Radiolabeled anti-CD20 antibodies produce responses in 60% to 95% of patients with relapsed non-Hodgkin lymphoma (NHL); however, absorbed radiation ratios between tumors and normal organs are relatively low, and many patients have relapses. In this study we compared the abilities of anti-CD45 (BC8) and anti-CD20 (1F5) antibodies to target human Ramos lymphoma xenografts in athymic mice. When direct radioiodination was performed with conventional methods, BC8 delivered 2- to 4-fold more radioiodine to tumors than 1F5, with tumor-to–normal organ ratios as high as 20:1 using radiolabeled BC8 compared with a maximal ratio of 9.8:1 using radioiodinated 1F5. To optimize the biodistribution of radioactivity, we performed studies following a pretargeting method using streptavidin (SA)–conjugated BC8 and 1F5. Injection of a synthetic clearing agent decreased the circulating level of conjugates by 80% to 90% within 1 hour. Pretargeting with BC8-SA resulted in a 2- to 4-fold greater tumor uptake of radiolabeled biotin than with 1F5-SA, with maximal tumor-to–normal organ ratios of more than 80:1 and approximately 16:1, respectively. Therapy experiments demonstrated that 400 μCi (14.8 MBq) or more of yttrium-90-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)–biotin cured 100% of mice treated with BC8-SA and more than 90% of mice pretargeted with 1F5-SA, with complete remission occurring 8 to 10 days sooner in mice receiving BC8-SA. After treatment with 200 μCi (7.4 MBq) 90Y-DOTA-biotin, 70% of the mice treated with BC8-SA were cured, but no mice were cured using 1F5-SA. Doses up to 800 μCi (29.6 MBq) 90Y-DOTA-biotin were delivered with minor toxicity using either antibody conjugate. These lymphoma xenograft data suggest that pretargeted radioimmunotherapy using either anti-CD20 or anti-CD45 conjugates is highly effective and minimally toxic.
Introduction
Non-Hodgkin lymphomas (NHLs) are collectively the most common hematopoietic neoplasms, with a predicted incidence of approximately 55 000 new cases per year in the United States and an annual increase in the incidence rate of 3% to 4% per year.1 Most patients with B-cell NHL cannot be cured with conventional treatments despite recent advances in radiation therapy and combination chemotherapy; thus, new strategies are needed to improve cure rates for this malignancy. The use of monoclonal antibodies (mAbs) conjugated to a β-emitting radioisotope is developing into an effective approach for the treatment of patients with NHL. Non-Hodgkin lymphomas are well suited for radioimmunotherapy (RIT) because of the expression of a number of well-defined surface antigens, the availability of antibodies that target many of these antigens, and the high radiosensitivity of lymphoma cells, even when resistant to chemotherapy. Furthermore, radiolabeled mAbs are not reliant on host immune effector systems, which are generally defective in NHL patients. Several trials have documented response rates of 60% to 95% using either a murine anti-CD20 antibody combined to iodine I-131 (tositumomab) or a conjugate between the anti-CD20 antibody 2B8 (ibritumomab) and the isotope 90Y using the tiuxetan chelate.2-7 However, most patients receiving conventional nonmyeloablative doses of these radioimmunoconjugates subsequently have relapses; median progression-free survival is usually less than 1 year.5 Further dose escalation is not feasible with conventional RIT without stem cell transplantation because of dose-limiting myelosuppression.2,3 Our group has used myeloablative doses of RIT with stem cell support to achieve 85% to 90% objective response rates with 75% to 80% complete remissions.2,3,8,9 Although these higher doses of RIT appear to be more efficacious, approximately half the patients treated with this approach still have relapses within 5 years.8 In addition, the tumor-to–normal organ ratios of absorbed radiation are usually low (1.5-10 times those of critical normal organs), resulting in relatively high nonspecific exposure of normal organs to radioactivity. Because non-Hodgkin lymphomas have a relatively steep dose-response curve with no apparent radiation threshold for cell injury,10 11 this therapy would be even more effective and less toxic if tumor-to–normal organ ratios could be improved.
One obstacle limiting the efficacy of RIT is the protracted circulating half-life of conventional radiolabeled antibodies. A pretargeting strategy using the high-affinity streptavidin (SA)–biotin system has been tested to improve cure rates and to diminish RIT-related toxicity. This pretargeting approach has been demonstrated to improve the ratios of radiation delivered to tumors compared with the doses delivered to normal organs in preclinical and clinical models.12-24Recent work using human carcinoma xenografts demonstrated that a single treatment of pretargeted90Y-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)– biotin resulted in rapid effective blood clearance of non–tumor-bound antibody, improved tumor-to–normal organ ratios of absorbed radioactivity, and superior objective and durable remissions with minimal toxicity.25
Synthetic clearing agents have been introduced as an additional refinement to pretargeting protocols to more efficiently deplete immunoconjugates lingering in the bloodstream before administration of the radioactive moiety.26,27 In a recent human trial, the use of a clearing agent in combination with the pretargeting system demonstrated removal of more than 95% of excess circulating antibodies, tumor-to-blood ratios of 65:1, visualization of all tumors, and minimal toxicity.22 Recent work from our group24 demonstrated reduced toxicity and markedly enhanced efficacy using pretargeted anti-CD20 antibody compared with the directly labeled anti-CD20 antibody in a mouse xenograft study.
Although the CD20 antigen has proved to be a promising target for RIT, one barrier limiting the efficacy of anti-CD20–directed RIT is the potential dissociation of labeled antibody from the cell surface. Earlier in vitro studies suggest that anti-CD45 antibodies may be more stably retained on the surfaces of human NHL cells than anti-CD20 antibodies and, therefore, may be a superior antigen for pretargeted RIT.28 CD45 is a tyrosine phosphatase expressed on virtually all cells of hematopoietic origin except erythrocytes and platelets.29 Most hematologic malignancies, including 85% to 90% of acute lymphoid and myeloid leukemias, also express CD45, but CD45 is not found on tissues of nonhematopoietic origin.30In general, CD20 and CD45 are expressed at a density of approximately 200 000 to 300 000 sites per cell on circulating leukocytes and malignant B cells, such as Burkitt lymphoma cells.31,32Encouraging results have recently been achieved using RIT targeting the CD45 antigen with an I-131–anti-CD45 antibody combined with chemotherapy, with or without total body irradiation (TBI), in a bone marrow transplantation (BMT) regimen for acute leukemia.33 34 These studies demonstrated that RIT using131I–anti-CD45 is safe and can deliver substantially greater doses of radiation to hematopoietic tissues than to critical normal organs such as the liver, lung, or kidney. To assess the relative merits of CD45 and CD20 pretargeting, we have performed comparative biodistribution and RIT experiments using human B-cell lymphoma xenografts implanted in athymic mice. Directly labeled and pretargeted mAbs were used to examine the biodistribution, toxicity, and therapeutic efficacy using an anti-CD45 mAb compared with an anti-CD20 antibody. In these studies we compared anti-CD45 immunoconjugate with anti-CD20 reagent and demonstrated significantly superior biodistribution and enhanced therapeutic efficacy with minimal toxicity using the anti-CD45 immunoconjugate.
Materials and methods
Cell lines
The human Ramos B-lymphoma cell line was obtained from American Type Culture Collection (Bethesda, MD). Cells were maintained in log-phase growth in RPMI 1640 medium (BioWhittaker, Walkersville, MD) supplemented with 10% heat-inactivated fetal calf serum, 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL fungizone, and 1 mM pyruvate at 37°C in a 5% carbon dioxide incubator. The viability of the cells used in the experiments described was greater than 95% by trypan blue exclusion.
Antibodies
Hybridoma cells secreting the murine immunoglobulin G 2a (IgG2a) mAb 1F5, which recognizes the human CD20 antigen, was a gift from Clay Siegall (Seattle Genetics, Seattle, WA). The murine IgG1 mAb BC8, which recognizes the CD45 antigen of all human isoforms, was a gift from Claudio Anasetti (Fred Hutchinson Cancer Research Center, Seattle, WA). Isotype-matched murine G3G6 mAb, an antiplatelet antibody that does not bind to Ramos cells, was used to block nonspecific binding of the IgG2a antibodies to Fc-receptors in the spleen, marrow, and liver for studies of biodistribution and therapy, as described previously.24Hybridoma culture supernatants from the 1F5, BC8, and G3G6 mAbs were produced using a hollow fiber bioreactor system in the Fred Hutchinson Cancer Research Center Monoclonal Antibody Production Facility (Seattle, WA). Murine IgG2a mAb NR-LU-10, which recognizes a widely expressed 40-kD epithelial antigen (Ep-CAM) on carcinoma cells, but not on lymphoma cells,35 served as a negative control antibody and was a gift from NeoRx Corporation (Seattle, WA).
Preparation and purification of mAb-streptavidin conjugates
Conjugates were prepared according to the method of Hylarides et al.36 Antibody thiols were generated with 20 mM dithiothreitol (DTT), followed by G25 chromatography. Maleimido–streptavidin was prepared by treatment at pH 8 with 3 M equivalents of succinimidyl 4-(N-malemidomethyl) cyclohexane-1-carboxylate (SMCC), also followed by G25 chromatography. Thiol levels on BC8 were determined by 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) analysis and maleimide levels on SA were determined by cysteine back-titration as described previously. The conjugation reaction between equimolar quantities of reduced BC8 and maleimido–streptavidin was allowed to proceed 21 minutes before the addition of sodium tetrathionate to quench remaining thiols.
Conjugate purification required 3 steps. Unconjugated BC8 was separated from the product by affinity chromatography on an iminobiotin affinity column using 50 mM glycine/500 mM NaCl, pH 9, as the loading/washing buffer and 200 mM NaOAc, pH 4, as the eluting buffer. Unconjugated SA was then separated from the product by affinity chromatography using a Protein G column (HiTrap; Pharmacia). The pH of the iminobiotin column eluate was raised to 6.5, and the material was applied to the Protein G column, which was then eluted with 500 mM HOAc, pH 3, in tubes containing 500 mM glycine, pH 9. The desired conjugate was separated from the high-molecular–weight byproduct by anion exchange chromatography using a Fractogel EMD TMAE column (EM Separations Technology, Gibbstown, NJ). The Protein G column eluate was repeatedly diluted and concentrated to exchange the buffer to 20 mM sodium phosphate, pH 6.5. The product was eluted with 20 mM sodium phosphate/60 mM NaCl, pH 6.5. BC8 and conjugates prepared from it showed a retarded elution from TosoHaas size exclusion chromatography columns; hence, analysis of this antibody and its conjugates was performed with 15% dimethyl sulfoxide (DMSO) in the elution buffer.
125I/131I labeling of mAbs
BC8 and 1F5 mAbs were iodinated with Na125I or Na131I (NEN Life Science Products, Boston, MA) by the chloramine T method, as previously published.37
Biotinylated clearing agent
A synthetic biotinylated clearing agent (CA), previously described and supplied by NeoRx Corporation,26,27containing 16 N-acetyl-galactosamine residues per dendrimeric molecule, was used to eliminate excess mAb-SA molecules from the circulation before the administration of radiolabeled biotin. The N-acetyl galactosamine residues have a high affinity for hepatic asialoglycoprotein receptors and thus facilitate the rapid hepatic clearance of residual mAb-SA conjugates from the bloodstream and their endocytosis into liver cells.38
111In and 90Y-DOTA-biotin radiolabeling
The bifunctional ligand DOTA-biotin was synthesized as described.39 Twenty microliters to 100 μL carrier-free indium In-111 in 0.04 M HCl or 90YCl3 in 0.05 M HCl was diluted with 2 M ammonium acetate, pH 5, to a total volume of 0.25 mL, and 1 mg DOTA-biotin was added. The solution was heated for 30 minutes at 80°C followed by the addition of 25 μL 100 mM DTPA to chelate any unbound radioisotope. Radiochemical purity was determined to be more than 99% by C18 reverse-phase gradient high-performance liquid chromatography (HPLC) (pump A, 5 mM aqueous DTPA; pump B, 50% acetonitrile in pump A) with flow-through gamma detection. Labeling efficiency was typically greater than 93%.
Mouse studies
Female BALB/c nude mice (Animal Technologics, Kent, WA), aged 6 to 8 weeks, were maintained under protocols approved by the Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee. Mice were injected with 1 × 107 Ramos cells subcutaneously in each flank to develop human lymphoma xenograft tumors. Mice with similar tumor sizes (approximately 100 mm3) were selected for experimentation. Mice were placed on a biotin-free diet (Harlan Teklad, Madison, WI) for 5 days before injection of mAb-SA conjugates and radiobiotin.
Biodistribution studies
For conventional and pretargeted biodistribution studies, mice were injected intraperitoneally with 1.4 nmol radioiodinated mAb (215 μg; 50 μCi [1.85 MBq]) or unlabeled mAb-SA (300 μg), respectively. In each experiment mice were also coinjected with 400 μg irrelevant IgG2a antibody (G3G6) to block nonspecific binding of the 1F5 and the NR-LU-10 antibodies to Fc-receptors.24Mice in pretargeted groups were subsequently administered 5.8 nmol CA (50 μg) intraperitoneally at 20 hours followed by intraperitoneal delivery of 1.2 nmol (1 μg) 111In- or90Y-DOTA biotin (50 μCi [1.85 MBq]) 2 hours after CA. Biodistribution studies were performed using groups of 5 mice killed at 24, 48, 72, 96, or 144 hours after injection of each radioiodinated mAb or unlabeled mAb-SA conjugate. At each time point, mice were bled from the retro-orbital venous plexus and killed, and tumors and normal organs (lungs, stomach, small intestine, colon, spleen, bone marrow, quadriceps muscle, kidneys, and liver) were excised. Organs and tumors were weighed and gamma counted for 131I, 125I,111In, or 90Y activity. The percentage injected dose of radioisotope per gram (% ID/g) tumor or organ was calculated (after correcting for radioactive decay using an aliquot of the injectate at each time point), as was the tumor-to–normal organ ratios of absorbed radioactivity. In double-label experiments, counts were corrected for 131I crossover into the 125I channel. Mean values and standard errors for each time point were plotted to generate biodistribution curves for each tissue. Control groups were injected with radiolabeled isotype-matched, nonbinding control mAb NR-LU-10 or NR-LU-10-SA conjugates.
Radioimmunotherapy studies
Comparisons of pretargeted anti-CD45 and anti-CD20 antibodies were conducted using groups of 10 tumor-bearing mice to assess differences in therapeutic efficacy and groups of 5 mice without tumor xenografts to assess toxicity. Mice were initially coinjected with 1.4 nmol (300 μg) unlabeled 1F5-SA, BC8-SA, or NR-LU-10-SA conjugate and 2 mg/mL (400 μg) G3G6 antibody, which was used to block nonspecific binding of the 1F5 and the NR-LU-10 antibodies to Fc-receptors. Twenty hours later, 5.8 nmol CA was administered; this was followed 2 hours later by the administration of 1.2 nmol 90Y-DOTA-biotin labeled with 200, 400, or 800 μCi (7.4, 14.8, 29.6 MBq, respectively)90Y. The total amount of antibodies delivered was the same for each animal in each experimental group, regardless of the radioactive dose of DOTA-biotin delivered. Mice were monitored every other day for general appearance, weight change, and tumor volume measurements. Mice were killed if tumors grew large enough to cause obvious discomfort or to impair ambulation. In the toxicity studies, blood was sampled weekly from the retro-orbital venous plexus of mice for analysis of leukocyte, hemoglobin, and platelet counts. In a separate toxicity experiment, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, and creatinine levels were also measured weekly. Averages and standard errors for hematology and chemistry data were collected and are reported for each group of mice studied.
Results
Comparative biodistributions of radioactivity after conventional 1-step RIT targeting CD20 and CD45 antigens
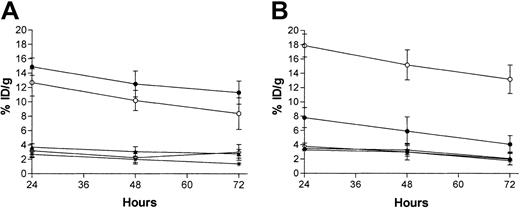
The biodistributions of radioiodinated BC8 (anti-CD45) and 1F5 (anti-CD20) antibodies were evaluated using conventional 1-step RIT in athymic mice bearing subcutaneous Ramos tumor grafts. Animals received simultaneous intraperitoneal injections of 1.4 nmol each of131I-1F5 and 125I-BC8. Mice were coinjected with a nonspecific IgG2a antibody, G3G6, to block the binding of IgG2a antibodies to Fc-receptors in the spleen and bone marrow as previously described.24 At 24, 48, and 72 hours after injection, groups of 5 mice were killed, and their tumors and organs were harvested, washed, weighed, and assayed for 131I and125I activity. Specific localization of antibody in tumors was documented in 3 separate experiments for 131I-1F5 and125I-BC8. At all time points, control animals injected with nonspecific 131I-NR-LU-10 antibody had negligible tumor uptake of radioiodine (0.63% ± 0.26% ID/g at 24 hours), demonstrating the specificity of targeting in these experiments. A representative study with 131I-1F5 and 125I-BC8 is shown in Figure 1. At 24 hours, 14.9% ± 1.4% injected dose of absorbed radionuclide per gram (% ID/g) was delivered to tumor using 125I-BC8 compared with 7.8% ± 1.2% ID/g delivered to tumor using 131I-1F5 (Figure 1). At 48 and 72 hours, the BC8-mediated uptake of absorbed radiation into tumors continued to exceed that observed using 1F5 antibody (Figure 1). Specific uptake of 125I-BC8 within tumors reached a maximum value of 14.9% ID/g tumor at 24 hours and was maintained at a high level (11.3% ID/g) at 72 hours after injection. In contrast, though the maximum uptake of 131I-1F5 into tumor was also seen at 24 hours (7.8% ID/g tumor), the 72-hour postinjection tumor uptake of this radioimmunoconjugate was only 4.1% ID/g tumor. The amount of absorbed radioactivity remained high in the blood, with 8.4% ID/g of blood using the BC8 antibody and 13.2% ID/g of blood using the 1F5 antibody at 72 hours (Figure 1). The tumor-to–normal organ ratios of radioactivity using the BC8 antibody ranged from 1.5:1 (blood) to almost 20:1 (small intestine) after 24 hours, and they were more favorable for all organs analyzed than the 1F5 antibody, which had tumor-to–normal organ ratios ranging from 0.4:1 (blood) to 9.8:1 (small intestine) (Table1).
Biodistributions of radioactivity in blood, tumor, kidney, liver, muscle, small intestine, colon, spleen, stomach, and lung.
Ramos xenograft-bearing athymic mice were injected with directly radiolabeled BC8 antibody (A) or 1F5 antibody (B). Mice were coinjected with 1.4 nmol conventional trace-labeled 125I-BC8 and 1.4 nmol 131I-1F5 antibodies. Groups of 5 mice were killed 24, 48, and 72 hours after injection of the radiolabeled antibodies and after the radioactivity in blood, tumor, kidney, liver, and lung was quantified by gamma counting and expressed as the percentage of the injected dose of radioactivity present per gram of tissue (% ID/g). Tumor (●), blood (○), kidney (▴), liver (*), lung (⋄).
Biodistributions of radioactivity in blood, tumor, kidney, liver, muscle, small intestine, colon, spleen, stomach, and lung.
Ramos xenograft-bearing athymic mice were injected with directly radiolabeled BC8 antibody (A) or 1F5 antibody (B). Mice were coinjected with 1.4 nmol conventional trace-labeled 125I-BC8 and 1.4 nmol 131I-1F5 antibodies. Groups of 5 mice were killed 24, 48, and 72 hours after injection of the radiolabeled antibodies and after the radioactivity in blood, tumor, kidney, liver, and lung was quantified by gamma counting and expressed as the percentage of the injected dose of radioactivity present per gram of tissue (% ID/g). Tumor (●), blood (○), kidney (▴), liver (*), lung (⋄).
Tumor-to–normal organ ratios using conventional RIT for131I-1F5 and 125I-BC8 at 24, 48, and 72 hours after the simultaneous injection of 131I-1F5 and125I-BC8 into athymic mice bearing Ramos tumors
| Organ . | . | Time, h . | ||
|---|---|---|---|---|
| 24 . | 48 . | 72 . | ||
| Blood | BC8 | 1.5 ± 0.5 | 1.3 ± 1.1 | 1.1 ± 0.7 |
| 1F5 | 0.44 ± 0.3 | 0.39 ± 0.3 | 0.31 ± 0.4 | |
| Kidney | BC8 | 3.4 ± 1.2 | 3.3 ± 1.6 | 3.6 ± 1.7 |
| 1F5 | 2.1 ± 0.8 | 1.6 ± 1.0 | 1.9 ± 1.3 | |
| Liver | BC8 | 4.7 ± 1.0 | 5.3 ± 1.3 | 6.5 ± 2.3 |
| 1F5 | 2.4 ± 0.6 | 2.0 ± 0.8 | 2.3 ± 1.2 | |
| Lung | BC8 | 4.0 ± 1.1 | 3.8 ± 1.4 | 3.5 ± 1.5 |
| 1F5 | 2.1 ± 0.5 | 2.0 ± 0.8 | 2.1 ± 1.1 | |
| Muscle | BC8 | 16.5 ± 1.7 | 14.3 ± 2.3 | 13.8 ± 0.9 |
| 1F5 | 8.7 ± 1.3 | 6.1 ± 2.0 | 4.4 ± 1.4 | |
| Small intestine | BC8 | 19.7 ± 2.6 | 16.9 ± 3.1 | 12.2 ± 2.2 |
| 1F5 | 9.8 ± 1.9 | 3.9 ± 1.3 | 5.2 ± 1.4 | |
| Colon | BC8 | 6.6 ± 2.3 | 4.2 ± 1.5 | 7.6 ± 1.9 |
| 1F5 | 2.0 ± 1.4 | 2.7 ± 1.4 | 4.6 ± 0.8 | |
| Spleen | BC8 | 3.5 ± 1.8 | 5.7 ± 2.4 | 5.5 ± 1.5 |
| 1F5 | 1.6 ± 0.7 | 1.7 ± 1.6 | 2.1 ± 0.5 | |
| Stomach | BC8 | 8.0 ± 2.2 | 6.6 ± 1.7 | 6.1 ± 1.4 |
| 1F5 | 3.6 ± 1.6 | 3.9 ± 1.4 | 2.4 ± 1.6 | |
| Marrow | BC8 | 4.0 ± 1.7 | 3.3 ± 2.0 | 5.2 ± 1.1 |
| 1F5 | 1.3 ± 0.6 | 1.1 ± 0.7 | 1.2 ± 0.3 | |
| Organ . | . | Time, h . | ||
|---|---|---|---|---|
| 24 . | 48 . | 72 . | ||
| Blood | BC8 | 1.5 ± 0.5 | 1.3 ± 1.1 | 1.1 ± 0.7 |
| 1F5 | 0.44 ± 0.3 | 0.39 ± 0.3 | 0.31 ± 0.4 | |
| Kidney | BC8 | 3.4 ± 1.2 | 3.3 ± 1.6 | 3.6 ± 1.7 |
| 1F5 | 2.1 ± 0.8 | 1.6 ± 1.0 | 1.9 ± 1.3 | |
| Liver | BC8 | 4.7 ± 1.0 | 5.3 ± 1.3 | 6.5 ± 2.3 |
| 1F5 | 2.4 ± 0.6 | 2.0 ± 0.8 | 2.3 ± 1.2 | |
| Lung | BC8 | 4.0 ± 1.1 | 3.8 ± 1.4 | 3.5 ± 1.5 |
| 1F5 | 2.1 ± 0.5 | 2.0 ± 0.8 | 2.1 ± 1.1 | |
| Muscle | BC8 | 16.5 ± 1.7 | 14.3 ± 2.3 | 13.8 ± 0.9 |
| 1F5 | 8.7 ± 1.3 | 6.1 ± 2.0 | 4.4 ± 1.4 | |
| Small intestine | BC8 | 19.7 ± 2.6 | 16.9 ± 3.1 | 12.2 ± 2.2 |
| 1F5 | 9.8 ± 1.9 | 3.9 ± 1.3 | 5.2 ± 1.4 | |
| Colon | BC8 | 6.6 ± 2.3 | 4.2 ± 1.5 | 7.6 ± 1.9 |
| 1F5 | 2.0 ± 1.4 | 2.7 ± 1.4 | 4.6 ± 0.8 | |
| Spleen | BC8 | 3.5 ± 1.8 | 5.7 ± 2.4 | 5.5 ± 1.5 |
| 1F5 | 1.6 ± 0.7 | 1.7 ± 1.6 | 2.1 ± 0.5 | |
| Stomach | BC8 | 8.0 ± 2.2 | 6.6 ± 1.7 | 6.1 ± 1.4 |
| 1F5 | 3.6 ± 1.6 | 3.9 ± 1.4 | 2.4 ± 1.6 | |
| Marrow | BC8 | 4.0 ± 1.7 | 3.3 ± 2.0 | 5.2 ± 1.1 |
| 1F5 | 1.3 ± 0.6 | 1.1 ± 0.7 | 1.2 ± 0.3 | |
Groups of 5 mice were used to generate mean values for each time point. Data were normalized for tissue weight and were corrected for radioactive decay.
Effects of synthetic biotinylated clearing agent on circulating anti-CD45 and anti-CD20 streptavidin conjugates
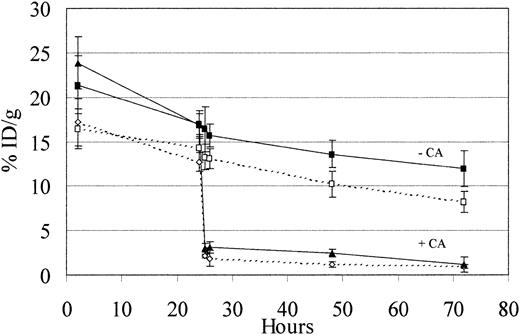
Despite the encouraging results using conventional RIT described above, the unbound radiolabeled BC8 and 1F5 antibodies were cleared from the circulation slowly. Consequently, comparative biodistribution experiments using the pretargeting RIT strategy first examined the effects on blood clearance of 131I-1F5-SA and131I-BC8-SA conjugates using a biotinylated polymeric, N-acetyl-galactosamine clearing agent (CA). In these experiments, athymic mice bearing subcutaneous Ramos tumor xenografts received 1.4 nmol 131I-1F5-SA or 131I-BC8-SA administered intraperitoneally, followed 24 hours later by 5.8 nmol CA, and blood was obtained from mice at 1, 2, 24, and 48 hours after CA injection. Figure 2 documents the rapid clearance of 80% to 90% of circulating 131I-BC8-SA and131I-1F5-SA immunoconjugates. Concentrations of131I-BC8-SA and 131I-1F5-SA in the blood decreased from 12.7% ± 1.1% and 16.8% ± 1.3% ID/g blood to 2.1% ± 0.2% and 3.0% ± 0.4% within 1 hour of CA administration, respectively (Figure 2). By 48 hours after CA injection, 1.2% ± 0.3% ID/g 131I-BC8-SA and 2.5% ± 0.4% ID/g 131I-1F5-SA was still circulating in blood. Three separate experiments replicated these results.
Effect of a biotinylated polymeric, N-acetyl-galactosamine–containing clearing agent on circulating BC8-SA and 1F5-SA conjugates.
Two groups of 10 BALB/c athymic mice were injected intraperitoneally with 1.4 nmol 131I-1F5-SA conjugate (closed symbols) or131I-BC8-SA conjugate (open symbols) at time 0. Five mice from each group were injected intraperitoneally 24 hours later with 5.8 nmol clearing agent (+CA; triangles), whereas the other 5 mice in each group did not receive clearing agent (−CA; squares). Serial blood samples were obtained from the retro-orbital venous plexus 25, 26, 48, and 72 hours after the injection of either mAb-SA conjugate and were analyzed by gamma counting.
Effect of a biotinylated polymeric, N-acetyl-galactosamine–containing clearing agent on circulating BC8-SA and 1F5-SA conjugates.
Two groups of 10 BALB/c athymic mice were injected intraperitoneally with 1.4 nmol 131I-1F5-SA conjugate (closed symbols) or131I-BC8-SA conjugate (open symbols) at time 0. Five mice from each group were injected intraperitoneally 24 hours later with 5.8 nmol clearing agent (+CA; triangles), whereas the other 5 mice in each group did not receive clearing agent (−CA; squares). Serial blood samples were obtained from the retro-orbital venous plexus 25, 26, 48, and 72 hours after the injection of either mAb-SA conjugate and were analyzed by gamma counting.
Biodistribution of radioactivity after pretargeted 2-step RIT comparing anti-CD45 and anti-CD20 antibodies
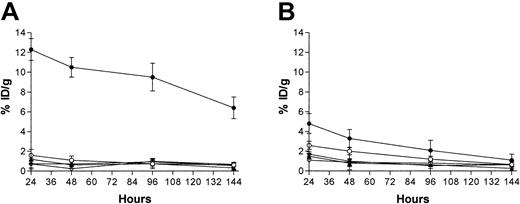
Biodistribution studies were performed using lymphoma-bearing mice injected with 1.4 nmol 1F5-SA or BC8-SA, followed 20 hours later by 5.8 nmol CA and 2 hours after that by 1.2 nmol111In-DOTA-biotin. These doses and time intervals were determined to be optimal in a previous study.24 Groups of 5 mice each were killed 24, 48, 96, and 144 hours after administration of the mAb-SA conjugate (Figure 3). Pretargeted BC8-SA resulted in biodistributions of radioactivity that were superior to those obtained with 1F5-SA at all time points assessed. The uptake of radioactivity in tumors of mice treated with pretargeted BC8-SA reached a maximum of 12.3% ± 1.1% ID/g 24 hours after the administration of 111In-DOTA-biotin and was still 6.4% ± 1.2% ID/g 144 hours later (Figure 3A). By contrast, mice that received pretargeted 1F5-SA conjugate reached a maximum tumor uptake of radioactivity of 4.8% ± 1.5% ID/g at 24 hours and the tumor content dropped to 1.1% ± 0.9% ID/g by 144 hours (Figure3B). At all time points assessed, the tumor-to–normal organ ratios were superior with the BC8-SA conjugate than were the ratios seen using the 1F5-SA conjugate (Table 2). At the 24-hour time point, the tumor-to-blood ratio exceeded 7:1 using pretargeted BC8-SA but was 1.8:1 at 24 hours with the 1F5-SA conjugate.
Biodistributions of radioactivity in blood, tumor, kidney, liver, and lung.
Ramos xenograft-bearing athymic mice were injected with either pretargeted BC8-SA conjugate (A) or pretargeted 1F5-SA conjugate (B). Mice were injected with 1.4 nmol unlabeled BC8-SA or 1.4 nmol unlabeled 1F5-SA, followed 20 hours later by 5.8 nmol clearing agent and 2 hours after that by 1.2 nmol 111In-DOTA-biotin. Groups of 5 mice were killed 24, 48, 96, and 144 hours after injection of each mAb-SA conjugate, and the radioactivity in blood, tumor, and normal organs were quantified by gamma counting and expressed as the percentage of the injected dose of radioactivity present per gram of tissue (% ID/g). Tumor (●), blood (○), kidney (▴), liver (*), lung (⋄).
Biodistributions of radioactivity in blood, tumor, kidney, liver, and lung.
Ramos xenograft-bearing athymic mice were injected with either pretargeted BC8-SA conjugate (A) or pretargeted 1F5-SA conjugate (B). Mice were injected with 1.4 nmol unlabeled BC8-SA or 1.4 nmol unlabeled 1F5-SA, followed 20 hours later by 5.8 nmol clearing agent and 2 hours after that by 1.2 nmol 111In-DOTA-biotin. Groups of 5 mice were killed 24, 48, 96, and 144 hours after injection of each mAb-SA conjugate, and the radioactivity in blood, tumor, and normal organs were quantified by gamma counting and expressed as the percentage of the injected dose of radioactivity present per gram of tissue (% ID/g). Tumor (●), blood (○), kidney (▴), liver (*), lung (⋄).
Tumor-to–normal organ ratios for pretargeted 1F5-SA (anti-CD20) and BC8-SA (anti-CD45) at 24, 48, 96, and 144 hours after the injection of 1.4 nmol 1F5-SA or BC8-SA
| Organ . | . | Time, h . | |||
|---|---|---|---|---|---|
| 24 . | 48 . | 96 . | 144 . | ||
| Blood | BC8 | 7.8 ± 1.4 | 9.6 ± 2.2 | 12.7 ± 2.8 | 10.3 ± 2.1 |
| 1F5 | 1.8 ± 0.8 | 1.7 ± 1.4 | 1.8 ± 0.5 | 1.7 ± 0.7 | |
| Kidney | BC8 | 9.9 ± 1.2 | 10.5 ± 2.5 | 12.7 ± 3.3 | 19.4 ± 2.6 |
| 1F5 | 3.2 ± 1.5 | 4.1 ± 2.0 | 3.2 ± 2.3 | 4.2 ± 1.4 | |
| Liver | BC8 | 16.4 ± 2.1 | 16.2 ± 3.0 | 10 ± 3.2 | 11.6 ± 2.1 |
| 1F5 | 2.8 ± 0.8 | 3.3 ± 1.6 | 3.8 ± 2.5 | 1.7 ± 0.5 | |
| Lung | BC8 | 16.8 ± 2.3 | 14.2 ± 2.8 | 9.5 ± 3.4 | 9.1 ± 2.1 |
| 1F5 | 4.4 ± 1.3 | 3.7 ± 1.9 | 2.6 ± 2.2 | 1.8 ± 0.7 | |
| Muscle | BC8 | 82 ± 4.6 | 42 ± 4.7 | 47.5 ± 4.9 | 64 ± 2.8 |
| 1F5 | 11.7 ± 1.8 | 6.6 ± 2.8 | 7.0 ± 3.5 | 5.5 ± 1.5 | |
| Small intestine | BC8 | 43.9 ± 3.3 | 31.8 ± 4.2 | 38 ± 4.4 | 17.8 ± 2.9 |
| 1F5 | 15 ± 2.5 | 16.5 ± 3.0 | 8.4 ± 3.6 | 4.6 ± 1.3 | |
| Colon | BC8 | 42.4 ± 4.8 | 19.4 ± 4.7 | 29.7 ± 3.2 | 20.6 ± 2.6 |
| 1F5 | 11.4 ± 2.0 | 8.3 ± 1.1 | 5.7 ± 2.1 | 4.4 ± 1.2 | |
| Spleen | BC8 | 34.2 ± 3.1 | 35 ± 4.5 | 38 ± 4.2 | 22.9 ± 3.1 |
| 1F5 | 11.2 ± 2.6 | 10 ± 2.4 | 7.8 ± 1.9 | 6.1 ± 2.0 | |
| Stomach | BC8 | 31.5 ± 2.4 | 15 ± 3.6 | 31.6 ± 3.2 | 24.6 ± 2.1 |
| 1F5 | 6.9 ± 2.6 | 5.5 ± 1.3 | 5.3 ± 2.0 | 3.5 ± 1.3 | |
| Marrow | BC8 | 14.3 ± 1.8 | 11.7 ± 2.5 | 13.2 ± 1.6 | 10 ± 1.1 |
| 1F5 | 4.5 ± 1.5 | 3.7 ± 1.4 | 3.0 ± 1.8 | 2.0 ± 0.6 | |
| Organ . | . | Time, h . | |||
|---|---|---|---|---|---|
| 24 . | 48 . | 96 . | 144 . | ||
| Blood | BC8 | 7.8 ± 1.4 | 9.6 ± 2.2 | 12.7 ± 2.8 | 10.3 ± 2.1 |
| 1F5 | 1.8 ± 0.8 | 1.7 ± 1.4 | 1.8 ± 0.5 | 1.7 ± 0.7 | |
| Kidney | BC8 | 9.9 ± 1.2 | 10.5 ± 2.5 | 12.7 ± 3.3 | 19.4 ± 2.6 |
| 1F5 | 3.2 ± 1.5 | 4.1 ± 2.0 | 3.2 ± 2.3 | 4.2 ± 1.4 | |
| Liver | BC8 | 16.4 ± 2.1 | 16.2 ± 3.0 | 10 ± 3.2 | 11.6 ± 2.1 |
| 1F5 | 2.8 ± 0.8 | 3.3 ± 1.6 | 3.8 ± 2.5 | 1.7 ± 0.5 | |
| Lung | BC8 | 16.8 ± 2.3 | 14.2 ± 2.8 | 9.5 ± 3.4 | 9.1 ± 2.1 |
| 1F5 | 4.4 ± 1.3 | 3.7 ± 1.9 | 2.6 ± 2.2 | 1.8 ± 0.7 | |
| Muscle | BC8 | 82 ± 4.6 | 42 ± 4.7 | 47.5 ± 4.9 | 64 ± 2.8 |
| 1F5 | 11.7 ± 1.8 | 6.6 ± 2.8 | 7.0 ± 3.5 | 5.5 ± 1.5 | |
| Small intestine | BC8 | 43.9 ± 3.3 | 31.8 ± 4.2 | 38 ± 4.4 | 17.8 ± 2.9 |
| 1F5 | 15 ± 2.5 | 16.5 ± 3.0 | 8.4 ± 3.6 | 4.6 ± 1.3 | |
| Colon | BC8 | 42.4 ± 4.8 | 19.4 ± 4.7 | 29.7 ± 3.2 | 20.6 ± 2.6 |
| 1F5 | 11.4 ± 2.0 | 8.3 ± 1.1 | 5.7 ± 2.1 | 4.4 ± 1.2 | |
| Spleen | BC8 | 34.2 ± 3.1 | 35 ± 4.5 | 38 ± 4.2 | 22.9 ± 3.1 |
| 1F5 | 11.2 ± 2.6 | 10 ± 2.4 | 7.8 ± 1.9 | 6.1 ± 2.0 | |
| Stomach | BC8 | 31.5 ± 2.4 | 15 ± 3.6 | 31.6 ± 3.2 | 24.6 ± 2.1 |
| 1F5 | 6.9 ± 2.6 | 5.5 ± 1.3 | 5.3 ± 2.0 | 3.5 ± 1.3 | |
| Marrow | BC8 | 14.3 ± 1.8 | 11.7 ± 2.5 | 13.2 ± 1.6 | 10 ± 1.1 |
| 1F5 | 4.5 ± 1.5 | 3.7 ± 1.4 | 3.0 ± 1.8 | 2.0 ± 0.6 | |
Clearing agent (5.8 nmol) was delivered at 19 hours after antibody-conjugate administration, followed 2 hours later by111In-biotin (50 μCi [1.85 MBq], 1.2 nmol). Groups of 5 mice/group were used to generate mean values. Data were normalized for tissue weight and corrected for radioactive decay.
It is important to recognize that the pretargeting approach generated biodistributions of radioactivity with each antibody superior to conventional radiolabeling (Tables 1, 2). Using the pretargeting approach with the BC8-SA conjugate, the tumor-to–normal organ ratios at 24 hours varied from 7.8:1 in blood to more than 80:1 in muscle (Table 2). At the same time point using conventional radiolabeled BC8, the tumor-to-blood ratio was 1.5:1 and the tumor-to-muscle ratio was less than 20:1 (Table 1). Similar results were seen in 2 other experiments. The specificity of tumor targeting by BC8-SA and 1F5-SA in these experiments was demonstrated by control groups treated with NR-LU-10-SA followed by CA and 111In-DOTA-biotin. These control mice did not exhibit any appreciable radiobiotin uptake into tumors at any time point (eg, 0.48% ± 0.21% ID/g at 24 hours; data not shown).
Pretargeted radioimmunotherapy with anti-CD20 versus anti-CD45 antibodies
In view of our results in the biodistribution experiments, we conducted radioimmunotherapy studies to determine whether the CD45 antigen would be superior to the CD20 antigen for pretargeted RIT. The therapeutic efficacy of pretargeted 90Y-DOTA-biotin was assessed following administration of either anti–CD20-SA or anti–CD45-SA conjugates in athymic mice bearing established Ramos lymphoma tumor xenografts. Treatment consisted of a single dose of pretargeted 90Y-DOTA-biotin delivered 10 to 14 days after the implantation of Ramos cells when palpable tumors (approximately 100 mm3) were present. Treatment groups of 10 animals each received 200, 400, or 800 μCi (7.4, 14.8, or 29.6 MBq, respectively)90Y-DOTA-biotin 2 hours after the administration of clearing agent and 22 hours after the administration of either BC8-SA or 1F5-SA conjugate or of control antibody conjugate NR-LU-10-SA. The results obtained are depicted in panels A to I of Figure4.
Regression of lymphoma xenografts after radioimmunotherapy comparing pretargeted anti-CD45 (BC8) and anti-CD20 1F5 antibodies.
Athymic BALB/c mice bearing Ramos lymphoma xenografts were injected intraperitoneally with 1.4 nmol BC8-SA (A-C) or 1F5-SA (D-F), followed 20 hours later by 5.8 nmol clearing agent and 2 hours after that with 800 (A, D), 400 (B, E), or 200 (C, F) μCi90Y-DOTA-biotin. Control mice bearing xenograft tumors were treated with 800 μCi (29.6 MBq) 90Y-DOTA-biotin alone (♦, G) or pretargeted NRLU-10-SA followed by 800 μCi (29.6 MBq)90Y-DOTA-biotin (×; H), or they were untreated (⋄, I). All mice treated with pretargeted BC8-SA are represented by closed triangles (▴), with the exception of 2 mice that had no tumor regression, (▵; C). Mice treated with pretargeted 1F5-SA are represented with open circles (○), except for 1 mouse that had no tumor regression (●, E). Mice that exhibited exponential tumor growth are depicted on a logarithmic scale (C, E-I). Each data point represents an individual animal. The numbers of mice that achieved complete xenograft regression (CR) are indicated relative to the number of mice in each treatment group.
Regression of lymphoma xenografts after radioimmunotherapy comparing pretargeted anti-CD45 (BC8) and anti-CD20 1F5 antibodies.
Athymic BALB/c mice bearing Ramos lymphoma xenografts were injected intraperitoneally with 1.4 nmol BC8-SA (A-C) or 1F5-SA (D-F), followed 20 hours later by 5.8 nmol clearing agent and 2 hours after that with 800 (A, D), 400 (B, E), or 200 (C, F) μCi90Y-DOTA-biotin. Control mice bearing xenograft tumors were treated with 800 μCi (29.6 MBq) 90Y-DOTA-biotin alone (♦, G) or pretargeted NRLU-10-SA followed by 800 μCi (29.6 MBq)90Y-DOTA-biotin (×; H), or they were untreated (⋄, I). All mice treated with pretargeted BC8-SA are represented by closed triangles (▴), with the exception of 2 mice that had no tumor regression, (▵; C). Mice treated with pretargeted 1F5-SA are represented with open circles (○), except for 1 mouse that had no tumor regression (●, E). Mice that exhibited exponential tumor growth are depicted on a logarithmic scale (C, E-I). Each data point represents an individual animal. The numbers of mice that achieved complete xenograft regression (CR) are indicated relative to the number of mice in each treatment group.
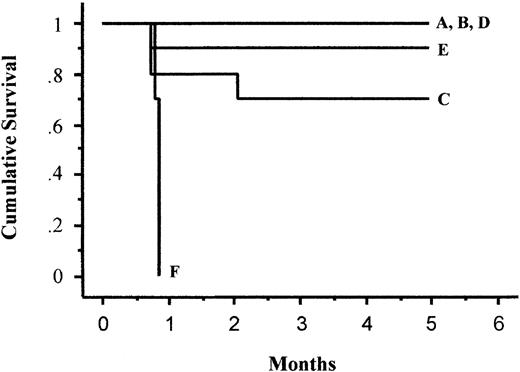
All control mice with untreated xenografts (Figure 4I), xenografts treated with 90Y-DOTA-biotin alone (Figure 4G), or control NR-LU-10-SA conjugate and 90Y-DOTA-biotin (Figure 4H) exhibited exponential growth of the lymphoma xenografts, necessitating humane killing by day 28. Animals treated with pretargeted 2-step RIT using anti–1F5-SA followed by 200 μCi (7.4 MBq)90Y-DOTA-biotin experienced similar exponential rates of tumor growth without any evidence of tumor regression (Figure 4F). Humane killing was required of all 10 mice in this group by day 26 after treatment (Figure 5). By comparison, 8 of 10 mice experienced complete tumor regression when treated with anti-BC8-SA conjugate followed by 200 μCi (7.4 MBq)90Y-DOTA-biotin (Figure 4C). Two mice in this group showed no response and tumors grew unabated, mandating humane killing on day 22. One of the 8 mice achieving complete remission with 200 μCi (7.4 MBq) 90Y-DOTA-biotin after anti-BC8-SA conjugate administration relapsed on day 46 and was killed on day 62 because of progressive tumor growth (Figure 5). Of the 7 remaining mice treated with anti-BC8-SA conjugate and 200 μCi (7.4 MBq)90Y-DOTA-biotin, xenograft tumors disappeared by day 24. These mice remained in complete remission for the duration of observation (more than 150 days following therapy) (Figure 4C).
Kaplan-Meier analysis of cumulative survival of mice bearing Ramos lymphoma xenografts treated with pretargeted radioimmunotherapy.
Groups of 10 mice bearing approximately 100 mm3 Ramos tumor xenografts were treated as described in the legend to Figure 4 and analyzed for survival as a function of time. Treatment groups included mice treated with (1) 1.4 nmol BC8-SA conjugate, followed 20 hours later by 5.8 nmol clearing agent, and 2 hours after that with 800 μCi (29.6 MBq) (A), 400 μCi (14.8 MBq) (B), or 200 μCi (7.4 MBq) (C)90Y-DOTA-biotin or (2) 1.4 nmol 1F5-SA conjugate, followed 20 hours later by 5.8 nmol clearing agent and 2 hours after that with 800 μCi (29.6 MBq) (D), 400 μCi (14.8 MBq) (E), and 200 μCi (7.4 MBq) (F) of 90Y-DOTA-biotin. The letters A-F in this figure correspond to treatment groups designated in panels A-F in Figure4.
Kaplan-Meier analysis of cumulative survival of mice bearing Ramos lymphoma xenografts treated with pretargeted radioimmunotherapy.
Groups of 10 mice bearing approximately 100 mm3 Ramos tumor xenografts were treated as described in the legend to Figure 4 and analyzed for survival as a function of time. Treatment groups included mice treated with (1) 1.4 nmol BC8-SA conjugate, followed 20 hours later by 5.8 nmol clearing agent, and 2 hours after that with 800 μCi (29.6 MBq) (A), 400 μCi (14.8 MBq) (B), or 200 μCi (7.4 MBq) (C)90Y-DOTA-biotin or (2) 1.4 nmol 1F5-SA conjugate, followed 20 hours later by 5.8 nmol clearing agent and 2 hours after that with 800 μCi (29.6 MBq) (D), 400 μCi (14.8 MBq) (E), and 200 μCi (7.4 MBq) (F) of 90Y-DOTA-biotin. The letters A-F in this figure correspond to treatment groups designated in panels A-F in Figure4.
Complete tumor responses were also seen in 9 mice receiving pretargeted 1F5-SA and 400 μCi (14.8 MBq) 90Y-DOTA-biotin, with a maximal response seen 26 days after treatment (Figure 4E). However, a xenograft rapidly grew in one mouse, leading to humane killing on day 24. In contrast, the 10 mice receiving pretargeted BC8-SA and 400 μCi (14.8 MBq) 90Y-DOTA-biotin achieved durable complete remission by day 16 (Figure 4B). All mice that received 800 μCi (29.6 MBq) 90Y-DOTA-biotin and either anti–BC8-SA or anti–1F5-SA conjugate experienced complete tumor regression, though regression of all tumors occurred 8 days sooner in mice that received anti–BC8-SA conjugate than in mice that received anti–1F5-SA conjugate (days 14, 22; Figure 4A, D, respectively). The mice in these groups were observed without any additional recurrences for 150 days after the beginning of the experiment and appear to be cured (Figure5).
Toxicity
Hematologic, hepatic, and renal toxicities were assessed in non–tumor-bearing mice treated with pretargeted RIT, to allow for sample collection at multiple time points without the confounding effects of humane killing. Groups of 5 mice received 200, 400, or 800 μCi (7.4, 14.8, and 29.6 MBq) 90Y-DOTA-biotin after injection of BC8-SA, 1F5-SA, or NR-LU-10-SA. Blood was obtained weekly from the retro-orbital venous plexus to determine leukocyte and platelet counts and hemoglobin values. Leukocyte counts reached nadirs 7 to 14 days after treatment with 800 μCi (29.6 MBq)90Y-DOTA-biotin in mice treated with the BC8-SA conjugate (48% ± 21% of initial leukocyte count) or the 1F5-SA conjugate (44% ± 22% of initial leukocyte count; Table3). In contrast, no90Y-DOTA-biotin dose-dependent hemoglobin nadir was observed using either mAb-SA conjugate. Mice that received 800 μCi (29.6 MBq) pretargeted 90Y-DOTA-biotin exhibited similar platelet nadirs 7 to 14 days after BC8-SA (52% ± 11% of the initial platelet count) or 1F5-SA (65% ± 12% of the initial platelet count). No effects on leukocyte, hemoglobin, or platelet counts were detected using lower doses of 90Y-DOTA-biotin (200 or 400 μCi [7.4 or 14.8 MBq]) after the BC8-SA or the 1F5-SA conjugate (Table 3).
Toxicity of pretargeted RIT in athymic mice
| . | Day . | Control . | 1F5-SA . | BC8-SA . | ||||
|---|---|---|---|---|---|---|---|---|
| — . | 200 μCi . | 400 μCi . | 800 μCi . | 200 μCi . | 400 μCi . | 800 μCi . | ||
| WBC (1000/μL) | 0 | 6.0 ± 0.9 | 5.3 ± 0.8 | 7.0 ± 0.4 | 6.5 ± 1.4 | 5.8 ± 0.3 | 6.2 ± 0.7 | 6.7 ± 1.4 |
| 7 | 5.9 ± 1.3 | 4.7 ± 1.1 | 5.2 ± 1.0 | 5.3 ± 1.3 | 2.9 ± 1.7 | 5.3 ± 0.8 | 4.8 ± 1.6 | |
| 14 | 4.5 ± 1.0 | 3.8 ± 0.5 | 3.4 ± 0.9 | 4.7 ± 2.6 | 5.4 ± 1.8 | 6.7 ± 0.8 | 4.5 ± 1.0 | |
| 21 | 7.2 ± 2.2 | 5.6 ± 2.3 | 5.3 ± 1.0 | 5.9 ± 1.9 | 7.0 ± 1.2 | 8.5 ± 2.0 | 5.6 ± 0.6 | |
| Hgb (g/dL) | 0 | 14.1 ± 1.2 | 14.0 ± 1.9 | 13.6 ± 2.2 | 13.7 ± 2.4 | 13.2 ± 2.0 | 14.3 ± 1.1 | 13.2 ± 2.0 |
| 7 | 13.8 ± 1.3 | 13.8 ± 0.7 | 12.4 ± 1.6 | 12.2 ± 1.4 | 14.1 ± 0.9 | 14.4 ± 0.6 | 12.2 ± 2.1 | |
| 14 | 13.4 ± 0.4 | 13.5 ± 1.8 | 13.2 ± 1.7 | 12.5 ± 2.1 | 13.1 ± 0.9 | 13.9 ± 0.7 | 14.8 ± 1.6 | |
| 21 | 13.3 ± 0.8 | 14.1 ± 0.9 | 13.8 ± 0.7 | 14.2 ± 0.6 | 13.7 ± 0.9 | 14.5 ± 0.4 | 14.0 ± 0.5 | |
| Platelets (1000/μL) | 0 | 1190 ± 149 | 1004 ± 61.5 | 1104 ± 135 | 1152 ± 188 | 1198 ± 199 | 1150 ± 201 | 1078 ± 49.7 |
| 7 | 1063 ± 136 | 961 ± 109 | 872 ± 121 | 775 ± 144 | 954 ± 172 | 990 ± 161 | 624 ± 142 | |
| 14 | 962 ± 56.1 | 845 ± 169 | 777 ± 192 | 718 ± 117 | 748 ± 192 | 970 ± 178 | 730 ± 175 | |
| 21 | 1038 ± 186 | 1076 ± 150 | 825 ± 186 | 995 ± 100 | 892 ± 145 | 1011 ± 171 | 1067 ± 158 | |
| AST (IU/L) | 0 | 147 ± 94.0 | 211 ± 95.1 | 197 ± 91.0 | 166 ± 92.9 | 164 ± 92.6 | 171 ± 98.1 | 188 ± 91.2 |
| 7 | 166 ± 79.5 | 255 ± 89.3 | 290 ± 100 | 316 ± 102 | 176 ± 88.0 | 220 ± 96.5 | 302 ± 79.8 | |
| 14 | 181 ± 29.9 | 248 ± 54.8 | 215 ± 97.7 | 322 ± 80.8 | 241 ± 96.0 | 192 ± 89.0 | 241 ± 94.6 | |
| 21 | 212 ± 62.2 | 248 ± 45.3 | 190 ± 67.2 | 355 ± 40.8 | 176 ± 47.1 | 171 ± 79.6 | 317 ± 88.0 | |
| ALT (IU/L) | 0 | 117 ± 84.2 | 155 ± 85.0 | 88.5 ± 47.8 | 115 ± 73.2 | 101 ± 58.0 | 79.6 ± 45.8 | 99 ± 62.0 |
| 7 | 83.0 ± 61.7 | 172 ± 72.3 | 104 ± 82.0 | 217 ± 71.4 | 148 ± 61.2 | 99.2 ± 61.1 | 191 ± 55.9 | |
| 14 | 71.8 ± 31.9 | 128 ± 57.5 | 150 ± 67.9 | 149 ± 77.0 | 93.0 ± 55.7 | 53.5 ± 26.5 | 63.5 ± 34.2 | |
| 21 | 67.5 ± 15.9 | 84.6 ± 31.7 | 121 ± 81.9 | 73.3 ± 34.8 | 72.5 ± 46.1 | 77.7 ± 50.4 | 60.6 ± 22.1 | |
| Creatinine (mg/dL) | 0 | 0.40 ± 0.1 | 0.30 ± 0.1 | 0.33 ± 0.2 | 0.33 ± 0.1 | 0.29 ± 0.1 | 0.33 ± 0.1 | 0.20 ± 0.1 |
| 7 | 0.22 ± 0.1 | 0.34 ± 0.1 | 0.21 ± 0.1 | 0.22 ± 0.1 | 0.22 ± 0.1 | 0.26 ± 0.1 | 0.24 ± 0.1 | |
| 14 | 0.25 ± 0.1 | 0.22 ± 0.1 | 0.20 ± 0.1 | 0.24 ± 0.1 | 0.24 ± 0.1 | 0.35 ± 0.1 | 0.35 ± 0.1 | |
| 21 | 0.30 ± 0.1 | 0.24 ± 0.1 | 0.27 ± 0.1 | 0.34 ± 0.1 | 0.32 ± 0.1 | 0.30 ± 0.1 | 0.41 ± 0.2 | |
| Weight (g) | 0 | 23.4 ± 1.8 | 23.0 ± 1.3 | 22.5 ± 1.9 | 22.9 ± 2.6 | 22.2 ± 1.7 | 23.4 ± 1.4 | 22.8 ± 1.8 |
| 7 | 25.0 ± 1.7 | 23.5 ± 1.4 | 23.5 ± 2.5 | 23.3 ± 2.5 | 22.6 ± 1.4 | 24.5 ± 1.3 | 22.9 ± 1.2 | |
| 14 | 24.9 ± 2.1 | 23.3 ± 1.5 | 23.3 ± 1.5 | 24.6 ± 2.4 | 23.1 ± 1.6 | 25.3 ± 0.9 | 23.7 ± 1.0 | |
| 21 | 24.8 ± 2.1 | 23.4 ± 2.1 | 23.4 ± 1.2 | 24.6 ± 2.2 | 23.7 ± 1.6 | 25.7 ± 1.6 | 23.8 ± 0.8 | |
| . | Day . | Control . | 1F5-SA . | BC8-SA . | ||||
|---|---|---|---|---|---|---|---|---|
| — . | 200 μCi . | 400 μCi . | 800 μCi . | 200 μCi . | 400 μCi . | 800 μCi . | ||
| WBC (1000/μL) | 0 | 6.0 ± 0.9 | 5.3 ± 0.8 | 7.0 ± 0.4 | 6.5 ± 1.4 | 5.8 ± 0.3 | 6.2 ± 0.7 | 6.7 ± 1.4 |
| 7 | 5.9 ± 1.3 | 4.7 ± 1.1 | 5.2 ± 1.0 | 5.3 ± 1.3 | 2.9 ± 1.7 | 5.3 ± 0.8 | 4.8 ± 1.6 | |
| 14 | 4.5 ± 1.0 | 3.8 ± 0.5 | 3.4 ± 0.9 | 4.7 ± 2.6 | 5.4 ± 1.8 | 6.7 ± 0.8 | 4.5 ± 1.0 | |
| 21 | 7.2 ± 2.2 | 5.6 ± 2.3 | 5.3 ± 1.0 | 5.9 ± 1.9 | 7.0 ± 1.2 | 8.5 ± 2.0 | 5.6 ± 0.6 | |
| Hgb (g/dL) | 0 | 14.1 ± 1.2 | 14.0 ± 1.9 | 13.6 ± 2.2 | 13.7 ± 2.4 | 13.2 ± 2.0 | 14.3 ± 1.1 | 13.2 ± 2.0 |
| 7 | 13.8 ± 1.3 | 13.8 ± 0.7 | 12.4 ± 1.6 | 12.2 ± 1.4 | 14.1 ± 0.9 | 14.4 ± 0.6 | 12.2 ± 2.1 | |
| 14 | 13.4 ± 0.4 | 13.5 ± 1.8 | 13.2 ± 1.7 | 12.5 ± 2.1 | 13.1 ± 0.9 | 13.9 ± 0.7 | 14.8 ± 1.6 | |
| 21 | 13.3 ± 0.8 | 14.1 ± 0.9 | 13.8 ± 0.7 | 14.2 ± 0.6 | 13.7 ± 0.9 | 14.5 ± 0.4 | 14.0 ± 0.5 | |
| Platelets (1000/μL) | 0 | 1190 ± 149 | 1004 ± 61.5 | 1104 ± 135 | 1152 ± 188 | 1198 ± 199 | 1150 ± 201 | 1078 ± 49.7 |
| 7 | 1063 ± 136 | 961 ± 109 | 872 ± 121 | 775 ± 144 | 954 ± 172 | 990 ± 161 | 624 ± 142 | |
| 14 | 962 ± 56.1 | 845 ± 169 | 777 ± 192 | 718 ± 117 | 748 ± 192 | 970 ± 178 | 730 ± 175 | |
| 21 | 1038 ± 186 | 1076 ± 150 | 825 ± 186 | 995 ± 100 | 892 ± 145 | 1011 ± 171 | 1067 ± 158 | |
| AST (IU/L) | 0 | 147 ± 94.0 | 211 ± 95.1 | 197 ± 91.0 | 166 ± 92.9 | 164 ± 92.6 | 171 ± 98.1 | 188 ± 91.2 |
| 7 | 166 ± 79.5 | 255 ± 89.3 | 290 ± 100 | 316 ± 102 | 176 ± 88.0 | 220 ± 96.5 | 302 ± 79.8 | |
| 14 | 181 ± 29.9 | 248 ± 54.8 | 215 ± 97.7 | 322 ± 80.8 | 241 ± 96.0 | 192 ± 89.0 | 241 ± 94.6 | |
| 21 | 212 ± 62.2 | 248 ± 45.3 | 190 ± 67.2 | 355 ± 40.8 | 176 ± 47.1 | 171 ± 79.6 | 317 ± 88.0 | |
| ALT (IU/L) | 0 | 117 ± 84.2 | 155 ± 85.0 | 88.5 ± 47.8 | 115 ± 73.2 | 101 ± 58.0 | 79.6 ± 45.8 | 99 ± 62.0 |
| 7 | 83.0 ± 61.7 | 172 ± 72.3 | 104 ± 82.0 | 217 ± 71.4 | 148 ± 61.2 | 99.2 ± 61.1 | 191 ± 55.9 | |
| 14 | 71.8 ± 31.9 | 128 ± 57.5 | 150 ± 67.9 | 149 ± 77.0 | 93.0 ± 55.7 | 53.5 ± 26.5 | 63.5 ± 34.2 | |
| 21 | 67.5 ± 15.9 | 84.6 ± 31.7 | 121 ± 81.9 | 73.3 ± 34.8 | 72.5 ± 46.1 | 77.7 ± 50.4 | 60.6 ± 22.1 | |
| Creatinine (mg/dL) | 0 | 0.40 ± 0.1 | 0.30 ± 0.1 | 0.33 ± 0.2 | 0.33 ± 0.1 | 0.29 ± 0.1 | 0.33 ± 0.1 | 0.20 ± 0.1 |
| 7 | 0.22 ± 0.1 | 0.34 ± 0.1 | 0.21 ± 0.1 | 0.22 ± 0.1 | 0.22 ± 0.1 | 0.26 ± 0.1 | 0.24 ± 0.1 | |
| 14 | 0.25 ± 0.1 | 0.22 ± 0.1 | 0.20 ± 0.1 | 0.24 ± 0.1 | 0.24 ± 0.1 | 0.35 ± 0.1 | 0.35 ± 0.1 | |
| 21 | 0.30 ± 0.1 | 0.24 ± 0.1 | 0.27 ± 0.1 | 0.34 ± 0.1 | 0.32 ± 0.1 | 0.30 ± 0.1 | 0.41 ± 0.2 | |
| Weight (g) | 0 | 23.4 ± 1.8 | 23.0 ± 1.3 | 22.5 ± 1.9 | 22.9 ± 2.6 | 22.2 ± 1.7 | 23.4 ± 1.4 | 22.8 ± 1.8 |
| 7 | 25.0 ± 1.7 | 23.5 ± 1.4 | 23.5 ± 2.5 | 23.3 ± 2.5 | 22.6 ± 1.4 | 24.5 ± 1.3 | 22.9 ± 1.2 | |
| 14 | 24.9 ± 2.1 | 23.3 ± 1.5 | 23.3 ± 1.5 | 24.6 ± 2.4 | 23.1 ± 1.6 | 25.3 ± 0.9 | 23.7 ± 1.0 | |
| 21 | 24.8 ± 2.1 | 23.4 ± 2.1 | 23.4 ± 1.2 | 24.6 ± 2.2 | 23.7 ± 1.6 | 25.7 ± 1.6 | 23.8 ± 0.8 | |
Hematologic, hepatic, and renal toxicities and weight as assessment of general health in untreated control mice or mice treated with 200, 400, or 800 μCi (7.4, 14.8, or 29.6 MBq) radiolabeled DOTA-biotin and pretargeted BC8-SA or IF5-SA conjugate.
Hepatic and renal toxicities were evaluated weekly by sampling blood for aspartate transaminase (AST), alanine transaminase (ALT), and creatinine levels. Slight increases in transaminase levels were detected in groups of mice treated with BC8-SA or 1F5-SA followed by 800 μCi (29.6 MBq) 90Y-DOTA-biotin (Table 3). One week after the injection of 800 μCi (29.6 MBq) of the radioisotope, AST and ALT levels were 61% and 92% above the initial levels, respectively, for the BC8-SA conjugate, and 90% and 89%, respectively, in mice treated with 1F5-SA. Transaminase levels were not significantly increased in mice treated with 200 μCi or 400 μCi (7.4 or 14.8 MBq) 90Y-DOTA-biotin and either BC8-SA or 1F5-SA. Table 3 demonstrates that creatinine levels were unaffected at all 90Y-DOTA-biotin dose levels up to 800 μCi (29.6 MBq) for all animals that received pretargeted BC8-SA or 1F5-SA conjugate. The weight of each mouse was measured on a weekly basis as a general assessment of overall animal health. Mouse weights were unaffected using the pretargeting strategy directed toward either the CD45 or the CD20 antigen at all doses tested up to 800 μCi (29.6 MBq)90Y-DOTA-biotin (Table 3).
Discussion
The efficacy of RIT depends on a number of variables, including properties of the targeted antigen (cell surface density, accessibility, shedding, and heterogeneity of expression) and the monoclonal antibody (binding-site specificity, immunoreactivity, in vivo stability, avidity, and affinity).40 Earlier in vitro studies examining various B-cell antigens on the surfaces of human lymphoma cells documented marked variation among several B-cell–specific monoclonal antibodies in their rates of internalization, degradation, and dissociation from the cell surface.28 Examination of the cell surface retention of these anti-lymphoma antibodies indicated that the 1F5 anti-CD20 antibody was minimally internalized but displayed a significant rate of passive dissociation from the surface of human lymphoma cells. In contrast, the BC8 anti-CD45 antibody was minimally internalized or shed from the cell surface, and, consequently, it exhibited a superior retention on the cell surface, compared with the 1F5 anti-CD20 antibody and other anti–B-cell antibodies tested. In this report, we compared conventional and pretargeted RIT and demonstrated superior delivery of radiation to tumors in vivo and improved tumor-to–normal organ ratios of absorbed radiation using an anti-CD45 antibody compared with an anti-CD20 antibody. Although the anti-CD20 antibody delivered significant doses of radiation to tumors, the anti-CD45 antibody consistently delivered 2- to 4-fold more radiation regardless of the method of RIT used. Specific uptake of directly radiolabeled anti-CD45 antibody by tumors reached a maximum value 24 hours after administration and was maintained at a high level 72 hours after injection. The maximum uptake of directly radiolabeled anti-CD20 antibody in tumors was also seen at 24 hours. However, after 72 hours, the amount of anti-CD20 radioimmunoconjugate in tumors decreased significantly. Moreover, the tumor-to–normal organ ratios of radioactivity obtained using the conventional radiolabeled anti-CD45 antibody were more favorable than the directly radiolabeled anti-CD20 antibody for all organs analyzed (Table 1).
Although the results of conventional RIT with the BC8 anti-CD45 antibody were encouraging, the tumor-to–normal organ ratios were still relatively low, and the high background levels of radiolabeled mAbs remaining in the blood were far from ideal. In the current study, we demonstrate that 90Y-DOTA-biotin delivered to lymphoma xenografts using anti-CD45 pretargeting resulted in improved tumor-to–normal organ ratios of absorbed radioactivity compared with conventional targeting using a directly labeled anti-CD45 antibody. Pretargeted RIT using the anti-CD45 antibody resulted in tumor-to-blood ratios greater than 7:1 after 24 hours and reached a maximum of almost 13:1 at 96 hours after injection of the unlabeled mAb conjugate (Table2). Tumor-to–normal organ ratios achieved using pretargeted anti-CD45 antibody were even more encouraging, ranging from 9:1 (lung) to more than 80:1 (muscle). Although the current studies confirm the promise of CD20 pretargeting,24 the tumor-to-blood and tumor-to–normal organ ratios seen using pretargeting with the anti-CD20-SA conjugate were inferior to those obtained with CD45 pretargeting. Pretargeted anti-CD20 antibody achieved tumor-to-blood ratios of 2:1 or less and maximal tumor-to–normal organ ratios of 15:1. The tumor-to–normal organ ratio data demonstrate that the anti-CD45 mAb is more stably retained at tumor sites in vivo, as predicted by our in vitro experiments.28
Tumor cell killing by RIT is directly related to the duration of exposure of cells to the radionuclide; therefore, rapid dissociation of the radioimmunoconjugate from target cells is undesirable. Prolonged retention of the anti-CD45 antibody may be particularly advantageous when using the pretargeted RIT approach by which radiolabeled biotin is delivered 24 to 48 hours after the pretargeted mAb-SA conjugate. The improved retention rates of the anti-CD45 conjugate were predicted to translate into improved response rates and survival rates. We performed comparative therapeutic pretargeting studies in mice treated with either anti-CD45-SA or anti-CD20-SA, followed by CA, and then increasing doses of 90Y-DOTA-biotin. The rates of tumor resolution were faster for mice treated with the anti-CD45-SA conjugate than for mice receiving the anti-CD20-SA conjugate and comparable doses of radio-biotin. For example, mice receiving 800 μCi (29.6 MBq)90Y-DOTA-biotin achieved complete remission 8 days earlier with anti-CD45 pretargeting than with anti-CD20 pretargeting. All tumors resolved by 14, 16, and 24 days in mice receiving anti-CD45-SA conjugate followed by 800, 400, or 200 μCi (29.6, 14.8, or 7.4 MBq)90Y-DOTA-biotin, respectively. In comparison, mice treated with the anti-CD20 conjugate and radio-biotin achieved complete remission 22 and 26 days after 800 and 400 μCi (29.6 and 14.8 MBq)90Y-DOTA-biotin, respectively. Treatment with anti-CD45-SA conjugate and 200 μCi (7.4 MBq) 90Y-DOTA-biotin cured 70% of mice, whereas no mice were cured after 200 μCi (7.4 MBq)90Y-DOTA-biotin pretargeting using the anti-CD20-SA conjugate. On the other hand, both the anti-CD20 and the anti-CD45 reagents cured at least 90% of mice treated with 400 or 800 μCi (14.8 or 29.6 MBq) 90Y-DOTA-biotin, indicating that both reagents merit clinical evaluation. Importantly, cures were reliably produced with pretargeted RIT at doses that caused minimal systemic, hematologic, renal, or hepatic toxicity.
Although pretargeted RIT appears promising using either anti-CD20 or anti-CD45 conjugates, we must acknowledge that there are limitations in the mouse xenograft model used in these experiments, and we stress that caution must be exercised in extrapolating mouse xenograft studies to humans. Considerable debate also exists regarding the selection of the optimal therapeutic radionuclide that should be used in mice. Yttrium-90 has a 5-mm path-length of β-emitting particles that is relatively long in relation to the sizes of mice and xenografts. This long path-length could lead to increased exposure of irradiation to normal tissues outside the xenograft and, thus, increase toxicity in the mouse model. It is possible, however, that the longer path-length of emitted β particles from 90Y-immunoconjugates may be preferred for humans with large tumors because a greater proportion of radioactivity may be deposited in tumors.24 25
Despite all the potential advantages of pretargeting, several disadvantages are evident. First, the pretargeting system is complex and requires multiple injections at precise intervals. Despite the complexity, however, a recent multi-institutional, nonmyeloablative, pretargeting phase 1 clinical trial using the anti-CD20-SA fusion protein has been performed demonstrating the safety and feasibility of the pretargeting approach.41 Second, the potential immunogenicity of SA may impede the ability to administer repetitive doses of Ab-SA conjugates. However, the limited production of human antimouse antibodies (HAMA) observed in lymphoma patients (10%-33%) suggests that this patient population will have a reduced antibody response because of the inherent immunosuppression associated with the disease.42 43 Third, endogenous biotin may bind SA and thus compete with 90Y-DOTA-biotin for binding to tumor-bound antibody-SA complexes. In our murine studies, we administered biotin-free diets before experimentation to eliminate significant amounts of endogenous biotin and to allow for successful targeting of radiolabeled biotin, but compliance may limit the efficacy of biotin-free diets in patients. Finally, the rapid renal clearance of90Y-DOTA-biotin and the renal uptake of SA, DOTA-biotin, or both may produce delayed radiation nephritis that may be dose-limiting.
One disadvantage of using anti-CD45 mAbs compared with anti-CD20 mAbs is that the CD45 antigen is expressed on most hematopoietic progenitor cells. Therefore, anti-CD45 RIT will probably always be associated with significant myelosuppression in clinical trials and may require stem cell rescue as part of the treatment approach. Although this is a significant disadvantage, even severe levels of myelosuppression would be acceptable if cure rates were markedly improved. Our group has shown that RIT using radiolabeled anti-CD45 antibody is feasible in patients with acute leukemia treated with 131I–anti-CD45 added to conventional bone marrow transplantation preparative regimens.33 34 These studies demonstrated that RIT using131I–anti-CD45 is safe and can deliver substantially greater doses of radiation to target tissues than liver, lung, or kidney without the use of preclearing doses of nonradioactive BC8 antibody.
In summary, the results of these mouse experiments demonstrate the promise of the pretargeting approach for treatment of hematologic malignancies expressing CD20 or CD45 antigens, and they suggest that antibodies that are stably retained on the cell surface (such as BC8) are more effective than those that are not. In comparison with pretargeting with the anti-CD20 antibody, anti-CD45 mAb pretargeting increases the tumor-to–normal organ concentrations of absorbed radioisotope, extends the retention time of radioisotope in tumors, and shortens the time to CR and improves CR rates and survival in tumor-bearing mice. Pretargeting with either antibody caused minimal toxicity to normal organs, including the bone marrow, in our model system, though it must be acknowledged that murine CD45 and CD20 do not bind the anti-human CD20 and anti-human CD45 antibodies used in these studies. Because the tumoricidal potential of RIT is directly proportional to the time antibody is bound on the cell surface, the superior cell surface retention seen with the anti-CD45 antibody suggests that the CD45 antigen may be a particularly advantageous therapeutic target allowing enhanced efficacy of RIT for lymphomas. Alternatively, the development of anti-CD20 antibodies with slower dissociation rates may allow improved targeting of B-cell malignancies and might produce less myelosuppression than targeting the CD45 antigen produces.
Prepublished online as Blood First Edition Paper, November 21, 2002; DOI 10.1182/blood-2002-03-0874.
Supported by National Institutes of Health grants RO1 CA76287 and T32 67-3166 and by the Penny E. Petersen Memorial Fund.
R.M., D.A., and L.J.T. are employees of the NeoRx Corporation, which has patented the clearing agent and streptavidin method used in these experiments.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John M. Pagel, Fred Hutchinson Cancer Research Center, D3-190, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: jpagel@fhcrc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal