Abstract
Extranodal marginal zone B-cell lymphomas (MZBCLs) arise on a background of chronic inflammation resulting from organ-specific autoimmunity, infection, or by unknown causes. Well-known examples are salivary gland MZBCL in Sjögren's sialadenitis and gastric MZBCL in Helicobacter pylori gastritis. MZBCLs express CXCR3, a receptor for interferon-γ–induced chemokines highly expressed in the chronic inflammatory environment. The immunoglobulin (Ig) variable heavy/light chain (IgVH/IgVL) gene repertoire of salivary gland and gastric MZBCL appears restricted and frequently encodes B-cell receptors with rheumatoid factor reactivity. Primary cutaneous marginal zone B-cell lymphomas (PCMZLs) are regarded as the skin-involving counterparts of extranodal MZBCLs. Although PCMZLs have been associated with Borrelia burgdorferi dermatitis, PCMZLs generally arise because of unknown causes. We studied an extensive panel of PCMZLs and show that PCMZLs do not conform to the general profile of extranodal MZBCL. Whereas most noncutaneous MZBCLs express IgM, PCMZLs in majority express IgG, IgA, and IgE and do not show an obvious immunoglobulin repertoire bias. Furthermore, the isotype-switched PCMZLs lack CXCR3 and seem to arise in a different inflammatory environment, compared with other extranodal MZBCLs.
Introduction
Extranodal marginal zone B-cell lymphomas (MZBCLs), also known as mucosa-associated lymphoid tissue (MALT) lymphomas, generally arise on a background of a chronic inflammation. Well-known examples are gastric MZBCLs associated with Helicobacter pylori infection, and salivary gland MZBCLs linked to Sjögren's syndrome.1 MZBCLs are composed of heterogeneous populations of small B lymphocytes, including centrocytic and monocytoid cells, plasma cells, and sometimes scattered immunoblasts and centroblasts. The tumor cells express CD20, CD22, CD79, and BCL2 and are typically CD5−, CD10−, and BCL6−.2 Like chronic lymphocytic leukemias, but in contrast to all other B-cell non-Hodgkin lymphomas, MZBCLs express the chemokine receptor CXCR3.3-6 Recurrent genetic aberrations in MZBCL include the t(11;18)(q21;q21) API2-MALT1 and t(14;18)(q32;q31) IgH-MALT1 translocations, as well as trisomies of chromosomes 3 and 18.7
MZBCLs resemble antigen-selected memory B cells, reflected in the expression of mutated immunoglobulin (Ig) genes, mostly of the IgM isotype, and generally with low replacement over silent (R/S) mutation ratios in the framework regions (FRs).3,8 By studying the complementary determining region 3 (CDR3) sequences of Ig variable heavy chain (IgVH) genes, we3 and others9 have shown that gastric and salivary gland MZBCLs express a restricted B-cell receptor repertoire with frequent homology to canonical, V1-69/JH4– or V3-7/JH3–encoded, rheumatoid factors. Recombinant expression of lymphoma-derived IgM antibodies confirmed this auto-reactivity for IgG in an enzyme-linked immunosorbent assay.3
Primary cutaneous marginal zone B-cell lymphomas (PCMZLs), by virtue of their histology and immunophenotype, are regarded as the skin-involving counterparts of MALT lymphomas.10-12 In certain areas of Europe, cases with PCMZLs were linked to previous infection with Borrelia burgdorferi; however, this was not found among PCMZLs from Asia or the United States.13-17 Overall, the etiology for most PCMZLs is unknown. Inconsistent findings have been reported for genomic aberrations in PCMZLs. Most remarkable was the near-complete absence of the t(11;18) API2-MALT1 translocation in the PCMZLs.7,14,18-22 Variable frequencies were found for the t(14;18) translocation, including one study reporting MALT1, but also BCL2 as the translocation partner of IgH.7,22,23 In addition, 2 cases have been reported by Streubel et al18 harboring a t(3;14) FOXP1/IgH translocation.
Previously, IgVH sequences of a total of 14 PCMLs have been analyzed by 4 independent groups.24-27 To extend our previous findings on Ig usage by extranodal MZBCLs, we conducted a detailed Ig gene analysis of an extensive cohort of PCMZLs. Furthermore, we analyzed the inflammatory environment in which the PCMZLs arise. The combined results indicate that the majority of PCMZLs essentially differ from their counterparts at other extranodal sites.
Methods
Patient material
Frozen as well as paraffin-embedded tissues of 17 PCMZLs were obtained from the Department of Dermatology from the Leiden University Medical Center (Leiden, The Netherlands) and paraffin-embedded tissue material of 25 PCMZLs was provided by the Department of Dermatology from the Medical University of Graz (Graz, Austria). Paraffin-embedded material of CM43 was obtained from the Department of Pathology and Laboratory Medicine, University Medical Center Groningen (Groningen, The Netherlands), and frozen material of CM44 was derived from the Department of Pathology at the Academic Medical Center (Amsterdam, The Netherlands). Diagnoses had been established by consensus of national panels of experts on cutaneous lymphoma, applying the World Health Organization-European Organization for Research and Treatment of Cancer classification. All lymphomas expressed CD20 (and CD79a), BCL-2, and lacked expression of CD10, and BCL-6 or CD5.10 Monoclonality had been confirmed in all cases either by immunohistochemistry, polymerase chain reaction (PCR), or both. Reverse-transcribed PCR (RT-PCR) for t(11;18) demonstrated the absence of the API-MALT1 translocation in 11 cases tested (CM01, CM03, CM04, CM05, CM07, CM08, CM11, CM15, CM17, CM35, and CM37). Light chain restriction was established for all cases: CM01, 02, 03, 04, 06, 07, 08, 09, 11, 14, 15, 16, 17, 18, 20, 22, 26, 31, 34, 35, 36, 37, 43, and 44 expressed kappa, whereas CM05, 10, 13, 19, 21, 23, 24, 25, 27, 28, 29, 30, 32, 33, 38, 39, 40, 41, and 42 expressed lambda.
The study was performed in accordance with the ethical standards and approved by the research code committee on human experimentation of the Academic Medical Center of Amsterdam.
RNA isolation and cDNA synthesis
Tissue sections from paraffin-embedded material were deparaffinized in xylol followed by ethanol. The dried pellet was dissolved and incubated with 500 μg/mL of Proteinase K (Roche Diagnostics, Almere, The Netherlands) in lysis buffer (10 mM of Tris-HCl, pH 8.0, 0.1 mM of ethylenediaminetetraacetic acid, pH 8.0, 2% sodium dodecyl sulfate, pH 7.3) at 56°C until complete lysis, after which RNA extraction was performed with TRIZOL. Preceding cDNA synthesis, this RNA was heated for 90 minutes at 70°C.28 Frozen material was directly dissolved in TRIZOL, and RNA isolation was performed according to the manufacturer's instructions. The RT mix contained the following: 0.1 mM Pd(N)6 random primers (GE Healthcare, Little Chalfont, United Kingdom), 8 U/μL of Moloney murine leukemia virus RT (Invitrogen, Carlsbad, CA), 1 mM of each dNTP, and 1.2 U/μL of RNase inhibitor (Roche Diagnostics) in 1× first strand buffer (Invitrogen). The reaction was performed for 1 hour at 37°C, followed by 10 minutes of inactivation at 95°C.
IgVH-CDR3 analysis
PCR amplification of the IgVH-CDR3 regions used a forward primer in framework region 3 (FR3) in combination with reverse primers specific for JH, Cμ, Cδ, Cγ, Cα,24 and Cϵ (5′-CGGAGGTGGCATTGGAGG-3′). Subsequently, a second PCR was performed in which the appropriate constant region primers for each tumor were combined with any of the VH gene family-specific primers. In 2 cases where cDNA quality was not sufficient to produce a VH-family PCR product, a part of the VH gene was amplified using an FR2 primer. Immunoglobulin light chain V (IgVL) genes were amplified, using Vκ-family specific primers in combination with a Cκ-primer.29 Reaction-mixture contents and the amplification programs for the PCRs were performed as described previously, with the exception that Taq Platinum (Invitrogen) was used as polymerase enzyme. Sequencing on both strands was performed using the big dye terminator v1.1 cycle sequencing kit (Applied Biosystems, Foster City, CA). Pseudo-clonality was excluded by a second independent PCR, confirming the Ig sequence of the tumor. The sequences were compared with the Vbase30 and IMGT/V31 Ig databases to obtain the VHDJH rearrangement and mutational status.32,33 IgVH-CDR3 amino acid sequences were compared with each other and blasted to GenBank (with the blastp-algorithm).34 A sequence was considered to be homologous when 1) sharing at least 75% amino acid sequence homology and 2) a length difference between the CDR3 sequences did not exceed 3 amino acids (maximum gap of 3 amino acids).3
Immunohistochemistry
CXCR3, CD20, and CD3 expression was visualized on frozen as well as paraffin-embedded tissue sections, with monoclonal antibodies against CXCR3 (clone 1C6, BD Biosciences PharMingen, San Diego, CA), CD20 (clone L26, DakoCytomation Denmark, Glostrup, Denmark), and CD3 (clone SP7, Lab Vision, Fremont, CA) in combination with the Powervision+poly-HRP detection system (Vision Biosystems, Norwell, MA). Heat-induced epitope retrieval was performed on the paraffin sections in Tris/ethylenediaminetetraacetic acid (tris(hydroxymethyl)aminomethane/ethylenediaminetetraacetic acid) buffer (10 mM/1 mM, pH 9.0) for 10 minutes at 100°C. 3-Amino-9-ethylcarbazole was used as chromogen and hematoxylin for nuclear counterstaining. Images were acquired on an Olympus BX51 microscope, using a UPlanApo 40×/0.85 objective, in combination with an Olympus DP70 digital camera and corresponding camera control software DPmanager and DPcontroller v.1.2.1.107 and .108, respectively (Olympus, Tokyo, Japan), and further processed with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA). Included in the analysis were only those cases in which the B and T cells, visualized with CD20 and CD3 stainings, were discernable as distinct populations, thus enabling an assessment of CXCR3 expression by the tumor B cells.
Cytokine RT-PCR
For semiquantitative RT-PCR, the cDNAs from frozen tissues were applied in 2 dilutions, usually 1:20 and 1:100. Based on satisfactory results in the actin RT-PCR, samples were selected for further analysis. PCR mixture contents were the same as for Ig-PCRs, combined with the following primer-pairs: interferon-γ (IFN-γ)-forward 5′-GCAGAGCCAAATTGTCTCCT-3′; IFN-γ-reverse 5′-ATGCTCTTCGACCTCGAAAC-3′; CXCL10-forward 5′-GGAACCTCCAGTCTCAGCACC-3′; CXCL10-reverse 5′-CAGCCTCTGTGTGGTCCATCC-3′; interleukin-12 (IL-12)-forward 5′-ATTGAGGTCATGGTGGATGC-3′; IL-12-reverse 5′-AATGCTGGCATTTTTGCGGC-3′; IL-4-forward 5′-TGCCTCCAAGAACACAACTG-3′; IL-4-reverse 5′-AACGTACTCTGGTTGGCTTC-3′; actin-forward 5′-CATGGACAAAATCTGGCACCACA-3′; actin-reverse 5′-CCACTGCACACTTCATGATGGAG-3′. After a hotstart for 4 minutes at 95°C, the first 10 cycles of amplification were performed: successively 60 seconds at 95°C, 30 seconds at 57°C, and 60 seconds at 72°C. The next 20 (CXCL10), 25 (actin), or 30 (IFN-γ, IL-12, IL-4) cycles of amplification consisted of 30 seconds at 95°C, 30 seconds at 55°C, and 60 seconds at 72°C. The reaction was completed for 6 minutes at 72°C. PCR products were densitometrically scored with Image-Pro Plus v5 (Media Cybernetics, Bethesda, MD) and categorized into 3 levels: no expression (< 5% of the maximally measured signal in the panel, per gene), little expression (5%-50% of maximum), and a high expression level (a signal > 50% of the maximum). By ranking these expression levels 0, 1, or 2, respectively, a maximum ranking of 4 was assigned per patient (being the sum of both dilutions). Statistical significance was determined with a Mann-Whitney rank-sum test.
Results
Immunoglobulin heavy and light chains
RT-PCRs were performed with IgVH-family–specific leader primers or FR2/FR3 primers in combination with JH6 or constant region primers. Of 21 PCMZLs, the IgVH-CDR3 sequence of the tumor clone was resolved. Three PCMZLs expressed IgM, of which2 coexpressed IgD. Ten PCMZLs expressed IgG and 2 PCMZLs expressed IgA. Furthermore, 3 PCMZLs expressed both IgG and IgA and 3 PCMZLs expressed both IgG and IgE. In each of these 6 cases, the isotype switch variants were derived of the same precursor clone, as judged by IgVH-CDR3 sequence and shared mutations. The VH germ line gene could be determined for 11 of 21 PCMZLs, demonstrating that 5 cases used VH1 and 6 used VH3. The results of the IgVH analyses are summarized in Table 1. Similarly, Vκ family leader primers combined with a Cκ primer identified the kappa-rearrangement for 7 PCMZLs: 3 used Vκ1, one used Vκ2, and 3 used Vκ3 (Table 2). Mutation frequencies within the IgVH gene varied between 4 and 43 mutations, with a mean number of mutations of 26, whereas 5 to 28 mutations (mean, 14) were found in the IgVL sequences. Analysis of the R/S ratios in the FR regions of IgVH according to Chang and Casali35 established that 3 of 8 PCMZLs were significantly below the ratio that would be expected in case of random mutation.
IgVH sequence analysis of cutaneous MZBCLs
| Patient no. . | Ig isotype (RT-PCR) . | IgVH rearrangement . | No. of mutations . | R/S ratio FR . | CDR3 sequence . | . | CDR3- length (aa) . |
|---|---|---|---|---|---|---|---|
| CM01 | Cγ4 | V1-69/D3-10/JH6 | 25 | 0.9* | CAR ATYSGSEQYDFDSSSYLDV | WGKG | 19 |
| Cα | V1-69/D3-10/JH6 | 33 | 2.6* | CAG VDYSDSGRHYSFSASYFDV | WGKG | 19 | |
| CM03 | Cγ1/Cα | V3-30/D2-8/JH4 | 20 | 2.6 | CAK GGMVSTIPIDY | WGQG | 11 |
| CM04 | Cμ/Cδ | V3-9/D2-8/JH4 | 19 | 2.3 | CAK DVDIVLMIFSFRSRGFDS | WGQG | 18 |
| CM06 | Cγ/Cϵ | ND | ND | ND | CAR GKTAVVGAPGYYFDY | WGQG | 15 |
| CM07 | Cγ/Cϵ | ND | ND | ND | CAT VRLDSPY(S/A)FAY | WGQG | 11 |
| CM08 | Cγ4 | V3-30/D3-9/JH4 | 43 | 1.3* | CAS LRMSIDRGFDC | WGQG | 11 |
| CM10 | Cγ | V1-08/D3-10/JH4 | 41 | 2.1* | CGR VLNTPAHRPIRGLIAY | WGQG | 16 |
| CM11 | Cα | V1-69/D6-6/JH4 | 4 | ND | CAR AVDFDSSSSYSF | WGQG | 12 |
| CM13 | Cμ | V3-72/D6-19/JH3 | 12 | 1.3 | CVR GYSIGWPYDALAR | WGQG | 13 |
| CM15 | Cγ1/Cϵ | V3-53/D2-2/JH3 | 24 | 3.6 | CAR ENPRHDVFDI | WGLG | 10 |
| CM19 | Cγ | ND | ND | ND | CAK ESGGAARMRGNYYYYYYMDV | WGQG | 20 |
| CM20 | Cγ | ND | ND | ND | CVR HSAEVADSVEED | WGQG | 12 |
| CM21 | Cγ | ND | ND | ND | CAR ETNYDSWTGSPSSHYFGLDF | WGQG | 20 |
| CM22 | Cα | ND | ND | ND | CAR GSGDYKVTKYYEDAFDI | WGQG | 17 |
| CM29 | Cγ | ND | ND | ND | CAR GVDFDYFDL | WGRG | 9 |
| CM34 | Cγ | ND | ND | ND | CAK DLLLRVVGCLDY | WGQG | 12 |
| CM35 | Cγ | V1-69/D1-14/JH5 | ND | ND | CAR GSHLIGGTIASFDP | WGQG | 14 |
| CM37 | Cγ/Cα | V1-02/D2-21/JH4 | ND | ND | CAA AL(S/P)ESSFPFTFHD | WGQG | 13 |
| CM42 | Cγ | ND | ND | ND | CAR LNYVLHLGRLIEGAGTNYGMDV | LGQG | 22 |
| CM43 | Cμ/Cδ | ND | ND | ND | CAR APFLGDVFFDP | WGQG | 11 |
| CM44 | Cγ | V3-48/D5-24/JH4 | 38 | 1.4 | CAK LQRRGLQLGYLEY | FGQG | 13 |
| Patient no. . | Ig isotype (RT-PCR) . | IgVH rearrangement . | No. of mutations . | R/S ratio FR . | CDR3 sequence . | . | CDR3- length (aa) . |
|---|---|---|---|---|---|---|---|
| CM01 | Cγ4 | V1-69/D3-10/JH6 | 25 | 0.9* | CAR ATYSGSEQYDFDSSSYLDV | WGKG | 19 |
| Cα | V1-69/D3-10/JH6 | 33 | 2.6* | CAG VDYSDSGRHYSFSASYFDV | WGKG | 19 | |
| CM03 | Cγ1/Cα | V3-30/D2-8/JH4 | 20 | 2.6 | CAK GGMVSTIPIDY | WGQG | 11 |
| CM04 | Cμ/Cδ | V3-9/D2-8/JH4 | 19 | 2.3 | CAK DVDIVLMIFSFRSRGFDS | WGQG | 18 |
| CM06 | Cγ/Cϵ | ND | ND | ND | CAR GKTAVVGAPGYYFDY | WGQG | 15 |
| CM07 | Cγ/Cϵ | ND | ND | ND | CAT VRLDSPY(S/A)FAY | WGQG | 11 |
| CM08 | Cγ4 | V3-30/D3-9/JH4 | 43 | 1.3* | CAS LRMSIDRGFDC | WGQG | 11 |
| CM10 | Cγ | V1-08/D3-10/JH4 | 41 | 2.1* | CGR VLNTPAHRPIRGLIAY | WGQG | 16 |
| CM11 | Cα | V1-69/D6-6/JH4 | 4 | ND | CAR AVDFDSSSSYSF | WGQG | 12 |
| CM13 | Cμ | V3-72/D6-19/JH3 | 12 | 1.3 | CVR GYSIGWPYDALAR | WGQG | 13 |
| CM15 | Cγ1/Cϵ | V3-53/D2-2/JH3 | 24 | 3.6 | CAR ENPRHDVFDI | WGLG | 10 |
| CM19 | Cγ | ND | ND | ND | CAK ESGGAARMRGNYYYYYYMDV | WGQG | 20 |
| CM20 | Cγ | ND | ND | ND | CVR HSAEVADSVEED | WGQG | 12 |
| CM21 | Cγ | ND | ND | ND | CAR ETNYDSWTGSPSSHYFGLDF | WGQG | 20 |
| CM22 | Cα | ND | ND | ND | CAR GSGDYKVTKYYEDAFDI | WGQG | 17 |
| CM29 | Cγ | ND | ND | ND | CAR GVDFDYFDL | WGRG | 9 |
| CM34 | Cγ | ND | ND | ND | CAK DLLLRVVGCLDY | WGQG | 12 |
| CM35 | Cγ | V1-69/D1-14/JH5 | ND | ND | CAR GSHLIGGTIASFDP | WGQG | 14 |
| CM37 | Cγ/Cα | V1-02/D2-21/JH4 | ND | ND | CAA AL(S/P)ESSFPFTFHD | WGQG | 13 |
| CM42 | Cγ | ND | ND | ND | CAR LNYVLHLGRLIEGAGTNYGMDV | LGQG | 22 |
| CM43 | Cμ/Cδ | ND | ND | ND | CAR APFLGDVFFDP | WGQG | 11 |
| CM44 | Cγ | V3-48/D5-24/JH4 | 38 | 1.4 | CAK LQRRGLQLGYLEY | FGQG | 13 |
R/S ratio FR indicates replacement/silent mutation ratio in IgVH framework regions; aa, amino acids; and ND, not determined.
Significant according to Chang and Casali.32
IgVL sequence analysis of cutaneous MZBCLs
| Patient no. . | IgVL rearrangement (IMGT/V) . | IgVL rearrangement (Vbase) . | No. of mutations . | CDR3 sequence . |
|---|---|---|---|---|
| CM01 | V3-15*01/J2*01 | DPK21/humkv328h5 | 6 | CQQ YNNWPPY TFGQG |
| CM03 | V1-5*03/J1*01 | L12a/PCRdil6-5+ of HK201 | 5 | CQQ YGTYSW TFGQG |
| CM04 | V3D-15*01/J2*01 | L16/humkv31es, DPK21/humkv328h5+ | 14 | CQQ YDTWPSY AFGQG |
| CM07 | V1-5*01/J3*01 | HK102/V1+ | 16 | CQQ FNTFPL TFGPG |
| CM08 | V2D-28*01/J2*02 | DPK15/A19. | 20 | CMQ GLQIPY TFGQG |
| CM11 | V3-20*01/J1*01 | DPK22/A27 | 28 | CHQ YGRPG TFGQG |
| CM15 | V1D-39*01/J5*01 | DPK9/O12 | 10 | CQQ SYSRPP TFGQG |
| Patient no. . | IgVL rearrangement (IMGT/V) . | IgVL rearrangement (Vbase) . | No. of mutations . | CDR3 sequence . |
|---|---|---|---|---|
| CM01 | V3-15*01/J2*01 | DPK21/humkv328h5 | 6 | CQQ YNNWPPY TFGQG |
| CM03 | V1-5*03/J1*01 | L12a/PCRdil6-5+ of HK201 | 5 | CQQ YGTYSW TFGQG |
| CM04 | V3D-15*01/J2*01 | L16/humkv31es, DPK21/humkv328h5+ | 14 | CQQ YDTWPSY AFGQG |
| CM07 | V1-5*01/J3*01 | HK102/V1+ | 16 | CQQ FNTFPL TFGPG |
| CM08 | V2D-28*01/J2*02 | DPK15/A19. | 20 | CMQ GLQIPY TFGQG |
| CM11 | V3-20*01/J1*01 | DPK22/A27 | 28 | CHQ YGRPG TFGQG |
| CM15 | V1D-39*01/J5*01 | DPK9/O12 | 10 | CQQ SYSRPP TFGQG |
IgVH -CDR3 repertoire
IgVH-CDR3 amino acid sequences from this study, but also those published by Bahler et al,27 Roggero et al,26 and 3 sequences obtained from our previous study,24 were compared with each other and blasted against GenBank, and analyzed according to previously defined criteria (“IgVH-CDR3 analysis”). These analyses revealed that there was no IgVH-CDR3 homology among the 33 PCMZLs. In total, 15 PCMZLs displayed IgVH-CDR3 amino acid sequence homology to those of other Ig sequences from GenBank, without any obvious bias. Most homologies matched to Ig sequences from healthy donors. Case 2 from the study of Bahler et al27 displayed IgVH-CDR3 homology to a rheumatoid factor, both with a V3–30/JH4 rearrangement. The IgVH-CDR3 sequence of CM21 was homologous to those of 5 chronic lymphocytic leukemia cases, which, according to homology criteria of Stamatopoulos et al,36 can be assigned to homology subset 7. Patient 7 from Aarts et al24 was homologous to a gastric MALT lymphoma, although the VH-gene rearrangement did not match (VH1-2 for patient 7 and VH3-30 for the gastric MALT lymphoma). A detailed overview of the results is provided as Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Table 3 summarizes the analysis on rheumatoid factor homology in PCMZLs compared with other extranodal MZBCLs.
Rheumatoid factor IgVH-CDR3 homology of cutaneous and other MZBCLs
| . | N . | RF homology,* no. (%) . |
|---|---|---|
| Gastric MZBCLs | 97 | 11 (11)† |
| Salivary gland MZBCLs | 32 | 13 (41) |
| Pulmonary MZBCLs | 19 | 0 (0) |
| Other extranodal MZBCLs | 4 | 0 (0) |
| Splenic MZBCLs | 32 | 1 (3) |
| Cutaneous MZBCLs | 33 | 1 (3) |
| . | N . | RF homology,* no. (%) . |
|---|---|---|
| Gastric MZBCLs | 97 | 11 (11)† |
| Salivary gland MZBCLs | 32 | 13 (41) |
| Pulmonary MZBCLs | 19 | 0 (0) |
| Other extranodal MZBCLs | 4 | 0 (0) |
| Splenic MZBCLs | 32 | 1 (3) |
| Cutaneous MZBCLs | 33 | 1 (3) |
N indicates number of sequences analyzed.
CDR3 amino acid sequence homology with previously published rheumatoid factors (RF).
Tumor environment
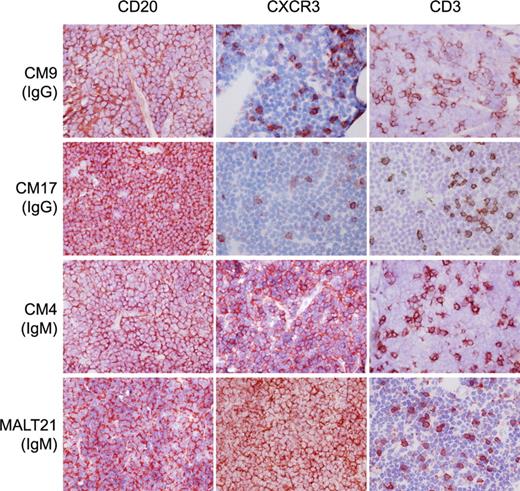
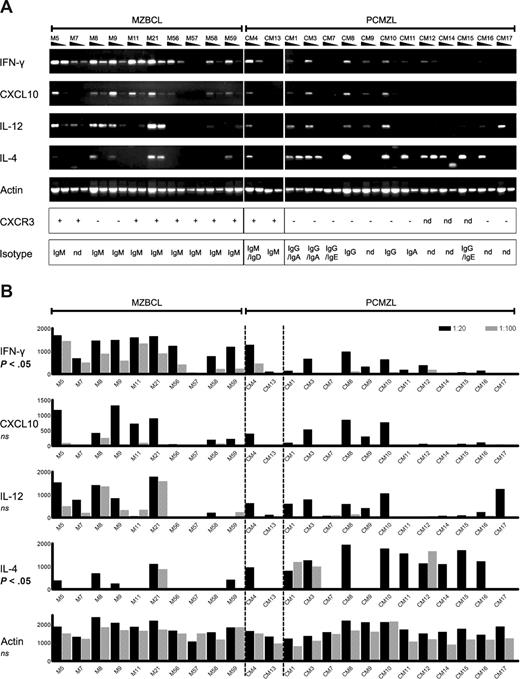
It has been established that virtually all extranodal and splenic MZBCLs express CXCR3.3-6,37 Immunohistochemical staining on PCMZL tissues revealed that a proportion of the infiltrating T cells express CXCR3; however, the tumor B cells were negative in all PCMZLs tested, except for CM04, CM13, and CM43 (Figure 1; Table 4). Next, we studied cytokine expression by performing semiquantitative RT-PCR. The results depicted in Figure 2A show that, in general, MZBCLs have a higher expression of the Th1 type cytokines IFN-γ, CXCL10, and IL-12, in contrast to the class-switched PCMZLs, which show more bias toward IL-4 expression, a typical Th2 cytokine. Mann-Whitney scoring of the arbitrary values, determined by densitometry of the PCR products, established that the differences between the 2 groups were statistically significant for IFN-γ (P = .001) and IL-4 (P = .028; Figure 2B).
IgG expressing PCMZLs do not express CXCR3. Frozen sections of CM09, CM17, CM04, and MALT21 stained with monoclonal antibodies (in red, 3-amino-9-ethylcarbazole) specific for CD20, CXCR3, and CD3 (original magnification × 400) and counterstained with hematoxylin. IgG-expressing PCMZLs CM09 and CM17 contain CXCR3+ T cells, whereas the neoplastic B cells in these tissues are CXCR3−. In contrast, CM04, an IgM-expressing PCMZL, is positive for CXCR3, like the salivary gland MZBCL “MALT21.”
IgG expressing PCMZLs do not express CXCR3. Frozen sections of CM09, CM17, CM04, and MALT21 stained with monoclonal antibodies (in red, 3-amino-9-ethylcarbazole) specific for CD20, CXCR3, and CD3 (original magnification × 400) and counterstained with hematoxylin. IgG-expressing PCMZLs CM09 and CM17 contain CXCR3+ T cells, whereas the neoplastic B cells in these tissues are CXCR3−. In contrast, CM04, an IgM-expressing PCMZL, is positive for CXCR3, like the salivary gland MZBCL “MALT21.”
PCMZLs develop in a distinct inflammatory environment. (A) Semiquantitative RT-PCR for IFN-γ, CXCL10, IL-12, IL-4, and actin, on whole tissue samples of 10 extranodal MZBCLs (left) and 14 PCMZLs (right); each sample was tested in 2 dilutions. The 2 cases in the middle, CM04 and CM13, represent the IgM+ CXCR3+ PCMZLs. The lower 2 panels depict the results of immunohistochemistry for CXCR3, and the Ig isotypes determined by RT-PCR. nd indicates not determined. (B) PCR band intensities as determined by densitometry (in arbitrary values). Differences between extranodal MZBCLs on the left and class-switched PCMZLs on the right were significant for IFN-γ (P = .001) and IL-4 (P = .028), as determined by a Mann-Whitney rank-sum test.
PCMZLs develop in a distinct inflammatory environment. (A) Semiquantitative RT-PCR for IFN-γ, CXCL10, IL-12, IL-4, and actin, on whole tissue samples of 10 extranodal MZBCLs (left) and 14 PCMZLs (right); each sample was tested in 2 dilutions. The 2 cases in the middle, CM04 and CM13, represent the IgM+ CXCR3+ PCMZLs. The lower 2 panels depict the results of immunohistochemistry for CXCR3, and the Ig isotypes determined by RT-PCR. nd indicates not determined. (B) PCR band intensities as determined by densitometry (in arbitrary values). Differences between extranodal MZBCLs on the left and class-switched PCMZLs on the right were significant for IFN-γ (P = .001) and IL-4 (P = .028), as determined by a Mann-Whitney rank-sum test.
Discussion
In this study, we show that PCMZLs, despite histologic resemblance, differ from other extranodal MZBCLs. A striking difference is the expression of class-switched Ig by 18 of 21 analyzed PCMZLs, in 6 cases with dual isotypes derived from the same clone. In contrast, only approximately 30% of MALT lymphomas express class-switched Ig.3 This finding is in accordance with previous immunohistochemical studies, although we are the first to demonstrate IgE expression in PCMZL.38-40
Five of the resolved PCMZL IgVH genes comprised a VH1 germ line genes and 6 contained a VH3 germ line gene. We24 previously reported the usage of VH1, VH3, and VH4 and Franco et al25 reported VH2 and VH6 usage in PCMZLs. These results do not confirm an exclusive usage of VH3 family members in the Ig rearrangements of PCMZLs, as was reported by Bahler et al.27 Analysis of the IgVH mutations showed that 3 of 8 PCMZLs had R/S ratios significantly less than those expected if mutation had been random.35 Including previously described cases,24,25 46% of the PCMZLs appear to be selected for maintenance of B-cell receptor structure, which is somewhat less than we have found in other extranodal MZBCLs (∼ 70%).3
A total of 33 IgVH-CDR3 sequences (ie, 21 obtained in this study, 3 from our previous study,24 1 from Roggero et al,26 and the 8 cases published by Bahler et al27 ) were analyzed for homology with each other and with sequences in GenBank. Within this relatively large panel of sequences, we were not able to detect an IgVH repertoire bias, as was found in salivary gland and gastric MZBCLs; there was no IgVH-CDR3 homology between the PCMZLs. Fifteen IgVH-CDR3 amino acid sequences matched IgVH sequences from GenBank, including one case that was homologous to an RF. Previous findings reported by Bahler et al27 on conserved PS/T or YG/T amino acids encoded by nontemplated N-nucleotide sequences within the CDR3 sequences, which would be a strong argument for similar antigen recognition, were not encountered within our panel of sequences.
Extranodal MZBCLs generally arise on a background of chronic inflammation, usually of the Th1 type. Th1 cytokines, such as IFN-γ and IL-2, are abundantly expressed in the initial chronically inflamed tissues as well as in the eventual tumor environment.38,39 In addition, IFN-γ–induced chemokines, such as CXCL9 and CXCL10, are expressed by the epithelial and endothelial cells, which attract more Th1 cells expressing CXCR3, the receptor for these chemokines.43-45 In agreement with the excess of IFN-γ in the tumor environment, the noncutaneous MZBCLs express CXCR3, a downstream target of the IFN-γ–induced transcription factor T-bet.3-5,37,46 Our analyses suggest that most PCMZLs develop in a distinct inflammatory environment. The majority of the PCMZLs (90%) lack expression of CXCR3. Of note, the CXCR3+ minority consisted of 4 IgM+ PCMZLs (CM04, CM13, CM43, and patient 6 of Aarts et al24 ). Interestingly, CM13 and CM43 had developed on a background of a B burgdorferi infection, and Roggero et al26 also reported a Borrelia-associated PCMZL expressing IgM. Like H pylori in the gastric mucosa, B burgdorferi evokes a Th1 type of response, supportive for the extranodal MZBCL-like phenotype of these 2 Borrelia-associated PCMZLs.47-49 The majority of PCMZLs, however, seem to reside in a Th2 type cytokine environment, as was supported by the cytokine RT-PCRs. Moreover, the fact that these lymphomas express IgG1, IgG4, IgA, and IgE, the latter 3 of which are typical Th2-dependent isotypes, is compatible with the Th2 inflammatory origin.50,51
The results presented here are suggestive of the existence of 2 types of PCMZL, most likely related to their pathogenesis. A small subgroup resembles noncutaneous MZBCLs, being CXCR3+ and IgM+ and potentially (Borrelia) infection associated. To confirm these results and to determine whether RF homology is more common among this type of PCMZLs, it would be interesting to study a larger panel of Borrelia-associated PCMZLs, if available. In contrast, most of the PCMZLs differ from other extranodal MZBCLs, as they possess switched Ig, lack of RF homology, do not express CXCR3, and have a cytokine profile more skewed toward the Th2 type. These differences in Ig repertoire and cytokine environment suggest that PCMZLs do not recognize a similar class of antigens. Further study on the clinical history of this type of PCMZLs might reveal an etiology in the large variety of Th2 type inflammatory conditions of the skin.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank E. J. M. Schilder-Tol, M. E. C. M. Oud, and A. A. Mulder for technical assistance, J. Aten for his help on statistics, and A. Iyer and P. K. Das for cytokine primer sequences.
Authorship
Contribution: F.v.M., R.J.B., R.v.D., and T.A.M.W. performed research and analyzed data; F.v.M., R.J.B., and C.J.M.v.N. wrote the paper and designed research; and P.M.K., R.W., and L.C. performed research, provided patient material, and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carel J. M. van Noesel, Department of Pathology, Academic Medical Center, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands; e-mail: c.j.vannoesel@amc.uva.nl.