Abstract
The increased use of hematopoietic progenitor cell (HPC) transplantation has implications and consequences for transfusion services: not only in hospitals where HPC transplantations are performed, but also in hospitals that do not perform HPC transplantations but manage patients before or after transplantation. Candidates for HPC transplantation have specific and specialized transfusion requirements before, during, and after transplantation that are necessary to avert the adverse consequences of alloimmunization to human leukocyte antigens, immunohematologic consequences of ABO-mismatched transplantations, or immunosuppression. Decisions concerning blood transfusions during any of these times may compromise the outcome of an otherwise successful transplantation. Years after an HPC transplantation, and even during clinical remission, recipients may continue to be immunosuppressed and may have critically important, special transfusion requirements. Without a thorough understanding of these special requirements, provision of compatible blood components may be delayed and often urgent transfusion needs prohibit appropriate consultation with the patient's transplantation specialist. To optimize the relevance of issues and communication between clinical hematologists, transplantation physicians, and transfusion medicine physicians, the data and opinions presented in this review are organized by sequence of patient presentation, namely, before, during, and after transplantation.
Introduction
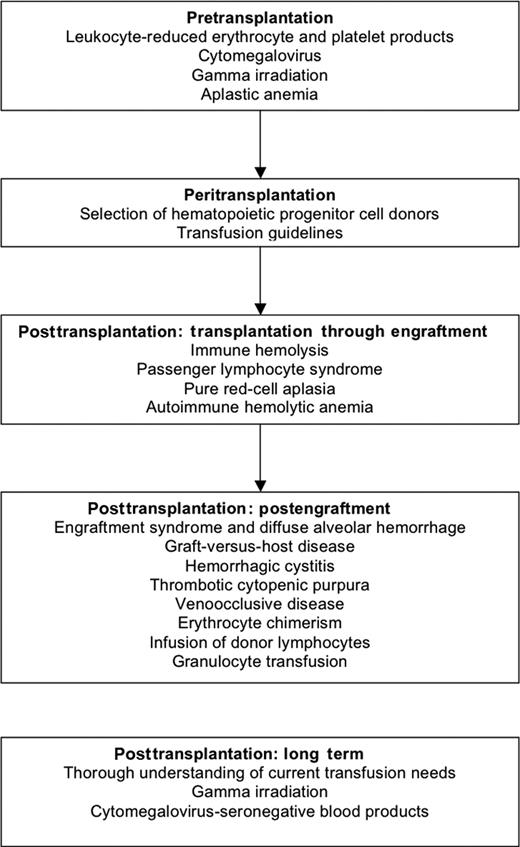
The improvements in clinical outcomes for hematopoietic stem cell or progenitor cell (HPC; both peripheral blood and bone marrow) transplantations have resulted in an increase in the number of procedures performed.1 HPC transplantations are used to treat hematologic malignancies, solid tumors, aplastic anemia/marrow-failure states, and a continuously expanding list of autoimmune and inherited metabolic and immunodeficiency diseases. The increase in the number of HPC transplantations performed has impacted transfusion services, not only in hospitals where transplantations are performed, but also in hospitals that do not perform transplantations but may become involved in managing patients before or after the procedure. Outcomes of the HPC transplantation may be compromised with transfusion because of (1) the alloimmunization from human leukocyte antigens (HLAs) with transfused products, (2) the immunohematologic consequences of ABO-mismatched transplantations,2,3 and (3) the impact of immunosuppression associated with the HPC procedure.4,5 The inability to measure continuously the degree of immunologic competence of HPC recipients creates a need for specialty blood products, which may not be available. When blood transfusion is urgent, the patient may require blood products locally, before a consult with the patient's transplantation specialist is possible. Because transplantation trials have focused on the efficacy of the procedure or treatment of its principal toxicities, few controlled trials of transfusion practice exist in this population. Transfusion practices are often derived from anecdotal reports of toxicities in this or similar populations, as well as from common practical sense based on presumed pathophysiology. Each institution that performs HPC transplantation typically experiences an experiential learning curve, which affects appropriate transfusion practice. In many comprehensive reviews of this topic, recommendations are solely supported by anecdotal reports or personal experience. Here, the limitations of the supporting evidence will be noted, and the data and opinions presented in this review are organized by sequence of patient presentation, namely, before, during, and after transplantation (Figure 1).
Before transplantation
As soon as a patient is identified as a candidate for an HPC transplantation, the patient's physician should communicate this information to the blood transfusion service. HPC transplantation candidates may require special blood components, such as leukocyte-reduced cellular, cytomegalovirus (CMV)-seronegative, and/or γ-irradiated components.4,6-8 Although certain regions of the United States encourage family donation of blood products for transfusion support in any type of patient (possible HPC recipient or not), concerns about this practice in potential HPC recipients exist because of the fear of sensitizing the recipient to minor HLA antigens and increasing the risk of graft rejection. In addition, HPC transplantation patients have a propensity to require a large number of transfused blood products, as a result of pancytopenia and organ and tissue damage sustained during the procedure.9 Optimal use of resources may be compromised before transplantation because of the acuity of the patient's condition or the scarcity of resources. Practices initiated before transplantation will continue throughout the transplantation course and beyond.
Leukocyte-reduced erythrocyte and platelet components
Many transfusion services in the United States routinely provide leukocyte-reduced erythrocyte and platelet components to all transfusion recipients to decrease the occurrence of febrile, nonhemolytic transfusion reactions. Reducing the exposure to leukocytes may also benefit potential HPC transplantation candidates (and other selected immunosuppressed patients) by decreasing both the incidence of alloimmunization to HLA antigens and the risk of transfusion-transmitted CMV infections. Minimizing the risk of HLA alloimmunization may reduce the incidence of complement-fixing HLA antibodies, which cause a positive serum crossmatch before HPC transplantation.8 Patients who receive an HLA crossmatch–positive HPC transplantation probably have primary graft rejection with an HLA-mismatched related donor and possibly, with an unrelated HLA phenotypically matched donor.8 Some centers use donor-recipient HLA or lymphocytotoxic crossmatches to select the optimal unrelated donor because positive crossmatches are associated with increased risk of graft rejection in allogeneic HPC transplantations.6-8 Some leukocytes and/or membrane fragments can pass through standard leukocyte reduction filters and alloimmunize recipients. In the multi-institutional Trial to Reduce Alloimmunization to Platelets study,6 a significant number of patients (17%-20%) developed HLA antibodies, although all of their erythrocyte and platelet components had been leukocyte reduced by stringently controlled leukocyte filtration. Results from studies in transfused solid organ transplantation patients showed a comparable incidence of HLA antibodies before and after implementation of leukocyte reduction, showing the incomplete effect of current methods of leukocyte reduction for preventing HLA alloimmunization. Despite the lack of 100% effectiveness in preventing HLA alloimmunization, we still recommend transfusing only leukocyte-reduced erythrocyte and platelet components to pre-HPC transplantation patients because it is one of the few tools available to potentially reduce the risk of platelet transfusion refractoriness.
Cytomegalovirus infection
Transfusion
CMV-seropositive rates among blood donors in different cities across the United States range from 40% to 80%,12,13 but very few CMV-seropositive donors have active CMV infections. Because immunosuppressed patients probably acquire a life-threatening CMV infection from a CMV-infective blood component,10 the use of CMV-seronegative blood components for transfusion is desired. However, the supply of CMV-seronegative erythrocyte and platelet components in certain metropolitan areas is inadequate to ensure that CMV-seronegative components will be available when needed. Leukocyte reduction by filtration reduces the risk that latent CMV infections will be spread by CMV-infected leukocytes to susceptible CMV-seronegative recipients.14,15
The current American Association of Blood Banks' Technical Manual16 states that a blood component with less than 5 × 106 residual leukocytes may significantly reduce, if not prevent, transfusion-transmitted CMV in transplantation recipients; much of these data were obtained from neonatal and hematologic malignancy patients and anecdotally applied to HPC transplantation recipients. As a result, most transfusion services in the United States now prevent transfusion-transmitted CMV infections by supplying leukocyte-reduced erythrocyte and platelet components.
Donor components
Transplantation donors must be tested for CMV seropositivity before donor selection and for proper HPC-product labeling; however, the screening test for prior CMV exposure for both donors and recipients may produce false negatives and is not completely reliable.17
Seropositive recipients
Although second-strain CMV infections have been identified in CMV-seropositive patients who were transplanted with organs from CMV-seropositive donors, no direct evidence that CMV infections in CMV-seropositive HPC recipients are caused by transfusion exist. For CMV-seropositive recipients, the most common cause of CMV disease is reactivation of latent CMV from prior exposure; therefore, the use of seronegative blood products has little impact.
Optimal survival in CMV-seropositive HLA-genotypic matched sibling and autologous HPC transplantation patients has been achieved with preemptive antiviral (ganciclovir or foscarnet) therapy (whereby therapy is based on changes in CMV load as measured by CMV antigenemia or CMV semiquantitative polymerase chain reaction), with or without high-dose intravenous immunoglobulin.12,13,18,19 CMV-seropositive recipients of T lymphocyte–depleted or cord blood allografts, and/or transplantations from unrelated, HLA-mismatched, or sibling-matched (matched other than genotypic) donors experience the highest incidence of CMV despite optimal preventative or preemptive CMV therapy; however, these patients often have less optimal outcomes because of incomplete prevention of direct and indirect immunomodulatory effects of CMV and drug toxicity. Therefore, most centers routinely administer anti-CMV prophylactic therapy to HPC transplantation recipients in whom immunocompetence is delayed (haploidentical, T-lymphocyte–depleted, and cord blood transplantations). This approach contrasts with the “watch and wait” method often used as a preemptive strategy for genotypic HLA-matched transplantations. Use of growth factor–mobilized peripheral blood HPC transplantation has improved survival times for recipients of HLA genotypic-matched sibling transplantations for the first 6 months to 1 year after transplantation.1 Whereas many authors have concluded that the improvement is a result of reduced relapse rates, another explanation is that the more robust graft of a peripheral blood HPC transplantation may allow for more optimal dosing of marrow suppressive CMV therapy and thereby fewer CMV infections resulting in death.
Seronegative recipients
The incidence of CMV infections is significantly reduced in potential HPC transplantation recipients who are CMV-seronegative and who are exposed only to CMV-seronegative blood products (including a CMV-seronegative HPC graft). Avoiding antiviral CMV treatment for select HPC recipients prevents exposure to therapy, which can suppress hematopoiesis or be nephrotoxic, and may improve HPC transplantation survival time.10,19 Leukocyte reduction filters, although helpful, have not convincingly demonstrated efficacy as a substitute for CMV-seronegative blood products. Access to CMV-seronegative blood products is problematic in certain regions, and urgent transfusions should not be delayed until CMV-seronegative blood products are available. Although no formal recommendations exist for how long CMV-seronegative patients should use CMV-seronegative blood, a prudent course may be to continue use until immunosuppression is withdrawn.
Gamma irradiation
Donor lymphocytes may be detected in recipients' circulation for months to years after the transfusion of freshly collected erythrocyte or platelet components (microchimerism).20 The lymphocytes may engraft in a susceptible immunosuppressed host and cause transfusion-associated graft-versus-host disease (TA-GVHD).21 As a consequence of immunosuppressive treatment, both autologous and allogeneic HPC transplantation recipients are at increased risk for the development of TA-GVHD.22,23 Limited data suggest that patients with cancer, bone marrow–failure states, and other diagnoses, such as inborn errors of metabolism and hemoglobinopathies, who may be treated by HPC transplantation, are also at increased risk.21,22,24 Although TA-GVHD is uncommon, the mortality rate is 88%. Clinical symptoms of TA-GVHD occur between 4 and 30 days after transfusion and may include fever, maculopapular rash, bloody diarrhea, and/or pancytopenia.21
Although washing, freeze-thawing, or filtering cellular blood components decreases the concentration of residual leukocytes, current filtration methods are not effective for preventing TA-GVHD.25,26 Gamma irradiation of all transfused erythrocyte and platelet components is the only reliable method for preventing TA-GVHD. Gamma irradiation induces chemical crosslinks in the DNA of irradiated donor lymphocytes, preventing their proliferation. The recommended dose is 2500 cGy to the internal midplane of a freestanding irradiation instrument canister, with a minimum of 1500 cGy at any other point within the canister. Gamma irradiation for all cellular blood components is prudent in the pre-HPC transplantation period, and its use should be instituted as soon as a patient is identified to be a potential HPC transplantation candidate. Gamma irradiation of all cellular blood components is also critical and standard practice in the peritransplantation and posttransplantation periods.23,27,28 Currently, no data exist to justify lifetime use of irradiated blood products after transplantation, but most centers recommend that all blood products be irradiated because no test that delineates complete immunologic reconstitution is available.
Despite the lack of clinical trials or anecdotal reports, many HPC physicians are concerned about the theoretical risk of causing TA-GVHD by administering nonirradiated blood products to an HPC donor during the collection phase. This theoretical risk is based on the idea that lymphocytes from a nonirradiated blood product may be transmitted to the donor and then become part of the HPC product being collected, thereby eventually exposing the HPC recipient to completely mismatched blood donor lymphocytes at the time they have been conditioned to accept a transplant. For allogeneic HPC donors, all efforts should be made to avoid transfusion of nonautologous blood components into normal donors. Avoiding this theoretical risk is easy by simply irradiating any allogeneic blood products that may be transfused into the HPC donor. That is why this represents the current policy of the National Marrow Donor Program.29
Experimental evidence indicates that psoralen and other treatments to reduce the risk of transfusion-transmitted agents may also induce cross-linking or dimerization of DNA, thereby potentially preventing TA-GVHD.30,31 Presently, no therapy has been shown to be as reliable as gamma irradiation, and none is recommended for the purpose of preventing TA-GVHD.
Aplastic anemia
Patients with severe aplastic anemia typically present with severe symptomatic pancytopenia. Rarely do these patients respond to administration of hematopoietic growth factors and, if they do, the response is usually delayed. Given the severe thrombocytopenia and anemia at presentation, the natural inclination of the initial examining physician is to transfuse erythrocytes and platelets. However, data from the International Bone Marrow Transplant Registry and the Fred Hutchinson Cancer Center show that multitransfused aplastic anemia patients have increased rates of graft rejection, which adversely impacts survival rates.32,33 Therefore, caution should be used when considering transfusion for these patients. For those aplastic anemia patients presenting with active bleeding or symptomatic anemia, transfusion of erythrocytes and platelets is a correct management strategy. Whenever possible, however, management of a new patient with possible aplastic anemia should be directed toward minimizing the number of blood transfusions until the diagnosis is clarified. Platelet components should be apheresis (single-donor) units, if available, to minimize the number of donor exposures.
Because of the higher risk of graft failure with aplastic anemia patients, use of blood components from family members or other potential donors before transplantation is discouraged for fear of immunologically sensitizing the recipient to the potential donor's HLAs or to other leukocyte antigens. However, this practice has never been sufficiently evaluated and has yet to be adequately addressed. In addition, whether the increased use of leukocyte-reduced blood components reduces the incidence of graft rejection has not been evaluated in this population. The risk of graft rejection even in heavily transfused patients, however, has been reduced with the administration of antithymocyte globulin as part of the preparative regimen, thereby diminishing the concern with pretransplantation transfusions.33
Because HLA-identical sibling transplantations (or a syngeneic transplantation, if available) are curative 80% to 90% of the time, physicians should HLA-type patients quickly, as well as their respective siblings, who present with pancytopenia. Because syngeneic transplantations need no preparative regimen or posttransplantation immunosuppression, transplantation can occur quickly once results of HLA typing and monozygosity study are available.34,35
Splenectomy
Anecdotal experience (J.L.G., unpublished data, June 3, 2008) has suggested that engraftment of HPC transplantations may be delayed or even fail because of severe hypersplenism (eg, spleen located below pelvic rim on physical examination), presumably as a consequence of splenic sequestration of transplanted HPCs. Whether reduced-intensity preparative regimens shrink the spleen as did the historical total body radiation regimen is unclear (J.G., unpublished data, June 3, 2008). Therefore, patients with massively enlarged spleens before transplantation may need to be evaluated and treated for hypersplenism.
Randomized trials of splenectomy in HPC transplantation candidates have not been conducted. However, clinical evidence suggests that splenectomy before HPC transplantation shortens the time to engraftment and has no influence on posttransplantation complications, such as late infections, acute or chronic GVHD, or overall survival rates.36,37 Splenectomy has the potential to increase the risk of infection from encapsulated bacteria, including Pneumococcus pneumonia, Haemophilus influenza, and Neisseria meningitis. Therefore, prophylactic presplenectomy immunization with polyvalent pneumococcal, H influenza B, and meningococcal vaccines are recommended for HPC transplantation candidates, but the efficacy of immunization in this situation has not been evaluated, and adults typically respond poorly to pneumococcal vaccines. Another treatment of hypersplenism, focused splenic irradiation, is rarely performed because of concerns about compromising left kidney function with radiation scatter.
Many patients with agnogenic myeloid metaplasia who are candidates for splenectomy may have some component of extramedullary (splenic) hematopoiesis. In these patients, splenectomy may result in postsplenectomy pancytopenia because of decreased hematopoiesis in the bone marrow. Li et al38 recently evaluated the impact of pretransplantation splenectomy in patients with agnogenic myeloid metaplasia (splenectomy, n = 11; no splenectomy, n = 15) on overall transplantation outcomes; they found no advantage of pretransplantation splenectomy on posttransplantation outcomes, including overall and disease-free survival.
Peritransplantation
The peritransplantation period begins with administration of the immunosuppressive preparative regimen, includes the HPC graft infusion, and extends until engraftment occurs. During this period, all pretransplantation considerations pertaining to blood transfusions remain in effect. Although redundant, we suggest that the transplantation service inform the transfusion service about the HPC transplantation to ensure that all cellular blood components are gamma-irradiated, regardless of whether the physician orders irradiated blood products, to prevent the potentially fatal error of transfusing nonirradiated blood components.
Selection of HPC donors
Potential HPC donors, whether autologous or allogeneic, must be screened and qualified according to blood-donor requirements and tissue-donation practices. Exceptions to certain infectious disease or other transfusable risk may be permissible if it is an autologous HPC donor or if only one well-matched allogeneic donor exists.17 In all exceptional cases, the basis for accepting such an HPC donor must be documented in the medical records of both donor and recipient. From the outset, persons screening potential HPC candidate donors must make it clear to all parties that the results of screening questions and laboratory tests are confidential. When confronted with these complex issues, staff must be sensitive to the competing ethical and privacy needs of family donors, as well as to the potential for family discord. Pediatric donors with only one identifiable parent, who is the intended recipient, require exceptional scrutiny.17,39-41
Selecting the optimal HPC donor includes consideration of (erythrocyte) blood group serology, as well as (leukocyte) HLA matching, CMV status, donor-recipient size disparity, and donor health. Optimally, donor and recipient ABO/Rh(D) types will be serologically compatible, if not identical. However, ABO/Rh(D) nonmatches do not exclude a candidate HPC donor. The outcomes of ABO/Rh(D)-mismatched transplantations have been mixed, with some studies associating mismatching with delayed engraftment of erythrocyte or platelet precursors and an increased incidence of acute GVHD and veno-occlusive disease (VOD); however, ABO/RH(D)-mismatched transplantations have usually not been associated with graft failure.3 The results of 2 retrospective studies evaluating approximately 200 to 300 HPC transplantation recipients each showed a decrease in survival time with either major or minor ABO-mismatched grafts,3,42 but results of other studies have shown no deleterious impact of donor recipient ABO/Rh(D) mismatching on survival rates.43,44 One explanation for the discrepancies in these study results is the use of bone marrow as the principal HPC source and methotrexate as a sole agent or T-cell depletion for GVHD prophylaxis. Another explanation is that treatment-related toxicity is exacerbated when the platelet depletion of incompatible plasma is not performed.42,45-47 Changing transfusion practice to avoid transfusion of ABO-incompatible plasma to HPC transplantation recipients eliminates the effect of ABO donor-recipient incompatibility on survival rates. Transplantation of HPC grafts from donors whose erythrocytes are serologically incompatible with the recipient's ABO/Rh(D) types may be performed using specific transplantation regimens. The procedures for preparing the HPC graft are described in standard cell-processing literature.48
Graft type
To optimize transplantation success, the decision about the type of donor product to collect (bone marrow vs granulocyte colony-stimulating factor [G-CSF]–mobilized peripheral blood HPCs) is usually based on assessment of the recipient's status. Although G-CSF–mobilized peripheral blood HPCs engraft quicker, the risk of chronic GVHD is increased, and the extent of its antitumor effect is controversial.49,50 Donor medical needs may also determine the type of product selected; a donor with underlying cardiac or vascular disease may be safer under the controlled environment of anesthesia.51
Major ABO incompatibility
If a major ABO incompatibility (ie, donor's erythrocytes are incompatible with recipient's plasma) exists, efforts should be made to minimize erythrocyte content in the HPC graft, even at the expense of a slightly lower HPC content. Because the volume of erythrocytes in the bone marrow HPC product may be significant (equal to or greater than that of a unit of blood), significant hemolysis is possible. To substantially mitigate this risk, erythrocytes may be removed from the donor's bone marrow by Hetastarch separation, mononuclear cell concentration by machine, or chemically (through density gradient separation).48 To minimize risk of exposure to incompatible erythrocyte volume with the collected product when there is major ABO incompatibility between donor and recipients, hematocrit should be kept to less than 2% during apheresis collection. If no major ABO incompatibility exists, the apheresis collection can maximize HPC yield and allow the hematocrit on apheresis product to be higher. However, apheresis machines rarely allow a hematocrit during collection to run higher than 4%.
Minor ABO incompatibility
If a minor ABO incompatibility (donor's plasma is incompatible with recipient's erythrocytes) exists, plasma may require reduction in the bone marrow product. The volume of plasma that is removed depends on the titer of the offending antibody(s) and the ratio of plasma-to-recipient erythrocyte volume. To minimize risk, many centers will plasma-deplete all minor erythrocyte-incompatible marrow products. HPC products collected by apheresis are already plasma- as well as erythrocyte-depleted.
An extended blood group phenotype of the donor's erythrocytes may be useful for assessing engraftment if the donor and recipient are ABO/Rh(D) identical. For ABO major-minor donor-recipient (bidirectional) mismatches, the bone marrow–derived HPC product must be depleted of erythrocytes and plasma, either through machine mononuclear cell concentration or by density gradient separation.
Occasionally, acute severe infusional toxicity, identical to a severe transfusion reaction, occurs with the infusion of a minimal amount of donor product. Such infusions should be immediately stopped, and donor and recipient identity, and erythrocyte phenotyping, and antibody screens immediately reviewed. Although infusional toxicity is caused by human error, donors and recipients occasionally have reactions to each other that could not have been predicted by antibody screen. Such cases require an immediate-density gradient, mononuclear cell preparation to ensure minimal plasma or erythrocyte contamination with the product.
Apheresis collection
The cryoprotectant dimethyl sulfoxide (DMSO) has been associated with infusion toxicity, as has the composition of the collected graft. Most DMSO toxicities (nausea, flushing, bradycardia, hypoxia) are mild and may be ameliorated by slowing the infusion rate. In addition, the use of ex vivo, T cell–depleted products or CD34+ selected products usually have a reduced volume and hence a lower amount of DMSO, thereby reducing the risk of DMSO infusion toxicities. With peripheral blood HPC products, the source of software, which monitors collection efficiency, may increase the risk of donor toxicity, in addition to the potential for erythrocyte and plasma contamination.
Sickle cell
Although patients with sickle cell disease are not commonly used as donors (only in rare, life-threatening situations), anecdotal reports of adverse events occurring during apheresis collections exist. Theoretically, the high leukocyte counts that follow G-CSF mobilization could mimic conditions associated with a low oxygen tension state and subsequently cause sickling.54 For that reason, the National Marrow Donor Program has decided to exclude donors with the sickle cell trait and sickle cell disease from apheresis collection and will only offer those donors the option of a conventional bone marrow harvest.29 However, this policy is in contrast to the results of 2 studies, a case report series of 5 patients with sickle cell disease55 and a small randomized study of patients with the sickle cell trait,56 which showed that G-CSF mobilization and collection of HPCs from these patients were feasible and safe. Therefore, clinicians should consider the risk and benefits of how best to harvest a donor who has either sickle cell trait or sickle cell disease.
Transfusion guidelines
Transfusion services in hospitals where HPC transplantations are performed require specific guidelines to address transfusion support during the peritransplantation period. Guidelines for transfusing pancytopenic patients with erythrocytes, platelets, or plasma are well established and are usually extended by inference to HPC patients because data on this population are lacking.57-61 Because hematopoiesis is expected to be decreased during this phase, replacement transfusion may be intensive, especially if active bleeding occurs.9,62
Platelet transfusion
Platelet counts of less than 10 000/μL have been associated with an increase in mortality.60 Therefore, conventional thresholds or “triggers” (eg, platelet count of 10 000/μL) for initiating platelet transfusions in HPC transplantation recipients57,58 are used by most centers. Bleeding in HPC patients is probably multifactorial, reflecting the active complications of HPC transplantation, such as mucositis, hemorrhagic cystitis, GVHD, VOD, and diffuse alveolar hemorrhage (DAH).58,60,63 Therefore, considering the patient's clinical situation as the “trigger” to initiating a platelet transfusion is recommended.
Response to platelet transfusions in the posttransplantation setting is often lower than that of chemotherapy-induced pancytopenia in the acute leukemia setting. Sometimes, simply increasing the platelet dose administered will improve posttransfusion platelet counts.60 However, HLA-matched or crossmatched apheresis platelets may be required for satisfactory responses. To accurately document the need for an HLA antibody workup and for more specialized platelet matching, a 30-minute postplatelet transfusion count is usually necessary.
Erythrocyte transfusion
Historically, HPC recipients were among the highest consumers of erythrocyte transfusions because of the high risk of hemorrhage associated with pancytopenia. However, this situation may have been ameliorated recently because of the increased use of peripheral HPC or possibly because of the increased use of white blood cell growth factors resulting from the earlier recovery of neutrophils associated with these practices,9 which may limit gastrointestinal bleeding as a result of earlier healing of mucositis. When severe hemorrhage does occur, few alternatives exist. Although results from studies supporting the administration of either recombinant factor VIIa or aminocaproic acid to quickly achieve hemostasis during severe hemorrhage64,65 are mostly anecdotal, both agents risk being too thrombogenic, particularly if access to transfused platelets is problematic. Optimally, agents with both risk and benefit would be evaluated in randomized trials, but given the rarity and suddenness of hemorrhagic events, such trials are difficult to implement.
Triggers for transfusing erythrocytes follow conventional guidelines: a hemoglobin of 8 g/dL for otherwise healthy persons, and higher for patients with coronary artery disease. HPC transplantations are increasingly performed in older patients with comorbid diseases, which may necessitate transfusion thresholds different from those for a patient with no comorbidities. Patients with ischemic heart disease may need a transfusion threshold higher than 8 g/dL as the neutropenic infections that often accompany transplantation may cause vasodilation and that, coupled with a low hemoglobin count, may trigger acute ischemia. Transfusion services may also want to track comorbid diseases for product-use justification.
ABO/Rh(D) matching
Because allogeneic HPC transplantations do not require blood group matching between donor and recipients, unique serologic challenges exist for transfusion services.42,46,62 Transfusion services should establish specific guidelines for transfusions that are ABO/Rh(D) compatible with both the donor and the recipient (Table 1). Because transfused erythrocytes may circulate for days to weeks, special consideration for selecting the ABO/Rh(D)-compatible blood components should be instituted as early as possible. Many institutions begin transfusing group O erythrocytes as soon as the blood bank is informed that a non–ABO-matched HPC transplantation is being considered. For ABO-identical transplantations, ABO-identical or ABO-compatible erythrocytes and plasma are appropriate. Criteria for platelet transfusion follow criteria for plasma transfusions. When units of rare erythrocytes and other blood products are required, transfusion services and clinicians must work together to prioritize the competing needs of various patients.61
Guidelines for selecting ABO blood group for erythrocyte- and plasma-containing components for patients undergoing HPC transplantation with ablative conditioning*
| Recipient RBC group . | Donor's RBC group . | Category of ABO mismatch† . | Erythrocyte transfusion . | Platelet or plasma transfusion . |
|---|---|---|---|---|
| A | O | Minor | O | A, AB |
| B | O | Minor | O | B, AB |
| AB | O | Minor | O | AB |
| AB | A | Minor | A, O | AB |
| AB | B | Minor | B, O | AB |
| O | A | Major | O | A, AB |
| O | B | Major | O | B, AB |
| O | AB | Major | O | AB |
| A | AB | Major | A, O | AB |
| B | AB | Major | B, O | AB |
| A | B | Minor and major | O | AB |
| B | A | Minor and major | O | AB |
| A | A | None | A, O | A, AB |
| B | B | None | B, O | B, AB |
| AB | AB | None | AB, A, B, O | AB |
| O | O | None | O | O, A, B, AB |
| Recipient RBC group . | Donor's RBC group . | Category of ABO mismatch† . | Erythrocyte transfusion . | Platelet or plasma transfusion . |
|---|---|---|---|---|
| A | O | Minor | O | A, AB |
| B | O | Minor | O | B, AB |
| AB | O | Minor | O | AB |
| AB | A | Minor | A, O | AB |
| AB | B | Minor | B, O | AB |
| O | A | Major | O | A, AB |
| O | B | Major | O | B, AB |
| O | AB | Major | O | AB |
| A | AB | Major | A, O | AB |
| B | AB | Major | B, O | AB |
| A | B | Minor and major | O | AB |
| B | A | Minor and major | O | AB |
| A | A | None | A, O | A, AB |
| B | B | None | B, O | B, AB |
| AB | AB | None | AB, A, B, O | AB |
| O | O | None | O | O, A, B, AB |
RBC indicates red blood cell; HPC, hematopoietic progenitor cell.
These guidelines apply from time of initiation of myeloablative therapy until reverse and forward erythrocyte typing of the donor.
Major (“forward”) mismatch occurs when donor's RBC-ABO group is serologically incompatible with recipient's plasma. Minor (“reverse”) mismatch occurs when recipient's RBC-ABO type is serologically incompatible with donor's plasma.
The most frequently needed plasma for ABO-mismatched HPC transplantation recipient is group AB (universal donor) plasma. However, only 4% of blood donors are group AB; therefore, many transfusion services carry an insufficient inventory of group AB plasma or platelets for large-volume or extended transfusions. If platelet components that are compatible with both the donor and recipient are unavailable, and if serologic or other evidence that donor-derived anti-A or -B is causing hemolysis exists, platelet components may be washed in 0.9% sodium chloride.66 Alternatively, the hemagglutinin titer(s) of platelet components may be measured, and if relatively low (< 32), volume-reduction or washing is unnecessary. For thrombocytopenic patients with life-threatening hemorrhage, the priority is getting the platelet count as high as possible, as soon as possible.
After transplantation
Transplantation through engraftment
In the immediate posttransplantation period, considerations for peritransplantation remain in effect, but additional requirements may apply.
Immune hemolysis
Potential immunohematologic complications after an ABO-mismatched allogeneic HPC transplantation include immediate hemolysis, delayed hemolysis, delayed erythrocyte engraftment, and pure red-cell aplasia.47,62,67-71 Immune hemolysis immediately after HPC transplantation infusion is from recipient-derived antierythrocyte antibodies (eg, major ABO mismatch), whereas delayed hemolysis is probably derived from donor blood-group antibodies (eg, minor ABO mismatch). Table 2 provides a guide to the differential diagnosis of hemolysis in patients after HPC transplantation.
Guide to the differential diagnosis of hemolysis in patients after HPC transplantation
| Diagnosis . | Pathophysiology . | Serologic findings . |
|---|---|---|
| HPC graft-related immune hemolysis | ||
| Major ABO incompatibility between HPC donor and recipient | Hemolysis of transfused erythrocytes, delay in erythrocyte engraftment | DAT positive for C3d, IgG, or both; anti-A and/or anti-B present in eluate |
| Minor ABO incompatibility between HPC donor and recipient | Hemolysis of patient's erythrocytes caused by transfused donor's plasma or by passenger lymphocyte-derived isohemagglutinins | DAT positive for C3d, IgG, or both anti-A and/or anti-B present in eluate |
| Major incompatibility: other blood group antigens | Hemolysis of transfused donor's erythrocytes | DAT positive for C3d, IgG, or both; antibody to non-ABO red blood cell antigen(s) identified in eluate and patient plasma |
| Minor incompatibility: other blood group antigens | Hemolysis of patient's erythrocytes caused by alloantibodies in transfused donor plasma or by passenger lymphocyte-derived alloantibodies | DAT positive for C3d, IgG, or both: antibody to non-ABO red blood cell antigen(s) identified in eluate and patient plasma |
| Transfusion-related immune hemolysis | ||
| Transfusion of erythrocytes incompatible with donor or patient | Hemolysis of transfused erythrocytes caused by patient's native or graft-derived antibodies | DAT positive for C3d, IgG, or both: anti-A and/or anti-B or antibody to other RBC antigen identified in eluate and patient plasma |
| Transfusion of plasma incompatible with donor or patient | Hemolysis of patient and/or donor erythrocytes | DAT positive for C3d, IgG, or both; anti-A and/or anti-B or antibody to other RBC antigen identified in eluate and patient plasma |
| Other causes of immune hemolysis | ||
| Autoimmune hemolytic anemia | Hemolysis and serologic incompatibility of (all) crossmatched donor erythrocytes | DAT positive for C3d, IgG or both; panagglutinin present in eluate and patient plasma |
| Drug-induced hemolytic anemia | Autoantibody formation induced by drug, hapten mechanism, or drug modification of erythrocyte membrane | DAT positive for C3d, IgG, or both; eluate may react with drug-treated erythrocytes |
| Nonimmune hemolysis | ||
| Thrombotic thrombocytopenic purpura | Microangiopathic hemolytic anemia | DAT and antibody screen negative |
| Infusion of cryopreserved stem cell products | Nonimmune hemolysis may be observed during infusion of DMSO-cryopreserved HPC preparations | DAT negative |
| Clostridium perfringens sepsis | C perfringens produces hemolysin toxins, resulting in nonimmune intravascular hemolysis | DAT negative |
| Diagnosis . | Pathophysiology . | Serologic findings . |
|---|---|---|
| HPC graft-related immune hemolysis | ||
| Major ABO incompatibility between HPC donor and recipient | Hemolysis of transfused erythrocytes, delay in erythrocyte engraftment | DAT positive for C3d, IgG, or both; anti-A and/or anti-B present in eluate |
| Minor ABO incompatibility between HPC donor and recipient | Hemolysis of patient's erythrocytes caused by transfused donor's plasma or by passenger lymphocyte-derived isohemagglutinins | DAT positive for C3d, IgG, or both anti-A and/or anti-B present in eluate |
| Major incompatibility: other blood group antigens | Hemolysis of transfused donor's erythrocytes | DAT positive for C3d, IgG, or both; antibody to non-ABO red blood cell antigen(s) identified in eluate and patient plasma |
| Minor incompatibility: other blood group antigens | Hemolysis of patient's erythrocytes caused by alloantibodies in transfused donor plasma or by passenger lymphocyte-derived alloantibodies | DAT positive for C3d, IgG, or both: antibody to non-ABO red blood cell antigen(s) identified in eluate and patient plasma |
| Transfusion-related immune hemolysis | ||
| Transfusion of erythrocytes incompatible with donor or patient | Hemolysis of transfused erythrocytes caused by patient's native or graft-derived antibodies | DAT positive for C3d, IgG, or both: anti-A and/or anti-B or antibody to other RBC antigen identified in eluate and patient plasma |
| Transfusion of plasma incompatible with donor or patient | Hemolysis of patient and/or donor erythrocytes | DAT positive for C3d, IgG, or both; anti-A and/or anti-B or antibody to other RBC antigen identified in eluate and patient plasma |
| Other causes of immune hemolysis | ||
| Autoimmune hemolytic anemia | Hemolysis and serologic incompatibility of (all) crossmatched donor erythrocytes | DAT positive for C3d, IgG or both; panagglutinin present in eluate and patient plasma |
| Drug-induced hemolytic anemia | Autoantibody formation induced by drug, hapten mechanism, or drug modification of erythrocyte membrane | DAT positive for C3d, IgG, or both; eluate may react with drug-treated erythrocytes |
| Nonimmune hemolysis | ||
| Thrombotic thrombocytopenic purpura | Microangiopathic hemolytic anemia | DAT and antibody screen negative |
| Infusion of cryopreserved stem cell products | Nonimmune hemolysis may be observed during infusion of DMSO-cryopreserved HPC preparations | DAT negative |
| Clostridium perfringens sepsis | C perfringens produces hemolysin toxins, resulting in nonimmune intravascular hemolysis | DAT negative |
DAT indicates direct antiglobulin test; C3D, complement factor 3D; DMSO, dimethyl sulfoxide; HPC, hematopoietic progenitor cell; IgG, immunoglobulin G; and RBC, red blood cell.
Distinguishing immune hemolysis from other posttransplantation complications, including VOD of the liver, GVHD, and thrombotic thrombocytopenic purpura, can cause an elevated bilirubin concentration and decreased hematocrit, obscuring conventional signs of immune hemolysis. ABO-related hemolysis after an ABO-mismatched HPC transplantation should be suspected when a positive direct antiglobulin test result is present, regardless of whether a nonreactive eluate is present. In this situation, neither anti-A nor anti-B antibody will be identified in an eluate of the circulating erythrocytes, unless the transfusion service has been alerted to the possibility, and group A and B reagent erythrocytes are added to the standard group O screening panel.
Passenger lymphocyte syndrome
A more severe form of immune hemolysis is passenger lymphocyte syndrome. Transient hemolysis may occur if donor-derived lymphocytes in the HPC graft remain viable and form blood group–specific antibodies, which are incompatible with the recipient's erythrocytes. Typically, the passenger lymphocyte syndrome involves ABO incompatibility, but hemolysis resulting from serologic incompatibility in the Rh, Kell, Duffy, or Kidd blood group systems has been reported.62,67-70 If hemolysis increases (rather than decreases) a few days after an HPC transplantation, the possibility of antibody production by the donor's lymphocytes should be considered.1 Because of the higher lymphocyte content in a peripheral blood HPC graft, the theoretical risk of passenger lymphocyte syndrome is higher than in bone marrow HPC grafts, but reports of passenger lymphocyte syndrome with peripheral blood grafts are mostly anecdotal. Additional risk factors for hemolysis resulting from passenger lymphocyte syndrome include the use of cyclosporine alone in the absence of an antiproliferative agent, such as methotrexate, for posttransplantation GVHD prophylaxis,62,72 and possibly the use of a reduced-intensity preparative regimen.
Usually, immune hemolysis related to passenger lymphocyte syndrome begins at the end of the first week or during the second week after transplantation. Hemolysis may be severe, persist for 5 to 10 days, and then subside as the patient's residual incompatible erythrocytes are eliminated and replaced by transfused or donor-derived erythrocytes. In some patients, hemolysis is more extensive than expected from serologic incompatibility with the patient's erythrocytes alone. In such cases, excess hemolysis has been attributed to hemolysis of transfused compatible erythrocytes (“bystander” immune hemolysis).2,62
In addition to keeping the hemoglobin level higher during the at-risk period (eg, 10 mg/dL instead of 8 mg/dL), use of group O erythrocytes during the pretransplantation period for transfusion support may minimize this risk. Erythrocyte exchange via apheresis may be considered for patients who receive a peripheral blood HCP transplantation with a minor ABO incompatibility, either prophylactically (when the risk is high for passenger lymphocyte syndrome) or in an effort to minimize bystander hemolysis after it has occurred. The results of a study conducted at the University of California at Los Angeles62 showed that erythrocyte exchange transfusion was unsuccessful in preventing passenger lymphocyte syndrome and subsequent reactive hemolysis. GVHD prophylaxis without methotrexate62,72 or other therapy to inhibit B lymphocyte proliferation after transplantation has been associated with a higher risk of passenger lymphocyte syndrome. Whether the larger number of B-lymphocytes infused with a minor-incompatible AB donor peripheral blood rather than bone marrow HPC product causes a higher risk of hemolysis is controversial, as the only reports of hemolysis after a peripheral blood HPC product are anecdotal. Speculative concerns for increased reactive hemolysis risk with reduced intensity compared with myeloablative preparative regimens exist because of the lack of recipient erythropoiesis suppression with reduced-intensity regimens. However, only anecdotal reports support this hypothesis. Single-center data are difficult to interpret, for new risks and registry data do not always provide the detailed transfusion history necessary to make observations with larger groups of patients. In addition, plasma reduction of the bone marrow or HPC product could be considered if the donor has high titer(s) of incompatible hemagglutinins. To prevent immediate reactions with infusion, many centers now opt for plasma reduction, rather than testing for hemagglutinins titers. Because of the rarity of this problem and the poor results for altering its course with early intervention, it is rarely monitored prophylactically. Clinical management concentrates on monitoring for signs of acute hemolysis (rising lactate dehydrogenase and indirect bilirubin levels with sudden decreasing hemoglobin levels in the absence of bleeding) and maintaining transfusions of compatible erythrocytes at a pace that at least matches the rate of hemolysis. Massive hemolysis, which in some cases has led to renal failure, may sometimes be treated by erythrocyte-exchange transfusion,73 using rituximab,74 or in one anecdote, by a single dose of methotrexate.62
Pure red-cell aplasia
Major ABO-mismatched HPC transplantations may result in immune-mediated pure red-cell aplasia.71,75 In this situation, the recipient's lymphocytes and/or plasma cells survive myeloablation and produce antibodies to the transplanted donor-derived erythrocytes, early or late (> 100 days) after transplantation. The recipient's antibodies suppress erythropoiesis by destroying erythroid progenitors, leading to anemia (with reticulocytopenia). The etiology of red-cell aplasia here remains unknown. Historically, delayed erythrocyte engraftment occurs in approximately 20% of patients after ABO-mismatched transplantation. Today, the more common use of peripheral blood progenitor cells as the HPC graft source may further reduce the occurrence of anemia. Some recipients may require erythrocyte transfusions for more than 1 year. A diagnosis of pure red-cell aplasia is established if reticulocytopenia persists for more than 60 days and erythrocyte precursors are absent in the bone marrow aspirate. An inverse correlation between ABO hemagglutinin titers and reticulocyte counts exists. In addition, some data suggest that erythrocyte engraftment is dependent on the loss of hemagglutinins that are incompatible with the donor erythrocytes. Anti-A or -B hemagglutinins may persist longer than 120 days after transplantation and have been detected as late as day + 605 after transplantation. In most cases, ABO hemagglutinins are undetectable by the second month after major ABO-incompatible HPC transplantation. The issues of persistent hemagglutinins causing posttransplantation problems are often clouded by the common use of high-dose immunoglobulin, which has not been commercially prepared to be ABO-blood type–specific. Not all patients with persistent incompatible ABO hemagglutinins experience overt hemolysis, and routine measurement of hemagglutinins has not been very helpful.
Before initiating red-cell aplasia therapy, red-cell aplasia resulting from parvovirus B19 (an infection acquired via the respiratory route or from a blood transfusion after transplantation) should be excluded by measuring immunoglobulin M antibodies or parvovirus DNA; clinical presentation of a parvovirus B19 infection may be obscured by the complex clinical and laboratory findings.
Treatments for red-cell aplasia not associated with parvovirus B19 infections include plasma exchange to remove the offending hemagglutinins and decreasing the myelosuppressive treatment to induce a graft-versus-host hematopoietic and lymphocyte effect. Plasma exchange has not been shown to be effective for reducing hemagglutinins titers because of its short effect and rapid rebound, presumably because of the extracellular reservoir of hemagglutinins.
Autoimmune hemolytic anemia
Autoimmune hemolytic anemia (AHA) occasionally develops in HPC transplantation recipients, occurring 2 months to 3 years after transplantation. The diagnosis should be considered if the direct antiglobulin test result becomes positive. The results of elution and absorption studies are indistinguishable from those of conventional AHA and may reveal a warm-type (immunoglobulin G) panagglutinin, cold-type (immunoglobulin M) agglutinin, or an antibody with relative serologic specificity for other blood-group antigens. Late AHAs have often had a deleterious impact on survival rates. Whether this deleterious impact is a result of T-cell dysregulation or viral infection or an early subtle sign of a pending relapse is unclear.76,77 Although reports are mostly anecdotal, late AHA may be more frequent in T cell–depleted transplantation recipients.
Postengraftment
Engraftment syndrome and DAH
HPC patients with engraftment are at risk for pulmonary complications similar to transfusion-related lung injury.64,78,79 This engraftment syndrome often presents with fever and hypoxia at initial white blood cell recovery and may progress to DAH. High-dose steroids are often the initial therapy, but if DAH occurs, platelets (along with either recombinant factor VIIa or aminocaproic acid) may be necessary. Again, the risk-benefit ratio must be considered, and the severity of the patient situation will dictate the course of action. Transfusion-related lung injury, usually associated with platelet transfusions, typically occurs in the bone marrow transplantation population. To rule out infection, clinicians should review the content of transfused product. If the lung injury is caused by donor-derived antibodies, future platelet products may need to be more closely HLA-matched or washed. Occasionally, peripheral blood HPC products collected with high granulocyte contamination or excessive amounts of DMSO may cause infusional pulmonary toxicities.
Graft-versus-host disease
Acute GVHD is a major, and frequent, complication of allogeneic HPC transplantations.1 HPC recipients with acute GVHD may develop cytopenias of 1 to 3 cell lines and an immune-based hemolytic anemia. Patients with gastrointestinal GVHD with a denuded bowel are prone to gastrointestinal bleeding, most probably to local ulcerations, but a recent report also documents clotting factor loss, and those patients with severe liver GVHD may also develop the clotting disturbances associated with end-stage liver disease.63 All of these patients may require transfusion support. In addition, clinical transplantation services showed biopsy patients with suspected GVHD despite low platelet counts because visceral (particularly viral) infections can often mimic GVHD symptoms, and the proper prompt treatment of either of these complications is necessary to prevent morbidity and mortality. Blood product support during the biopsy procedures is essential.9
Hemorrhagic cystitis
Hemorrhagic cystitis is a common bleeding complication after transplantation, usually occurring in the short-term postengraftment period and more commonly after allogeneic transplantation.80 Historically, hemorrhagic cystitis was thought to be a toxicity associated with cyclophosphamide; however, several viral etiologies, including adenovirus and polyoma viruses (such as B-K virus), have been reported.80 Because of the magnitude of erythrocyte loss and patient discomfort, patients with hemorrhagic cystitis require a large number of platelet products. Procoagulants have rarely been used because of concerns about possible clotting within the ureters (although, in a recent study of recombinant factor VIIa, clotting was not observed).81 Bladder irrigation and platelet transfusion are the staple of therapy, but if B-K virus is found and the patient has no GVHD, immune suppression tapering should be considered.
Veno-occlusive disease
Incompatible isoagglutinins to ABH blood-group antigens on hepatic sinusoidal endothelial cells may cause toxic injury initiating a sequence of biologic events that may lead to circulatory compromise of centrilobular hepatocytes, fibrosis, and obstruction of blood flow, resulting in VOD, a major cause of posttransplantation mortality.82,83 The results of one study report that transfusions of platelet concentrates containing ABO-incompatible plasma is also associated with hepatic VOD in children after busulfan-conditioned autologous HPC transplantations.84 Whether one should conclude from this article that all platelet transfusions after transplantation then should be ABO-compatible with both donor and recipient blood types is debatable and probably a manner of personal opinion, particularly because the availability of platelet transfusion products is problematic, especially if AB-type platelets are the only product compatible with both donor and recipient.
Platelet transfusion requirements are often higher in VOD patients than in patients without VOD, and the requirement may become apparent by day 0 (day of HPC infusion), even before the onset of VOD-related symptoms. Platelet consumption is increased during VOD because the disease promotes activation of coagulation, including cytokine-induced endothelial cell damage, activation of von Willebrand factor, and low levels of natural anticoagulants (and subsequent increased thrombin formation). Portal hypertension with increased splenic sequestration of platelets also contributes to thrombocytopenia, further increasing the need for platelet transfusions in these patients. Indications for platelet transfusion in VOD patients are similar to those with liver disease.
Erythrocyte (blood group) chimerism
Blood group chimerism is an intrinsic characteristic of HPC transplantation. Transfusion services have reported varied incidences of ABO-grouping discrepancies (so-called forward- or reverse-typing discrepancies) or mixed field-agglutination reactions, depending on whether the donor and recipient are ABO-identical. Many nonmyeloablative, as well as T cell–depleted, transplantation recipients remain in the microchimeric state for a prolonged time period after neutrophil recovery. Once the patient's blood becomes full-donor chimera, with disappearance of recipient-derived antierythrocyte antibodies, the use of blood products consistent with donor ABO typing is logical. Transfusion services should monitor for recurrence of recipient ABO-group erythrocytes after non–ABO-identical allogeneic HPC engraftment. After establishment of full donor engraftment, the onset of mixed erythrocyte chimerism (circulating erythrocytes typing with mixed field-donor recipient ABO groups) may be signaling an impending relapse and/or graft failure.85
Infusion of donor lymphocytes
Increasingly, infusions of lymphocytes from the HPC donor are used to treat early relapse after allogeneic transplantations, as well as Epstein-Barr virus infections.86,87 The same considerations that apply for serologic matching and manipulation of apheresis HPC products apply to preparing lymphocyte infusions. Usually, pancytopenia develops 4 to 6 weeks after a donor lymphocyte infusion. Clinical response typically occurs at 3 to 4 months after the infusion but may occur later, especially if chemotherapy is administered concomitantly. Little has been published about transfusion issues related to lymphocyte infusion. Often, for patients with relapse of the primary disease, the red cells have returned to recipient typing, and donor red-cell typing emerges after the antitumor response. Changes in the status of HPC engraftment will be observed by the transfusion service at the onset of mixed-field agglutination in ABO-blood grouping.
Granulocyte transfusion
Whereas transfusions of granulocyte concentrates are not widely used for management of infections in neutropenic HPC recipients and remain controversial, some transplantation services use granulocyte transfusions, with or without G-CSF–stimulated donors.88,89 Granulocyte transfusions have also been used to treat refractory fungal infections in immunocompromised patients. However, the role of granulocyte transfusions in managing fungal infections has been decreased with improved antifungal agents.
Several factors must be considered when selecting granulocyte-infusion donors. Granulocyte transfusions have a risk of transmitting CMV and, usually, potential donors who are CMV-seropositive are excluded.90,91 Because growth factors in animal models have been associated with fetal demise, prospective donors of childbearing potential must be screened for an unknown pregnancy. Finally, apheresis collections of granulocytes, in sufficient number to be clinically effective, usually require the donors to be pretreated with G-CSF or corticosteroids to increase circulating granulocyte counts. Complications of G-CSF stimulation are not insignificant and may include considerable bone pain or splenic rupture; therefore, the donor should be informed of these possible complications.
Granulocyte transfusions increase the risk for development of HLA antibodies; if this occurs, subsequent granulocyte concentrates should be obtained from HLA-matched donors.92 Finally, all granulocyte concentrates must be gamma-irradiated before administration to prevent transfusion-associated GVHD.
Long term
Although most patients will not require posttransplantation transfusions if transplantation is successful, a thorough understanding of posttransplantation transfusion issues is necessary. Little has been published on long-term transfusion management issues for HPC recipients. Long-term management of post–HPC transplantation patients requires ready access to pertinent blood transfusion–related medical records. However, often patients return to communities distant from the transplantation center where their most current medical reports are unavailable. Although most hospital transfusion services have computerized records that specify special requirements for their own previously treated patients, these records often do not include special requirements for gamma irradiation, CMV-seronegative components, or group O erythrocytes for HPC patients who require an urgent transfusion.
Uninformed transfusion services can easily overlook obvious transfusion needs for transplantation patients. For example, given the possibility of blood-group chimerism, a subtle mixed-field agglutination reaction could be overlooked in an urgent situation and potentially incompatible erythrocytes could be transfused. In addition, unawareness could prevent prompt detection of an early relapse (eg, detection of a rising volume of recipient type erythrocytes) that could be quickly and possibly effectively treated if an urgent marrow evaluation and consideration for additional intervention with donor cells were coordinated by the transfusion service. To ensure optimal matching of erythrocytes for transfusion, as well as provision of special transfusion needs for their patients, transplantation physicians have an obligation to communicate to their patients the importance of providing information about their HPC transplantation to local healthcare providers, including transfusion services, and to consider wearing transfusion requirement identification on their bodies in case of an emergency.
Because no reliable measures of full immunocompetence recovery after transplantation exist, most transplantation services prefer not to enforce time limitations on the receipt of irradiated blood products, but all patients treated with immunosuppressive agents should receive only irradiated blood products. Based on personal experience (J.L.G., T.R.K.), nonirradiated blood products have been administered to long-term survivors (off immunosuppression) without harm. Because the major risk for the development of CMV is immediately after transplantation, once CMV-seronegative patients are off immunosuppression, the need for CMV-seronegative blood products diminishes.
Acknowledgments
The authors thank the Murphy, Mayer, Galt, and Goldsmith families for their philanthropic donations and Lisa Holle for editorial services. J.L.G. acknowledges, in this work, the mentorship of H. Bender of the University of Notre Dame (South Bend, IN), R. Kozera of Temple University (Philadelphia, PA), and K. DeBenedictis of Reading Hospital (West Reading, PA).
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the US Government (V.V.J., Department of Pathology and Laboratory Medicine, National Naval Medical Center).
Authorship
Contribution: J.L.G., V.V.J., S.G.S., T.R.K., and A.S. contributed to the writing and editing of this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James L. Gajewski, Oregon Health & Science University, Center for Hematologic Malignancies, Mail Code UHN73C, 3181 SW Sam Jackson Park Road, Portland, OR 97239; e-mail: gajewski@ohsu.edu.