In this issue of Blood, De Groot and colleagues report the identification and functional characterization of Tregitopes, which are Treg-activating regions in the Fc portion of the IgG molecule. This important finding has the potential to bring understanding about a number of phenomena related to Ig, including tolerance to Ab variable regions, the tolerogenic properties of immunoglobulin-Ag conjugates, the weak immunogenicity of Fc fusion proteins, and the therapeutic and regulatory effects of clinical preparations of IVIg on autoimmune and inflammatory diseases.
Immunoglobulin (Ig) has long been known to have tolerogenic properties. Thus, antigens (Ags) conjugated to Ig elicit tolerance rather than immunity,1,2 and intravenous administration of pooled Ig from multiple donors, known as intravenous immunoglobulin (IVIg), is used in clinical practice to treat autoimmune and inflammatory diseases.3 The reason for these tolerogenic effects of Ig is not understood, but recently IVIg has been shown to enhance human regulatory T cells (Tregs).4,5 This, together with the observation that Fc fusion proteins of soluble receptors and other bioactive molecules are either poorly or nonimmunogenic, and antibody (Ab) variable regions (to which central tolerance should not exist) do not elicit robust autoimmune responses, led De Groot et al to postulate that the Ig mol-ecule must contain regions or epitopes that are stimulatory to Tregs (ie, Tregitopes).
Using computational epitope mapping, the authors looked for consensus 9 amino acid regions in the human Ig molecule that would bind to multiple HLA class II molecules (on the premise that most Tregs are CD4-restricted). They identified 2 such clusters of major histocompatibility complex (MHC) binding motifs in the Fc molecule that could be presented to T cells. Predicted human Tregitope (hTregitope) sequences 167 and 289 were synthesized and were indeed shown to bind to multiple MHC class II molecules. Using a variety of Ags and culture conditions, the authors presented evidence that these Tregitope peptides activate as well as expand Tregs. The authors conclude that both natural Tregs (nTregs) and Ag-specific adaptive Tregs are affected. However, due to limitations of the experimental setup and the complexities of the human system, the distinction between effects on natural versus adaptive Tregs (as in humans, CD4+CD25high cells are a mixture of both) and between the expansion of preexisting FoxP3+cells versus their de novo conversion from conventional T cells is not always clear.
In the next step, the functional effects of Tregitopes on Ag-induced cytokine production and surface activation markers are documented using depletion experiments and Ag-MHC tetramers. The authors use a pool of immunogenic peptides derived from the complement component C3d (an autologous T-cell target) and birch pollen allergen tetramers to demonstrate that, in the presence of Tregitopes, the proinflammatory and allergic responses are attenuated, whereas the antiinflammatory cytokines are enhanced. Similarly, surface markers associated with regulatory function are enhanced, whereas markers associated with effector function are attenuated. Finally, the authors support their studies in vivo by using HLA-Tg mice immunized with house dust mite allergen and showing that coadministration of the murine equivalents of hTregitopes attenuates induction of responses to house dust mite allergen.
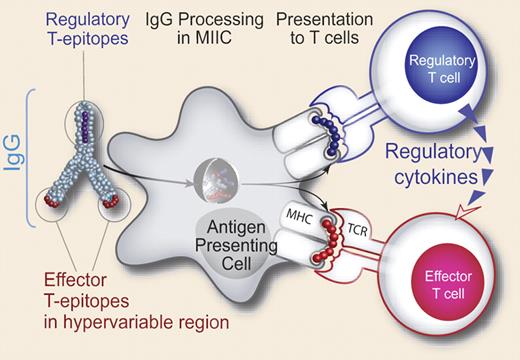
Hypothesized tolerizing mechanism of IgG. Conserved T-cell epitopes in IgG that engage nTregs have been discovered. The authors hypothesize that antibody-derived Treg epitopes (dark blue epitope) activate Tregs, leading to suppression of effector T cells that recognize effector epitopes (red epitope), like those of IgG hypervariable regions to which central tolerance does not exist. Whether this suppression is mediated by regulatory cytokines alone, or whether contact-dependent signaling also plays a role, has yet to be determined. See the complete figure in the article beginning on page 3303.
Hypothesized tolerizing mechanism of IgG. Conserved T-cell epitopes in IgG that engage nTregs have been discovered. The authors hypothesize that antibody-derived Treg epitopes (dark blue epitope) activate Tregs, leading to suppression of effector T cells that recognize effector epitopes (red epitope), like those of IgG hypervariable regions to which central tolerance does not exist. Whether this suppression is mediated by regulatory cytokines alone, or whether contact-dependent signaling also plays a role, has yet to be determined. See the complete figure in the article beginning on page 3303.
The authors hypothesize that Ab-derived Tregitope sequences presented on MHC class II+Ag-presenting cells activate Tregs, leading to down-regulation of effector cell activation and function via regulatory cytokines, as shown in the figure. The model does not take into account Ag dependence on activation/expansion of Ag-specific adaptive Tregs and does not address (but does not negate) the reported role of cell to cell contact in suppression by nTregs. Although it leaves open many questions, this is an important paper that helps to shed light on the well documented but poorly understood phenomena associated with the tolerogenic effects of immunoglobulins.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal