The signaling pathway vivisection of MM by Zöllinger and colleagues has elegantly isolated the Akt pathway using specific genetic and pharmacologic inhibitors and shown that some but not all MMs are critically dependent on akt signaling.
The signaling alchemists are searching for the philosopher's stone that divines a base signal for normal cells as a critical signal for tumor cells. Unfortunately, there appears to be a hopelessly intricate web of diverse signaling pathways (eg, Akt, MAPK, STAT3), many of which have previously been implicated as critical for the survival of multiple myeloma (MM). Instead of presenting a confusogram that unsuccessfully attempts to diagram and reconcile this complexity, the authors have adopted a reductionist approach and dichotomized MM into those that depend on Akt signaling and those that do not. The important clinical implication to their work is that some fraction of patients with MM will be expected to respond to treatment with an Akt kinase inhibitor, such as the selective, non-ATP–competitive inhibitor (Akti-1/2) used in their studies. Importantly, in order to identify patients with sensitive tumors, Zöllinger et al use a clinically useful marker: levels of phospho-Akt in MM cells detected by immunohistochemistry or flow cytometry.
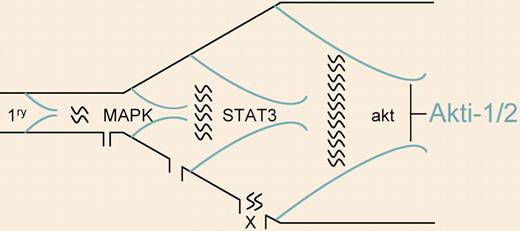
Signaling cascades in the progression of multiple myeloma. Following a primary event (eg, chromosome translocation involving immunoglobulin genes), activation of a variety of secondary signaling cascades (MAPK, STAT3, and Akt are shown in random order) is associated with tumor progression. In certain tumors, blockade of 1 pathway (by the Akt inhibitor akti-1/2, for example) inhibits growth and induces apoptosis. Given intratumor heterogeneity, it seems likely that alternative pathways associated with drug resistance (denoted by an X) will eventually be activated and that combined blockade of the signaling cascades may be more effective.
Signaling cascades in the progression of multiple myeloma. Following a primary event (eg, chromosome translocation involving immunoglobulin genes), activation of a variety of secondary signaling cascades (MAPK, STAT3, and Akt are shown in random order) is associated with tumor progression. In certain tumors, blockade of 1 pathway (by the Akt inhibitor akti-1/2, for example) inhibits growth and induces apoptosis. Given intratumor heterogeneity, it seems likely that alternative pathways associated with drug resistance (denoted by an X) will eventually be activated and that combined blockade of the signaling cascades may be more effective.
Although the authors exclude genetic inactivation of PTEN, further studies will be required to define the mechanism underlying the Akt activation seen in MM. The fact that it is seen in some but not all MM suggests that it does not represent a feature of normal plasma cell biology and more likely represents a tumor-specific transforming event. Intriguingly, the simultaneous analysis of multiple signaling pathways (Akt, MAPK, STAT3) in primary MM cells has shown that several tumors independent of Akt were likewise independent of MAPK and STAT3. The identities of the signals, if any, on which these latter tumors depend remain to be identified.
The limitations of this study are common to all preclinical functional studies in MM. The field is severely limited by the lack of faithful preclinical models, and in particular, by the inability to culture primary MM cells in vitro. Assays performed on primary cells, either alone or in contact with stromal cells, are restricted to the measurement of a change in the intrinsic rate of apoptosis that begins from the moment the tumors cells are extracted from the patient. It is not possible to routinely stimulate proliferation or expansion of primary cells in vitro. Although the use of stromal cells is helpful, it is not possible to duplicate in vitro the complex microenvironment of a human bone marrow. Myeloma cell lines are useful tools for discovery, but they differ significantly from primary tumor cells. Not only are they much more proliferative, but they also contain a variety of additional genetic changes, some of which are quite uncommon in primary patient tumors (eg, mutational inactivation of PTEN). Finally, the authors have not performed xenograft studies, in which the same cell lines that are responsive in vitro are grown and treated in immunodeficient mice. These studies would not have helped to define the role of the Akt pathway in MM, the main point of this paper, but would have spoken to the relative therapeutic index of Akt inhibition in vivo, which has been predicted to simulate a phenotype of insulin resistance.1
Unfortunately, the preclinical evaluation of drugs in MM, although very helpful in functionally defining important pathways, only rarely correlates with clinical activity in MM. Among recently approved drugs, thalidomide and lenalidomide have marginal activity in preclinical models of MM, and bortezomib, although very active in these models, has similar preclinical activity in models of other cancers that, in contrast, do not show clinical response. Conversely, a number of drugs have activity in preclinical models of MM, but lack clinical activity in patients. Dysregulation of Abl is a disease-inititating event in chronic myeloid leukemia, and its inhibition results in sustained disease control. On the other hand, it is likely that activation of the Akt pathway is a secondary event in MM, and that its inhibition in sensitive tumors may result in a transient response with eventual emergence of resistant tumor clones dependent on other pathways, as shown in the figure. Ultimately, combination signaling inhibition may prove the most effective means for providing long-term disease control.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal