IgG (both from Dianova, Hamburg, Germany). Expression of FoxP3, CD4, and CD25 was analyzed by use of the FoxP3 Staining Buffer Set (no. 130-093-142; Miltenyi Biotec) and antibodies specific for FoxP3 (APC-conjugated; no. 130–093-013), CD4 (FITC-conjugated; no. 130-080-501), and CD25 (PE-conjugated; no. 130-091-024; all from Miltenyi Biotec). Expression of CD127 was analyzed by a use of a PE-conjugated CD127-specific antibody (no. 557938; BD Pharmingen, San Diego, CA). Immunofluorescence was analyzed using a FACSCalibur flow cytometer and CELLQuest software (Becton Dickinson, Heidelberg, Germany).
Proliferation assays and analysis of apoptosis
DNA synthesis was determined by [3H]-thymidine incorporation assays using standard protocols. The percentage of viable and apoptotic cells was determined by annexin V–fluorescein isothiocyanate (FITC)/propidium iodide (PI) double-staining according to the manufacturer′s recommendation (Bender MedSystems, Vienna, Austria). Cells were stained with FITC-conjugated annexin V and PI, and samples were analyzed using a FACSCalibur flow cytometer and CELLQuest software (Becton Dickinson). Annexin V–FITC-negative and PI-negative cells were considered as viable cells.
RNA preparation and RT-PCR analysis
Total RNA was prepared using the RNeasy kit (Qiagen, Hilden, Germany). For reverse transcriptase–polymerase chain reaction (RT-PCR) analyses, first strand cDNA-synthesis was performed by use of the first-strand cDNA synthesis Kit (AMV; Roche, Mannheim, Germany) adding oligo-p(dT)15 primer according to the manufacturer's recommendation. Primers used for RT-PCR analyses were as follows: GAPDH sense 5′-ATGCTGGCGCTGAGTAC, GAPDH antisense 5′-TGAGTCCTTCCACGATAC; IL21R sense 5′-GATGTATTCCACTTCATGGCC, IL21R antisense 5′-TGACAAGCAGGAGGAGAAGC; IL21 sense 5′-GGATTGTCATCTGTCTGATGG, IL21 antisense 5′-TCACTTCCGTGTGTTCTAGAGG; MCL1 sense 5′-GAGGAGGAGGAGGACGAGTT, MCL1 antisense 5′-ACCAGCTCCTACTCCAGCAA; MIP-3α sense 5′-GAAGGCTGTGACATCAATGC, MIP-3α antisense 5′-TTGGACAAGTCCAGTGAGGC; and IL6 sense 5′-CCACTCACCTCTTCAGAACG, IL6 antisense 5′-TGACCAGAAGAAGGAATGCC. All PCR products were verified by sequencing.
Preparation of whole-cell and nuclear extracts, EMSA, and Western blot analysis
Preparation of whole-cell and nuclear extracts were performed as described.3 For electrophoretic mobility shift assay (EMSA) analyses, 5 μg whole-cell extract per lane were used. The buffer used for EMSA analysis of STAT3 was 20 mM HEPES (pH 7.9), 60 mM KCl, 5 mM dithiothreitol, 1 mM EDTA, 4% Ficoll, 0.5 mg/mL bovine serum albumin (BSA), and 0.1 μg/mL poly-deoxy-inosinic-deoxy-cytidylic acid (poly[d(I-C)]). The sequences of the STAT3 oligonucleotides were as follows: STAT3 sense 5′-AGCTTCATTTCCCGTAAATCCCTA and STAT3 antisense 5′- AGCTTAGGGATTTACGGGAAATGA. Following annealing, oligonucleotides were end-labeled with [α-32P]dCTP using Klenow fragment. Positions of the complexes were visualized by autoradiography. For Western blot analyses, 20 to 50 μg whole-cell extract or 5 μg nuclear extract per lane were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose filters (Schleicher and Schuell, Dassel, Germany). The filters were blocked (1% nonfat dry milk, 0.1% Triton X-100, 150 mM NaCl, 50 mM Tris [pH 7.5]) and incubated with the following primary antibodies: goat polyclonal anti–PARP-1 (sc-1561; Santa Cruz Biotechnology), rabbit polyclonal anti–phospho-STAT3 (Tyr705; no. 9131), rabbit polyclonal anti-STAT3 (no. 9132; both from Cell Signaling, Frankfurt/Main, Germany), rabbit polyclonal anti–IL-21 (500-P191; PeproTech, Eching, Germany), and monoclonal anti–β-actin (Sigma-Aldrich, Taufkirchen, Germany). Filters were incubated with horseradish peroxidase–conjugated secondary antibodies. Bands were visualized using the enhanced chemiluminescence system (Amersham Pharmacia Biotech, Freiburg, Germany).
Measurement of the secreted amount of MIP-3α, IL-6, and IL-21 by ELISA
Enzyme-linked immunosorbent assay (ELISA) was performed with supernatants of the cell lines indicated in the figure legends. For the generation of supernatants, cells were plated in a density of 0.8 × 106/mL, and supernatants were collected after 48 hours. The amount of MIP-3α in the supernatant was measured using the MIP-3α DuoSet ELISA Development kit (DY360; R&D Systems) according to the manufacturer's recommendations. For measurement of secreted IL-6, no. MAB206 was used as capture antibody and no. BAF206 as detection antibody (both from R&D Systems); for IL-21, no. 500-P191 was used as capture antibody and no. 500-P191Bt as detection antibody (both from PeproTech). As a standard, rhIL-6 (no. 206-IL; R&D Systems) or rhIL-21 (Biosource) were used. Optical density was determined at 450 nm (corrected for optical imperfections of the plate measured at 570 nm).
Chemotaxis assay
Chemotaxis assays were performed with cell culture supernatants of the cell lines KM-H2, L591, and Namalwa. Supernatants were generated by culture of cells in standard medium containing 0.2% FCS for 48 hours. Peripheral mononuclear cells (PMNCs) from the peripheral blood of different donors were purified using Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). Control medium containing 0.2% FCS, cell culture supernatant with or without a neutralizing MIP-3α antibody (AF360; R&D Systems), or the respective isotype control antibody (each at 5 μg/mL) was added to 24-well plates. A total of 106 purified PMNCs were added to 3-μm pore size transwell inserts (Corning, Munich, Germany), and plates were incubated for 2.5 hours at 37°C. Thereafter, inserts were removed and migrated cells were enriched and counted in a Neubauer chamber. Expression of CCR6, CD4, CD25, FoxP3, and CD127 was analyzed by FACS analysis as described. All assays were performed in triplicate.
Immunohistology
All cases were drawn from the files of the Consultation and Reference Center for Haematopathology at the Institute of Pathology, Campus Benjamin Franklin, Medical University Berlin. Diagnoses were established according to the World Health Organization (WHO) criteria. Immunohistologic analyses were performed on 4 μm sections obtained from formalin-fixed and paraffin-embedded material. The HL specimens analyzed consisted of 6 cases of mixed cellularity and 4 cases of nodular sclerosing subtype. In 2 of the cases, the HRS cells expressed the latent membrane protein-1 of the Epstein-Barr virus. The primary antibodies used were anti–IL-21 (500-P191; PeproTech) and anti–MIP-3α (sc-9775; Santa Cruz Biotechnology). For evaluating the specific immunostaining, appropriate negative control experiments involving isotype control immunostains were performed. Prior to incubation with primary antibodies, the dewaxed sections were subjected to an antigen retrieval protocol consisting of a brief, high-temperature heating of the sections immersed in a citrate buffer (10 mM; pH 6.0) in a high-pressure cooker. Bound antibodies were made visible using the streptavidin-biotin–alkaline phosphatase method and FastRed as chromogen (all from Dako, Glostrup, Denmark).
Image acquisition
Specimens were studied using an Olympus Provis AX70 microscope equipped with a range of plan-apochromatic objective lenses (4×/0.13 NA; 10×/0.40 NA; 20×/0.70 NA; 40×/0.95 NA) and coupled with a multicontrol U-MCB box that enabled zooming function (all from Olympus Optical, Tokyo, Japan). Original magnification of panels as automatically calculated by the multicontrol U-MCB box were 110×. Images were captured directly by a KY-F40 digital camera (Victor, Yokohama, Japan) and were processed with Diskus Program 4.20 (Hilgers Technical, Koenigswinter, Germany), which converted and exported images in tagged-image file format.
Results
Aberrant expression of IL-21 and functional IL-21R in HRS cell lines
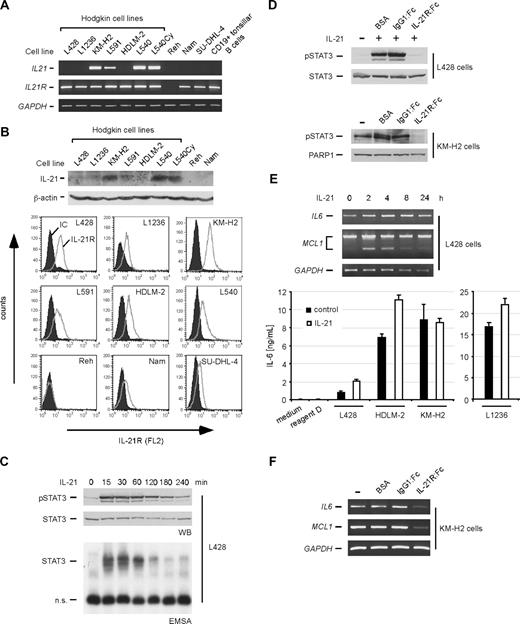
In search of factors which are usually not expressed in B lineage–derived cells, we analyzed expression of the cytokine IL-21 in a panel of Hodgkin- and non-Hodgkin cell lines as well as purified CD19+ tonsillar B cells (Figure 1A,B). mRNA expression of IL21 was not detectable in the non-Hodgkin, B cell–derived cell lines Reh, Namalwa, and SU-DHL-4 or CD19+ primary B cells, confirming that IL-21 is usually not expressed in the B-cell compartment (Figure 1A). In contrast, IL21 mRNA was expressed in 4 of 7 HRS cell lines, including the B cell–derived cell lines KM-H2 and L591, as revealed by RT-PCR analysis. In accordance with the mRNA expression, IL-21 protein expression was detectable by Western blot analysis in KM-H2, L591, L540, and L540Cy cells (Figure 1B top panels). We could measure the secreted amount of IL-21 in the supernatant (SN) of L540 and KM-H2 cells by an IL-21–specific ELISA (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), whereas sensitivity of the ELISA was apparently too low to measure IL-21 in the SN of L591 cells. mRNA expression of IL21R was detectable in all investigated cell lines apart from Reh cells (Figure 1A). In general, HRS cell lines displayed strong IL-21R mRNA expression compared with the non-HRS control cell lines. At the protein level, all cell lines except Reh cells expressed IL-21R, and expression was most prominent in the majority of HRS cell lines (Figure 1B bottom panels).
Expression and functional activity of the IL-21/IL-21R system in HRS cell lines. (A) RT-PCR analyses of IL21 and IL21R mRNA expression in Hodgkin (L428, L1236, KM-H2, L591, HDLM-2, L540, L540Cy) and non-Hodgkin (Reh, Namalwa, SU-DHL-4) cell lines and purified CD19+ tonsillar B cells. Expression of GAPDH was analyzed as a control. (B) Protein expression of IL-21 and IL-21R in various cell lines. Top panels show protein expression of IL-21 in the indicated cell lines, which was analyzed by Western blot in whole-cell extracts. Expression of β-actin was analyzed as a control. Bottom panels show analysis of IL-21R expression by flow cytometry in HRS (L428, L1236, KM-H2, L591, HDLM-2, L540) and non-Hodgkin (Reh, Namalwa, SU-DHL-4) cell lines. IC indicates isotype control. (C) Induction of STAT3 activity in L428 cells following treatment with rhIL-21. L428 cells were cultured for 12 hours in medium containing 0.5% FCS. Thereafter, cells were stimulated with 100 ng/mL rhIL-21, and whole-cell extracts were prepared at the indicated times. Extracts were analyzed by Western blot for phospho-STAT3 (pSTAT3) and, as a control, STAT3 expression (top panels). STAT3 DNA-binding activity was analyzed in whole-cell extracts by EMSA (bottom panel). (D) Inhibition of IL-21 by IL-21R:Fc. Top panels: to verify functionality of IL-21R:Fc, HRS L428 cells were left untreated or treated for 15 minutes with 20 ng/mL rhIL-21 preincubated with BSA (carrier for the :Fc constructs), or with 30 μg/mL of the control IgG1:Fc or the specific IL-21R:Fc construct. Thereafter, whole-cell extracts were analyzed by Western blot for pSTAT3 and STAT3 expression. Bottom panels: KM-H2 cells with constitutive STAT3 phosphorylation were left untreated, incubated with BSA, or treated with 50 μg/mL IgG1:Fc (control) or IL-21R:Fc, as indicated. After 6 hours, nuclear extracts were prepared and analyzed by Western blot for pSTAT3 and, as a control, PARP-1 expression. (E) Regulation of STAT3 target genes following stimulation of HRS cell lines with rhIL-21. Top panels: L428 HRS cells were stimulated with rhIL-21 as described in panel D. At the indicated times, mRNA was prepared and analyzed by RT-PCR for expression of IL6, MCL1, and, as a control, GAPDH. Bottom panel: measurement of secreted IL-6 by ELISA following stimulation with rhIL-21. The cell lines L428, L1236, HDLM-2, and KM-H2 were cultured in 1% FCS and stimulated every 8 hours with rhIL-21. After 24 hours, supernatants were analyzed by an IL-6–specific ELISA. The amount of IL-6 in the supernatants is shown in nanograms per milliliter. As controls, standard medium and the reagent diluent for the standard (reagent D) were included. Error bars denote SD. (F) KM-H2 cells were treated as described in panel D. After 6 hours, mRNA was prepared and analyzed by RT-PCR for expression of IL6, MCL1, and, as a control, GAPDH.
Expression and functional activity of the IL-21/IL-21R system in HRS cell lines. (A) RT-PCR analyses of IL21 and IL21R mRNA expression in Hodgkin (L428, L1236, KM-H2, L591, HDLM-2, L540, L540Cy) and non-Hodgkin (Reh, Namalwa, SU-DHL-4) cell lines and purified CD19+ tonsillar B cells. Expression of GAPDH was analyzed as a control. (B) Protein expression of IL-21 and IL-21R in various cell lines. Top panels show protein expression of IL-21 in the indicated cell lines, which was analyzed by Western blot in whole-cell extracts. Expression of β-actin was analyzed as a control. Bottom panels show analysis of IL-21R expression by flow cytometry in HRS (L428, L1236, KM-H2, L591, HDLM-2, L540) and non-Hodgkin (Reh, Namalwa, SU-DHL-4) cell lines. IC indicates isotype control. (C) Induction of STAT3 activity in L428 cells following treatment with rhIL-21. L428 cells were cultured for 12 hours in medium containing 0.5% FCS. Thereafter, cells were stimulated with 100 ng/mL rhIL-21, and whole-cell extracts were prepared at the indicated times. Extracts were analyzed by Western blot for phospho-STAT3 (pSTAT3) and, as a control, STAT3 expression (top panels). STAT3 DNA-binding activity was analyzed in whole-cell extracts by EMSA (bottom panel). (D) Inhibition of IL-21 by IL-21R:Fc. Top panels: to verify functionality of IL-21R:Fc, HRS L428 cells were left untreated or treated for 15 minutes with 20 ng/mL rhIL-21 preincubated with BSA (carrier for the :Fc constructs), or with 30 μg/mL of the control IgG1:Fc or the specific IL-21R:Fc construct. Thereafter, whole-cell extracts were analyzed by Western blot for pSTAT3 and STAT3 expression. Bottom panels: KM-H2 cells with constitutive STAT3 phosphorylation were left untreated, incubated with BSA, or treated with 50 μg/mL IgG1:Fc (control) or IL-21R:Fc, as indicated. After 6 hours, nuclear extracts were prepared and analyzed by Western blot for pSTAT3 and, as a control, PARP-1 expression. (E) Regulation of STAT3 target genes following stimulation of HRS cell lines with rhIL-21. Top panels: L428 HRS cells were stimulated with rhIL-21 as described in panel D. At the indicated times, mRNA was prepared and analyzed by RT-PCR for expression of IL6, MCL1, and, as a control, GAPDH. Bottom panel: measurement of secreted IL-6 by ELISA following stimulation with rhIL-21. The cell lines L428, L1236, HDLM-2, and KM-H2 were cultured in 1% FCS and stimulated every 8 hours with rhIL-21. After 24 hours, supernatants were analyzed by an IL-6–specific ELISA. The amount of IL-6 in the supernatants is shown in nanograms per milliliter. As controls, standard medium and the reagent diluent for the standard (reagent D) were included. Error bars denote SD. (F) KM-H2 cells were treated as described in panel D. After 6 hours, mRNA was prepared and analyzed by RT-PCR for expression of IL6, MCL1, and, as a control, GAPDH.
Next, we investigated the functionality of the IL-21R on HRS cell lines. For this purpose, HRS cell lines that expressed the IL-21R but no IL-21 cytokine (L428, HDLM-2, L1236) were stimulated with rhIL-21 (Figures 1C, S2). Following stimulation, we analyzed the activation status of IL-21–responsive signaling pathways.5 We did not observe a modulation of MAPK/AP-1 or PI3K activity by Western blot or EMSA (data not shown). However, robust STAT3 activity was rapidly induced in all investigated HRS cell lines (Figures 1C, S2A) as measured by detection of phosphorylated STAT3 protein by Western blot (Figure 1C top panel) and enhanced STAT3 DNA binding activity by EMSA analysis (Figure 1C bottom panel; Figure S2A). In contrast to recently published data,25 we did not detect a rhIL-21–mediated activation of STAT5 in HRS cells (data not shown), most probably due to different culture conditions. With the exception of HDLM-2 cells, in which STAT proteins are constitutively activated due to SOCS mutation and JAK2 amplification,26,27 constitutive STAT3 activation was most prominent in HRS cell lines with IL-21 production (KM-H2, L591, L540, L540Cy; detection of pSTAT3 by Western blot, Figure S2B), which suggested an autocrine stimulation loop in these cell lines. We therefore investigated STAT3 activity in HRS cells following inhibition of IL-21 by use of an IL-21R:Fc inhibitory protein, which neutralizes IL-21. IL-21R:Fc completely abolished rhIL-21–induced activation of STAT3 in L428 cells (Figure 1D; top panel), confirming the functionality of the IL-21R:Fc construct. Importantly, treatment of KM-H2 cells with IL-21R:Fc resulted in a decrease of constitutive STAT3 activity (Figure 1D; bottom panel), indicating that IL-21 is involved in the activation of STAT3 in HRS cells.
Having shown an induction of STAT3 activity by rhIL-21, we reasoned that STAT3 target genes might be activated. We therefore performed RT-PCR analyses of the known STAT3 target genes IL6 and the antiapoptotic gene MCL1,28 which are highly expressed in HRS cells, following stimulation of L428, L1236, and HDLM-2 cells with rhIL-21. We observed an IL-21–induced increased expression of these genes in all 3 cell lines (Figure 1E top panel; Figure S2C). Regarding MCL1, the short variant in particular was induced. Induction of IL-6 by rhIL-21 in L428, L1236, and HDLM-2 cells was confirmed at the protein level by an ELISA (Figure 1E bottom panel). Production of IL-6 could not be further enhanced by rhIL-21 in KM-H2 cells, suggesting full activation of this signaling pathway by an autocrine stimulation loop in these cells (Figure 1E; lower panel). Furthermore, expression of MCL1 and IL6 in KM-H2 cells was down-regulated by treatment with IL-21R:Fc (Figure 1F). These data indicated that IL-21 modulates gene expression in HRS cells at least in part by modulation of STAT3 activity.
IL-21 protects HDLM-2 cells from CD95-induced apoptosis
IL-21 can deliver costimulatory signals for B cells stimulated with CD40.7 We reasoned that IL-21 might modify effects of the TNFR family members CD40, CD30, or CD95, which are highly expressed on HRS cell lines. We did not observe an effect on [3H]-thymidine incorporation following rhIL-21 stimulation together with activation of CD40 or CD30 activation in the cell lines L428, L1236, or HDLM-2 (data not shown). However, rhIL-21 significantly rescued the reduction of [3H]-thymidine incorporation following incubation of HDLM-2 HRS cells with the agonistic anti-CD95 antibody CH-11 (Figure 2 top panel). This effect was dose dependent regarding the concentration of the agonistic anti-CD95 antibody (compare 10 with 20 ng/mL) as well as the concentration of rhIL-21 (compare 25 with 50 ng/mL). Further analyses of the cells by annexin V–FITC/PI staining revealed that rhIL-21 significantly protected HDLM-2 cells from CH-11–induced apoptosis (Figure 2 bottom panel). Again, this effect was dose dependent with respect to CH-11 or rhIL-21 concentrations. Other HRS cell lines were not suitable for these analyses, since they are resistant to CD95-induced cell death due to constitutive expression of c-FLIP proteins.24
Rescue of HDLM-2 cells from CD95 death receptor–induced apoptosis. Top panel: HDLM-2 cells were left untreated or treated with 10 or 20 ng/mL of the agonistic CD95 antibody CH-11 (□) or the respective IgM control (■) together with the indicated amounts of rhIL-21 for 20 hours. Thereafter, cells were pulsed with 0.037 MBq (1 μCi) [3H]-thymidine per well for a further 20 hours, and [3H]-thymidine incorporation was determined. cpm indicates counts per minute. Error bars denote SD. Bottom panel: cells were treated as described for panel A. After 48 hours, the percentage of apoptotic cells was determined by annexin V–FITC/PI staining and FACS analysis. Shown is the percentage of viable, annexin V–FITC/PI− cells. Error bars denote SD; P values were calculated using the Student t test. All assays were performed in triplicate.
Rescue of HDLM-2 cells from CD95 death receptor–induced apoptosis. Top panel: HDLM-2 cells were left untreated or treated with 10 or 20 ng/mL of the agonistic CD95 antibody CH-11 (□) or the respective IgM control (■) together with the indicated amounts of rhIL-21 for 20 hours. Thereafter, cells were pulsed with 0.037 MBq (1 μCi) [3H]-thymidine per well for a further 20 hours, and [3H]-thymidine incorporation was determined. cpm indicates counts per minute. Error bars denote SD. Bottom panel: cells were treated as described for panel A. After 48 hours, the percentage of apoptotic cells was determined by annexin V–FITC/PI staining and FACS analysis. Shown is the percentage of viable, annexin V–FITC/PI− cells. Error bars denote SD; P values were calculated using the Student t test. All assays were performed in triplicate.
HRS cells display high level expression of the IL-21 target gene MIP-3α, which attracts Treg cells
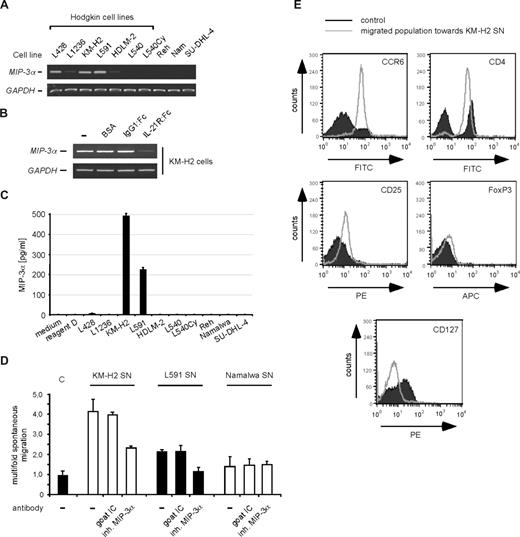
Recently, the immunmodulatory chemokine MIP-3α has been identified as IL-21 target gene.17 We therefore analyzed MIP-3α mRNA expression in Hodgkin compared with non-Hodgkin cell lines by RT-PCR (Figure 3A). Whereas MIP-3α was undetectable in the 3 tested non-Hodgkin cell lines (Reh, Namalwa, SU-DHL-4), MIP-3α expression was detected in 5 of 7 HRS cell lines. Importantly, we observed the highest expression level of MIP-3α in KM-H2 and L591 HRS cells, which displayed high levels of IL-21 cytokine and receptor expression (Figure 1A,B). We therefore asked, whether IL-21 signaling might be linked to MIP-3α up-regulation in HRS cells. Indeed, we observed a decrease of MIP-3α mRNA expression following IL-21R:Fc–mediated IL-21 inhibition in KM-H2 cells (Figure 3B). The fact that MIP-3α is also expressed in cells lacking IL-21 expression (eg, L428), and that in these cells MIP-3α was not further induced by stimulation with rhIL-21 (data not shown), indicated that MIP-3α is also regulated by other factors in HRS cells.
Functional activity of MIP-3α produced by HRS cells. (A) RT-PCR analysis of MIP-3α mRNA expression in various Hodgkin and non-Hodgkin cell lines, as indicated. Expression of GAPDH was analyzed as a control. (B) MIP-3α is regulated by IL-21. KM-H2 cells were left untreated, incubated with BSA (carrier for the :Fc constructs), or treated with 50 μg/mL of the IgG1:Fc control or the specific IL-21R:Fc construct, as indicated. After 6 hours, mRNA was prepared and analyzed by RT-PCR for expression of MIP-3α. Expression of GAPDH was analyzed as a control. (C) Measurement of secreted MIP-3α by ELISA. For the analysis of the secreted amount of MIP-3α by the various cell lines, supernatants were analyzed by a MIP-3α–specific ELISA. The amount of MIP-3α in the supernatants is shown in picograms per milliliter. As controls, standard medium and the reagent diluent for the standard (reagent D) were included. Error bars denote SD. (D) Chemotaxis assay of PMNCs toward SN of the HRS cell lines KM-H2 (□) and L591 (■) and the non-Hodgkin cell line Namalwa (□), as indicated. The migration of freshly isolated PMNCs toward SNs, each without (−) or with preincubation with a neutralizing MIP-3α antibody or the respective control antibody, is shown as fold migration relating to the spontaneous migration toward medium, which was set at 1.0. All assays were performed in triplicate. Error bars denote SD. (E) MIP-3α–induced migration of Treg cells toward KM-H2 cells. Migrated cells and cells incubated in KM-H2 SN for the time of the assay (control) were analyzed by FACS for expression of CCR6, CD4, CD25, FoxP3, and CD127, as indicated.
Functional activity of MIP-3α produced by HRS cells. (A) RT-PCR analysis of MIP-3α mRNA expression in various Hodgkin and non-Hodgkin cell lines, as indicated. Expression of GAPDH was analyzed as a control. (B) MIP-3α is regulated by IL-21. KM-H2 cells were left untreated, incubated with BSA (carrier for the :Fc constructs), or treated with 50 μg/mL of the IgG1:Fc control or the specific IL-21R:Fc construct, as indicated. After 6 hours, mRNA was prepared and analyzed by RT-PCR for expression of MIP-3α. Expression of GAPDH was analyzed as a control. (C) Measurement of secreted MIP-3α by ELISA. For the analysis of the secreted amount of MIP-3α by the various cell lines, supernatants were analyzed by a MIP-3α–specific ELISA. The amount of MIP-3α in the supernatants is shown in picograms per milliliter. As controls, standard medium and the reagent diluent for the standard (reagent D) were included. Error bars denote SD. (D) Chemotaxis assay of PMNCs toward SN of the HRS cell lines KM-H2 (□) and L591 (■) and the non-Hodgkin cell line Namalwa (□), as indicated. The migration of freshly isolated PMNCs toward SNs, each without (−) or with preincubation with a neutralizing MIP-3α antibody or the respective control antibody, is shown as fold migration relating to the spontaneous migration toward medium, which was set at 1.0. All assays were performed in triplicate. Error bars denote SD. (E) MIP-3α–induced migration of Treg cells toward KM-H2 cells. Migrated cells and cells incubated in KM-H2 SN for the time of the assay (control) were analyzed by FACS for expression of CCR6, CD4, CD25, FoxP3, and CD127, as indicated.
Next, we assessed secretion of MIP-3α in the SNs of the various cell lines by an ELISA (Figure 3C). This assay largely confirmed the RT-PCR data. The amount of MIP-3α produced by L1236 and HDLM-2 cells was apparently too low to be detected by the ELISA. MIP-3α has been shown to attract CCR6+ cells.18 To address the question whether MIP-3α secreted by HRS cells can attract CCR6+ cells and thereby contribute to the composition of the reactive infiltrate in HL-affected lymph nodes, we analyzed the functional activity of MIP-3α in the SN from KM-H2 and L591 cells in a migration assay with freshly isolated PMNCs (Figure 3D). As a control, we included SN from Namalwa cells, which do not produce MIP-3α. Compared with the medium control, to which 4% of the input cells were attracted, KM-H2 and L591 SN significantly attracted mononuclear cells (16.6% and 8.6% of input cells, respectively). This effect was dependent on MIP-3α, since KM-H2 and L591 SN-induced chemoattraction was abolished by a MIP-3α inhibitory antibody (reduction to 9.4% and 4.8% of input cells, respectively; Figure 3D), whereas addition of the isotype control (IC) antibody did not affect migration of the cells. In contrast, Namalwa SN only slightly increased chemoattraction (from 3.1% migration of input cells toward the medium control to 4.2% toward Namalwa SN), and addition of a MIP-3α inhibitory antibody did not affect this chemoattraction (Figure 3D). To analyze the nature of the cells migrated toward HRS cell SN, we performed CCR6, CD4, CD25, FoxP3 and CD127 FACS analyses of cells migrated toward KM-H2 SN (Figure 3E). These analyses revealed that migrated cells were CCR6+, confirming dependence of the migration on MIP-3α. Furthermore, these cells showed a CD4+CD25+FoxP3+CD127lo phenotype (Figure 3E), defining them as Treg cells.29 The analysis of nonmigrated cells cultured in KM-H2 SN for the time of the assay is shown as a control (Figure 3E).
Analysis of IL-21 and MIP-3α in primary lymphoma cases
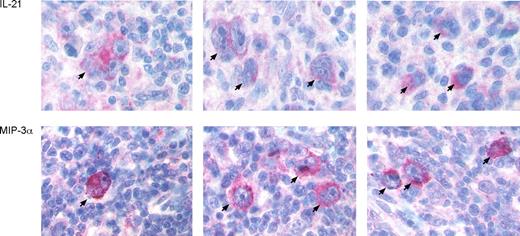
To study the expression of IL-21 in primary lymphoma cases, we performed immunhistochemical staining of various B cell–derived lymphomas, including 10 classical HLs, 14 diffuse large B-cell lymphomas, 10 B-cell chronic lymphocytic leukemias, 6 mantle cell lymphomas, 10 follicular lymphomas, and 2 Burkitt lymphomas (Table 1). Whereas 9 of 10 HLs revealed aberrant IL-21 protein expression, which confirmed recently published data in HRS cells using single-cell PCR and immunohistochemistry (IHC),25 IL-21 expression was found in only 3 of 42 B cell–derived non-Hodgkin lymphomas. This showed that expression of IL-21 among these lymphomas is largely restricted to HL. Furthermore, we analyzed expression of MIP-3α in 10 primary HL samples (Table 1). This analysis revealed an expression of MIP-3α in a variable proportion of the HRS cells of 9 of 10 HL samples, independent of the Epstein-Barr virus (EBV)–association status. Representative lymphoma specimens are shown in Figure 4. Together, these data were in accordance with our in vitro analyses.
Summary of IL-21 and MIP-3α immunohistochemistry results
| . | Positive . | Negative . |
|---|---|---|
| IL-21 staining, no. cases | ||
| Classic Hodgkin lymphoma | 9* | 1 |
| Diffuse large B-cell lymphoma | 1† | 13 |
| B-cell chronic lymphocytic leukemia | 2† | 8 |
| Mantle cell lymphoma | 0 | 6 |
| Follicular lymphoma (grades 1 and 2) | 0 | 10 |
| Burkitt lymphoma | 0 | 2 |
| MIP-3α staining, no. cases | ||
| Classic Hodgkin lymphoma | 9‡ | 1 |
| . | Positive . | Negative . |
|---|---|---|
| IL-21 staining, no. cases | ||
| Classic Hodgkin lymphoma | 9* | 1 |
| Diffuse large B-cell lymphoma | 1† | 13 |
| B-cell chronic lymphocytic leukemia | 2† | 8 |
| Mantle cell lymphoma | 0 | 6 |
| Follicular lymphoma (grades 1 and 2) | 0 | 10 |
| Burkitt lymphoma | 0 | 2 |
| MIP-3α staining, no. cases | ||
| Classic Hodgkin lymphoma | 9‡ | 1 |
In 3 cases, fewer than 50% of HRS cells were stained; in 6 cases, more than 50% of HRS cells were stained.
Staining was observed in more than 50% of tumor cells.
In 4 cases, fewer than 50% of HRS cells were stained; in 5 cases, more than 50% of HRS cells were stained.
Immunohistology of IL-21 and MIP-3α in primary HL cases. Immunohistology of representative HL specimens stained for IL-21 (top row) and MIP-3α (bottom row) is shown. HRS cells are marked by arrows. Magnification, 110×.
Immunohistology of IL-21 and MIP-3α in primary HL cases. Immunohistology of representative HL specimens stained for IL-21 (top row) and MIP-3α (bottom row) is shown. HRS cells are marked by arrows. Magnification, 110×.
Discussion
In this study, we describe a lineage-inappropriate expression of the Th2 cytokine IL-21 in B cell–derived HRS cells. Initially, IL-21 expression was shown to be restricted to CD4+ Th2 cells, and only very recently IL-21 expression has also been described in stimulated NK-T cells.4 Here, we demonstrate expression of IL-21 in a human malignant disease, which confirmed data of a recent study published during the review process of this manuscript.25 The analysis of different human B-cell lymphoma–derived cell lines, primary tonsillar B cells, and tissue specimens of various B cell–derived non-Hodgkin lymphomas by IHC showed that IL-21 expression was largely restricted to HRS cells in the normal or malignant B-cell compartment. The simultaneous expression of IL-21 and its receptor in the majority of HRS cell lines suggested an autocrine stimulation loop. Indeed, specific neutralization of IL-21 by use of an IL-21R:Fc chimeric protein in HRS cells expressing both cytokine and receptor resulted in inhibition of constitutive activation of transcription factor STAT3,30,31 and concomitant down-regulation of its target genes. In line with these results, IL-21 stimulation of HRS cell lines with expression of IL-21R but no IL-21 cytokine induced robust STAT3 activation and target gene induction. Due to the fact that HL-bearing lymph nodes are in most cases resected for diagnostic purposes, and that HRS cells are very rare in the affected lymph nodes and might not—if the purification of HRS cells would be possible in any HL case—retain their viability, we were not able to perform functional experiments with primary HRS cells. However, our data clearly suggest that IL-21 is the key cytokine required for the commonly deregulated STAT3 activity in HRS cells.31 Although IL-6, which is commonly expressed in HRS cells, is not able to modulate STAT3 activity in HRS cells in vitro,30 it might further enhance IL-21–induced STAT3 activation in vivo. Furthermore, IL-21 protected HDLM-2 HRS cells from CD95-induced cell death, the mechanism of which has to be elucidated in future studies. This effect might be important for HRS cells in which c-FLIP24 does not fully protect these cells from death receptor–induced apoptosis. In HRS cells, a lineage-inappropriate expression of T cell–associated transcription factors like GATA3, TCF-1, and T-bet has been shown.3 IL-21 expression is regulated by T cell–associated factors like nuclear factor of activated T cell (NFAT).32 HRS cells are known to produce other Th2-associated cytokines such as IL-13 or IL-5.33 Even though transcription factor NF-κB is involved, for example, in the up-regulation of IL-13 in HRS cells,34 lineage-inappropriate gene expression might provide the basis for this unusual gene expression pattern. Our data regarding the functional activity of IL-21 in HRS cells support the concept that B lineage–inappropriate genes play an important role for the biology of these cells.
IL-21 has immunostimulatory effects, and therefore has been introduced into tumor immunotherapy protocols.4,5 However, increasing data reveal that IL-21 is involved in the pathogenesis of autoimmune diseases in mouse models,13,35,36 and elevated levels of IL-21 have been found in human chronic inflammatory diseases like rheumatoid arthritis (RA)12 or inflammatory bowel disease (IBD).11 In addition, IL-21 is a growth factor for lymphoid malignancies including acute T-cell leukemia9 or multiple myeloma,10 and regulates important genes involved in HL, as shown by our work. It is of particular interest that we could show a direct involvement of IL-21 in MIP-3α up-regulation in HRS cells, which has also been found in gut epithelial cells.17 Via MIP-3α and subsequent attraction of Treg cells, IL-21 might indirectly mediate immunosuppressive effects. These data suggest that administration of IL-21 could have severe side effects, and therefore its application in clinical trials should be performed with caution.
Regarding the expression of MIP-3α in HRS cell lines without IL-21 expression, transcription factor NF-κB or other cytokines produced by HRS cells might regulate MIP-3α expression.19,37 Although EBV proteins are involved in the regulation of MIP-3α in lymphoid cells,38 we did not observe a correlation of MIP-3α expression and the presence of EBV in cell lines or in primary HL cases. MIP-3α serves as an autocrine growth factor for tumor cells expressing MIP-3α and its receptor CCR6.39,40 HRS cells do not express CCR6 (unpublished data), precluding that MIP-3α is a direct growth factor for HRS cells. However, MIP-3α secreted by HRS cells is a key chemoattractant protein for CCR6+ lymphoid cells migrating toward HRS cells, as demonstrated by specific inhibition of migration toward HRS cell line SNs following blockade of MIP-3α.
The infiltrate of benign cells surrounding HRS cells pre-dominantly consists of CD4+ Th2 cells. In particular, CD4+CD25+FoxP3+ Treg cells might suppress an effective immune response against HRS cells.22,23 Whereas the nature of the cellular infiltrate in lymph nodes affected by HL becomes more and more characterized, mechanisms leading to the formation of this cellular infiltration are less well defined. HRS cells might induce apoptotic cell death of activated T cells, in particular Th1 cytotoxic T cells, by production of CD95L.41 HRS cells themselves escape the T-cell–mediated immune attack by high-level expression of c-FLIP proteins, which also protects them from CD95 death receptor–induced apoptosis.24,41,42 As shown in our work, IL-21 might support death receptor resistance. Very recently, it has been shown that HRS cells overexpress the glycan-binding protein galectin-1 (Gal1), which is able to increase the number of immunosuppressive CD4+CD25highFoxP3+ Treg cells at the expense of Th1 cells.43 Our results suggest that MIP-3α in lymph nodes affected by HL attracts CCR6+CD4+CD25+FoxP3+CD127lo cells, which are defined as Treg cells.29 Thus, in primary HL cases, immunosuppressive Treg cells might be selectively expanded with a resulting altered Th1/Th2-Treg balance,43 but they might also be attracted by HRS cell–secreted MIP-3α from the surrounding tissue and peripheral blood toward HRS cell–bearing lymph nodes.
Together, our data describe the endogenous production of IL-21 by human, B cell–derived tumor cells. IL-21 exerts direct effects on HRS cells and influences the composition of the cellular infiltration surrounding HRS cells. Thus, inhibition of IL-21, but also of MIP-3α, might provide a new treatment strategy for HL that would not only directly target HRS cells but might also enforce a HRS cell–directed immune attack.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Simone Kressmann (Berlin, Germany) and Franziska Hummel (Berlin, Germany) for excellent technical assistance. We thank Karl Köchert (Berlin, Germany) for help with statistical analyses and Ariane Buchal and Benedikt Sedlmaier (both Berlin, Germany) for providing human tonsil material.
This work was supported in part by grants of the Wilhelm Sander-Stiftung, the Berliner Krebsgesellschaft, and the Deutsche Forschungsgemeinschaft.
Authorship
Contribution: B.L. designed and performed research, analyzed and interpreted data, and contributed to writing the manuscript; S.K. performed research and analyzed data. I.A., K.J., and H.S. performed, analyzed, and interpreted IHC analyses; F.J. contributed to writing the manuscript; G.M. performed experiments and analyzed data; M.J. and B.D. analyzed and interpreted data and contributed to writing the manuscript; and S.M. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: G.M. filed a patent entitled “Interleukin-21 (IL-21) binding proteins and methods of making and using same” (European patent application No. 08425294.9). All other authors declare no competing financial interests.
Correspondence: Stephan Mathas, Max-Delbrück-Center for Molecular Medicine and Charité, Hemtology/Oncology, CVK, Medical University Berlin, Robert-Rössle-Str 10, 13125 Berlin, Germany; e-mail: stephan.mathas@charite.de.

![Figure 2. Rescue of HDLM-2 cells from CD95 death receptor–induced apoptosis. Top panel: HDLM-2 cells were left untreated or treated with 10 or 20 ng/mL of the agonistic CD95 antibody CH-11 (□) or the respective IgM control (■) together with the indicated amounts of rhIL-21 for 20 hours. Thereafter, cells were pulsed with 0.037 MBq (1 μCi) [3H]-thymidine per well for a further 20 hours, and [3H]-thymidine incorporation was determined. cpm indicates counts per minute. Error bars denote SD. Bottom panel: cells were treated as described for panel A. After 48 hours, the percentage of apoptotic cells was determined by annexin V–FITC/PI staining and FACS analysis. Shown is the percentage of viable, annexin V–FITC/PI− cells. Error bars denote SD; P values were calculated using the Student t test. All assays were performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/8/10.1182_blood-2008-01-134783/4/m_zh80200825710002.jpeg?Expires=1766123199&Signature=rSWTujXuPPZLU~vFwUsU2eK4Yzr1EAahbdffK9O2hI5mE36tmZLvmo4Df1AASynqD2Ek6KXdqdQ48RZJuC~YGuNGL93IoxH7amaJAu10Kg1SRqbz~nSMK75ckyfhc1XAAwhnSLp9CYXrW3GdLSVZ07e4qvAZf-ZHlh8wzMVCUBIfUmJ6WHy8NjeXqga7bm9kt7Re3mbD0ClPaCh87cWuq1F1ltEa7N4xgn31KKiVvc4ba7TcLb86SonoTQaw5eM1IQ~ia3EzNLRzkkygfCasrIHKObT4UEGVxOuFqSZ09J2dUdtBHWAgMkdsKuUjnZhRTqwlukOFEcfo7CP5a4lgTw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal