Abstract
The cancer testis antigen (CTA) preferentially expressed antigen of melanoma (PRAME) is overexpressed in many hematologic malignancies, including chronic myeloid leukemia (CML). The sensitivity of CML to donor lymphocyte infusion after allogeneic stem cell transplantation suggests this tumor can be highly susceptible to cellular immunotherapy targeted to tumor associated antigens. We therefore tested whether functional PRAME-specific cytotoxic T lymphocytes (PRAME CTLs) could be generated and expanded from healthy donors and CML patients, or whether the limited immunogenicity of this CTA coupled with tumor-associated anergy would preclude this approach. Using optimized culture conditions and HLA-A*02–restricted PRAME-peptides, we have consistently generated PRAME CTLs from 8/9 healthy donors and 5/6 CML patients. These CTLs released IFNγ in response to PRAME peptides (between 113 ± 8 and 795 ± 23 spot forming cells/105 T cells) and lysed PRAME peptide–loaded cells (45 ± 19% at an effector:target [E:T] ratio of 20:1) in a MHC-restricted fashion. Importantly, these CTLs recognized and had cytotoxic activity against HLA-A*02+/PRAME+ tumor cell lines, and could recognize and respond to primary CML cells. PRAME CTLs were generated almost exclusively from the naive T-cell compartment, and clonal analysis showed these cells could have high αβTCR-peptide avidity. PRAME CTLs or vaccines may thus be of value for patients with CML.
Introduction
Achievement of complete cytogenetic remission in chronic myeloid leukemia (CML) has dramatically improved with the introduction of imatinib.1,2 Nonetheless, allogeneic hematopoietic stem cell transplantation (HSCT) remains a valid option for patients losing or not achieving cytogenetic response with imatinib.3-6 This benefit is mediated largely by T lymphocytes in the donor graft, and donor lymphocyte infusion can rescue 80% of CML patients who develop cytogenetic relapse after allogeneic HSCT.6-8 Unfortunately, the advantages of this graft-versus-leukemia (GVL) effect are frequently offset by the occurrence of severe graft-versus-host disease (GVHD).9 Considerable efforts have therefore been directed toward separating GVL from GVHD by identifying tumor-associated antigens (TAAs), such as proteinase-3 (PR3)10-12 or p210 bcr/abl proteins,13,14 and generating T lymphocytes that specifically recognize these antigens. Encouragingly, recent studies using whole-cell vaccines or peptides have successfully generated tumor-specific cytotoxic T lymphocytes (CTLs) in vivo and showed evidence for disease control.12,14
Cancer testis antigens (CTAs) represent a class of potential target antigens expressed on leukemic cells.15 One such antigen is preferentially expressed antigen of melanoma (PRAME),16 which is overexpressed in many acute and chronic leukemias.17 In malignant melanoma cells, PRAME overexpression makes a significant contribution to oncogenesis by inhibiting retinoic acid receptor signaling, which is crucial for development and cell differentiation.18 Although PRAME's contribution to leukemogenesis is not yet clear, its broad expression in CML cells and their known sensitivity to appropriately targeted effector T cells encouraged us to explore the generation of PRAME-specific cytotoxic T lymphocytes (PRAME CTLs) in healthy donors and in patients with CML.
Several obstacles must be addressed in effort to prepare TAA-specific CTLs for adoptive immunotherapy in cancer patients. First, with the exception of viral-associated antigens, such as Epstein-Barr virus (EBV)–derived antigens,19 most TAAs are only weakly stimulatory to the immune system. Second, many TAAs, including CTAs such as PRAME, are self-antigens, and responding T cells may be anergized or clonally deleted, leaving only small numbers of low-affinity or unresponsive circulating T cells available for expansion.20 Third, many patients with advanced hematologic malignancy have been treated extensively and may have few or abnormal professional antigen-presenting cells (APCs) capable of recruiting an immune response against such weakly stimulatory antigens. Finally, aberrant antigen processing mechanisms in tumor cells may preclude presentation even of highly expressed TAAs in a way that can be recognized by major histocompatibility class (MHC) I– and II–restricted CTLs.
In this paper we describe the use of an artificial APC line to overcome the need for large numbers of professional APCs to generate tumor-specific T cells, and the development of culture conditions that expand PRAME CTLs. We found that PRAME CTLs generated from normal donors and from patients with CML are specific for HLA-A*02–restricted PRAME peptides, and respond in a MHC-restricted manner to HLA-A*02+ /PRAME+ tumor cell lines and autologous CML blasts. These data suggest that PRAME is a suitable target for CML immunotherapy.
Methods
Cell lines and tumor cells
The following tumor cell lines were used: KT1 (CML) kindly provided by Dr Fujita (First Department of Internal Medicine, School of Medicine, Ehime University, Japan); BV173 (CML), L428 and HDLM-2 (Hodgkin lymphoma) and ME1 (acute myelogenous leukemia) from German Collection of Cell Cultures (DSMZ, Braunschweig, Germany); and U266B1 and ARH77 (multiple myeloma), K562 (erythroleukemia), and MRC-5 (normal human fetal lung fibroblasts) from ATCC (Rockville, MD). Cells were maintained in culture with RPMI 1640 medium (Hyclone, Logan, UT) containing 10% fetal bovine serum (FBS; Hyclone), 2 mM l-glutamine (GIBCO-BRL Invitrogen, Gaithersburg, MD), 25 IU/mL penicillin, and 25 mg/mL streptomycin (Lonza Walkersville, Walkersville, MD) in a humidified atmosphere containing 5% CO2 at 37°C.
Samples from CML patients and healthy donors
Bone marrow samples were collected from 50 CML patients after signed informed consent was obtained in accordance with the Declaration of Helsinki and after approval by the Institutional Review Board (IRB) of University of Naples Federico II (Table 1). For 8 CML patients (7 HLA-A*02+ and 1 HLA-A*02−) and 9 HLA-A*02+ healthy donors, peripheral blood was collected according to the local IRB-approved protocol (Baylor College of Medicine, Houston, TX). This protocol was approved for collecting anonymized CML samples (stripped of identifiers), as the major goal of this study was to evaluate the feasibility of generating PRAME CTLs.
PRAME mRNA expression in CML samples from patients at different stage of disease
| Pt . | Disease stage . | Treatment . | Copy number of PRAME, average ± SD (range) . | Level of PRAME mRNA expression . | |||
|---|---|---|---|---|---|---|---|
| N < 0.1 . | 0.1 < N < 1 . | 1 < N < 10 . | N > 10 . | ||||
| 20 | Dx | None | 3.46 ± 4.34 (0-18.3) | 1 (5%) | 4 (20%) | 14 (70%) | 1 (5%) |
| 14 | CP | < 60 mo IFN-α | 11.17 ± 22.79 (0-83.12) | 1 (7%) | 4 (29%) | 6 (43%) | 3 (21%) |
| 6 | CP | > 60 mo IFN-α | 24.31 ± 46.60 (0.4-118.8) | 0 | 1 (16%) | 3 (50%) | 2 (34%) |
| 8 | CP | < 60 mo IM | 17.85 ± 25.80 (0-65.83) | 1 (12%) | 3 (38%) | 1 (12%) | 3 (38%) |
| 10 | BC | IFN-α/IM | 95.97 ± 163.49 (1.19-515.98) | 0 | 0 | 3 (30%) | 7 (70%) |
| Pt . | Disease stage . | Treatment . | Copy number of PRAME, average ± SD (range) . | Level of PRAME mRNA expression . | |||
|---|---|---|---|---|---|---|---|
| N < 0.1 . | 0.1 < N < 1 . | 1 < N < 10 . | N > 10 . | ||||
| 20 | Dx | None | 3.46 ± 4.34 (0-18.3) | 1 (5%) | 4 (20%) | 14 (70%) | 1 (5%) |
| 14 | CP | < 60 mo IFN-α | 11.17 ± 22.79 (0-83.12) | 1 (7%) | 4 (29%) | 6 (43%) | 3 (21%) |
| 6 | CP | > 60 mo IFN-α | 24.31 ± 46.60 (0.4-118.8) | 0 | 1 (16%) | 3 (50%) | 2 (34%) |
| 8 | CP | < 60 mo IM | 17.85 ± 25.80 (0-65.83) | 1 (12%) | 3 (38%) | 1 (12%) | 3 (38%) |
| 10 | BC | IFN-α/IM | 95.97 ± 163.49 (1.19-515.98) | 0 | 0 | 3 (30%) | 7 (70%) |
Pt indicates patient number; N, normalized mRNA copy number (mRNA copy PRAME/Gusb*104); Dx, CML at diagnosis; CP, CML in chronic phase; BC, CML in blastic phase; IFN-α, interferon-α; and IM, imatinib (STI-571).
Professional and artificial APCs
Professional APCs: dendritic cells.
To generate dendritic cells (DCs), peripheral blood mononuclear cells (PBMCs) were selected for the CD14+ population using anti-CD14 beads (Miltenyi Biotech, Auburn, CA) and cultured in media (CellGenix, Antioch, IL) with IL-4 (1000 U/mL) and GM-CSF (800 U/mL; R&D Systems, Minneapolis, MN). On day 5, cells were matured with IL-6 (1 μg/mL), TNF-α (1 μg/mL), IL-1β (1 μg/mL), and PGE (1 μg/mL; all from R&D Systems) for 48 hours and then used to prime T cells after loading them with specific peptides.
Professional APCs: CD40-activated B cells.
To generate CD40-activated B lymphocytes, PBMCs were cocultured with MRC-5 cell line engineered to stably express human CD40L (> 90% positive) in AIM-V media (Invitrogen, Carlsbad, CA) supplemented with 5% human AB serum (Valley Biomedical, Winchester, VA) in the presence of IL-4 (500 U/mL) and Cyclosporine-A (1 mg/mL; Sandoz Pharmaceutical, Washington, DC).21 After 7 days of culture, the cells were plated under the same conditions. On day 14 of culture, more than 90% of the cells were CD19+ based on phenotypic analysis, and after loading them with specific peptides they were used to prime T cells.
Artificial APCs.
To generate artificial APCs (aAPCs), the K562 cell line was modified to express human HLA-A*02, CD80, CD40L, and OX40L22 molecules using sequential transduction with retroviral vectors. After transduction, K562 cells were selected by drug resistance (puromycin and neomycin), and single-cell fluorescence-activated cell–sorted (CD40L and OX40L) to obtain a K562/HLA-A*02+/CD80+/CD40L+/OX40L+ clone (K562/aAPCs). This clone was then expanded, and the expression of the transgenic molecules was monitored by fluorescence-activated cell sorting (FACS) analysis over time.
Generation and expansion of specific CTLs
CTL lines were generated from PBMCs obtained from 9 HLA-A*02+ healthy donors and 6 HLA-A*02+ CML patients.
Generation of virus-specific CTLs.
PBMCs were stimulated with K562/aAPCs (PBMCs:K562/aAPCs ratio 20:1) loaded either with NLVPMVATV (pp65-cytomegalovirus (CMV) or CLGGLLTMV (LMP-2 EBV) HLA-A*02–restricted peptides at 5 μM for 2 hours, then washed twice in complete media [RPMI 1640 45%, Click medium (Irvine Scientific, Santa Ana, CA) 45%, supplemented with 5% human AB serum (hABS) and 2 mmoL L-glutamine]. T cells were collected and stimulated weekly with K562/aAPCs loaded with the same peptides. After second round of stimulation, cells were expanded using IL-2 (50 U/mL; Proleukin, Chiron, Emeryville, CA) and hABS in the complete media was replaced with FBS.
Generation of PRAME peptide–specific CTLs.
The previously identified HLA-A*02–restricted PRAME peptides used in this study included ALYVDSLFFL, VLDGLDVLL, SLYSFPEA and SLLQHILGL.16 CD8+ cells were selected using magnetic antibodies (Miltenyi). These cells were primed with professional APCs (pAPCs; T cells:APCs ratio 20:1) loaded with tumor-associated HLA-A*02–restricted peptides, in complete media and in the presence of IL-7 (10 ng/mL), IL-12 (1 ng/mL), and IL-15 (2 ng/mL; all from R&D Systems). For peptide loading, pAPCs and aAPCs were incubated with a pool of the 4 HLA-A*02–restricted PRAME-peptides, each at 5 μM. This optimal peptide concentration was determined in preliminary titration experiments (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). After 2 hours incubation at 37°C in 5% CO2, cells were washed twice in PBS-1× to remove unbound peptides, resuspended in the appropriate media, and then used as stimulator cells for T lymphocyte activation. In a second set of experiments we used PBMCs from healthy donors and generated CTL lines using pAPCs and aAPCs loaded with the HLA-A*02– restricted ALY-peptide, which consistently recruits specific T cells from healthy donors.
After the first stimulation, T cells were collected and stimulated weekly with K562/aAPCs loaded with the same peptides. IL-7, IL-12, and IL-15 cytokines were added for the second stimulation. Subsequently, cells were expanded using IL-2 (50 U/mL) and complete media with FBS.
The same culture conditions were also used for additional HLA-A*02– restricted TAA, including ELAGIGILTV (from MART-1),23 RMFPNAPYL (from Wilms tumor-1, WT-1),12 VLQELNVTV (PR1 peptide from PR3 protein),11 KVAELVHFL (from MAGE-A3),24 ILAKFLMWL and RLVDDFLLV (from human telomerase, hTERT)25,26 and YMDGTMSQV (from tyrosinase, TYR).27 All peptides were obtained from Genemed Synthesis (San Antonio, TX).
In selected experiments, PRAME ALY-specific CTLs were generated using CD8+CD45RO+or CD8+CD45RA+ T cells obtained by negative immunomagnetic sorting (Miltenyi Biotech).28
Isolation and expansion of PRAME peptide–specific T-cell clones
Polyclonal T-cell lines obtained after 3 stimulations with K562/aAPCs loaded with ALY peptide were cloned by limiting dilution assay in 96-well plates in the presence of IL-2 (100 U/mL), CD3 antibody (50 ng/mL OKT3; Ortho Biotech, Bridgewater, NJ), and irradiated (4000 rad) allogeneic PBMCs as feeder cells, as previously described.19 The specificity of the T-cell clones was evaluated by measuring the IFNγ release in response to the specific PRAME peptide (ELIspot assay), and their cytotoxic activity was determined with a standard 51Cr-release assay against autologous phytohaemagglutinin (PHA)–activated blasts loaded with the specific PRAME peptide.
Immunophenotyping
Phycoerythrin (PE)–conjugated, fluorescein isothiocyanate (FITC)–conjugated and periodin chlorophyll protein (PerCP)–conjugated CD3, CD4, CD8, and CD56 monoclonal antibodies were used to stain T lymphocytes. Control samples labeled with an appropriate isotype-matched antibody were included in each experiment. Cells were analyzed by a FACScan (BD Biosciences, San Jose, CA) equipped with the filter set for 4 fluorescence signals. CTL lines were also analyzed for binding with specific tetramers, as previously described.29 Tetramers were prepared by Baylor College of Medicine core facility. For each sample, a minimum of 100 000 cells were analyzed using a FACSCalibur with the CellQuest software (BD Biosciences). To determine whether samples from normal donors and CML patients were HLA-A*02+, we used a specific FITC-labeled antibody. To evaluate the percentage of circulating malignant cells in CML samples, we used CD33 and CD34 monoclonal antibodies. All antibodies were from BD Biosciences.
ELIspot assay
The IFNγ ELIspot assay was performed as previously described.30 T cells were plated in triplicate and serially diluted from 105 to 104 cells/well, and then each PRAME peptide (5 μM) was added. In all experiments, T cells were also incubated with an irrelevant peptide to show the specificity of IFNγ release. As positive control, T cells were stimulated with 25 ng/mL phorbol myristate acetate (PMA) and 1 μg/mL ionomycin (Iono; Sigma-Aldrich, St Louis, MO). To measure the αβTCR avidity, CTL lines and clones were stimulated with specific peptides from 200 to 0.02 nM and IFNγ+ SFC enumerated (Zellnet Consulting, Fort Lee, NJ).
Chromium release assay
The cytotoxic specificity of T cells was evaluated using a standard 4-hour 51Cr release assay, as previously described.29 Target cells incubated in media alone or in 1% Triton X-100 (Sigma-Aldrich) were used to determine spontaneous and maximum 51Cr release, respec-tively. The mean percentage of specific lysis of triplicate wells was calculated as follows: [(test counts − spontaneous counts)/(maximum counts − spontaneous counts)] × 100.
Western blot analysis
Tumor cell line lysates, obtained from 5 × 106 cells, were resolved on a 7.5% SDS-PAGE. PRAME protein was detected by immunoblot using a rabbit polyclonal antibody (Ab32185; AbCAM, Cambridge, MA). Immunoblots were developed using enhanced chemoluminescence detection reagents (GE Healthcare, Little Chalfont, United Kingdom). Membranes were reprobed using the monoclonal anti-GAPDH Ab (Sigma-Aldrich).
Real-time qRT-PCR and fluorescence in situ hybridization analysis
PRAME mRNA was measured by real-time quantitative reverse transcription–polymerase chain reaction (qRT-PCR) analysis. Total RNA was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA) following the manufacturer's instructions. One microgram total RNA was reverse transcribed using SuperScript First-strand Synthesis System (Invitrogen) following the manufacturer's instructions. cDNAs from PRAME and beta-glucuronidase (GUSb, as housekeeping control) were separately amplified using primer and probe sequences that were designed, tested, and standardized by Applied Biosystems (Foster City, CA; HS00196132_m1 and Hs99999908_m1, respectively). All reactions were amplified in triplicate on an ABI Prism 7700 Sequence Detector (Perkin-Elmer, Foster City, CA) and the mean Ct values were used to calculate the transcript copy number by the formula: mRNA copy number = power (10,(Ct − 39.7)/− 3.47), where 39.7 and 3.47 are the intercept and the slope, respectively, of the appropriate plasmid standard curve. The normalized copy number for PRAME was determined as follows: (mRNA Copy number PRAME/mRNA copy number GUSb)*104. The threshold was systematically set at 0.1 to avoid problems of baseline creeping. The sensibility of PRAME detection by the described method is 10−1 normalized copies.
Statistical analysis
All in vitro data are presented as means plus or minus SD. Student t test was used to determine the statistical significant differences between samples, and P at less than .05 was accepted as indicating a significant difference.
Results
Functional validation of K562/HLA-A*02/CD80/CD40L/OX40L aAPC line (K562/aAPCs)
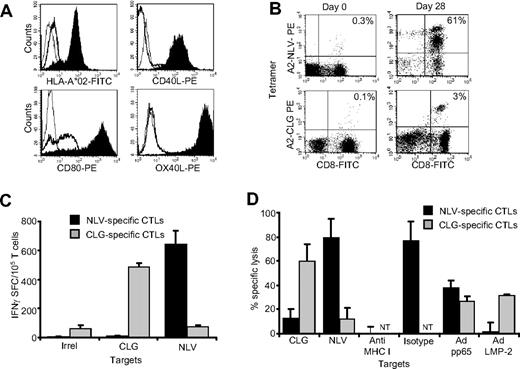
Many efforts to generate TAA-specific CTLs are limited by their requirement for large numbers of autologous professional APCs for repeated target cell stimulations. To overcome this problem, we prepared aAPCs derived from the K562 cell line that does not express MHC molecules. We engineered this line to express the HLA-A*02 molecule, and improved its costimulatory properties by coexpressing CD80, CD40L and OX40L molecules as described in “Methods.” This aAPC line stably expressed transgenic HLA-A*02, CD80, CD40L, and OX40L for more than 6 months (Figure 1A). In addition, expression was stable after several freeze-thaw cycles (data not shown).
Generation and validation of artificial antigen-presenting cells. The K562 cell line was modified using retroviral vectors to stably express transgenic HLA-A*02, CD80, CD40L, and OX40L. (A) The expression of the transgenic molecules in modified (K562/aAPC; filled profiles) and nontransduced cells (bold lines). Isotype controls are illustrated as thin lines. To assess functionality, K562/aAPCs loaded with either NLV or CLG peptides were used to reactivate NLV- or CLG-specific CTLs, respectively, from PBMC of CMV- or EBV-seropositive HLA-A*02 healthy donors. (B) Tetramer staining illustrates the enrichment of expanded CTLs in NLV and CLG T-cell precursors (right panels) after 28 days of culture, compared with PBMCs before ex vivo culture (left panels). (C) Frequency of CTLs responding to NLV (■) or CLG peptides ( ) as assessed by IFNγ ELIspot assay in a representative donor after 3 stimulations with aAPCs loaded with the specific peptides. IFNγ production in response to an irrelevant peptide (ELA peptide) was negligible. (D) Cytotoxic activity of these CTLs using a standard 51Cr release assay at an effector:target ratio of 20:1. Target cells were autologous PHA blasts loaded either with NLV or CLG-peptides, and autologous EBV lymphoblastoid cell lines (LCL) transduced with an adenoviral vector encoding the full protein of either pp6533 or LMP-2.34 Specific killing was MHC-restricted because it was inhibited by preincubation of PHA blasts with class I MHC blocking antibodies, but not with isotype. Shown is 1 representative experiment of 5 donors. NT indicates not tested.
) as assessed by IFNγ ELIspot assay in a representative donor after 3 stimulations with aAPCs loaded with the specific peptides. IFNγ production in response to an irrelevant peptide (ELA peptide) was negligible. (D) Cytotoxic activity of these CTLs using a standard 51Cr release assay at an effector:target ratio of 20:1. Target cells were autologous PHA blasts loaded either with NLV or CLG-peptides, and autologous EBV lymphoblastoid cell lines (LCL) transduced with an adenoviral vector encoding the full protein of either pp6533 or LMP-2.34 Specific killing was MHC-restricted because it was inhibited by preincubation of PHA blasts with class I MHC blocking antibodies, but not with isotype. Shown is 1 representative experiment of 5 donors. NT indicates not tested.
Generation and validation of artificial antigen-presenting cells. The K562 cell line was modified using retroviral vectors to stably express transgenic HLA-A*02, CD80, CD40L, and OX40L. (A) The expression of the transgenic molecules in modified (K562/aAPC; filled profiles) and nontransduced cells (bold lines). Isotype controls are illustrated as thin lines. To assess functionality, K562/aAPCs loaded with either NLV or CLG peptides were used to reactivate NLV- or CLG-specific CTLs, respectively, from PBMC of CMV- or EBV-seropositive HLA-A*02 healthy donors. (B) Tetramer staining illustrates the enrichment of expanded CTLs in NLV and CLG T-cell precursors (right panels) after 28 days of culture, compared with PBMCs before ex vivo culture (left panels). (C) Frequency of CTLs responding to NLV (■) or CLG peptides ( ) as assessed by IFNγ ELIspot assay in a representative donor after 3 stimulations with aAPCs loaded with the specific peptides. IFNγ production in response to an irrelevant peptide (ELA peptide) was negligible. (D) Cytotoxic activity of these CTLs using a standard 51Cr release assay at an effector:target ratio of 20:1. Target cells were autologous PHA blasts loaded either with NLV or CLG-peptides, and autologous EBV lymphoblastoid cell lines (LCL) transduced with an adenoviral vector encoding the full protein of either pp6533 or LMP-2.34 Specific killing was MHC-restricted because it was inhibited by preincubation of PHA blasts with class I MHC blocking antibodies, but not with isotype. Shown is 1 representative experiment of 5 donors. NT indicates not tested.
) as assessed by IFNγ ELIspot assay in a representative donor after 3 stimulations with aAPCs loaded with the specific peptides. IFNγ production in response to an irrelevant peptide (ELA peptide) was negligible. (D) Cytotoxic activity of these CTLs using a standard 51Cr release assay at an effector:target ratio of 20:1. Target cells were autologous PHA blasts loaded either with NLV or CLG-peptides, and autologous EBV lymphoblastoid cell lines (LCL) transduced with an adenoviral vector encoding the full protein of either pp6533 or LMP-2.34 Specific killing was MHC-restricted because it was inhibited by preincubation of PHA blasts with class I MHC blocking antibodies, but not with isotype. Shown is 1 representative experiment of 5 donors. NT indicates not tested.
To measure the function of these aAPCs, we first used 2 virus-derived antigens, pp65 (from CMV) and LMP-2 (from EBV). K562/aAPCs were loaded either with HLA-A*02–restricted NLV (CMV) or CLG (EBV) peptides and used to stimulate unfractionated PBMCs or CD8+ T cells obtained from CMV- or EBV-seropositive HLA-A*02+ healthy donors. As shown in Figure 1B, after 3 weeks of culture, a significant percentage of T cells was specific for either NLV or CLG peptides, as assessed by tetramer staining. These T-cell lines were also functional since they produced IFNγ on exposure to either NLV or CLG peptides (Figure 1C), and were cytotoxic to autologous PHA blasts loaded with NLV or CLG peptides and to autologous EBV-lymphoblastoid cell lines (EBV-LCL) transduced with an adenoviral vector to express pp6533 or LMP-2 proteins34 (Figure 1D). These data confirmed that K562/aAPCs can be used to generate and expand functional CTLs derived from effector-memory T cells and directed to immunogenic viral antigens.
Characterization of PRAME CTLs generated from HLA-A*02+ healthy donors
Having generated an unlimited source of biologically active aAPCs, we determined whether these cells were sufficiently potent to generate PRAME CTLs from the PBMCs of healthy individuals.
Initial priming with pAPCs and IL-15 is required to generate PRAME CTLs.
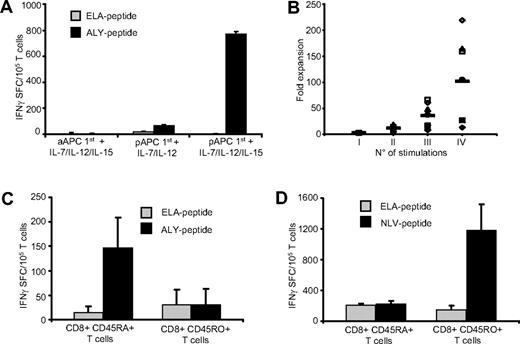
We stimulated CD8+ cells from healthy HLA-A*02+ donors with aAPCs loaded with PRAME peptides, using the same methodology validated for the generation of viral specific CTLs. In contrast to viral-specific CTL generation, however, K562/aAPCs loaded with PRAME peptides (ALY, VLD, SLY, and SLL) were insufficient to generate PRAME CTLs from PBMC (data not shown). We therefore substituted pAPCs (DCs or CD40-activated B cells) loaded with PRAME-peptides, and we also added a combination of cytokines that included IL-7, IL-12, and IL-15. As shown in Figure 2A, priming with pAPCs in combination with IL-7 and IL-12 cytokines induced the generation of PRAME CTLs. The addition of IL-15 to the cytokine cocktail, but not IL-15 alone, significantly improved the generation of these CTLs. We also found that both DCs and CD40L-activated B cells were equally efficient in generating PRAME CTLs (data not shown). Once generated, PRAME CTL lines could then be significantly expanded to numbers potentially useful for clinical application by substituting PRAME-peptides loaded K562/aAPCs and IL-2 in subsequent cultures to produce a median 102-fold expansion after 3 rounds of K562/aAPCs exposure (range, 13- to 218-fold; Figure 2B).
PRAME CTLs derive from the naive T-cell subset. To generate PRAME CTLs from healthy donors, CD8+ T cells were stimulated weekly with peptide-loaded K562/aAPCs in the presence of IL-7, IL-12, and IL-15, or primed with peptide-loaded autologous pAPCs in the presence of IL-7 and IL-12 with or without IL-15, then expanded with peptide-loaded K562/aAPCs. (A) Frequency of T cells responding (in an IFNγ ELIspot assay) to PRAME peptides; shown is ALY as representative peptide, ■) or an irrelevant peptide (ELA,  ). The number of IFNγ+ SFC was negligible when T cells were stimulated weekly only with peptide loaded K562/aAPCs. Although IFNγ+ SFC were detectable when T cells were primed with peptide-loaded pAPCs, only the addition of IL-15 to the cytokine cocktail significantly increased the generation of ALY-specific CTLs. Shown is 1 representative of 4 donors. (B) Fold expansion of PRAME CTLs primed with autologous pAPCs and stimulated with aAPCs for 3 weeks. Each symbol represents one of the 8 individual PRAME CTL lines and the horizontal lines represent the mean group value. (C) IFNγ+ SFC in response to ALY-peptide (■) or ELA-irrelevant peptide (
). The number of IFNγ+ SFC was negligible when T cells were stimulated weekly only with peptide loaded K562/aAPCs. Although IFNγ+ SFC were detectable when T cells were primed with peptide-loaded pAPCs, only the addition of IL-15 to the cytokine cocktail significantly increased the generation of ALY-specific CTLs. Shown is 1 representative of 4 donors. (B) Fold expansion of PRAME CTLs primed with autologous pAPCs and stimulated with aAPCs for 3 weeks. Each symbol represents one of the 8 individual PRAME CTL lines and the horizontal lines represent the mean group value. (C) IFNγ+ SFC in response to ALY-peptide (■) or ELA-irrelevant peptide ( ) of ALY-specific CTLs expanded from 3 donors from sorted naive (CD45RA+) and memory (CD45RO+) T-cell subsets. T cells producing IFNγ in response to the ALY peptide were significantly higher in CTL lines that originated from the naive (CD45RA+) T-cell subset. In contrast, CTLs generated against the viral peptide NVL (from the pp65 protein of CMV), were originated predominantly from the memory (CD45RO+) T-cell subset (D).
) of ALY-specific CTLs expanded from 3 donors from sorted naive (CD45RA+) and memory (CD45RO+) T-cell subsets. T cells producing IFNγ in response to the ALY peptide were significantly higher in CTL lines that originated from the naive (CD45RA+) T-cell subset. In contrast, CTLs generated against the viral peptide NVL (from the pp65 protein of CMV), were originated predominantly from the memory (CD45RO+) T-cell subset (D).
PRAME CTLs derive from the naive T-cell subset. To generate PRAME CTLs from healthy donors, CD8+ T cells were stimulated weekly with peptide-loaded K562/aAPCs in the presence of IL-7, IL-12, and IL-15, or primed with peptide-loaded autologous pAPCs in the presence of IL-7 and IL-12 with or without IL-15, then expanded with peptide-loaded K562/aAPCs. (A) Frequency of T cells responding (in an IFNγ ELIspot assay) to PRAME peptides; shown is ALY as representative peptide, ■) or an irrelevant peptide (ELA,  ). The number of IFNγ+ SFC was negligible when T cells were stimulated weekly only with peptide loaded K562/aAPCs. Although IFNγ+ SFC were detectable when T cells were primed with peptide-loaded pAPCs, only the addition of IL-15 to the cytokine cocktail significantly increased the generation of ALY-specific CTLs. Shown is 1 representative of 4 donors. (B) Fold expansion of PRAME CTLs primed with autologous pAPCs and stimulated with aAPCs for 3 weeks. Each symbol represents one of the 8 individual PRAME CTL lines and the horizontal lines represent the mean group value. (C) IFNγ+ SFC in response to ALY-peptide (■) or ELA-irrelevant peptide (
). The number of IFNγ+ SFC was negligible when T cells were stimulated weekly only with peptide loaded K562/aAPCs. Although IFNγ+ SFC were detectable when T cells were primed with peptide-loaded pAPCs, only the addition of IL-15 to the cytokine cocktail significantly increased the generation of ALY-specific CTLs. Shown is 1 representative of 4 donors. (B) Fold expansion of PRAME CTLs primed with autologous pAPCs and stimulated with aAPCs for 3 weeks. Each symbol represents one of the 8 individual PRAME CTL lines and the horizontal lines represent the mean group value. (C) IFNγ+ SFC in response to ALY-peptide (■) or ELA-irrelevant peptide ( ) of ALY-specific CTLs expanded from 3 donors from sorted naive (CD45RA+) and memory (CD45RO+) T-cell subsets. T cells producing IFNγ in response to the ALY peptide were significantly higher in CTL lines that originated from the naive (CD45RA+) T-cell subset. In contrast, CTLs generated against the viral peptide NVL (from the pp65 protein of CMV), were originated predominantly from the memory (CD45RO+) T-cell subset (D).
) of ALY-specific CTLs expanded from 3 donors from sorted naive (CD45RA+) and memory (CD45RO+) T-cell subsets. T cells producing IFNγ in response to the ALY peptide were significantly higher in CTL lines that originated from the naive (CD45RA+) T-cell subset. In contrast, CTLs generated against the viral peptide NVL (from the pp65 protein of CMV), were originated predominantly from the memory (CD45RO+) T-cell subset (D).
As a control, we also generated T-cell lines from HLA-A*02+ donors using K562/aAPCs without PRAME peptide loading. None of these T-cell lines had measurable specific response to PRAME peptides or PRAME-positive target cells (data not shown). To further validate the culture protocol optimized for the generation and expansion of PRAME CTLs, we used the same in vitro condition to generate CTLs specific for other TAAs such as WT1, MART-1, PR3, MAGE-A3, hTERT, and Tyr. These results are summarized in Figure S2, and they confirm that this approach was applicable to a variety of TAAs.
PRAME CTLs originate from a different T-cell subset to viral-specific CTLs.
We next investigated the mechanism responsible for the observed difference in APC requirements between CTLs specific for viral antigens versus the cancer testis antigen PRAME. Effector T cells specific for CMV/EBV derived viral antigens are primarily generated from memory T-cell subsets.35 We assessed whether CTLs specific for the self-antigen PRAME had the same origin. We prepared ALY-peptide specific CTLs from 3 healthy donors using sorted CD45RO+ and CD45RA+ T-cell fractions and the same culture conditions and cytokines described above. As shown in Figure 2C, ALY-peptide–responsive T cells were detected only in CTL lines originating from the naive (CD45RA+) T-cell subsets. In contrast, CTLs specific for the CMV-derived NLV peptide generated using the same culture conditions, were preferentially expanded from the memory (CD45RO+) T-cell population (Figure 2D). Hence antigen-dependent expansion of the (naive) PRAME-specific T-cell population has more rigorous requirements for costimulation during initial antigen presentation than (memory) viral-specific T cells.
Specificity and functionality of PRAME CTLs
We used multiple approaches to ensure that the PRAME CTLs expanded from the naive subset of T cells were specific and active against antigen-expressing targets.
Cytokine release
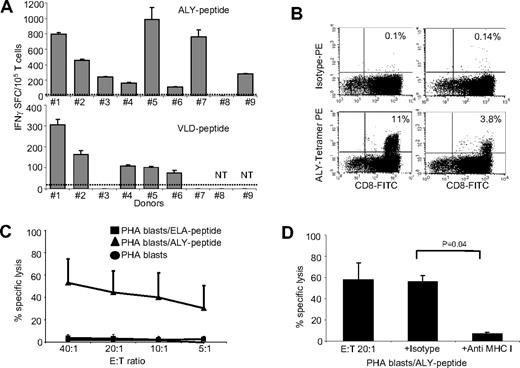
CTL lines generated using the peptide pool were tested for IFNγ release in response to each peptide individually, using a specific ELIspot assay. As shown in Figure 3A, we found that the majority of the expanded PRAME CTLs released IFNγ in response to at least 2 PRAME peptides (ALY and VLD in 8/9 and 5/7 donors, respectively). In addition, 2 CTL lines released IFNγ in response to SLY- or SLL-PRAME peptides. CTL line #2 recognized ALY, VLD, and SLY peptides and CTL line #1 responded to ALY, VLD, and SLL peptides. This observation suggests that ALY and VLD peptides are more dominant.16 IFNγ production was specific, as fewer than 25 IFNγ SFC/105 cells were produced when PRAME CTLs were incubated with media alone or with an irrelevant peptide (Figure 3A). Although IFNγ+ T cells were detectable in8 of 9 ALY-specific CTL lines, we could clearly identify ALY-tetramer+ cells in only 2 CTL lines (Figure 3B). To evaluate whether this discrepancy between IFNγ release and tetramer staining was due to variable affinity/avidity of the αβTCRs, we compared the numbers of IFNγ+ SFCs produced in response to decreasing concentrations of ALY-peptide (ranging from 200 to 0.02 nM) between ALY-tetramer+ and ALY-tetramer− CTLs. We found that ALY-tetramer+ CTLs produced more than 600 IFNγ+ SFC/105 cells when pulsed with 0.2 nM of peptide, whereas only 35 (± 8) IFNγ+ SFC/105 were produced by ALY-tetramer− CTLs (Figure S3A), suggesting that only CTLs with high avidity for ALY peptide were detected by staining with the specific tetramer.
PRAME CTLs can be reproducibly generated from HLA-A*02+ healthy donors. (A) illustrates the frequency of IFNγ + T cells responding to 2 PRAME peptides (ALY and VLD peptides) for PRAME CTL lines generated from 9 HLA-A*02+ healthy donors after 3 stimulations, as assessed by ELIspot assay. PRAME CTLs from donors 1 and 2 also targeted SLL and SLY peptides, respectively (see “Cytokine release”). Fewer than 25 IFNγ+ SFC were counted when CTL lines were pulsed with the ELA-irrelevant peptide (indicated by ----). (B) Staining with the ALY-specific tetramer of 2 PRAME CTL lines generated from healthy donors. (C) PRAME CTLs expanded from healthy donors were evaluated for their cytotoxic activity, using a standard 4-hour 51Cr release assay, against autologous PHA blasts loaded with ALY peptide (▴), ELA-irrelevant peptide (■), or no peptide (●). Data represent the means plus or minus SD of PRAME CTLs from 5 healthy donors. (D) Killing (20:1 E:T ratio) of ALY-loaded autologous PHA blasts by CTLs was significantly inhibited by preincubation of the targets with MHC class I antibody, but not by an isotype control, indicating MHC restricted killing. NT indicates not tested.
PRAME CTLs can be reproducibly generated from HLA-A*02+ healthy donors. (A) illustrates the frequency of IFNγ + T cells responding to 2 PRAME peptides (ALY and VLD peptides) for PRAME CTL lines generated from 9 HLA-A*02+ healthy donors after 3 stimulations, as assessed by ELIspot assay. PRAME CTLs from donors 1 and 2 also targeted SLL and SLY peptides, respectively (see “Cytokine release”). Fewer than 25 IFNγ+ SFC were counted when CTL lines were pulsed with the ELA-irrelevant peptide (indicated by ----). (B) Staining with the ALY-specific tetramer of 2 PRAME CTL lines generated from healthy donors. (C) PRAME CTLs expanded from healthy donors were evaluated for their cytotoxic activity, using a standard 4-hour 51Cr release assay, against autologous PHA blasts loaded with ALY peptide (▴), ELA-irrelevant peptide (■), or no peptide (●). Data represent the means plus or minus SD of PRAME CTLs from 5 healthy donors. (D) Killing (20:1 E:T ratio) of ALY-loaded autologous PHA blasts by CTLs was significantly inhibited by preincubation of the targets with MHC class I antibody, but not by an isotype control, indicating MHC restricted killing. NT indicates not tested.
Cytotoxic activity
We evaluated the cytotoxic activity of PRAME CTLs using standard 4-hour 51Cr release assays with autologous PHA blasts loaded with ALY peptide (as the response to this peptide was the most consistently detectable) or irrelevant peptide as target cells. As shown in Figure 3C, PRAME CTLs lysed PHA-blasts loaded with the ALY peptide (average 45% ± 19%; effector:target [E:T] ratio, 20:1; P < .001), but not PHA blasts loaded with an irrelevant peptide (< 10%). The killing mediated by PRAME CTLs was MHC class I–restricted since it was significantly inhibited by preincubation of target cells with anti-HLA class I antibody (7% ± 1.5%; E:T ratio 20:1), but not by preincubation with the appropriate isotype control antibody (56% ± 6%; E:T ratio 20:1; P = .04; Figure 3D). Notably, not only CTL lines with high avidity but also CTL lines with low avidity for PRAME peptides were cytotoxic to peptide-loaded PHA blasts (data not shown).
PRAME CTLs recognize naturally processed PRAME peptides
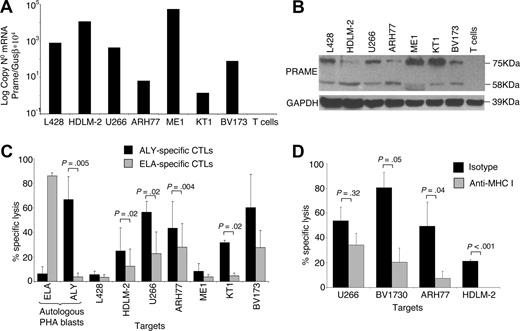
The capacity of PRAME CTLs to lyse target cells pulsed with peptides may not predict their recognition and lysis of tumor cells that are naturally expressing and processing PRAME antigens. Therefore, we measured the cytotoxic activity of PRAME CTLs against PRAME-expressing tumor cell lines. As shown in Figure 4A,B, screening of multiple HLA-A*02+ tumor cell lines using both qPCR and Western blot analysis demonstrated that HDLM-2, U266-B1, ARH77, ME1, KT1, and BV173 tumor cell lines expressed PRAME. We then used these tumor cell lines as targets to determine the cytotoxic activity of PRAME CTLs in a standard 4-hour 51Cr release assay. Because CTLs recognizing ALY peptide were consistently generated from healthy donors, we also generated CTLs in which pAPCs and aAPCs were loaded with ALY peptide exclusively. Cytotoxic activity against HLA-A*02+/PRAME+ tumor cell lines was evaluated in CTLs generated using pAPCs and aAPCs loaded either with the peptide pool (data not shown) or with the ALY peptide alone (ALY-specific CTL lines). CTL lines expanded using APCs loaded with the ALY peptide showed more consistent cytotoxic activity. As shown in Figure 4C, killing of HDLM-2, U266-B1, KT1, and BV173 tumor cells by ALY-specific CTL lines was higher than that mediated by CTLs reactivated against ELA peptide from MART-1 (lysis at 40:1 E:T ratio was 25% ± 19% vs 12% ± 14% for HDLM-2, P = .02; 57% ± 9% vs 23% ± 18% for U266-B1, P = .02; 49.4% ± 21.8% vs 27.9% ± 19% for ARH-77, P = .004; 8% ± 6% vs 4% ± 2% for ME-1, P = .5; 32% ± 2% vs 5% ± 2% for KT1, P = .02; 60% ± 27% vs 28% ± 14% for BV173, P = .088; Figure 4C). Killing of autologous PHA blasts loaded with an irrelevant peptide and killing of the PRAME+ but HLA-A*02− L428 cell line were both less than 10%. Killing mediated by CTLs was MHC class I–restricted (Figure 4D), as it was inhibited by preincubation of target cells with an anti-HLA class I antibody (U266-B1: 34% ± 13%; BV173: 21% ± 16%; ARH77: 8% ± 8%; HDLM-2: 0% ± 5%), but not with the appropriate isotype control antibody (U266-B1: 54% ± 15%, P = .32; BV173: 80% ± 16%, P = .05; ARH77: 50% ± 26%, P = .04; HDLM-2: 22% ± 5%, P = .001).
ALY-specific CTLs are cytotoxic to PRAME+ tumor cell lines. (A) Expression of PRAME in several tumor cell lines as assessed by qPCR. (B) Expression of PRAME in the same cell lines by Western blot analysis. In addition to the predicted band (58 kDa), another band of approximately 75kDa was observed, likely originated from posttranscriptional modifications. (C) Cytotoxic activity of ALY-specific CTLs (■) toward tumor cell lines, evaluated using a standard 51Cr release assay. As negative controls, we used (i) CTLs specific for another HLA-A2*02 tumor-restricted epitope (ELA from MART-1 protein;  ); (ii) autologous PHA blasts loaded with ELA-irrelevant peptide; (iii) the L428 tumor cell line (PRAME+ but HLA-A*02−). As positive control we used autologous PHA blasts loaded with the ALY peptide. Data at a CTLs:tumor cells ratio of 40:1 are shown. Overall, killing of PRAME+/HLA-A*02+ was significantly higher than control cell killing. (D) Killing of PRAME+/HLA-A*02+ cell lines was inhibited by preincubation with MHC class I antibody but not with isotype control, confirming MHC class I restriction.
); (ii) autologous PHA blasts loaded with ELA-irrelevant peptide; (iii) the L428 tumor cell line (PRAME+ but HLA-A*02−). As positive control we used autologous PHA blasts loaded with the ALY peptide. Data at a CTLs:tumor cells ratio of 40:1 are shown. Overall, killing of PRAME+/HLA-A*02+ was significantly higher than control cell killing. (D) Killing of PRAME+/HLA-A*02+ cell lines was inhibited by preincubation with MHC class I antibody but not with isotype control, confirming MHC class I restriction.
ALY-specific CTLs are cytotoxic to PRAME+ tumor cell lines. (A) Expression of PRAME in several tumor cell lines as assessed by qPCR. (B) Expression of PRAME in the same cell lines by Western blot analysis. In addition to the predicted band (58 kDa), another band of approximately 75kDa was observed, likely originated from posttranscriptional modifications. (C) Cytotoxic activity of ALY-specific CTLs (■) toward tumor cell lines, evaluated using a standard 51Cr release assay. As negative controls, we used (i) CTLs specific for another HLA-A2*02 tumor-restricted epitope (ELA from MART-1 protein;  ); (ii) autologous PHA blasts loaded with ELA-irrelevant peptide; (iii) the L428 tumor cell line (PRAME+ but HLA-A*02−). As positive control we used autologous PHA blasts loaded with the ALY peptide. Data at a CTLs:tumor cells ratio of 40:1 are shown. Overall, killing of PRAME+/HLA-A*02+ was significantly higher than control cell killing. (D) Killing of PRAME+/HLA-A*02+ cell lines was inhibited by preincubation with MHC class I antibody but not with isotype control, confirming MHC class I restriction.
); (ii) autologous PHA blasts loaded with ELA-irrelevant peptide; (iii) the L428 tumor cell line (PRAME+ but HLA-A*02−). As positive control we used autologous PHA blasts loaded with the ALY peptide. Data at a CTLs:tumor cells ratio of 40:1 are shown. Overall, killing of PRAME+/HLA-A*02+ was significantly higher than control cell killing. (D) Killing of PRAME+/HLA-A*02+ cell lines was inhibited by preincubation with MHC class I antibody but not with isotype control, confirming MHC class I restriction.
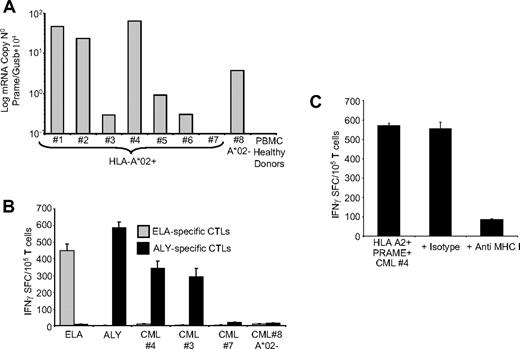
PRAME CTLs target PRAME+ CML tumor cells
Because PRAME has been reported previously to be overexpressed in CML tumor cells,17 we have analyzed its expression in 58 samples obtained from CML patients in different stages of disease.
As shown in Table 1, PRAME mRNA was broadly detectable using qPCR assay in our group of patients, since 46% of them showed between 1/104 and 10/104 copies of PRAME/GUSb mRNA, and 27% more than 10/104 copies. For 7 HLA-A*02+ CML patients (Figure 5A), sufficient PBMCs were available to perform culture experiments. To assess disease stage in these patients we evaluated the percentage of circulating CD33+ and CD34+ cells36,37 and FISH analysis.31,32 Samples from patients 1, 2, and 5 lacked circulating CD33+ or CD34+ cells, and cytogeneic analysis showed 100% of Philadelphia (Ph)–negative metaphases. In patient samples 3 and 6, circulating CD33+ cells were 41% and 44%, respectively, whereas CD34+ cells were 0% and 1.5%, respectively, suggesting that these patients were in chronic phase. For the sample obtained from patient 3, cytogenetic analysis showed 80% Ph+ metaphases. In patient sample 4 the percentages of circulating CD33+ cells and CD34+ cells were 86% and 50%, respectively, suggesting that this patient was in accelerated/blastic phase. Because CML samples 3 and 4 contained significant numbers of CD33+ cells, we used these samples as a target for PRAME CTLs generated from HLA-A*02+ healthy donors, measuring the CTLs response by IFNγ ELIspot assays. As controls, we used PRAME+ CML blasts from an HLA-A*02− patient (no. 8) with circulating blasts (CD33+ :77% and CD34+ :3%), or incubated MART-1-ELA-peptide specific CTLs with autologous CML blasts. As shown in Figure 5B, ALY-specific CTLs responded both to ALY peptide and to PRAME+/HLA-A*02+ CML blasts. In contrast, few IFNγ+ SFC were detected in response to CML blasts which lacked significant PRAME expression or which were PRAME+ but HLA-A*02− (Figure 5B). ELA-specific CTLs from the same donors also failed to respond to autologous CML cells (Figure 5B). Furthermore, blocking experiments using anti-HLA class I antibody showed that the production of IFNγ by ALY-specific CTLs in response to CML blasts was MHC restricted (Figure 5C).
PRAME CTLs can target PRAME+ CML tumor cells. (A) Expression of PRAME mRNA in PBMCs isolated from 8 patients with CML and from a pool of 4 representative healthy donors. In 1 of the HLA-A*02+ CML patients, PRAME mRNA was undetectable (< 10−1 normalized copies) as in healthy donors. (B) shows IFNγ production by PRAME CTLs (■) or by ELA-specific CTLs ( ) generated from healthy donors against blasts obtained from CML patients. Significant numbers of IFNγ + SFC were detected in response to PRAME+/HLA-A*02+ CML cells (patients 3 and 4) but not in response to HLA-A*02+ CML cells with undetectable PRAME expression (patient 7) or PRAME+/HLA-A*02− CML cells (patient 8). Negligible numbers of IFNγ+SFC were released by ELA-specific CTLs. (C) IFNγ production by PRAME CTLs in response to PRAME+/HLA-A*02+ CML cells is reduced by MHC class I antibody.
) generated from healthy donors against blasts obtained from CML patients. Significant numbers of IFNγ + SFC were detected in response to PRAME+/HLA-A*02+ CML cells (patients 3 and 4) but not in response to HLA-A*02+ CML cells with undetectable PRAME expression (patient 7) or PRAME+/HLA-A*02− CML cells (patient 8). Negligible numbers of IFNγ+SFC were released by ELA-specific CTLs. (C) IFNγ production by PRAME CTLs in response to PRAME+/HLA-A*02+ CML cells is reduced by MHC class I antibody.
PRAME CTLs can target PRAME+ CML tumor cells. (A) Expression of PRAME mRNA in PBMCs isolated from 8 patients with CML and from a pool of 4 representative healthy donors. In 1 of the HLA-A*02+ CML patients, PRAME mRNA was undetectable (< 10−1 normalized copies) as in healthy donors. (B) shows IFNγ production by PRAME CTLs (■) or by ELA-specific CTLs ( ) generated from healthy donors against blasts obtained from CML patients. Significant numbers of IFNγ + SFC were detected in response to PRAME+/HLA-A*02+ CML cells (patients 3 and 4) but not in response to HLA-A*02+ CML cells with undetectable PRAME expression (patient 7) or PRAME+/HLA-A*02− CML cells (patient 8). Negligible numbers of IFNγ+SFC were released by ELA-specific CTLs. (C) IFNγ production by PRAME CTLs in response to PRAME+/HLA-A*02+ CML cells is reduced by MHC class I antibody.
) generated from healthy donors against blasts obtained from CML patients. Significant numbers of IFNγ + SFC were detected in response to PRAME+/HLA-A*02+ CML cells (patients 3 and 4) but not in response to HLA-A*02+ CML cells with undetectable PRAME expression (patient 7) or PRAME+/HLA-A*02− CML cells (patient 8). Negligible numbers of IFNγ+SFC were released by ELA-specific CTLs. (C) IFNγ production by PRAME CTLs in response to PRAME+/HLA-A*02+ CML cells is reduced by MHC class I antibody.
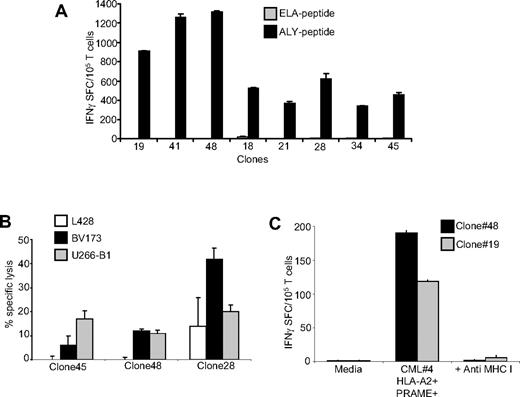
PRAME CTL single-cell clones
Because PRAME CTLs generated from healthy donors could be matched with primary PRAME+ cell lines or CML blasts only for HLA-A*02, to ensure that the reactivity we observed was genuinely PRAME-specific and not a manifestation of residual alloreactivity in the CTL lines, we cloned ALY-specific T lymphocytes from the bulk CTL cultures by limiting dilution. We were able to identify several clones (66/111 of the expanded clones) that produced IFNγ in response to the ALY peptide but not to an irrelevant peptide (Figure 6A). As shown in Figure 6B, we also confirmed that these clones were cytotoxic to HLA-A*02+PRAME+ cell lines (such as BV173 and U266-B1), but not to the HLA-A*02−PRAME+ cell line L428. In addition, we found that these clones could target primary CML blasts as assessed by IFNγ ELISpost assay. Figure 6C shows the response of 2 clones to CML blasts. Recognition was mediated through the conventional TCR as it was inhibited by preincubation with MHC class I antibodies.
Analysis of clones derived from PRAME CTLs. (A) Specificity of representative single-cell clones targeting the ALY peptide, generated by limiting dilution assay. These representative CTL clones produced significant amounts of IFNγ in response to the ALY peptide but not to ELA-irrelevant peptide. (B) Functional specificity of 3 representative ALY-specific T-cell clones against PRAME+ tumor cell lines. These clones lysed HLA-A*02+/PRAME+ cell lines (BV173, ■ and U266-B1,  ) but not the HLA-A*02−/PRAME+ cell line (L428, □). (C) Two representative ALY-specific T-cell clones produce significant amounts of IFNγ in response to blasts isolated from a HLA-A*02+/PRAME+ CML patient (patient 4) in a MHC class I–restricted fashion.
) but not the HLA-A*02−/PRAME+ cell line (L428, □). (C) Two representative ALY-specific T-cell clones produce significant amounts of IFNγ in response to blasts isolated from a HLA-A*02+/PRAME+ CML patient (patient 4) in a MHC class I–restricted fashion.
Analysis of clones derived from PRAME CTLs. (A) Specificity of representative single-cell clones targeting the ALY peptide, generated by limiting dilution assay. These representative CTL clones produced significant amounts of IFNγ in response to the ALY peptide but not to ELA-irrelevant peptide. (B) Functional specificity of 3 representative ALY-specific T-cell clones against PRAME+ tumor cell lines. These clones lysed HLA-A*02+/PRAME+ cell lines (BV173, ■ and U266-B1,  ) but not the HLA-A*02−/PRAME+ cell line (L428, □). (C) Two representative ALY-specific T-cell clones produce significant amounts of IFNγ in response to blasts isolated from a HLA-A*02+/PRAME+ CML patient (patient 4) in a MHC class I–restricted fashion.
) but not the HLA-A*02−/PRAME+ cell line (L428, □). (C) Two representative ALY-specific T-cell clones produce significant amounts of IFNγ in response to blasts isolated from a HLA-A*02+/PRAME+ CML patient (patient 4) in a MHC class I–restricted fashion.
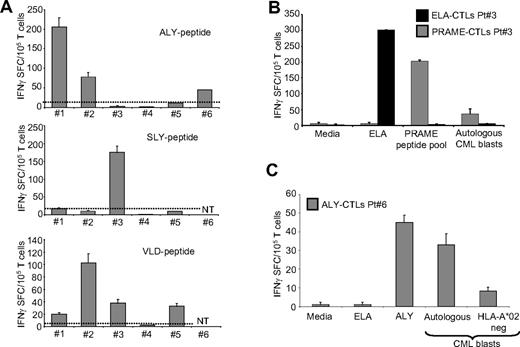
Generation of PRAME CTLs from CML patients
We next obtained T cells from the peripheral blood of 6 HLA-A*02+ CML patients, and primed them with autologous pAPCs loaded with the pool of PRAME-derived peptides (ALY, VLD, SLY, and SLL) in the presence of IL-7, IL-12, and IL-15. Subsequently we stimulated these cells with peptide loaded K562/aAPCs and IL-2. We were able to detect and expand PRAME CTLs from 5 of the 6 patients tested.
As shown in Figure 7A, in 3 patients we expanded T cells that produced significant amount of IFNγ in response to 2 different PRAME-peptides [ALY (205 ± 24 and 78 ± 11 IFNγ SFC/105 T cells) and VLD (20 ± 2 and 103 ± 14 IFNγ SFC/105 T cells), or SLY (175 ± 18 IFNγ SFC/105 T cells) and VLD (33 ± 6 IFNγ SFC/105 T cells)]. In 2 other patients, T cells generated responded to a single PRAME peptide, either ALY (45 ± 4 IFNγ SFC/105 T cells) or VLD (33 ± 2 IFNγ SFC/105 T cells), but not in response to an irrelevant peptide (all < 5 IFNγ SFC/105 T cells) as assessed by the specific ELIspot assay.
PRAME CTLs can be generated from HLA-A*02+ CML patients. (A) Frequency of IFNγ CTLs specific for ALY (top graph), SLY (middle graph), and VLD peptides (bottom graph) in 6 HLA-A*02+ CML patients. (B) PRAME CTLs produced IFNγ+ SFC in response to PRAME peptides and to autologous CML blasts. In addition, CTLs reacting against the ELA peptide (■) generated from the same patient did not produce IFNγ+ SFC when cocultured with autologous CML blasts. (C) PRAME CTLs produced IFNγ in response to autologous CML blasts but not to CML blasts from a PRAME+/HLA-A*02− patient (patient 8).
PRAME CTLs can be generated from HLA-A*02+ CML patients. (A) Frequency of IFNγ CTLs specific for ALY (top graph), SLY (middle graph), and VLD peptides (bottom graph) in 6 HLA-A*02+ CML patients. (B) PRAME CTLs produced IFNγ+ SFC in response to PRAME peptides and to autologous CML blasts. In addition, CTLs reacting against the ELA peptide (■) generated from the same patient did not produce IFNγ+ SFC when cocultured with autologous CML blasts. (C) PRAME CTLs produced IFNγ in response to autologous CML blasts but not to CML blasts from a PRAME+/HLA-A*02− patient (patient 8).
These PRAME CTLs obtained from CML patients also recognized autologous tumor cells. As shown in Figure 7B,C, we found that PRAME CTLs from the 2 donors tested produced IFNγ+ SFC when incubated with autologous PRAME+ CML blasts (56 ± 11 IFNγ+ SFC/105 T cells for patient 3 and 33 ± 6 IFNγ+ SFC/105 T cells for patient 6) but not with PRAME− CML blasts (Figure 7C). In contrast, CTLs generated from the same patient against the ELA peptide did not produce IFNγ when incubated with PRAME peptides (< 5 SFC/105 T cells) or with autologous CML blasts (3 ± 2 IFNγ+ SFC/105 T cells; Figure 7B). Unlike those from healthy donors, PRAME CTLs expanded from leukemic patients had low avidity for PRAME-derived peptides, as CTLs did not recognize PRAME tetramers (data not shown) and produced low amounts of IFNγ+ SFC when exposed to low concentrations (<2 nM) of specific peptides (Figure S3B).
Discussion
CML is highly susceptible to control by the immune system, and effector T cells recognizing TAAs overexpressed in tumor cells may play an important role in this process.6-8 The identification and validation of candidate antigens with clinical promise is crucial for developing immunotherapies for CML that can be translated to clinical trials. Here, we show that the cancer testis antigen PRAME is one such target. A combination of professional and artificial APCs and cytokines allows PRAME CTLs to be obtained from healthy donors and from CML patients with active disease. These PRAME CTLs recognize and kill tumor cell lines expressing PRAME and also specifically recognize blasts from CML patients expressing endogenous PRAME.
Our results suggest that PRAME may be a suitable target antigen for immunotherapy aimed at control of CML. The 2 most widely considered means of obtaining such an immune response are the use of peptide vaccination to recruit CTLs in vivo (“active” immunity), and the preparation of CTLs ex vivo for adoptive transfer (“passive” immunity). Both strategies are currently being evaluated in patients with hematologic malignancies and solid tumors using other putative target antigens.12,14,38,39
Although both active and passive approaches can induce objective clinical responses, adoptive transfer of tumor-specific CTLs, may be better suited than vaccination for the control of advanced disease, at least in patients with melanoma40 and EBV-associated malignancies.19,41-43 But although tumor-specific CTLs targeting viral antigens, such as those derived from EBV, are consistently expanded ex vivo from a pool of memory T cells,44 it has proved harder to consistently prepare CTLs specific for nonviral TAAs such as PRAME. The frequency of CTL precursors specific for TAA/CTA-derived peptides is usually very low, and these cells are rarely detectable in the peripheral blood of healthy individuals.12 Although it has been reported that PRAME CTL precursors can be detected in PBMCs freshly isolated from healthy donors,45 we found the frequency to be too low to be quantitated by IFNγ ELIspot assay in our cohort of healthy donors. Moreover, these low frequency cells may reside in the naive T-cell subpopulation, or be anergized, so that their recruitment and expansion require the use of potent APCs in combination with several cytokines. Our results show that PRAME CTLs were derived from naive CD45RA+/CD8+ T cells and that the requirement for pAPCs could not be overcome even by substituting an aAPC with a broad array of costimulatory components (CD80, CD40L, and OX40L), so that pAPCs provide more effective priming for naive T cells. We used B cells exposed to CD40L21 as our pAPCs because these cells could be reproducibly generated in sufficient numbers from the limited amount of blood available for the study. Although we did not perform a formal comparison between the effectiveness of CD40-activated B cells and DCs, in 1 case in which DCs and CD40-activated B cells were both available, we found no apparent difference. IL-15, a cytokine that plays an important role in breaking immunotolerance and in restoring the function of T cells that have been anergized or tolerized,46 was also essential for optimal expansion of PRAME CTLs from both healthy donors and CML patients. Fortunately, however, once this initial priming step is overcome, our results show that subsequent expansion steps have less stringent requirements and can be accomplished with an artificial APC and IL-2, simplifying implementation in clinical practice where autologous pAPCs may be limited in number.
The antitumor effect of adoptive T-cell therapies is likely to be enhanced when the infused T cells recognize more than one epitope of viral and/or tumor-associated antigens,19 because the risk of tumor escape is reduced.47 Therefore, we determined whether our PRAME responses were directed to more than one of the previously identified HLA-A*02–restricted PRAME epitopes.16 We found that CTL lines from both healthy donors and CML patients were directed against at least 2 PRAME-derived peptides, ALY and VDL. These CTL lines and clones were functional and could target not only peptide-loaded cells, but also tumor cell lines expressing PRAME and PRAME+ primary CML tumor cells. Hence, the PRAME peptides targeted by these CTL lines are naturally processed and presented in the context of MHC class I even by tumor cells.
In patients with leukemia, CTLs with the highest avidity for tumor-associated antigens such as PR1 may be deleted.20 Therefore, we examined whether CTL lines generated from healthy donors and CML patients had a different range of avidities for PRAME-derived peptides. We found that PRAME CTLs generated from CML patients with active disease were characterized by a lower avidity for PRAME-derived peptides than CTLs from normal donors. Thus, selective depletion of high-avidity CTLs in patients with leukemia may occur not only for PR1-specific CTLs,20 but also for PRAME CTLs, and may be a general immune evasion strategy. Our study was not designed to correlate PRAME expression specifically with disease stage, cytogenetic response, or treatment, so we cannot correlate these parameters with the generation of PRAME CTLs from CML patients. Of note, PRAME expression by CML cells may be regulated by epigenetic mechanisms,48 so that treatment with IFN and/or imatinib may alter PRAME expression and further modify the ability to expand PRAME CTLs from patients in any given stage of disease.
Given our results showing the antileukemic effects of PRAME CTLs, the administration of PRAME peptides to generate T cells in vivo represents a simple, and hence appealing, alternative to the preparation of PRAME CTLs for adoptive transfer ex vivo. Vaccine trials using peptides derived from other leukemia-associated antigens including p210 bcr/abl, PR1, and WT1 have shown promise, although their overall potency may be lower than that of adoptively transferred CTLs.12,14,20 However, the predominance of low-avidity CTLs in patients with active disease suggests that achievement of minimal residual disease with more conventional therapies before peptide vaccination may be required to allow the generation of T cells with higher avidity for the antigen.20
In conclusion, we have confirmed that PRAME is expressed by a significant proportion of CML samples17 and represents a potential target antigen both for adoptive T-cell therapy and for vaccination of CML patients. Because the highest-avidity CTLs are obtained from healthy donors, this antigen may be of particular value for generating a selective GvL effect after allogeneic stem cell transplantation. Ultimately, CTL administration may be used in sequence with peptide vaccines to maintain long-term immune surveillance.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Linda Chapman Golden Funds for the support and Dr Malcolm Brenner for critical revision of the manuscript.
This work was supported in part by the Leukemia & Lymphoma Society Specialized Center of Research (SCOR; grant no. 7018) and Leukemia & Lymphoma Society Translational Research grants (G.D. and B.S.), and by PRIN, MIUR-Rome PS35-126/IND Grants (Rome, Italy), Progetto Integrato Oncologia, Ministero Salute (Rome, Italy), Regione Campania.
Authorship
Contribution: C.Q., B.S., B.D.A., V.H., and G.D. designed and performed experiments; C.Q., G.D., F.P., and B.S. designed the research and analyzed the data; L.L., F.P., and M.M. provided CML samples; H.E.H. and C.M.R. provided expertise in T-cell generation and analyzed the data; C.Q., G.D., F.P., and B.S. wrote the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Barbara Savoldo, MD, Center for Cell and Gene Therapy, Baylor College of Medicine, 6621 Fannin Street, MC 3-3320, Houston, TX 77030; e-mail: bsavoldo@bcm.tmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal