T(11;18)(q21;q21) is the most common structural abnormality in extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) leading to the fusion of the apoptosis inhibitor-2 (API2) gene and the MALT lymphoma-associated translocation (MALT1) gene. In 2 patients with MALT lymphoma of the liver and skin, respectively, t(14;18)(q32;q21) was observed by cytogenetic analysis. Subsequent fluorescence in situ hybridization (FISH) studies disclosed that the immunoglobulin heavy-chain locus (IGH) and the MALT1 gene were rearranged by this translocation. In order to screen a large series of MALT lymphomas for this aberration, a 2-color interphase FISH assay was established. Among a total of 66 cases, t(14;18)(q32;q21) involving IGH and MALT1 was detected in MALT lymphomas of the liver (4 of 4), skin (3 of 11), ocular adnexa (3 of 8), and salivary gland (2 of 11), but did not occur in MALT lymphomas of the stomach (n = 10), intestine (n = 9), lung (n = 7), thyroid (n = 4), or breast (n = 2). In total, 12 of 66 (18%) MALT lymphomas harbored t(14;18)(q32;q21); 7 additional cases of splenic marginal zone lymphoma tested negative. All of the 12 MALT lymphomas featuring the t(14;18)(q32;q21) were negative for t(11;18)(q21;q21) by reverse transcriptase–polymerase chain reaction (RT-PCR). However, trisomy 3 and/or 18 was found in 4 of 12 cases, suggesting that the t(14;18)(q32;q21) does not occur as the sole genetic abnormality. This study identifies IGH as a new translocation partner of MALT1 in MALT lymphomas, which tend to arise frequently at sites other than the gastrointestinal tract and lung. In contrast to t(11;18)(q21;q21)+ MALT lymphomas, those with t(14;18)(q32;q21) may harbor additional genetic abnormalities.
Introduction
Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is listed as a distinct clinicopathologic entity in the recently published World Health Organization (WHO) classification of malignant lymphomas.1 According to the International Lymphoma Study Group, MALT lymphoma comprises 7.6% of all non-Hodgkin lymphomas (NHLs) and represents 1 of the 6 most common NHLs.2 The majority of MALT lymphomas occur in the stomach, but this type of lymphoma may affect virtually every organ in the human body, including the ocular adnexa, lung, salivary glands, thyroid, skin, and intestine.3 MALT lymphomas are indolent (low-grade) lymphomas despite the fact that in about one third of the cases dissemination to other mucosal sites, bone marrow, or multiple lymph nodes is found at diagnosis.4 Histologically, MALT lymphomas are characterized by a proliferation of neoplastic marginal zone–related cells that invade epithelial structures to generate lymphoepithelial lesions and colonize reactive lymphoid follicles.5 The MALT lymphoma concept suggests that these tumors correspond to cells of postfollicular differentiation stages and usually arise from lymphoid tissue acquired by chronic antigenic stimulation triggered by persistent infections and/or autoimmune processes.6,7 This lymphoid tissue becomes genetically unstable with the acquisition of abnormalities such as trisomy 3, trisomy 18,8 p16 deletion,9t(1;14)(p22;q32),10 and t(11;18)(q21;q21),11,12 leading to transformation into MALT lymphoma. T(11;18)(q21;q21) is the most frequent structural chromosomal abnormality in MALT lymphoma, resulting in the fusion of the apoptosis inhibitor-2 (API2) gene and the MALT lymphoma-associated translocation (MALT1) gene at the 11q21 and 18q21 breakpoints, respectively.13-15 Moreover, t(11;18)(q21;q21) is restricted to MALT lymphomas and has not been detected in nodal or splenic marginal zone lymphomas, diffuse large B-cell lymphomas, or other non-Hodgkin lymphomas.16-19
We report on a novel recurrent translocation in MALT lymphoma involving IGH at 14q32 and MALT1 at 18q21. The frequency of this translocation was investigated by a 2-color fluorescence in situ hybridization (FISH) assay in a large series of MALT lymphomas of various sites and in several splenic marginal zone lymphomas.
Materials and methods
Cases
Sixty-six MALT lymphoma cases and material from 7 patients with splenic marginal zone lymphoma were studied for the presence of t(14;18)(q32;q21) involving IGH and MALT1. For inclusion in this study, the cases were required to fulfill the histologic and immunohistologic criteria defined for the respective entities in the WHO classification of tumors of hematopoietic and lymphoid tissues.1 20 The immunophenotype of the tumor cells, as assessed on paraffin sections, was CD20+, cyclin D1−, CD23−, CD5−, bcl-6−, CD10−. Among 64 MALT lymphomas tested, 46 showed light chain restriction; in the remaining samples the lymphoma cells did not stain for either κ or λ light chains or the staining result was not clearly interpretable. All samples used for analysis were derived from the primary site of disease presentation. Formalin-fixed and paraffin-embedded tissue samples of biopsies or surgical resection specimens were retrieved from the files of the Department of Pathology, Vienna General Hospital, except for 1 hepatic, 5 cutaneous, and 4 intestinal MALT lymphoma cases that were provided by the Institute of Pathology in Würzburg, the Department of Dermatology in Graz, and the Department of Pathology, Klinikum Bayreuth, respectively. Among the total of 66 MALT lymphoma cases, 11 cases each were of salivary gland and cutaneous origin; 10 involved the stomach; 9, the intestine; 8, the ocular adnexa; 7, the lung; 4, the liver; 4, the thyroid; and 2, the breast. The presence of tumor cells was evaluated in each tissue block on hematoxylin and eosin–stained slides cut before and after the sections used for fluorescence in situ hybridization (FISH) or reverse transcriptase–polymerase chain reaction (RT-PCR) analyses.
Cytogenetic analysis
In 2 cases, cytogenetic analyses were performed on short-term cultures (24-72 hours) of fresh tumor samples. Methods of cell cultivation and of chromosome preparation and staining by a modified GAG-banding technique have been described previously.21The constitutional karyotype of both patients was determined on metaphases from phytohemagglutinin-stimulated lymphocytes. The karyotypes were described according to the International System for Human Cytogenetic Nomenclature (ISCN) (1995).22
FISH analysis
In all cases, except the 2 in which fresh tissue was available, formalin-fixed paraffin-embedded tissue was used. For a reliable interpretation of the hybridization signals, we preferred the analysis of single-cell suspensions to thin sections. After deparaffinization in xylene, two 30-μm–thick slices were incubated for 25 minutes in 4% pepsin, pH 1.5, at 37°C. After a rapid wash in phosphate-buffered saline, cells were incubated for 30 minutes in 0.075 M KCl and fixed twice in methanol/acetic acid (3:1) and dropped on slides.
In all 73 cases FISH was performed on interphases with a probe spanning the MALT1 gene and flanking regions (PAC 152M5)17 and an IGH probe (BAC158A2)23 picked from a bacterial artificial chromosome (BAC) library. The IGH probe was directly labeled with SpectrumGreen, the MALT1 probe with SpectrumOrange by nick translation (Vysis, Downer's Grove, IL). The cutoff value for the diagnosis of a rearrangement involving IGH andMALT1 was 5.3%, which is above the mean percentage of cells with a false-positive signal constellation plus 3 standard deviations, as assessed on tissue from 20 reactive lymph nodes processed as described for the lymphomas. Moreover, IGH dual color break-apart rearrangement probes (Vysis) were applied to cells of all t(14;18)(q32;q21)+ lymphomas. The SpectrumGreen–labeled LSI IGHV probe covers the entire IGH variable region, the SpectrumOrange–labeled probe lies completely 3′ to the IGHlocus. As a result of this probe design, any translocation with a breakpoint at the J segments or within switch sequences should produce separate orange and green signals. Finally, FISH experiments using 2 P1 artificial chromosomes (PACs) flanking MALT1 were performed on interphases of t(14;18)(q32;q21)+ and t(11;18)(q21;q21)+ tumors. The orange-labeled PAC 117B5 is centromeric; the green-labeled PAC 59N7, telomeric toMALT1.17 A rearrangement involvingMALT1 should therefore result in separated orange and green signals. Additionally, FISH with centromere-specific probes for the chromosomes 3 and 18 (Vysis) was done in t(14;18)(q32;q21)+and t(11;18)(q21;q21)+ cases. In the 2 cases of which fresh tissue was available FISH with LSI IGH/BCL2 Dual Color, Dual Fusion Translocation probe (Vysis) was performed on metaphases and interphases. The probes are designed to detect the juxtaposition of theIGH locus and BCL2 sequences by 2 fusion signals, one on the derivative chromosome (der)(14) and one on the der(18). In one case each FISH with whole chromosome painting probes for the chromosomes 1, 6, and 13 and for the chromosomes 11, 18, 21, and 22, respectively, were used to determine the karyotype.BCL6 rearrangement was evaluated in 4 hepatic MALT lymphomas using BCL6 dual-color break-apart probe covering all breakpoints (Vysis). FISH procedure was performed according to published standard methods.21
RT-PCR forAPI2-MALT1 fusion transcripts
RNA was isolated from archival formalin-fixed, paraffin-embedded lymphoma tissues of the gastric and pulmonary MALT lymphoma cases and in all cases positive for the t(14;18). Total RNA was extracted from 10-μm sections with a high pure RNA paraffin kit (Roche Diagnostics, Mannheim, Germany). First-strand cDNA was synthesized from 1 μg total RNA with a superscript first-strand synthesis system (Invitrogen, Carlsbad, CA) using random hexamers as primers. RT-PCR was performed according to Inagaki et al24 with one modification: first-round RT-PCR products were amplified in a second round separately and not as multiplex nested PCRs.
Results
FISH analyses of 2 MALT lymphomas with t(14;18)(q32;q21) identify rearrangements of IGH and MALT1
In 2 cases of histopathologically typical primary MALT lymphomas of the liver and skin, respectively, cytogenetic analyses surprisingly revealed a t(14;18)(q32;q21) as usually found in follicular lymphoma (Table 1).
Karyotype of 2 MALT lymphomas
| Site . | Karyotype . |
|---|---|
| Liver | 46,XX[16]/50,XX,+3,+4,+13,t(14;18)(q32;q21),+18[12]/51,XX,+X,+3, +4,+13,t(14;18)(q32;q21),+18[2] |
| Skin | 46,XY,dup(1)(q21q42),del(6)(q23),dup(13)(q22q34),t(14;18)(q32;q21)[8] /46,XY[7]. |
| Site . | Karyotype . |
|---|---|
| Liver | 46,XX[16]/50,XX,+3,+4,+13,t(14;18)(q32;q21),+18[12]/51,XX,+X,+3, +4,+13,t(14;18)(q32;q21),+18[2] |
| Skin | 46,XY,dup(1)(q21q42),del(6)(q23),dup(13)(q22q34),t(14;18)(q32;q21)[8] /46,XY[7]. |
Several FISH studies on metaphases were performed to characterize the breakpoints in 14q32 and 18q21. This approach is described in detail for the hepatic MALT lymphoma case below.
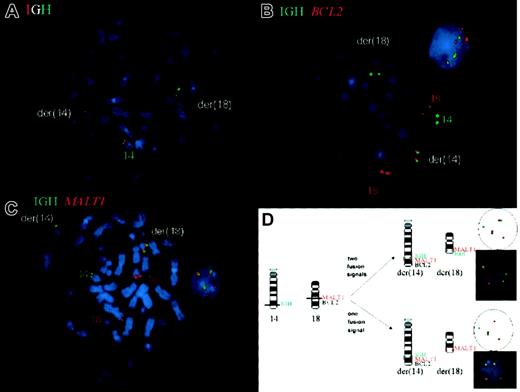
The dual break-apart probe for IGH confirmed the rearrangement of the IGH locus, showing the SpectrumOrange signal on the der(14) and the SpectrumGreen signal on the der(18) (Figure 1A). The next FISH experiment was done to investigate whether BCL2 is indeed the translocation partner of IGH. The green signal highlighting theIGH locus was seen on the normal chromosome 14 as well as on the der(14) and der(18), again confirming the IGHtranslocation. The red signal for BCL2 was observed on the normal chromosome 18 and the der(14), but not, as to be expected for the t(14;18) involving the BCL2 locus, on the der(18). Moreover, the interphases did not show IGH/BCL2 fusion signals (Figure 1B). These results indicated that the breakpoint in 18q21 was located centromeric of BCL2.
Fluorescence in situ hybridization (FISH) for the detection of t(14;18)(q32;q21) involving IGH and MALT1 on metaphase chromosomes and interphase nuclei of a hepatic MALT lymphoma.
(A) The dual break-apart probe for IGH confirmed the rearrangement of the IGH locus showing the red signal on the der(14) and the green signal on the der(18), whereas the normal chromosome 14 carries the colocalization signal. By using probes forIGH and BCL2 (B), a split green signal forIGH is seen on der(14) and der(18), confirming the IGH translocation, however, the red signal for BCL2 is observed only on the der(14), indicating that the breakpoint is centromeric toBCL2. Moreover, the interphase nucleus does not showIGH/BCL2 fusion signals. The translocation ofMALT1 is demonstrated in (C), featuring a split signal for the red MALT1 probe in the aberrant metaphase on the der(14) and der(18) and fusion signals in both the metaphase and interphase. The interphase FISH assay schematically shown in (D) usesIGH and MALT1 probes for the detection of the t(14;18)(q32;q21) in isolated nuclei of routinely processed tissue.
Fluorescence in situ hybridization (FISH) for the detection of t(14;18)(q32;q21) involving IGH and MALT1 on metaphase chromosomes and interphase nuclei of a hepatic MALT lymphoma.
(A) The dual break-apart probe for IGH confirmed the rearrangement of the IGH locus showing the red signal on the der(14) and the green signal on the der(18), whereas the normal chromosome 14 carries the colocalization signal. By using probes forIGH and BCL2 (B), a split green signal forIGH is seen on der(14) and der(18), confirming the IGH translocation, however, the red signal for BCL2 is observed only on the der(14), indicating that the breakpoint is centromeric toBCL2. Moreover, the interphase nucleus does not showIGH/BCL2 fusion signals. The translocation ofMALT1 is demonstrated in (C), featuring a split signal for the red MALT1 probe in the aberrant metaphase on the der(14) and der(18) and fusion signals in both the metaphase and interphase. The interphase FISH assay schematically shown in (D) usesIGH and MALT1 probes for the detection of the t(14;18)(q32;q21) in isolated nuclei of routinely processed tissue.
To determine whether the MALT1 gene, which is located centromeric of BCL2 in band q21, is targeted by the translocation, the following FISH experiment employed probes forIGH and MALT1. A split signal for theMALT1 probe was observed in the aberrant metaphases on the der(14) and der(18), and fusion signals were found in both the meta- and interphases, confirming the translocation of MALT1(Figure 1C). This experimental design also was applied in the cutaneous MALT lymphoma, revealing the same hybridization patterns and therefore the involvement of IGH and MALT1 in t(14;18).
The frequency of t(14;18)(q32;q21) involving IGH andMALT1 depends on MALT lymphoma site
A FISH assay employing probes for MALT1 andIGH, as schematically shown in Figure 1D, was used to screen a large series of MALT lymphomas of various sites and several splenic marginal zone lymphomas for the presence of t(14;18)(q32;q21).
As listed in Table 2, 12 of 66 (18%) MALT lymphomas harbored t(14;18)(q32;q21). In all t(14;18)+cases the rearrangement of IGH was confirmed by the dual-color break-apart probes for IGH (not shown).
Frequency of t(14;18)(q32;q21) involving IGHand MALT1 relative to lymphoma site
| Diagnosis . | Cases analyzed, no. . | t(14;18)+cases, no. (%) . |
|---|---|---|
| MALT lymphoma | 66 | 12 (18) |
| Liver | 4 | 4 (100) |
| Ocular adnexa | 8 | 3 (38) |
| Skin | 11 | 3 (27) |
| Salivary gland | 11 | 2 (18) |
| Stomach | 10 | 0 (0) |
| Intestine | 9 | 0 (0) |
| Lung | 7 | 0 (0) |
| Thyroid | 4 | 0 (0) |
| Breast | 2 | 0 (0) |
| Splenic marginal zone lymphoma | 7 | 0 (0) |
| Diagnosis . | Cases analyzed, no. . | t(14;18)+cases, no. (%) . |
|---|---|---|
| MALT lymphoma | 66 | 12 (18) |
| Liver | 4 | 4 (100) |
| Ocular adnexa | 8 | 3 (38) |
| Skin | 11 | 3 (27) |
| Salivary gland | 11 | 2 (18) |
| Stomach | 10 | 0 (0) |
| Intestine | 9 | 0 (0) |
| Lung | 7 | 0 (0) |
| Thyroid | 4 | 0 (0) |
| Breast | 2 | 0 (0) |
| Splenic marginal zone lymphoma | 7 | 0 (0) |
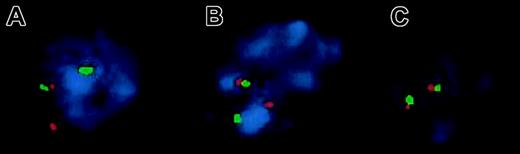
Further confirmation for the involvement of MALT1 was obtained by FISH experiments using PAC probes flankingMALT1. As expected, interphases of all 11 t(14;18)+ lymphomas tested (no tissue was left of the 12th case) revealed a split signal for the 2 PACs, confirming the rearrangement of MALT1 (Figure2A). Similarly, interphases of all t(11;18)+ tumors detected by RT-PCR (outlined below) showed the same hybridization pattern (Figure 2B). In contrast, interphases of 5 normal control lymph node suspensions exhibited 2 fusion signals (Figure 2C). The clinical data of these 12 patients are shown in Table3.
Two-color FISH to interphase nuclei isolated from paraffin-embedded tissue for the detection of the MALT1translocation.
The orange PAC 117B5 (centromeric to MALT1) and green PAC 59N7 (telomeric to MALT1) show separate signals on interphases of a t(14;18)(q32;q21)+ hepatic MALT lymphoma, confirming MALT1 rearrangement (A). The same hybridization pattern is observed in a pulmonary t(11;18)(q21;q21)+ MALT lymphoma (B), whereas fusion signals are found in normal control nuclei (C).
Two-color FISH to interphase nuclei isolated from paraffin-embedded tissue for the detection of the MALT1translocation.
The orange PAC 117B5 (centromeric to MALT1) and green PAC 59N7 (telomeric to MALT1) show separate signals on interphases of a t(14;18)(q32;q21)+ hepatic MALT lymphoma, confirming MALT1 rearrangement (A). The same hybridization pattern is observed in a pulmonary t(11;18)(q21;q21)+ MALT lymphoma (B), whereas fusion signals are found in normal control nuclei (C).
Clinical data of 12 cases of extranodal MALT lymphoma characterized by t(14;18)(IGH;MALT1)
| Site . | Age/sex . | Stage3-150 . | Clinical history . | Additional sites involved . | Clinical course . | |
|---|---|---|---|---|---|---|
| At presentation . | During course . | |||||
| Liver | 72/F | Not staged | Colorectal adenocarcinoma; solitary hepatic nodule detected during follow-up | Not known | Not known | Died 1 month after liver biopsy |
| Liver | 61/F | EII1 | Rheumatoid arthritis; MALT lymphoma diagnosed at autopsy | Portal lymph nodes | None | Died of pulmonary embolism |
| Liver (multifocal) | 58/F | EI1 | None | None | None | Chemotherapy followed by resection, alive + 37 months (CR) |
| Liver | 62/F | EI1 | Breast cancer; solitary hepatic nodule detected during staging after modified mastectomy | None | None | Resection, tamoxifen; alive +9 months after resection (CR) |
| Lacrimal gland | 76/M | IV | Uneventful | Lacrimal gland (bilateral) | None | Chemotherapy; alive +32 months (CR) |
| Lacrimal gland | 81/M | EI | Uneventful | None | Not known | Chemotherapy, lost to follow-up |
| Orbit | 51/F | IV | Uneventful | Colon | None so far | Alive +4 months |
| Parotid gland | 50/F | EI1 | Sjögren syndrome; Hashimoto thyroiditis | None | None | Radiotherapy, alive +31 mos (CR) |
| Parotid gland | 36/F | IV | Sjögren syndrome | Parotid gland (contralateral) | None | Chemotherapy followed by radiation, alive +28 months (CR) |
| Skin, grouped lesions on left arm | 67/M | EI | Uneventful | None | None | Radiotherapy, alive +156 months (CR); died of unrelated cause |
| Skin, few lesions on arms and legs | 30/M | IV | Uneventful | None | None | Intralesional interferon, alive +60 months (CR) |
| Skin, more than 50 nodules on trunk | 44/M | IV | Uneventful | None | None | Rituximab, alive +16 months (CR) |
| Site . | Age/sex . | Stage3-150 . | Clinical history . | Additional sites involved . | Clinical course . | |
|---|---|---|---|---|---|---|
| At presentation . | During course . | |||||
| Liver | 72/F | Not staged | Colorectal adenocarcinoma; solitary hepatic nodule detected during follow-up | Not known | Not known | Died 1 month after liver biopsy |
| Liver | 61/F | EII1 | Rheumatoid arthritis; MALT lymphoma diagnosed at autopsy | Portal lymph nodes | None | Died of pulmonary embolism |
| Liver (multifocal) | 58/F | EI1 | None | None | None | Chemotherapy followed by resection, alive + 37 months (CR) |
| Liver | 62/F | EI1 | Breast cancer; solitary hepatic nodule detected during staging after modified mastectomy | None | None | Resection, tamoxifen; alive +9 months after resection (CR) |
| Lacrimal gland | 76/M | IV | Uneventful | Lacrimal gland (bilateral) | None | Chemotherapy; alive +32 months (CR) |
| Lacrimal gland | 81/M | EI | Uneventful | None | Not known | Chemotherapy, lost to follow-up |
| Orbit | 51/F | IV | Uneventful | Colon | None so far | Alive +4 months |
| Parotid gland | 50/F | EI1 | Sjögren syndrome; Hashimoto thyroiditis | None | None | Radiotherapy, alive +31 mos (CR) |
| Parotid gland | 36/F | IV | Sjögren syndrome | Parotid gland (contralateral) | None | Chemotherapy followed by radiation, alive +28 months (CR) |
| Skin, grouped lesions on left arm | 67/M | EI | Uneventful | None | None | Radiotherapy, alive +156 months (CR); died of unrelated cause |
| Skin, few lesions on arms and legs | 30/M | IV | Uneventful | None | None | Intralesional interferon, alive +60 months (CR) |
| Skin, more than 50 nodules on trunk | 44/M | IV | Uneventful | None | None | Rituximab, alive +16 months (CR) |
CR indicates complete remission.
Staging is given according to the Ann Arbor System, as modified by Radaszkiewicz et al for gastrointestinal lymphomas.25Standard staging procedures consisted of computed tomography scanning of thorax and abdomen; gastroscopy with mapping biopsies; endosonography of the upper gastrointestinal tract; enteroclysma; colonoscopy; bone marrow biopsy; imaging of salivary and lacrimal glands by means of magnetic resonance imaging or ultrasound; and ear, nose, and throat investigation. All lesions suggestive of lymphoma involvement were subjected to biopsy.
The frequency of the cases positive for the translocation depended on the localization and was most striking for hepatic MALT lymphoma, with 4 of 4 cases positive. By descending order of frequency, the translocation was detected in MALT lymphomas of the ocular adnexa (3 of 8), skin (3 of 11), and salivary gland (2 of 11), but not in cases of gastrointestinal and pulmonary origin. Similarly, 4 and 2 cases of the thyroid and breast, respectively, tested negative, as did all 7 splenic marginal zone lymphomas.
MALT lymphomas harboring the t(14;18)(q32;q21) have additional chromosomal aberrations
MALT lymphomas with t(11;18)(q21;q21) are known to only rarely display secondary aberrations as opposed to their t(11;18)(q21;q21) negative counterparts.8 26 To investigate whether this is also the case in MALT lymphomas positive for t(14;18)(q32;q21), the 12 lymphomas harboring the translocation were screened by FISH using centromere-specific probes for chromosomes 3 and 18. Two hepatic and 2 MALT lymphomas of the ocular adnexa exhibited trisomy 3, one of the hepatic cases additionally exhibited trisomy 18. Therefore, together with the cytogenetic findings of the cutaneous lymphoma shown in Table1, 5 of the 12 t(14;18)(q32;q21)+ MALT lymphomas showed additional chromosomal aberrations.
Previous cytogenetic analysis of a hepatic MALT lymphoma demonstrated t(3;14)(q27;32), suggestive of BCL6 involvement, as the sole genetic abnormality.27 We therefore investigated the 4 hepatic MALT lymphomas for BCL6 rearrangement by FISH but did not find evidence for BCL6 translocation (not shown).
T(14;18)(q32;q21) and t(11;18)(q21;q21) are mutually exclusive
In order to exclude a hidden t(11;18)(q21;q21) among the t(14;18)(q32;q21)+ MALT lymphomas, RT-PCR studies were performed, which were all negative. In contrast, API2-MALT1fusion transcripts indicative of the t(11;18)(q21;q21) were detected in 4 of 10 gastric and in 4 of 7 pulmonary MALT lymphomas, all of which tested negative for t(14;18)(q32;q21), as outlined above. These findings indicate that the 2 translocations are mutually exclusive.
Discussion
Different subtypes of NHLs have been characterized by distinct recurrent chromosomal aberrations, the most notorious example being t(14;18)(q32;q21) found in follicular lymphoma.28Recently, screening of larger MALT lymphoma series revealed the presence of t(11;18)(q21;q21) in 18% to 35% of the cases, indicating that the translocation represents the most frequent structural chromosomal aberration in this NHL subtype.12,16-18,29 The oncogenic potential of t(11;18)(q21;q21) in MALT lymphoma is underlined by its association with advanced gastric tumors and with the high frequency of t(11;18)(q21;q21) in gastric MALT lymphomas resistant toHelicobacter pylori eradication therapy.30However, the majority of MALT lymphomas do not harbor this translocation, although there is considerable variation relative to published data and to anatomic site. Pulmonary and gastric MALT lymphomas have been reported to feature t(11;18)(q21;q21) in 44% to 62% and 12% to 48% of the cases, respectively, and the intestine may also frequently give rise to t(11;18)(q21;q21)+ MALT lymphomas, but the number of cases analyzed is very small.16-18,29,31 At other MALT lymphoma sites t(11;18)(q21;q21) is rarely found (ocular adnexa, thyroid, salivary glands) or has not been reported at all (skin, breast).11 32
In the present study we have identified a novel structural chromosomal aberration in MALT lymphoma involving the IGH locus in 14q32 and MALT1 in 18q21. By screening a series of 66 MALT lymphomas, t(14;18)(q32;q21) was detected in 12 cases (18%). Interestingly, the anatomic distribution of t(14;18)(q32;q21)+ MALT lymphomas was complementary to that reported for t(11;18)(q21;q21)+ cases, that is, they presented as tumors of the ocular adnexa, the skin, or the salivary glands, but not as gastrointestinal or pulmonary lesions. Whether this polarization reflects different pathogenesis is highly speculative. However, MALT lymphomas of the salivary glands and ocular adnexa are often associated with autoimmune disease, whereas those arising in the stomach are clearly linked to an infectious agent (H pylori).
MALT lymphomas with t(11;18)(q21;q21) usually do not have additional genetic aberrations,8,26 which is in contrast to t(14;18)(q32;q21)+ cases, as demonstrated by karyotyping in 2 and FISH studies using centromere-specific probes for chromosomes 3 and 18 in all 12 of our MALT lymphomas. Aneuploidy was shown in 5 of the 12 cases, suggesting that probably the majority of t(14;18)(q32;q21)+ MALT lymphomas harbor additional genetic aberrations. Clinical follow-up and survival studies of larger series are clearly needed to determine whether t(14;18)(q32;q21) confers a risk for more aggressive disease. It is tempting to speculate that the significantly shorter time to progression observed in nongastrointestinal MALT lymphomas33 could at least in part be attributed to this novel genetic abnormality.
The most striking finding was the close association of t(14;18)(q32;q21) with hepatic disease, as all 4 MALT lymphomas arising in this organ harbored the translocation. Even considering a case report on a hepatic MALT lymphoma demonstrating t(3;14)(q27;q32) as the sole genetic abnormality,27 t(14;18)(q32;q21) might be a characteristic feature of hepatic MALT lymphoma. Similarly noteworthy is the observation of t(14;18)(q32;q21) in cutaneous MALT lymphoma, as no recurrent genetic abnormality has been reported to date for this disease manifestation.
Although our study does not provide insight into oncogenetic pathways involved with t(14;18)(q32;q21), which could be different from that reported for t(11;18)(q21;q21),34 these 2 structural aberrations might constitute a unifying feature of MALT lymphoma, withMALT1 being the common genetic denominator. Considering the frequencies of t(11;18)(q21;q21) and t(14;18)(q32;q21) found in MALT lymphoma, that is, 18% to 35%, and 18%, respectively, roughly 40% to 50% demonstrate MALT1 rearrangement. The involvement ofMALT1 in MALT lymphomas of all typical anatomic sites reinforces the validity of the MALT lymphoma concept proposed almost 20 years ago based on histopathologic features alone.35 36
We thank H. Avet-Loiseau for providing a FISH probe forIGH.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-09-2963.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andreas Chott, Department of Pathology, Vienna General Hospital, Vienna, Währinger Guertel 18-20, A-1090 Vienna, Austria; e-mail:andreas.chott@akh-wien.ac.at.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal