Abstract
Primary myelofibrosis (PMF) is the rarest and the most severe Philadelphia-negative chronic myeloproliferative syndrome. By associating a clonal proliferation and a mobilization of hematopoietic stem cells from bone marrow to spleen with profound alterations of the stroma, PMF is a remarkable model in which deregulation of the stem cell niche is of utmost importance for the disease development. This paper reviews key data suggesting that an imbalance between endosteal and vascular niches participates in the development of clonal stem cell proliferation. Mechanisms by which bone marrow niches are altered with ensuing mobilization and homing of neoplastic hematopoietic stem cells in new or reinitialized niches in the spleen and liver are examined. Differences between signals delivered by both endosteal and vascular niches in the bone marrow and spleen of patients as well as the responsiveness of PMF stem cells to their specific signals are discussed. A proposal for integrating a potential role for the JAK2 mutation in their altered sensitivity is made. A better understanding of the cross talk between stem cells and their niche should imply new therapeutic strategies targeting not only intrinsic defects in stem cell signaling but also regulatory hematopoietic niche–derived signals and, consequently, stem cell proliferation.
Introduction
Myeloproliferative disorders (MPDs) are a heterogeneous group of related diseases that classically includes chronic myeloid leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF).1 MPDs are clonal malignant hemopathies that arise from the transformation of hematopoietic stem cells/progenitors (HSCs/HPs) with no real growth autonomy and no marked alteration in differentiation. They share several features including abnormal proliferation of hematopoietic cells from one or several cell lineages that is driven by a hypersensitivity to regulatory growth factors.2,3 In contrast to CML, the molecular mechanisms leading to the progression of Philadelphia-negative (Ph−) MPDs remained unclear until the discovery in 2005 of a single point mutation in the tyrosine kinase JAK2 (JAK2V617F) in virtually all patients with PV, in about 70% with ET, and in half of those with PMF.4-7 This finding has transformed our understanding of the pathogenesis of these diseases. However, the fact that the JAK2+ syndromes exhibit various clinical features raises the question of how a single mutation can generate different diseases and strongly suggests that other acquired events are required for the development of ET and PMF. The presence of activating mutations affecting the thrombopoietin receptor MPL (MPLW515L and MPLW515K) in 5% to 7% of PMF and 2% to 4% of ET patients is in agreement with this assumption.8-11
JAK2V617F as well as MPLW515 mutations have been reported to affect cytokine signaling, survival, and G1-S cell-cycle transition, through a constitutive activation of JAK-STAT signaling.4,12-14 The role of alterations of kinase pathways in the development of myeloproliferative disorders has been confirmed in murine models in which JAK2V617F or MPLW515 mutations were overexpressed by retroviral transduction or by transgenesis. Whereas these results indicate that activated tyrosine kinases play a critical role in mediating the hematopoietic stem cell/progenitor proliferation that characterizes MPDs, several experimental and clinical findings suggest that changes within the hematopoietic environment might also be of crucial importance for the development of the pathological process and in disease progression. The first experimental proofs of this concept come from recent studies by Walkley et al in RAR gamma– and in RB-null mice15,16 showing that myeloproliferative diseases could not result simply from hematopoietic cell–intrinsic defects, but were heavily influenced and even caused by alteration(s) and/or mutation(s) in the hematopoietic microenvironment.
According to Spradling et al, “The true nature of stem cells can be learned only by discovering how they are regulated”17 (p98). The concept of regulatory niches as a specific site where stem cells reside and undergo self-renewal and differentiation was first proposed by Schofield.18 Fewer than 10 years later, we proposed a model based on interactions between tumor/leukemic stem cells and their ecosystem/microenvironment. This model, in which the “tumor niche” is responsible for a nongenetically determined enhanced cell proliferation and for response to therapies,19 strengthens and widens the concept of Schofield to neoplastic processes. In the present review, we will take the paradigm of PMF, the rarest and most severe Ph− MPD, to shed a new and challenging light on these hemopathies as diseases involving a defect in the stem cell niche as an important contributing factor for disease progression.
Hematopoietic stem cells and their regulatory niches
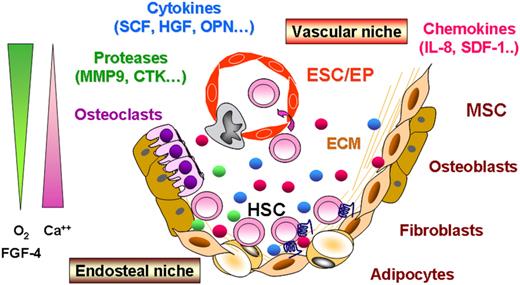
In adults, hematopoiesis relies on the ordered self-renewal and differentiation of HSCs within the bone marrow. This process involves intrinsic and extrinsic cues including both cellular and humoral regulatory signals generated by the hematopoietic niches.20,21 Besides HSCs themselves, these niches are composed of stromal cells including fibroblasts, osteocytes, and adipocytes derived from mesenchymal stem cells (MSCs), hematopoietic-derived osteoclasts and of endothelial stem cells (ESCs), all mesodermal in origin. HSCs are engaged in a constant cross talk with their niche and are maintained in close contact with stromal cells via adhesion molecules within the proximity of the endosteal surface and the perivascular space (Figure 1). Until recently, evidence was accumulating that 2 types of niches existed: the endosteal and the vascular niches.22-24 The endosteal niche, a site of a relative hypoxia in close vicinity to the bone edge, has been proposed to play a major role in maintaining the HSC pool in quiescence through interactions with a subpopulation of immature osteoblasts expressing the N-cadherin.23 The vascular niche, consisting of a network of thin-walled and fenestrated sinusoidal vessels in which the oxygen level is higher than in the area close to the endosteum, is suggested to take part in the HSC proliferation/differentiation and mobilization processes.25,26 However, it remains uncertain whether endosteal and vascular niches are distinct or they both contribute to a common hematopoietic niche. Data from Kiel et al27 and Kiel and Morisson,28 showing that quiescent murine HSCs can be found close to endothelial cells, strengthen the fact that it is difficult to currently attribute a specific role for each niche. Among signals emanating from these different niches, the main actors of this complex signaling regulatory process are (1) combined cell-intrinsic regulatory mechanisms (Tie2/angiopoietin-1, Notch/Jagged, Frizzled/Wnt, sonic Hedgehog signaling, etc), (2) adhesion molecule interactions (VLA4, VLA5, CD44, N-cadherin, etc), and (3) extracellular matrix components and environmental elements such as calcium, oxygen concentration, hormones (parathyroid hormone), bone morphogenic proteins (BMP-2/-4), cytokines (FGF-4, SCF, VEGF, TPO, etc), and chemokines (CXCL12, IL8, etc).29-31
A simplistic model of hematopoietic stem cell niches. In adults, hematopoiesis occurs in the bone marrow where hematopoietic stem cells (HSCs) are engaged in a constant cross talk within specific niches at the proximity of the endosteal surface (endosteal niche) and of the perivascular space (vascular niche). These niches are composed of (1) stromal cells derived from mesenchymal stem cells (MSCs), able to generate fibroblasts, osteoblasts/osteocytes, and adipocytes, (2) osteoclasts derived from HSCs, and (3) endothelial cells derived from endothelial stem cells (ESCs). Regulatory signals emanating from these different niches include cell-intrinsic regulatory mechanisms, adhesion molecule interactions, extracellular matrices (ECMs), and environmental components such as calcium (Ca++), oxygen (O2) concentration, proteases, as well as humoral factors including cytokines and chemokines.
A simplistic model of hematopoietic stem cell niches. In adults, hematopoiesis occurs in the bone marrow where hematopoietic stem cells (HSCs) are engaged in a constant cross talk within specific niches at the proximity of the endosteal surface (endosteal niche) and of the perivascular space (vascular niche). These niches are composed of (1) stromal cells derived from mesenchymal stem cells (MSCs), able to generate fibroblasts, osteoblasts/osteocytes, and adipocytes, (2) osteoclasts derived from HSCs, and (3) endothelial cells derived from endothelial stem cells (ESCs). Regulatory signals emanating from these different niches include cell-intrinsic regulatory mechanisms, adhesion molecule interactions, extracellular matrices (ECMs), and environmental components such as calcium (Ca++), oxygen (O2) concentration, proteases, as well as humoral factors including cytokines and chemokines.
A subtle balance between the 2 types of microenvironmental niches orchestrates hematopoietic homeostasis, which results from an accurate equilibrium between HSC dormancy, activation, and differentiation. Alterations of this balance can lead to uncontrolled cellular proliferation and ultimately to the promotion of leukemias and MPDs. However, it remains to be elucidated how the hematopoietic microenvironment contributes to the deregulation of normal hematopoiesis and/or in the development of the neoplastic clone. Whether a niche may participate in tumorigenesis as a leukemic niche or may promote metastasis of hematopoietic neoplastic cells is also of concern.30,32 Recently, the concept of asymmetric cell divisions as another control instance in hematopoietic homeostasis has emerged, thus suggesting that defects in the process of asymmetric cell divisions within the niches might also participate in the transformation of normal HSCs/HPs into leukemic cells.33
The primary myelofibrosis paradigm: are MPDs diseases of intrinsic stem cell signaling and/or of the cross talk between stem cells and their regulatory niches?
Primary myelofibrosis is an uncommon chronic myeloproliferative disorder (MPD), the incidence of which was estimated to be 0.73 and 0.40 per 100 000 persons per year in males and females, respectively.34 It is characterized by a leukoerythroblastic blood picture, teardrop poikilocytosis, and extramedullary hematopoiesis with progressive hepatosplenomegaly partly due to the prominent mobilization of hematopoietic progenitors from bone marrow to spleen and liver. Such an alteration of hematopoiesis is constantly associated with profound modifications of the stroma within bone marrow and spleen as demonstrated by the presence of myelofibrosis, osteosclerosis, and neoangiogenesis.1,2
Whereas molecular defect(s) associated with the development of PMF have been described, its pathogenesis is not thoroughly elucidated. This accounts for the lack of adequately targeted therapeutic approaches. Several abnormalities that could explain the myeloproliferation have been reported: (1) the multipotency of the hematopoietic clonal cell with myeloid and lymphoid differentiation,35-37 although an absolute lymphopenia has been described in peripheral blood38 ; (2) the progressive dominance of clonal hematopoiesis over normal polyclonal hematopoiesis, resulting in the overproduction of one or more of the mature elements of blood; (3) the hypersensitivity of hematopoietic progenitors to growth factors39,40 ; (4) a striking involvement of the megakaryocytic (MK) lineage, with hyperplasia and dysplasia resulting in an excessive production of several cytokines and chemokines3,41 ; (5) the presence of mutations in the JAK2 and in the thrombopoietin receptor MPL genes associated with an inappropriate activation of JAK/STAT proteins that participate in the proliferation and hypersensitivity of HSC/HP to growth factors, and (6) the lack of a consistent cytogenetic abnormality.34
Whereas most of these cellular abnormalities could be shared by other Ph− MPDs, PMF is defined by unique clinical features such as HSC/HP trafficking and myelofibrosis that are the hallmarks of the disease. The number of circulating CD34+ cells is considerably elevated in PMF patients compared with other Ph− chronic myeloproliferative disorders (PV and ET) and is presently considered to be a marker of clinical importance for the diagnosis and the outcome of the disease.42,43 As the genuine signature of PMF, myelofibrosis results from a complex process including alterations of fibroblasts, leading to a modified expression of adhesion molecules and to an increased deposition of extracellular matrix components. This accumulation is thought to be the consequence of an excessive release/leakage of growth factors within the bone marrow by cells from the pathological hematopoietic clone and especially by necrotic megakaryocytes.44-48 Among them, platelet factor 4 (PF4), platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), transforming growth factor-beta (TGF-β),49-52 and vascular endothelial growth factor (VEGF)53,54 are thought to activate mesenchymal cells, leading to myelofibrosis, as well as endothelial cells, contributing to angiogenesis. It has been recently hypothesized that an increased production of osteoprotegerin by stromal and endothelial cells might also contribute to the unbalanced osteoblast production, resulting in the osteosclerosis frequently associated with myelofibrosis in patients55,56 and in murine models.57
Thus, in PMF, although the primitive molecular event is still unknown, anomalies of intrinsic stem cell signaling, partly resulting from JAK2 and MPL mutations, likely play a role in the hematopoietic progenitor proliferation and hypersensitivity to growth factors. However, the “specificity” of the pathological process would result from alterations in the cross talk between hematopoietic and stromal cells (Figure 2). Stromal cells are conditioned by the malignant hematopoietic cells and reciprocally, by acquiring new properties, stromal cells create a pathologic microenvironment that participates in the maintenance of the clone, leading to an imbalance that compromises normal hematopoiesis. Therefore, ignoring the environmental cues that control HSC compartment size during homeostasis, neoplastic HSCs can survive at anatomical sites (ie, spleen and liver) unable to support normal adult hematopoiesis.
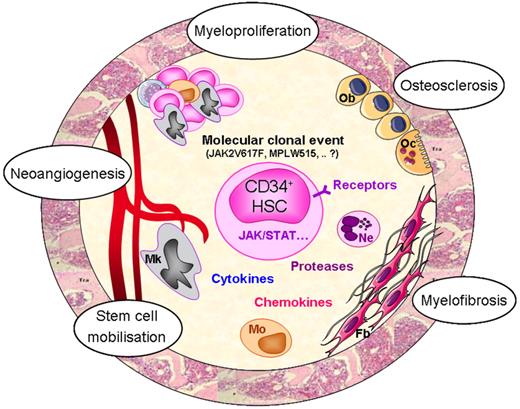
Putative mechanisms responsible for PMF pathogenesis. Whereas the initial molecular defect at the origin of the pathological clone is still unknown, alteration of tyrosine kinase–related signalization likely participates in the amplification of the hematopoietic clone as attested by the presence of mutations in the JAK2 and MPL genes in a proportion of PMF patients. Altered interactions between clonal CD34+ cells and stromal cells in the hematopoietic environment would result in an increased cytokine production by the clonal hematopoietic cells and especially by dystrophic megakaryocytes and monocytes. Increased production of hematopoietic, fibrogenic, and angiogenic growth factors would maintain hematopoietic cell proliferation (myeloproliferation) and stimulate myelofibrosis, osteosclerosis, and neoangiogenesis through activation of stromal and endothelial cells. Egress of CD34+ cells from the bone marrow (stem cell mobilization) would result from several mechanisms including chemokines/receptor alterations and protease release from activated neutrophils. HSC indicates hematopoietic stem cell; Mk, megakaryocyte; Mo, monocyte; Ne, neutrophil; Fb, fibroblast; Ob, osteoblast; and Oc, osteoclast.
Putative mechanisms responsible for PMF pathogenesis. Whereas the initial molecular defect at the origin of the pathological clone is still unknown, alteration of tyrosine kinase–related signalization likely participates in the amplification of the hematopoietic clone as attested by the presence of mutations in the JAK2 and MPL genes in a proportion of PMF patients. Altered interactions between clonal CD34+ cells and stromal cells in the hematopoietic environment would result in an increased cytokine production by the clonal hematopoietic cells and especially by dystrophic megakaryocytes and monocytes. Increased production of hematopoietic, fibrogenic, and angiogenic growth factors would maintain hematopoietic cell proliferation (myeloproliferation) and stimulate myelofibrosis, osteosclerosis, and neoangiogenesis through activation of stromal and endothelial cells. Egress of CD34+ cells from the bone marrow (stem cell mobilization) would result from several mechanisms including chemokines/receptor alterations and protease release from activated neutrophils. HSC indicates hematopoietic stem cell; Mk, megakaryocyte; Mo, monocyte; Ne, neutrophil; Fb, fibroblast; Ob, osteoblast; and Oc, osteoclast.
Interestingly, a proportion of patients with polycythemia vera also develops increasing splenomegaly but still with hypercellular bone marrow and moderate reticulin fibrosis. This disease stage may last for several years but eventually the patient may develop anemia with huge splenomegaly when entering the advanced post–polycythemia vera myelofibrosis stage. Therefore, in these cases, alterations of the stroma could also be part of the pathophysiological process, raising the possibility that alterations of hematopoietic niches could reflect a biologic continuum within the MPDs, with the most pronounced alterations in stem cell niche interactions in PMF and in patients with “myelofibrosis with myeloid metaplasia” following ET or PV.
When a deregulation of stem cell niches participates in the leukemic process
Based on these observations, PMF could be considered as a disease in which the development of the pathological clone is deeply influenced by alterations of the microenvironmental niches. According to this hypothesis, an imbalance between endosteal and vascular niches within the bone marrow would take part in the pathogenesis of the disease. Such disequilibrium would favor the proliferation of stem cells including hematopoietic, mesenchymal, and endothelial stem cells together with their mobilization from the bone marrow to the blood. This leads to an increase in circulating stem cells and a progressive displacement of medullary hematopoiesis. These stem cells would then migrate to the spleen, and likely to the liver, in newly created or “reinitialized” vascular niches, which would favor their homing, proliferation, and differentiation, whereas normal HSCs could not survive in such a pathologic microenvironment. This process would result in extramedullary hematopoiesis with enlargement of spleen and liver (Figure 3).
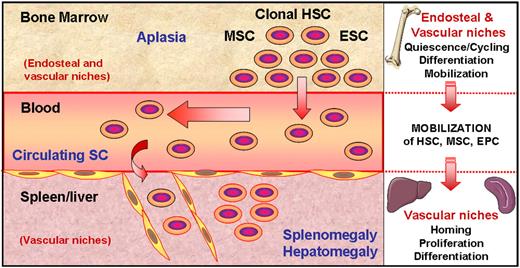
Stem cells moving from bone marrow to spleen/liver niches in PMF. As a consequence of a still unknown molecular event, clonal hematopoietic stem cells proliferate and generate differentiated cells, resulting in the medullar hyperproliferative stage described in early disease. Among these cells, dystrophic megakaryocytes produce or release several growth factors and proteases within the bone marrow environment, leading to myelofibrosis and to an imbalance between endosteal and vascular niches. This imbalance would favor proliferation of stem cells (SCs) including hematopoietic, endothelial, and likely mesenchymal stem cells (HSCs, ESCs, and MSCs, respectively) and their mobilization through blood, resulting in a higher number of circulating SCs and in a terminal bone marrow aplasia. These SCs colonize the spleen in which newly created or reinitialized vascular niches would favor their homing and differentiation, leading to the splenomegaly/hepatomegaly that characterizes PMF.
Stem cells moving from bone marrow to spleen/liver niches in PMF. As a consequence of a still unknown molecular event, clonal hematopoietic stem cells proliferate and generate differentiated cells, resulting in the medullar hyperproliferative stage described in early disease. Among these cells, dystrophic megakaryocytes produce or release several growth factors and proteases within the bone marrow environment, leading to myelofibrosis and to an imbalance between endosteal and vascular niches. This imbalance would favor proliferation of stem cells (SCs) including hematopoietic, endothelial, and likely mesenchymal stem cells (HSCs, ESCs, and MSCs, respectively) and their mobilization through blood, resulting in a higher number of circulating SCs and in a terminal bone marrow aplasia. These SCs colonize the spleen in which newly created or reinitialized vascular niches would favor their homing and differentiation, leading to the splenomegaly/hepatomegaly that characterizes PMF.
Several clinical and experimental arguments support our hypothesis
(1) PMF is clinically characterized by leukoerythroblastosis associated with extramedullary hematopoiesis—myeloid metaplasia—and consequently hepatosplenomegaly. Its association with the bone marrow hypoplasia suggests a displacement of hematopoiesis from bone marrow to the spleen and liver, 2 organs that are hematopoietic sites during embryonic life.58 This supports the concept that microenvironmental niches are plastic and that their initial regulatory functions can be reinitialized by the pathologic process. In contrast to primary mesenchymal stem cells/fibroblasts purified from the spleen of healthy subjects, spleen fibroblasts isolated from PMF patients are able to support the proliferation of autologous patients' CD34+ cells but not that of their normal counterparts.59,60 These data confirm that the in vitro growth of PMF HSC/HPs is not autonomous; more importantly, the data also demonstrate the specific ability of PMF spleen fibroblasts to sustain adult neoplastic hematopoiesis and illustrate the stem cell microenvironmental plasticity. Of significance, these coculture experiments also show that PMF spleen fibroblasts participate in megakaryocytic proliferation to the detriment of the lymphoid one, thus reproducing the major megakaryocytic and lymphoid changes observed in patients.59-61 Therefore, by displaying distinct expression patterns of adhesion molecules, extracellular matrix elements, growth factors, and chemokines compared with their normal counterparts,62 PMF spleen mesenchymal stem cells/fibroblasts most probably contribute to create a microenvironment favorable to neoplastic HSC proliferation and differentiation.
(2) In concert with endothelial cells, mesenchymal stem cells are known to play a major role in the HSC regulatory process within the niche. An elegant study from Rafii's group (Avecilla et al63 ) has reported the contribution of chemokine-mediated interactions of hematopoietic progenitors with the vascular niches in their megakaryocytic differentiation. In PMF patients, clusters of dystrophic and dysmature MKs are frequently observed close to endothelial sinuses in bone marrow and spleen.64 Our recent demonstration of an implication for the IL-8/CXCR1-2 chemokine axis in MK deregulation41 strongly suggests that vascular niches play a crucial role in the dysmegakaryopoiesis that features PMF. The osteogenic capacity for stromal cell–derived osteoprotegerin (OPG)55,56 associated with the demonstration of its involvement in the vascular complications frequently associated with MPD65 further support the critical role of stromal and endothelial components of the niches in PMF pathogenesis.
(3) The huge mobilization of hematopoietic progenitors from bone marrow to spleen and liver has been documented by an up to 200-fold increase in the circulating CD34+ cell count42,43 and by their presence in large numbers in the spleen (M.-C.L.B.-K., personal results, August 27, 2008). This regularly observed HSC/HP egress strengthens the hypothesis of an abnormal migration and location of hematopoiesis in anatomic sites other than bone marrow. Interestingly, elevated numbers of endothelial precursors66,67 and mesenchymal stem cells can also be detected in the blood and spleen of PMF patients. This observation extends the HSC/HP migration process to a more common stem cell mobilization mechanism likely involved in the development of myeloid metaplasia and angiogenesis in the spleen. It is proposed that PMF stem cell mobilization results from an impairment of their adherence to bone marrow stroma allowing them to egress from their niches, to migrate into the circulation, and to colonize the spleen, the liver, and other extramedullary sites. This process could be related to several mechanisms; among them, an altered expression of membrane adhesion molecules and integrins,60,62 a reduced CXCR4 expression on CD34+ cells68,69 shown to be related to a hypermethylation of the CXCR4 promoter,70 and a disruption of CXCR4/SDF-1 axis by a bone marrow proteolytic environment resulting from altered production of proteases71,72 have been proposed. The JAK/STAT pathway has been reported to be involved in the activation of genes responsible for metalloprotease synthesis, suggesting that the JAK2V617F mutation could take part in CD34+ cell mobilization. Recent results in MPD patients, showing that JAK2V617F may constitutively activate granulocytes and by this means mobilize CD34+ cells,73 are in agreement with such hypothesis. In contrast, in PMF, the aberrant collagenase expression has been reported not to be influenced by the JAK2 mutation status but to be predominantly related to the stage of disease.74 These data suggest that in PMF the matrix-modeling gene modulation is not directly dependent of an underlying JAK2 mutation and that molecular mechanisms other than the JAK2V617F mutation are responsible for this mobilization process. Therefore, involvement of the JAK2 pathway and especially of JAK2 mutation in bone marrow microenvironmental changes warrants further evaluation.
(4) PMF is an inflammatory disease characterized by elevated levels of circulating cytokines and chemokines that participate in HSC/HP proliferation and mobilization (SDF-1, HGF, IL-6, IL-8, SCF, VEGF, etc) as well as in promotion of fibrosis and angiogenesis (bFGF, TGF-β, PF4, VEGF, etc). These molecules are produced mainly by hematopoietic cells from the clone including CD34+ cells,41,50 megakaryocytes,47,50,72 and monocytes75,76 as well as by stromal cells within bone marrow and spleen.60-62 Thus, it is most likely that these cytokines and chemokines contribute to the regulatory humoral changes occurring within the medullar and spleen niches.
These considerations may also apply for the precursor stages of primary myelofibrosis, ET, and PV, implying a biologic continuum from JAK2V617F-positive ET over PV to the advanced myelofibrosis stage,77-80 with a steady increase in the JAK2V617F mutation load within this spectrum of disease phenotypes. However, the prognostic relevance of V617F allele dose in PMF remains controversial and suggests that additional individual variables act in concert, especially in nonmutated patients.80
Do cancer-initiating cells exist in PMF?
In PMF, although increasing evidence suggests that cells constituting the hematopoietic stem cell niches are altered, leading to marked modifications in the behavior of clonal neoplastic HSCs, the nature of the stem cell and the primitivity level of the initial oncogenic event are still unknown. This raises the questions of whether cancer-initiating cells exist in this disease and, if so, whether these cells are hematopoietic.
The existence of leukemic stem cells in acute myeloid leukemia (AML) and during the acute phase of chronic myeloid leukemia has been clearly demonstrated81 ; however, their existence in Ph− myeloproliferative disorders and especially in PMF is a fully open issue. Generation of leukemia in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice after transplantation of PMF CD34+ cells is a rare event that is associated with progression of the disease to acute leukemia.82 Several hypotheses are proposed to explain these results. First, the slow rate of the PMF hematopoietic clone could take much longer time to engraft compared with AML or CML cells. Second, additional somatic mutations or epigenetic events occurring in HSC/HPs are needed for the development of fully malignant disease. Third, it is also likely that specific interaction between HSCs and stromal cells are essential to this process. Actually, the dialogue between the NOD/SCID microenvironment and PMF CD34+ cells could be complicated, and even impossible, due to differences between murine environment and human niches. Very recently, Saito et al83 have shown that transplantation of PMF CD34+CD38− HSCs could recapitulate PMF in newborn NOD/SCID/IL2rg-null mice. Their demonstration that initiating cells were contained within the CD34+CD38− fraction and that this stem cell fraction possessed differentiation capacity to fibroblast strengthened the importance of stromal cells in the development of the disease. Therefore, it could not be excluded that yet unknown mutations/alterations within the microenvironment components could also be required to promote/maintain the myeloproliferative-like disease phenotype as suggested in RAR gamma– or in RB-null mice.15,16 Whatever the underlying mechanisms, altogether these data strongly support the importance of humoral and/or cellular components of the niche(s) and of interactions between hematopoietic and stromal cells in the development and progression of PMF.
Although the clonality of hematopoietic stem cells in PMF is obvious,13,37,84,85 the contribution of mesenchymal and/or endothelial stem cells—being part of the hematopoietic niche—to the malignant clone is still a matter of debate. Several arguments, including the presence of the JAK2 mutation, suggest that, like in CML, a bipotent hemangioblastic stem cell could participate in the neoplastic process.86 However, there is no evidence indicating that mesenchymal stem cells belong to the malignant clone.35,87 Therefore, do unknown mutations within the microenvironment components and do cancer-initiating cells exist in PMF? Until now, the absence of recurrent genomic abnormalities in PMF has not allowed for a conclusion on this challenging issue.
Beside the potential conceptual importance of the existence of cancer-initiating cells in PMF, their roles in the development of the disease and their impact on treatment strategies could be questionable. Actually, is the presence of cancer stem cells required to label a disease as cancer/leukemia with a fatal prognosis and, as questioned by Abbott, “Is targeting cancer stem cells a way to finish tumors off once and for all?”88 (p742)
Unanswered questions and future directions
Progress in the understanding of the role of hematopoietic microenvironment in PMF is obvious. However, several concerns still remain to be addressed.
Among the questions are the following: Are bone marrow niches specifically altered and why is bone marrow stem cell homing changed? Are spleen niches newly created or reinitialized and how is spleen stem cell homing developed? Why are bone marrow/endosteal niches disadvantaged to the benefit of spleen/endothelial niches? Do PMF HSCs have different sensitivity with respect to the 2 types of niches and, if so, is there a role for the JAK2 and MPL mutations in this altered sensitivity? Are there correlations between clinical phenotype (in terms of degree of CD34+/immature circulating cells and of splenomegaly) and alterations of the niches? Are niche-initiating stem cells (MSCs, ESCs) mobilized? Mobilization of ESCs in the peripheral blood of patients is restricted to an early biologic phase of the disease and precedes that of CD34+ hematopoietic cells.67 This suggests that migration of ESCs and likely that of MSCs are important in setting up premetastatic niches for hematopoietic stem/progenitor cells in the spleen and liver. Taking into account the importance of endothelial cells in the pathogenesis of MPDs, it would be of importance to analyze the potential alterations of the hematopoietic microenvironment in PV and ET patients and to compare them with those of PMF patients. Recent results from Xing et al89 indicate that stroma–stem cell interactions are dynamic over a lifetime and result in physiologically relevant changes in the biology of primitive hematopoietic cells together with age. As most of PMF patients are older than 60 years, it could be therefore questioned whether age would influence changes in the niche microenvironment. With regard to the fact that the JAK2V617F dose increases with disease progression,90 the influence of time in the pathological process could be of concern in PMF, as well as in JAK2-mutated PV and ET patients.
Histomorphometric and immunologic studies on bone marrow and spleen sections are currently in progress to demonstrate the existence of niche alterations and to characterize these changes. Recent results from Schmidt et al showing that an uncoupling of bone remodeling with an increase in bone formation rate is evidenced in patients91 are in agreement with an alteration of the endosteal niche in PMF. In vitro hematopoietic niche models, in which interactions between the different cellular and/or environmental components of both endosteal and vascular niches could be analyzed in tridimensional cultures, are currently under development. They should facilitate dissecting intimate mechanisms of interactions between HSCs and their niches as well as their role in the pathogenesis of PMF. Finally, the use of experimental animal models, especially of JAK2 and MPL transgenic mice,8,92-95 should put into perspective the potential contribution of these mutations in the altered interactions of HSCs with their niches in terms of proliferation and homing.
Conclusions and therapeutic challenge
Are stem cell niche alterations an inescapable requisite for PMF development?
PMF is a complex disease in which the physiopathological mechanism involves many types of stem cells, some of them being clonal. Hematopoietic stem cells are known to belong to the pathological clone, and remissions observed in young patients subsequent to a hematopoietic cell transplantation96 strongly support the assumption that genetic lesion(s) of HSCs take part in the genesis of the disease. As a consequence, PMF could be considered a hematopoietic stem cell disease.
However, as outlined in this review, evidence is increasing that environmental cells participating in HSC regulation, whether or not they belong to the pathological clone, contribute in a crucial way to the development of the disease through specific and mutually dependent interactions with pathological HSCs. Therefore, the possibility exists that during allogeneic transplantation, the normal donor hematopoietic cells permit the recipient microenvironment to recover and function normally. Actually, the aberrant clonal megakaryocyte proliferation is considered to have a major role in regulating niche cells by the release of several growth factors that are mitogenic for fibroblast and endothelial proliferation. Some evidence also supports the fact that microenvironmental cells and especially endothelial cells could be transplanted successfully and participate in the repair of the marrow vascular niche following stem cell transplantation.97,98 Therefore, by participating in the recovery of a physiological regulatory environment, cells—including mesenchymal and/or endothelial cells provided by the healthy donor—would favor extinction of the clonal hematopoiesis to the benefit of the normal hematopoiesis.
Besides the conceptual importance of this issue, new insights into the possible role of hematopoietic niche deregulation in the pathogenesis of PMF should open innovative therapeutic strategies for patients whose treatment has been largely palliative until now. The lack of efficient treatment is most likely because chemotherapy cannot eradicate malignancies derived from pluripotent stem cells but can only temporarily reduce the leukemic clone.99 This lack could also be linked to the complexity of the disease, resulting in the plurality of potential therapeutic targets.
Obviously, therapies must target the pathological hematopoietic clone, and trials testing the efficacy of agents directed against the underlying cellular and genetic lesions that lead to this disorder are in progress. Small-molecule inhibitors of JAK2, which are currently being studied, appear to be promising therapeutic agents. Actually, several reports have demonstrated their ability to inhibit the aberrant JAK2V617F along with the wild-type JAK2.100,101 However, the effect of JAK2 inhibition on disease progression and especially on blast transformation in patients, whether or not they harbor the JAK2 mutation, is not yet elucidated, thus inciting a careful and long-term follow up of treated patients. It is of interest that in CML, imatinib and dasatinib are very efficient kinase targeted therapies that can control the disease for very long periods of time but without definitively curing the disease.
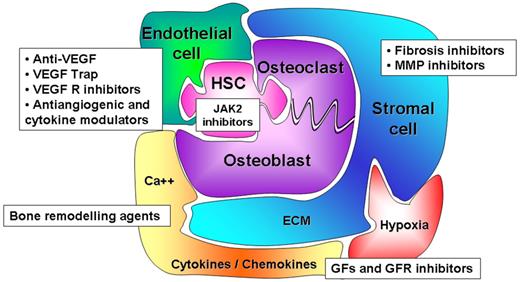
Therapeutic research in PMF must also target hematopoietic niches to manipulate the competitive balance between endosteal and vascular niches, thereby modifying stem cell proliferation, trafficking, and homing. Indeed, drugs that would force specific niches to reassume a normal function would likely limit the proliferation and dissemination of the malignant clone while restoring normal hematopoiesis.102 According to this hypothesis, several drugs targeting either cellular or acellular components of the hematopoietic niches, altered in PMF patients, are currently being assessed (Figure 4). Among them, NF-κB/inhibitors of proteasome, specific antibodies or soluble receptors, inhibiting either the production and/or the immunomodulatory activity of fibrogenic and angiogenic cytokines such as TGF-β and VEGF have been tested in murine models103 and in patients.100,101
Hematopoietic niches: a new therapeutic target for PMF? New therapeutic strategies must target HSCs, and JAK-STAT is a legitimate target pathway as demonstrated by recent results with JAK2 inhibitors. However, they must also target the different cellular and/or acellular components of hematopoietic niches to manipulate the balance of competition between endosteal and vascular niches and therefore to modify the stem cell outcome. Actually, therapeutic strategies such as anti–TGF-β, anti-VEGF or inhibitors of their receptors, bone remodeling drugs, fibrosis and metalloprotease (MMP) inhibitors are in progress in PMF.
Hematopoietic niches: a new therapeutic target for PMF? New therapeutic strategies must target HSCs, and JAK-STAT is a legitimate target pathway as demonstrated by recent results with JAK2 inhibitors. However, they must also target the different cellular and/or acellular components of hematopoietic niches to manipulate the balance of competition between endosteal and vascular niches and therefore to modify the stem cell outcome. Actually, therapeutic strategies such as anti–TGF-β, anti-VEGF or inhibitors of their receptors, bone remodeling drugs, fibrosis and metalloprotease (MMP) inhibitors are in progress in PMF.
Other experimental therapeutics including (1) chromatin-modifying agents targeting the epigenetic mechanisms104 supposed to be involved in silencing genes impeded in CD34+ cell proliferation and mobilization and (2) small-molecule inhibitors of proteases, participating in the CD34+ mobilization and, consequently, in the extramedullary hematopoiesis are also in development.100,101 Recently, several studies have also shown that bisphosphonates are able to induce apoptosis in cancer cells. Among bisphosphonates, the antiproliferative, antiapoptotic, and bone-remodeling effects of zoledronic acid may be further enhanced by combining it with statins.105 Besides their tumor-apoptotic effects, statins inhibit macrophage activation and secretion of various proteolytic enzymes, including metalloproteinases. They also down-regulate the expression of urokinase-type plasminogen activator receptor (u-PAR), which modulates the bone marrow proteolytic environment. Zoledronic acid may also inhibit malignant cell proliferation through enhancement of immune-mediated cytotoxicity against the tumoral cells.106,107 Accordingly, as a result of their numerous biologic activities on hematopoietic microenvironment, statins and zoledronic acid are promising therapeutic agents that deserve to be tested in PMF patients.105
In contrast to CML in which tyrosine kinase inhibitors used alone have considerably increased the survival of patients,108 the complexity of the pathophysiological process that underlies the development of PMF implies a more global therapeutic approach. Such strategies should include combination treatment with drugs targeting the molecular hematopoietic stem cell defect but also targeting the numerous cellular, humoral, and environmental factors that regulate the proliferation and dissemination of the pathological clone. It is unlikely that chemotherapy or biotherapies could definitively cure PMF patients, for whom the prognostic is poor, but they could offer them a longer survival and a better quality of life. Associated with a tolerable immune-specific treatment, targeted biotherapies could challenge allogeneic transplantation, which is currently the only treatment allowing a cure for some young patients. Actually, since many patients with PMF are older than 60 years, this approach is rarely possible.
In conclusion, whereas the concepts of “stem cell niches” and “tumor niches” are quite old, direct evidence of their in vivo role in human hematopoiesis is still missing. The demonstration of a role for hematopoietic niche alterations in the development of a myeloproliferative disease such as PMF would transform a concept into reality and would make these niches a new challenging therapeutic target!
The online version of this article contains a data supplement.
Acknowledgments
The authors are very grateful to Dr Dominique Bonnet for a critical review of the paper.
M.-C.L.B.-K. is indebted to Dr Hugo R. Castro-Malaspina for having had the privilege to work with him and to share his knowledge and his expertise on primary myelofibrosis.
We apologize to authors whose papers have not been cited here due to space limitations.
This work was supported by grants from the Association Nouvelles Recherches Biomédicales (ANRB, Villejuif, France), the Association pour la Recherche contre le Cancer (ARC, Villejuif, France) no. 3232, The Institut National du Cancer (INCA, Paris, France) no. PL054 and 2007-1-PL5-Inserm11-1, the Groupement d'Intérêt Scientifique (GIS, Paris, France)–Institut des Maladies Rares no. 03/GIS/PB/SJ/no. 35, the Laurette Fugain Association (Paris, France) no. 922, the Fondation de France (Paris, France) no. 032145/2005004819, and the European Union-EUMNET Project (QLRT-2001-01123).
This work has been performed as part of the European Leukemia-Net WP9 within the 6th European Community Framework Program for Research and Technological Development.
The French Inserm and the European EUMNET networks on Myelofibrosis can be found in the Supplemental Appendix, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Authorship
Contribution: J.-J.L., H.C.H., M.-C.M., C.J., and M.-C.L.B.-K. did the required background research for this paper and wrote the paper; O.P.-L. participated in discussions and in figure preparation; G.U. participated in discussions and checked the final version; and all the authors have read the paper, concur with its content, and state that it has not been submitted elsewhere.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marie-Caroline Le Bousse-Kerdilès, Inserm U602, André Lwoff Institute, Paul Brousse Hospital, 14, Avenue Paul-Vaillant Couturier, 94807 Villejuif Cedex, France; e-mail: caroline.le-bousse-kerdiles@inserm.fr.