Abstract
Production of a red blood cell's hemoglobin depends on mitochondrial heme synthesis. However, mature red blood cells are devoid of mitochondria and rely on glycolysis for ATP production. The molecular basis for the selective elimination of mitochondria from mature red blood cells remains controversial. Recent evidence suggests that clearance of both mitochondria and ribosomes, which occurs in reticulocytes following nuclear extrusion, depends on autophagy. Here, we demonstrate that Ulk1, a serine threonine kinase with homology to yeast atg1p, is a critical regulator of mitochondrial and ribosomal clearance during the final stages of erythroid maturation. However, in contrast to the core autophagy genes such as atg5 and atg7, expression of ulk1 is not essential for induction of macroautophagy in response to nutrient deprivation or for survival of newborn mice. Together, these data suggest that the ATG1 homologue, Ulk1, is a component of the selective autophagy machinery that leads to the elimination of organelles in erythroid cells rather that an essential mechanistic component of autophagy.
Introduction
Erythroid differentiation involves progression through morphologically distinct nucleated precursor stages, from proerythroblast to orthochromatic erythroblasts, prior to enucleation and maturation of the nascent reticulocyte. This process results in a successive reduction in cell volume, massive increase in hemoglobin production and conversion to a purely glycolytic pathway for energy production. Unlike most cells, mature red blood cells have no nucleus or organelles. Following enucleation of orthochromatic erythroblasts, nascent reticulocytes mature over the course of 48 to 72 hours and are cleared of all intracellular organelles, including mitochondria and ribosomes. Approximately 30% of red cell hemoglobin is produced in reticulocytes, and since heme is synthesized in the mitochondria these organelles are among the last to be eliminated (reviewed in Geminard et al1 ). The programmed clearance of mitochondria that occurs in reticulocytes makes it an ideal system for studying the molecular pathways involved in mitochondrial degradation. Understanding the process by which cells degrade mitochondria is important not only for red cell maturation, but also for other cell types as accumulation of damaged or dysfunctional mitochondria has been implicated in aging and various pathologic processes such as diabetes, cancer, neurodegeneration, and α1 antitrypsin disease (reviewed in Pieczenik and Neustadt2 ).
The molecular pathways involved in mitochondrial degradation in reticulocytes are unknown, although several important insights have been made into this process. 15-Lipoxygenase is a lipid-peroxidizing enzyme whose expression peaks in reticulocytes shortly before organelle degradation.3 It integrates into organelle membranes, disrupts mitochondrial membrane integrity allowing release of proteins from the organelle lumen and proteasome-dependent degradation of lumenal and integral membrane proteins.4,5 Chemical inhibition of 15-lipoxygenase impairs mitochondrial clearance in cultured reticulocytes.6 Recently, using a knockout mouse model, we demonstrated that the BH3-only protein, Nix, plays a critical role in mitochondrial degradation in reticulocytes.7 The presence of mitochondria in lysosomes and autophagic structures of maturing erythroid cells has long suggested that autophagy is involved in mitochondrial clearance8-10 ; however, the role of autophagy in this process has not been established.
Autophagy is a catabolic process through which cellular contents are delivered to lysosomes for degradation. There are several forms of autophagy. The best studied, macroautophagy, is a process in which cytosolic components (including organelles) are nonspecifically sequestered in double-membrane–bound vesicles, or autophagosomes, that mediate delivery to lysosomes (reviewed in Kundu and Thompson11 ). In contrast, in selective autophagy, specific components are targeted by autophagosomes. Although mitochondria may enter lysosomes via nonspecific macroautophagy, or through a selective autophagy-related process, the distinction between these pathways has only recently been made in yeast through characterization of the 32 δuth1 mutant.12,13 Generally, the processes involved in delivering mitochondria to lysosomes are referred to collectively as “mitochondrial autophagy” or “mitophagy.” Signals that may contribute to targeting mitochondria for degradation by the autophagy-lysosomal pathway include yeast mitochondrial proteins such as uth1p12 and aup1,14 the BH3-only protein Nix,7 oxidation of mitochondrial lipids by reactive oxygen species,15 and loss of mitochondrial membrane potential,16,17 which in some cases may be associated with mitochondrial fission and reduced OPA1 expression.18
Many of the genes involved in all forms of autophagy have been characterized in Saccharomyces cerevisiae. Several of these, including ATG1, ATG5, ATG6, ATG8, ATG9, ATG12, and ATG13, appear to contribute to mitochondrial autophagy,13,17,19,20 although there is some controversy regarding the role of ATG5 and ATG12.21 In yeast, atg1p (encoded by ATG1) is the only serine-threonine kinase in the autophagy pathway and a critical regulator in the induction of autophagy downstream of the Tor kinase, which coordinates cellular protein translation in response to nutrient availability (reviewed in Kamada et al22 ). The Tor kinase regulates the interactions of atg1p with other components of the autophagy machinery to switch from selective autophagy-related processes to nonselective macroautophagy under starvation conditions.23,24 The induction of macroautophagy helps to restore free pools of amino acids when nutrient availability is limiting.25 During this switch from selective to nonselective autophagy, atg1p recruits proteins to promote formation of larger autophagosomes.26,27 In addition, atg1p is also involved in the shuttling of an integral membrane protein, atg9p, which may contribute to recycling autophagosome membranes.28
Mouse and human ULK1 and ULK2 are ubiquitously expressed serine-threonine kinases that are evolutionarily related to S cerevisiae atg1p and Caenorhabditis elegans Unc51.29-31 Unc51 was initially identified based on the uncoordinated phenotype of the C elegans mutant after which it was named.32 Unc51, Ulk1, and Ulk2 are all implicated in the regulation of neurotransmitter trafficking and axonal growth,33-37 perhaps in part by modulating autophagy.38 Although Ulk1 and Ulk2 may share some functional redundancy, an RNAi screen performed in HEK293 cells expressing LC3-GFP identified ULK1, but not ULK2, as an important regulator of autophagy.39 In this study, we generate a new knockout mouse model, which provides the first genetic evidence that the mammalian atg1p homologue, Ulk1, plays an important role in clearance of mitochondria and RNA-containing ribosomes in reticulocytes, but does not play an essential role in starvation-induced autophagy.
Methods
All of the animal uses in these studies have been approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania.
Friend leukemia virus cultures
Erythroid proliferation was induced in CD1 mice by infection with the anemia-inducing strain of Friend leukemia virus (FVA). FVA cells were cultured as described previously.40 Briefly, erythroid precursors were isolated from the enlarged spleens of infected mice and induced to differentiate ex vivo by culturing with erythropoietin (EPO). Cultured cells were harvested and analyzed morphologically by May-Grünwald and benzidine stains. Slides were examined on a Leica Microsystems microscope (DM2500) using the 100× oil objective, and images were captured using a Leica DFC420 camera and Leica imaging software (Bannockburn, IL). Differentials were performed using previously described morphologic criteria.41
Quantitative reverse-transcription–polymerase chain reaction and Northern blot analysis
Total RNA was isolated from cells using TRIzol Reagent (Invitrogen, Frederick, MD). RNA (1 μg) was used to prepare cDNA using SuperScript II Reverse Transcriptase (Invitrogen) according to the manufacturer's instructions. All samples were normalized to 18S RNA transcript levels. Murine ulk1, ulk2, and map1lc3b primers and probes were purchased from Applied Biosystems (Foster City, CA). Samples were run on a 7900HT Sequence Detection System (Applied Biosystems) and analyzed using SDS 2.1 (Applied Biosystems). Northern blots were probed with a 1.1-kb SalI-PstI fragment of Ulk1 cDNA (IMAGE clone; Invitrogen) containing exons 1 to 15.
Western blot analysis and antibodies
Protein extracts were prepared by lysing cells in radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM EDTA, and 50 mM Tris·HCl, pH 8.0) supplemented with Complete EDTA-free protease inhibitor cocktail tablets (Roche Applied Science, Indianapolis, IN) and phosphatase inhibitor cocktails (Sigma-Aldrich, St Louis, MO). Cleared lysates were separated on 14% Tris-glycine gels or 4% to 12% Bis-Tris gels (Invitrogen) followed by transfer onto nitrocellulose. After blocking in 5% skim milk, blots were probed using the following primary antibodies: Ulk1 (N17; Santa Cruz Biotechnology, Santa Cruz, CA), mitochondrial Complex IV subunit IV (MS407; MitoSciences, Eugene, OR), LC3,42 and actin (AC-74; Sigma-Aldrich). Bands were detected using horseradish peroxidase–labeled secondary antibodies and enhanced chemiluminescence detection kit (Amersham, Arlington Heights, IL).
Electron microscopy
Samples for electron microscopy were fixed in 2.5% glutaraldehyde and 2.0% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.4, overnight at 4°C and processed as described previously.42 Sections were examined with a JEOL 1010 electron microscope (JEOL USA, Peabody, MA) fitted with a Hamamatsu digital camera system (Hamamatsu Photonics, Okayama City, Japan).
Generation of ulk1 floxed mice and murine embryonic fibroblasts
A targeting construct including a neomycin resistance cassette (neo) and a 5.6-kb EcoRI fragment containing exons I to III each flanked by loxP sites was generated. This construct was electroporated into R1 embryonic stem (ES) cells.43 Electroporation, selection, and maintenance of ES cells have been previously described.44 Southern blotting was used to identify clones that had undergone homologous recombination. Heterozygous ulk+/FL (C57B/6 and 129Sv mixed) mice were mated with EIIa-Cre (FVB/N) transgenic mice (The Jackson Laboratory, Bar Harbor, ME) to generate ulk+/− mice. These mice were mated and viable ulk−/− offspring were generated at expected Mendelian frequency. Primary murine embryonic fibroblasts (MEFs) were generated from matings of ulk1 heterozygous mice and immortalized by transfection with SV40 DNA as previously described.45 MEFs were grown in DMEM supplemented with 10% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine. RNA was prepared from ulk1+/+ and ulk1−/− MEFs, and Northern blots were probed with an ulk1 cDNA probe.
Complete blood count and reticulocyte cultures
Peripheral blood was analyzed on an Advia 2120 Hematology System (Siemens, New York, NY) calibrated daily in the clinical hematology laboratory at Children's Hospital of Philadelphia. Reticulocytosis was induced by daily intraperitoneal injection of 40 mg/kg phenylhydrazine (Sigma-Aldrich) for 5 days as previously described.8 Reticulocytes were harvested on day 7 or 8, and cultured in IMDM supplemented with 20% BIT (StemCell Technologies, Vancouver, BC), 0.1% monothioglycerol, 100 units/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine, and 0.5 mM adenine hemisulfate.
Flow cytometry
Reticulocytes and murine embryonic fibroblasts were stained with Mitotracker Green FM, Mitotracker Red FM, TMRM (all Invitrogen), Retic-Count/Thiazole-Orange (BD Biosciences, San Jose, CA), and/or antimouse CD71-PE (BD Biosciences) and analyzed using a FACSCalibur (BD Biosciences). Data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Measurement of citrate synthase activity
The specific activity of citrate synthase in whole-cell extracts prepared from MEFs was measured at 412 nm minus 360 nm (13.6 mM−1 cm−1) using 5,5-dithio-bis(2-nitrobenzoic acid) to detect free sulfhydryl groups in coenzyme A as described previously.46
Results
Induction of Ulk1 expression correlates with the onset of autophagic clearance of mitochondria during erythroid maturation
Infection of mice with an anemia-inducing strain of Friend leukemia virus (FVA) results in uncontrolled erythroid proliferation and splenic hypertrophy within 2 weeks. Erythroid precursors were harvested from the spleens of animals 2 weeks after infection and induced to differentiate by culturing in the presence of erythropoietin.40 After 1 hour, the cultures consisted predominantly of cells morphologically similar to proerythroblasts (PEs) and basophilic erythroblasts (BEs) (Figure 1A left panel), which differentiated through to the reticulocyte stage by 48 hours (Figure 1A center panel). The FVA culture system can be used to follow gene expression during erythroid maturation.47 Indeed, the role of the BH3-only protein, Nix, in mitochondrial clearance in reticulocytes was initially identified using this system, where its expression peaks at 24 to 36 hours in culture.7,48 To begin exploring the role of autophagy in mitochondrial clearance during erythroid maturation, we isolated RNA from cultures at 1, 24, 36, and 48 hours and determined the expression pattern of several genes involved in autophagy by quantitative reverse-transcription–polymerase chain reaction (RT-PCR) analysis. Genes exhibiting a pattern of expression similar to nix included mammalian ATG1 homologue, ulk1, and the ATG8 homologue, map1lc3b.
Induction of Ulk1 expression correlates with onset of autophagy and loss of mitochondria during terminal erythroid maturation. Erythroid maturation was examined using the FVA culture system described previously.40 (A) May-Grünwald and benzidine (inset) stains of splenic erythroblasts cultured in the presence of erythropoietin (EPO) for 1 hour (left panel) and 48 hours (center panel). The right panel shows the relative distribution of cells at various stages of erythroid maturation after 1, 24, 36, and 48 hours in culture from a representative experiment. Differentials were performed on more than 300 cells per time point. PE indicates proerythroblast; BE, basophilic erythroblast; PCE, polychromatophilic erythroblast; OCE, orthochromatic erythroblast; and retic, reticulocyte. (B) Quantitative RT-PCR (TaqMan, Applied Biosystems) analysis was performed in triplicate using RNA isolated from cells after 1, 24, 36, and 48 hours in culture. Levels of ulk1 and ulk2 mRNA were normalized to that of 18S RNA. The expression of ulk1 and ulk2 relative to levels in control 3T3 cells is shown in the graph. Error bars represent standard deviation. (C) Northern blot analysis of cells after 1, 24, 36, and 48 hours in culture using an ulk1 cDNA probe (bottom panel). The top panel shows levels of ribosomal RNA on an ethidium bromide–stained agarose gel. (D) Western blot analysis of cells after 1, 24, 36, and 48 hours in culture. (E) Quantitative RT-PCR (TaqMan) analysis was performed in triplicate using RNA isolated from cells after 1, 24, 36, and 48 hours in culture. Levels of lc3 RNA levels were normalized to 18S RNA. The graph shows lc3 RNA levels relative to the 1-hour time point. Error bars represent standard deviation. (F) Representative electron micrographs of autophagosomes containing mitochondria (highlighted by ↗) in a reticulocyte.
Induction of Ulk1 expression correlates with onset of autophagy and loss of mitochondria during terminal erythroid maturation. Erythroid maturation was examined using the FVA culture system described previously.40 (A) May-Grünwald and benzidine (inset) stains of splenic erythroblasts cultured in the presence of erythropoietin (EPO) for 1 hour (left panel) and 48 hours (center panel). The right panel shows the relative distribution of cells at various stages of erythroid maturation after 1, 24, 36, and 48 hours in culture from a representative experiment. Differentials were performed on more than 300 cells per time point. PE indicates proerythroblast; BE, basophilic erythroblast; PCE, polychromatophilic erythroblast; OCE, orthochromatic erythroblast; and retic, reticulocyte. (B) Quantitative RT-PCR (TaqMan, Applied Biosystems) analysis was performed in triplicate using RNA isolated from cells after 1, 24, 36, and 48 hours in culture. Levels of ulk1 and ulk2 mRNA were normalized to that of 18S RNA. The expression of ulk1 and ulk2 relative to levels in control 3T3 cells is shown in the graph. Error bars represent standard deviation. (C) Northern blot analysis of cells after 1, 24, 36, and 48 hours in culture using an ulk1 cDNA probe (bottom panel). The top panel shows levels of ribosomal RNA on an ethidium bromide–stained agarose gel. (D) Western blot analysis of cells after 1, 24, 36, and 48 hours in culture. (E) Quantitative RT-PCR (TaqMan) analysis was performed in triplicate using RNA isolated from cells after 1, 24, 36, and 48 hours in culture. Levels of lc3 RNA levels were normalized to 18S RNA. The graph shows lc3 RNA levels relative to the 1-hour time point. Error bars represent standard deviation. (F) Representative electron micrographs of autophagosomes containing mitochondria (highlighted by ↗) in a reticulocyte.
Ulk1 (Unc51-like kinase 1) is a serine threonine kinase that is evolutionarily related C elegans Unc51 and yeast atg1p.30 Ultrastructural studies of erythroblasts from rat bone marrow have suggested that autophagic clearance of mitochondria begins during enucleation of the orthochromatic erythroblasts and continues in reticulocytes.9 An increase in the percentage of orthochromatic erythroblasts and reticulocytes was observed in the FVA cultures at 36 and 48 hours (Figure 1A left panel), and reticulocytes showed frequent autophagic vacuoles and lysosomal structures containing organelles, consistent with previous reports (Figure 1F).8-10 There was a dramatic increase in ulk1 mRNA expression at 36 and 48 hours in culture by quantitative RT-PCR analysis using primer/probe sets specific for ulk1 (Figure 1B) and Northern blot analysis (Figure 1C), which correlated with the increased percentage of orthochromatic erythroblasts and reticulocytes. Ulk1 shares significant homology with Ulk2,31 however, the relatively low basal level and lack of a differentiation-induced expression of ulk2 as shown in Figure 1B suggests that ulk2 does not play a significant role in erythroid maturation. Ulk1 protein levels also increased in culture (Figure 1D). The increase in Ulk1 protein and RNA levels correlated with the loss of mitochondrial proteins, including Cox IV subunit IV. Concomitantly there was biochemical evidence of induction of autophagy, as indicated by the appearance of the lipid (phosphatidyl-ethanolamine)–conjugated form of Map1LC3, known as LC3-II, at 36 and 48 hours. Map1LC3 is a ubiquitin-like molecule that is conjugated to phosphatidyl-ethanolamine upon induction of autophagy; this modification appears to promote association of LC3 with the autophagosome membrane, an important step in autophagosome formation and expansion. LC3 remains bound to the surface of the autophagosome membrane upon completion of the vesicle. Transcription of map1lc3 also increases with stimulation of autophagy (reviewed in Tanida et al49 ). Accordingly, we observed increased lc3 RNA levels during erythroid differentiation (Figure 1E).
Ulk1 knockout animals are viable
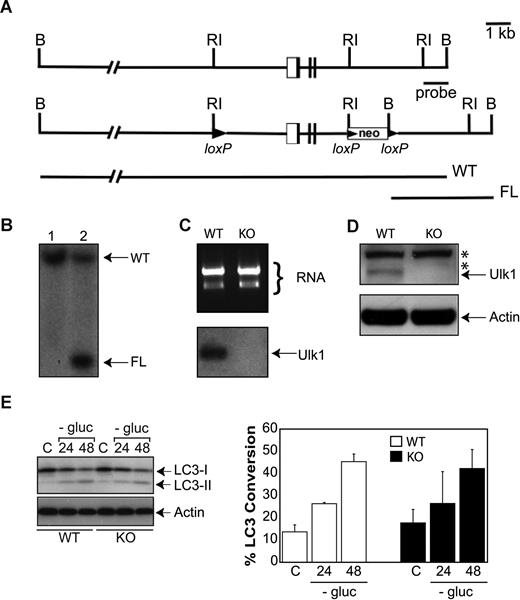
Because deficiency of core autophagy genes such as atg5 or atg7 leads to perinatal lethality from starvation and inability to maintain critical levels of amino acids and glucose in critical tissues such as liver, heart, and skeletal muscle,50,51 and Ulk1 shows high levels of expression in these tissues,30 we decided to generate a conditional knockout mouse model to examine the role of ulk1 in erythroid maturation in vivo. The targeting strategy for generating the ulk1 conditional knockout (Figure 2A) and a representative Southern blot of correctly targeted ES cells (Figure 2B) are shown. Animals harboring floxed ulk1 alleles (ulk1+/flox) were crossed with animals transgenic for EIIA-Cre to generate animals harboring germ-line deletions within the ulk1 locus (ulk1+/−). Murine embryonic fibroblasts (MEFs) were generated from ulk1−/− animals and showed no detectable ulk1 mRNA or Ulk1 protein expression (Figure 2C,D). In contrast to Atg5- or Atg7-deficient cells,50,51 Ulk1-deficient MEFs showed a level of LC3 conversion in response to glucose withdrawal comparable to wild-type, indicating that autophagy could be induced in the absence of Ulk1 (Figure 2E). Consistent with the induction of autophagy in Ulk1-deficient MEFs, ulk1−/− animals were viable and showed no overt developmental defects.
Generation of viable ulk1−/− mice without significant defect in autophagy. (A) The first panel shows the genomic organization of the 5′ end of the ulk1 gene. The second panel depicts the targeting construct that introduces an ulk1 allele where loxP sites flank a 5.6-kb EcoRI fragment containing exons I to III. The third panel shows the expected sizes of the wild-type (WT) and floxed (FL) ulk1 alleles, following digestion of DNA with BamHI after probing with the 1-kb EcoRI-BamHI probe (indicated by the bar). (B) Southern blot analysis of BamHI digested DNA from representative wild-type (WT) and floxed (FL) ulk1 clones probed with the EcoRI-BamHI probe. This probe generates an approximately 19-kb fragment in WT mice, but as a result of cloning into to the loxP vector a BamHI restriction site is introduced that produces a 4.3-kb fragment in clones containing a homologously recombined floxed ulk1 allele. (C) Northern blot analysis of ulk1+/+ (WT) and ulk1−/− (KO) murine embryonic fibroblasts (MEFs). (D) Western blot analysis of ulk1+/+ (WT) and ulk1−/− (KO) MEFs. The asterisk (*) denotes nonspecific bands. (E) Western blot analysis of ulk1+/+ (WT) and ulk1−/− (KO) MEFs cultured in the presence (C) or absence of glucose (−gluc) for 24 or 48 hours. The experiment was performed in triplicate. Error bars represent standard deviation. Percentage LC3 conversion was calculated using the following formula: 100*LC3-I/total LC3.
Generation of viable ulk1−/− mice without significant defect in autophagy. (A) The first panel shows the genomic organization of the 5′ end of the ulk1 gene. The second panel depicts the targeting construct that introduces an ulk1 allele where loxP sites flank a 5.6-kb EcoRI fragment containing exons I to III. The third panel shows the expected sizes of the wild-type (WT) and floxed (FL) ulk1 alleles, following digestion of DNA with BamHI after probing with the 1-kb EcoRI-BamHI probe (indicated by the bar). (B) Southern blot analysis of BamHI digested DNA from representative wild-type (WT) and floxed (FL) ulk1 clones probed with the EcoRI-BamHI probe. This probe generates an approximately 19-kb fragment in WT mice, but as a result of cloning into to the loxP vector a BamHI restriction site is introduced that produces a 4.3-kb fragment in clones containing a homologously recombined floxed ulk1 allele. (C) Northern blot analysis of ulk1+/+ (WT) and ulk1−/− (KO) murine embryonic fibroblasts (MEFs). (D) Western blot analysis of ulk1+/+ (WT) and ulk1−/− (KO) MEFs. The asterisk (*) denotes nonspecific bands. (E) Western blot analysis of ulk1+/+ (WT) and ulk1−/− (KO) MEFs cultured in the presence (C) or absence of glucose (−gluc) for 24 or 48 hours. The experiment was performed in triplicate. Error bars represent standard deviation. Percentage LC3 conversion was calculated using the following formula: 100*LC3-I/total LC3.
Ulk1 deficiency results in a novel population of CD71− red blood cells retaining mitochondria
To characterize the effect of Ulk1 deficiency on erythropoiesis, peripheral blood was collected from wild-type (WT) and Ulk1-deficient knockout (KO) mice (ages 8 weeks to 5 months), and complete blood counts were performed using an Advia 2120 Hematology System (Table 1). A number of changes were observed in the ulk1−/− mice, including increases in the absolute reticulocyte count (389.7 ± 48.8 × 109 cells/L in WT vs 1735.7 ± 401.6 × 109 cells/L in KO) and reticulocyte percentage (.037 ± .005 [3.7% ± 0.5%] in WT vs .179 ± .043 [17.9% ± 4.3%] in KO). There was also an increase in the mean corpuscular volume (MCV) from 49.9 ± 1.8 fL in wild-type to 54.5 ± 1.8 fL in Ulk1-deficient mice that reflected increases in the MCVs of both mature red blood cells and reticulocytes. There were no significant differences in white blood cell or platelet (Plt) counts (data not shown); and the mild decrease in red blood cell (RBC) count in the ulk1−/− mice (10.6 ± 1.3 × 1012/L in WT vs 9.8 ± 0.7 × 1012/L in KO) did not reach statistical significance. Total hemoglobin (Hb) was not significantly altered, however, mean corpuscular hemoglobin (MCH, Hb/RBC) was increased in the ulk1−/− mice (15.5 ± 0.6 pg in WT vs 17.1 ± 0.7 pg in KO), correlating with the increased hemoglobin content in both mature red blood cells (CHm's) (15.4 ± 0.6 pg in WT vs 16.9 ± 0.7 pg in KO) and reticulocytes (CHr's) (17.0 ± 0.7 pg in WT vs 18.7 ± 0.7 pg in KO). The increase in red cell distribution width (RDW) (12.1% ± 0.6% in WT vs 15.1% ± 1.3% in KO) in the ulk1−/− mice reflected an increased RDW of mature red cells; there was a concomitant decrease in the RDW of reticulocytes (16.6% ± 0.9% in WT vs 12.1% ± 1.1% in KO). The RDW and MCV findings together suggest a defect in reticulocyte maturation specifically affecting the normal loss of cytoplasmic content.
Red cell indices in wild-type (WT, ulk1+/+) and knockout (KO, ulk1−/−) mice
| . | WT, n = 10 . | KO, n = 10 . |
|---|---|---|
| RBC, ×1012/L | 10.6 ± 1.3 | 9.8 ± 0.7 |
| HGB, g/L | 164 ± 19 | 166 ± 13 |
| HCT | 0.529 ± 0.60 | 0.532 ± 0.045 |
| MCV, fL | 49.9 ± 1.8 | 54.5 ± 1.8* |
| MCH, pg | 15.5 ± 0.6 | 17.1 ± 0.7* |
| MCHC, g/L | 310 ± 5 | 314 ± 10 |
| RDW, % | 12.1 ± 0.6 | 15.1 ± 1.3* |
| Retic, % | 0.037 ± 0.05 | 0.179 ± 0.043 |
| Retic, ×109 cells/L | 389.7 ± 48.8 | 1735.7 ± 401.6* |
| MCV mature, fL | 51.1 ± 1.8 | 53.8 ± 1.4* |
| CHCM mature, g/dL | 30.1 ± 0.6 | 31.6 ± 1.2* |
| CH mature, pg | 15.4 ± 0.6 | 16.9 ± 0.7* |
| RDW mature, % | 11.4 ± 0.6 | 14.2 ± 1.1* |
| MCV retic, fL | 62.3 ± 1.9 | 65.1 ± 1.3* |
| CHCM retic, g/dL | 27.5 ± 0.5 | 28.8 ± 1.1* |
| CH retic, pg | 17.0 ± 0.7 | 18.7 ± 0.7* |
| RDW retic, % | 16.6 ± 0.9 | 12.7 ± 1.1* |
| . | WT, n = 10 . | KO, n = 10 . |
|---|---|---|
| RBC, ×1012/L | 10.6 ± 1.3 | 9.8 ± 0.7 |
| HGB, g/L | 164 ± 19 | 166 ± 13 |
| HCT | 0.529 ± 0.60 | 0.532 ± 0.045 |
| MCV, fL | 49.9 ± 1.8 | 54.5 ± 1.8* |
| MCH, pg | 15.5 ± 0.6 | 17.1 ± 0.7* |
| MCHC, g/L | 310 ± 5 | 314 ± 10 |
| RDW, % | 12.1 ± 0.6 | 15.1 ± 1.3* |
| Retic, % | 0.037 ± 0.05 | 0.179 ± 0.043 |
| Retic, ×109 cells/L | 389.7 ± 48.8 | 1735.7 ± 401.6* |
| MCV mature, fL | 51.1 ± 1.8 | 53.8 ± 1.4* |
| CHCM mature, g/dL | 30.1 ± 0.6 | 31.6 ± 1.2* |
| CH mature, pg | 15.4 ± 0.6 | 16.9 ± 0.7* |
| RDW mature, % | 11.4 ± 0.6 | 14.2 ± 1.1* |
| MCV retic, fL | 62.3 ± 1.9 | 65.1 ± 1.3* |
| CHCM retic, g/dL | 27.5 ± 0.5 | 28.8 ± 1.1* |
| CH retic, pg | 17.0 ± 0.7 | 18.7 ± 0.7* |
| RDW retic, % | 16.6 ± 0.9 | 12.7 ± 1.1* |
CH indicates corpuscular hemoglobin; CHCM, CH concentration mean; HGB, hemoglobin; HCT, hematocrit; MCH, mean corpuscular hemoglobin; MCHC, MCH concentration (calculated); MCV, mean corpuscular volume; RBC, red blood cells; RDW, red cell distribution width; and Retic, reticulocyte.
P < .005.
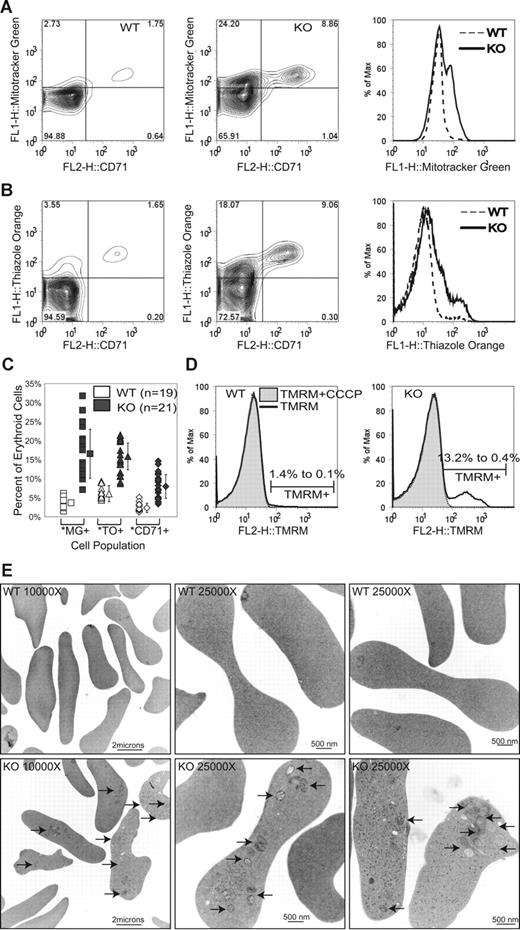
To characterize red cell populations in more detail, we used flow cytometry to examine the relationship between surface CD71 expression, and presence of mitochondria and RNA, parameters that have been used to distinguish between reticulocytes and mature red blood cells.52 CD71, the transferrin receptor, is found on the surface of reticulocytes, but is eliminated from red cell membranes by exocytosis as mitochondria are cleared and hemoglobin production ceases (reviewed in de Gassart et al53 ). RNA staining using thiazole orange is an established method of enumerating reticulocytes in clinical laboratories.54 Mitochondria can be detected by fluorescent dyes, including Mitotracker Green FM55 and Mitotracker Red 580, which accumulate in mitochondria independent of mitochondrial membrane potential, as well as potentiometric dyes, such as tetramethyl rhodamine methyl ester (TMRM) that accumulate only in intact polarized mitochondria. Examination of peripheral blood from Ulk1-deficient mice and wild-type littermates by flow cytometry revealed a variable increase in the percentage of reticulocytes (CD71+, Mitotracker positive, TO positive), as well as the appearance of a population of red cells with no detectable CD71 expression that retained mitochondria and variable amounts of RNA (Figure 3A-C). This population of CD71− red cells retaining mitochondria was observed in the Ulk1-deficient mice, but not detected in wild-type animals or thalassemic animals with elevated reticulocyte counts (data not shown). The mitochondrial membrane potential of these retained mitochondria appeared to be relatively well maintained, as they were detectable with the potentiometric dye TMRM (Figure 3D) and the signal was abolished by addition of carbonyl cyanide m-chloro phenyl hydrazone (CCCP), a potent mitochondrial uncoupling agent that causes loss of mitochondrial membrane potential. Electron microscopy was performed on peripheral blood samples obtained from Ulk1-deficient animals, and showed the presence of mitochondria in the red blood cells (Figure 3E) with frequencies similar to those obtained using the fluorescent mitochondria-specific dyes. Ribosomes were also observed by electron microscopy.
Retention of mitochondria in mature red blood cells of ulk1−/− mice. Peripheral blood from 21 ulk1+/+ (WT) and 19 ulk1−/− (KO) mice ranging in age from 8 weeks to 5 months was analyzed by fluorescence-activated cell sorting (FACS) analysis. (A) Representative contour plot of WT (left panel) and KO (center panel) cells stained with Mitotracker Green FM (y-axis) and CD71-PE (x-axis), with histogram (right panel) showing direct comparison of Mitotracker Green fluorescence (FL1) of the 2 populations (WT vs KO). (B) Representative contour plot of WT (left panel) and KO (center panel) cells stained with thiazole orange (y-axis) and CD71-PE (x-axis), with histogram (right) showing direct comparison of thiazole orange fluorescence (FL1) of the 2 populations. (C) Graph showing percentage of erythroid cells in individual WT and KO animals staining positively with Mitotracker Green FM (MG+), thiazole orange (TO+), and CD71 (CD71+). The population means and standard deviations (error bars) are graphically depicted and show the following statistically significant differences in percentage of Mitotracker Green–positive (3.7% ± 1.2% in WT vs 16.5% ± 6.4% in KO), thiazole orange–positive (7.0% ± 2.1% in WT vs 15.8% ± 4.4% in KO), and CD71+ (3.1% ± 1.2% in WT vs 8.0% ± 3.4% in KO) erythroid cells. Statistically significant differences (P < .001) between WT and KO populations were identified by Student t test analysis and are marked by an asterisk (*). (D) Representative histograms of WT (left panel) and KO (right panel) red blood cells stained with TMRM. The markers denote positive TMRM staining. Each TMRM-stained sample was split into 2 tubes, and the mitochondrial decoupler, CCCP, was added to one of the tubes immediately prior to analysis. The percentage of TMRM-positive cells before (1.4% in WT vs 13.2% in KO) and after (0.1% in WT vs 0.4% in KO) incubation in 50 μM CCCP is shown. (E) Representative electron micrographs of WT (top panel) and KO (bottom panel) red blood cells. ↗ point to intact mitochondria. Ribosomes are seen in a subset of the red cells (bottom right panel).
Retention of mitochondria in mature red blood cells of ulk1−/− mice. Peripheral blood from 21 ulk1+/+ (WT) and 19 ulk1−/− (KO) mice ranging in age from 8 weeks to 5 months was analyzed by fluorescence-activated cell sorting (FACS) analysis. (A) Representative contour plot of WT (left panel) and KO (center panel) cells stained with Mitotracker Green FM (y-axis) and CD71-PE (x-axis), with histogram (right panel) showing direct comparison of Mitotracker Green fluorescence (FL1) of the 2 populations (WT vs KO). (B) Representative contour plot of WT (left panel) and KO (center panel) cells stained with thiazole orange (y-axis) and CD71-PE (x-axis), with histogram (right) showing direct comparison of thiazole orange fluorescence (FL1) of the 2 populations. (C) Graph showing percentage of erythroid cells in individual WT and KO animals staining positively with Mitotracker Green FM (MG+), thiazole orange (TO+), and CD71 (CD71+). The population means and standard deviations (error bars) are graphically depicted and show the following statistically significant differences in percentage of Mitotracker Green–positive (3.7% ± 1.2% in WT vs 16.5% ± 6.4% in KO), thiazole orange–positive (7.0% ± 2.1% in WT vs 15.8% ± 4.4% in KO), and CD71+ (3.1% ± 1.2% in WT vs 8.0% ± 3.4% in KO) erythroid cells. Statistically significant differences (P < .001) between WT and KO populations were identified by Student t test analysis and are marked by an asterisk (*). (D) Representative histograms of WT (left panel) and KO (right panel) red blood cells stained with TMRM. The markers denote positive TMRM staining. Each TMRM-stained sample was split into 2 tubes, and the mitochondrial decoupler, CCCP, was added to one of the tubes immediately prior to analysis. The percentage of TMRM-positive cells before (1.4% in WT vs 13.2% in KO) and after (0.1% in WT vs 0.4% in KO) incubation in 50 μM CCCP is shown. (E) Representative electron micrographs of WT (top panel) and KO (bottom panel) red blood cells. ↗ point to intact mitochondria. Ribosomes are seen in a subset of the red cells (bottom right panel).
Ulk1 deficiency results in delayed mitochondrial clearance in reticulocytes
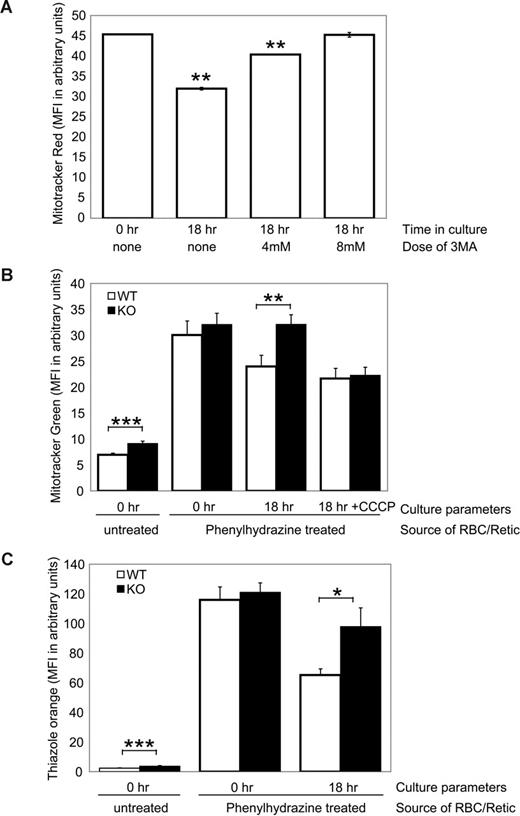
Ulk1-deficient mice showed mild splenomegaly compared with wild-type littermates (1.7 ± 0.4-fold increase in weight; n = 8 of each genotype). Although there was no consistent histologic or flow cytometric evidence of increased extramedullary hematopoiesis (data not shown), it is possible that stress erythropoiesis may contribute to the increase in reticulocytes observed in Ulk1-deficient animals. To specifically determine whether immature reticulocytes (also known as “stress” or “shift” reticulocytes) clear mitochondria by autophagy and if Ulk1 deficiency results in a defect in mitochondrial clearance in reticulocytes, we isolated reticulocytes from wild-type and Ulk1-deficient mice and followed reticulocyte maturation in culture. Toward that end, mice were treated with phenylhydrazine to induce a hemolytic anemia and compensatory reticulocytosis. Peripheral blood isolated from Ulk1-deficient mice and wild-type littermates 2 days after the last injection showed greater than 70% reticulocytes, reflected in the dramatic increase in Mitotracker (Figure 4B, untreated compared with phenylhydrazine treated at 0 hour), thiazole orange (Figure 4C, untreated compared with phenylhydrazine treated at 0 hour), and CD71 (data not shown) staining. After 18 hours in culture, reticulocytes from wild-type animals showed a 20% loss of mitochondrial staining by Mitotracker Green FM or Mitotracker Red 580 (Figure 4A,B) and greater than 40% loss of thiazole orange staining (Figure 4C) and CD71 (data not shown), indicating clearance of mitochondria and RNA and maturation of reticulocytes. Mitochondrial clearance was inhibited by 3 methyl-adenine (3-MA, 10 mM; Figure 4A), a well-established inhibitor of autophagy, and 1 μM hydroxychloroquine (data not shown), indicating that autophagy is important in mitochondrial clearance during terminal erythroid maturation.
Ulk1 deficiency impairs mitochondrial clearance in reticulocytes. (A) Peripheral blood from wild-type phenylhydrazine-treated mice consisted of greater than 70% CD71+/Retic-Count–positive reticulocytes. The graph shows mean fluorescence intensity (MFI) of Mitotracker Red FM–stained cells at the start of the culture (0 hours) and after 18 hours in culture with or without the indicated doses of the autophagy inhibitor, 3-methyladenine (3-MA). (B) The graph shows mean fluorescence intensity (MFI) of Mitotracker Green–stained red blood cells from untreated (PBS-injected) and phenylhydrazine (PHZ)–treated ulk1+/+ (WT, n = 3 in each group) and ulk1−/− (KO, n = 3 in each group) mice at the start of the culture (0 hours) and PHZ treated after 18 hours in complete media with or without 50 μM CCCP. (C) The graph shows mean fluorescence intensity (MFI) of thiazole orange–stained samples described in panel B. Statistically significant differences between WT and KO populations were identified by Student t test analysis and are noted with asterisks: *P < .05; **P < .01; ***P < .005.
Ulk1 deficiency impairs mitochondrial clearance in reticulocytes. (A) Peripheral blood from wild-type phenylhydrazine-treated mice consisted of greater than 70% CD71+/Retic-Count–positive reticulocytes. The graph shows mean fluorescence intensity (MFI) of Mitotracker Red FM–stained cells at the start of the culture (0 hours) and after 18 hours in culture with or without the indicated doses of the autophagy inhibitor, 3-methyladenine (3-MA). (B) The graph shows mean fluorescence intensity (MFI) of Mitotracker Green–stained red blood cells from untreated (PBS-injected) and phenylhydrazine (PHZ)–treated ulk1+/+ (WT, n = 3 in each group) and ulk1−/− (KO, n = 3 in each group) mice at the start of the culture (0 hours) and PHZ treated after 18 hours in complete media with or without 50 μM CCCP. (C) The graph shows mean fluorescence intensity (MFI) of thiazole orange–stained samples described in panel B. Statistically significant differences between WT and KO populations were identified by Student t test analysis and are noted with asterisks: *P < .05; **P < .01; ***P < .005.
Mitochondrial clearance was impaired in reticulocytes derived from Ulk1-deficient animals compared with wild-type littermates (Figure 4B). This was not due to a complete inability to clear mitochondria as this defect could be overcome by culturing in the presence of the mitochondrial uncoupler, CCCP (Figure 4B). It should be noted that Mitotracker Green staining is unaltered by the addition of CCCP, unlike TMRM that is released from cells with loss of membrane potential (data not shown). These findings suggest that although Ulk1 plays a role in mitochondrial clearance, there are redundant Ulk1-independent mechanisms that ultimately clear RBCs of mitochondria. Loss of RNA was also impaired in Ulk1-deficient mice (Figure 4C) and with high (20 mM) doses of 3-MA (data not shown), suggesting that autophagy also plays a role in clearing RNA-bound ribosomes.
Ulk1-deficient murine embryonic fibroblasts (MEFs) show an increase in mitochondrial mass
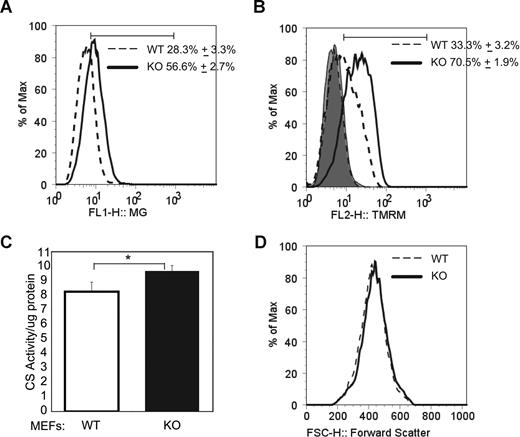
To determine whether the role of Ulk1 in mitochondrial clearance was specific to red blood cells, we examined mitochondrial mass in murine embryonic fibroblasts derived from Ulk1-deficient embryos by 2 independent techniques. First, we observed increased Mitotracker Green FM staining, and a corresponding increase in TMRM staining by flow cytometry (Figure 5A,B). Next, extracts were prepared from wild-type and Ulk1-deficient MEFs and analyzed for citrate synthase activity (normalized to total protein). Citrate synthase is a mitochondrial matrix protein whose activity has been shown to correlate well with mitochondrial mass.56 Figure 5C shows a statistically significant increase in citrate synthase activity in Ulk1-deficient MEFs compared with wild type. In the absence of any appreciable difference in cell size (as approximated by forward scatter, Figure 5D), the reproducible increase in citrate synthase activity together with the increase in mitochondrial staining by flow cytometric analysis indicates an increase in mitochondrial mass in Ulk1-deficient MEFs and suggests that Ulk1's role in regulating mitochondrial clearance is not restricted to RBCs.
Increased mitochondrial mass in ulk1−/− murine embryonic fibroblasts (MEFs). (A) MEFs derived from ulk1+/+ (WT) and ulk1−/− (KO) were stained with Mitotracker Green FM and analyzed by FACS. The histogram shows the distribution of Mitotracker staining in both populations. Mean (n = 5) and standard deviation of the percentage of cells within the marker are shown. The marker was set to include all points above the intersection of the 2 plots to highlight the difference in staining between the 2 populations. (B) MEFs derived from ulk1+/+ (WT) and ulk1−/− (KO) were stained with TMRM. The histogram shows the distribution of TMRM staining in both populations before and after incubation with in 50 μM CCCP. Mean (n = 5) and standard deviation of the percentage of cells within the marker are shown. After treatment with CCCP mean FL2 fluorescence was reduced to 5.8 (± 0.3) in WT MEFs and 5.7 (± 0.1) in KO MEFs. (C) Graph showing citrate synthase activity in whole-cell extracts prepared from wild-type (WT) and Ulk1-deficient (KO) MEFs normalized to total protein. The mean (n = 5) and standard deviations are shown. Statistical significance (P < .01) was calculated by Student t test analysis and is marked with an asterisk (*). (D) Representative histogram showing forward scatter distribution of MEFs derived from ulk1+/+ (WT) and ulk1−/− (KO).
Increased mitochondrial mass in ulk1−/− murine embryonic fibroblasts (MEFs). (A) MEFs derived from ulk1+/+ (WT) and ulk1−/− (KO) were stained with Mitotracker Green FM and analyzed by FACS. The histogram shows the distribution of Mitotracker staining in both populations. Mean (n = 5) and standard deviation of the percentage of cells within the marker are shown. The marker was set to include all points above the intersection of the 2 plots to highlight the difference in staining between the 2 populations. (B) MEFs derived from ulk1+/+ (WT) and ulk1−/− (KO) were stained with TMRM. The histogram shows the distribution of TMRM staining in both populations before and after incubation with in 50 μM CCCP. Mean (n = 5) and standard deviation of the percentage of cells within the marker are shown. After treatment with CCCP mean FL2 fluorescence was reduced to 5.8 (± 0.3) in WT MEFs and 5.7 (± 0.1) in KO MEFs. (C) Graph showing citrate synthase activity in whole-cell extracts prepared from wild-type (WT) and Ulk1-deficient (KO) MEFs normalized to total protein. The mean (n = 5) and standard deviations are shown. Statistical significance (P < .01) was calculated by Student t test analysis and is marked with an asterisk (*). (D) Representative histogram showing forward scatter distribution of MEFs derived from ulk1+/+ (WT) and ulk1−/− (KO).
Discussion
Here we demonstrate that a critical regulator of autophagy, Ulk1, plays a major role in organelle clearance during reticulocyte maturation. Ulk1-deficient mice exhibit a number of statistically significant changes in red cell parameters, including increases in reticulocytes (percentage and absolute numbers), mean cell volume (MCV), mean corpuscular hemoglobin content (MCH), and relative distribution width (RDW) of mature red blood cells. Together, these findings suggest that Ulk1 deficiency results in a defect in reticulocyte maturation, which impairs the clearance of cellular components. The retention of mitochondria and RNA-bound ribosomes in a population of Ulk1-deficient RBCs may allow increased hemoglobin production, and may contribute to the increased hemoglobin content of both mature RBCs (CHm's) and reticulocytes (CHr's). Consistent with the hypothesis that Ulk1 plays an important role in the efficient clearance of organelles from maturing RBCs, flow cytometric analysis and electron microscopy demonstrate that up to 35% of RBCs in the peripheral blood of Ulk1-deficient mice retain intact mitochondria; many of these RBCs also contain ribosomes. In addition, clearance of mitochondria and RNA (presumably bound to ribosomes) from immature reticulocytes is impaired with chemical inhibition of autophagy and Ulk1 deficiency. Together, these findings implicate Ulk1 in the autophagic clearance of mitochondria, as well as ribosomes. It is interesting to note that a recent study has implicated autophagy in the selective degradation of ribosomes in yeast.57
Although these findings indicate that Ulk1 deficiency leads to a defect in organelle clearance, ultimately, the majority of red cells clear their mitochondria and other organelles. Thus, additional system(s) or low-level expression of the related protein, Ulk2, can lead to clearance of organelles in the absence of functional Ulk1. Indeed, the defect in mitochondrial clearance in ulk1−/− reticulocytes can be overcome in vitro by treating reticulocytes with CCCP, a mitochondrial uncoupler that increases production of reactive oxygen species (ROSs) and leads to mitochondrial membrane depolarization.58 Since loss of mitochondrial membrane potential and ROS-induced mitochondrial lipid peroxidation have been independently implicated in mitochondrial autophagy,15-17 CCCP may provide multiple signals to increase the efficiency of mitochondrial autophagy in an Ulk1-independent manner. The enzyme 15-lox catalyzes mitochondrial lipid peroxidation, and both Nix and 15-Lox can contribute to loss of mitochondrial membrane potential,59,60 therefore, either or both of these may provide the necessary physiologic signals to promote mitochondrial autophagy in Ulk1-deficient reticulocytes. The relationship of Ulk1 to other mediators of mitochondrial clearance remains to be elucidated.
Other ubiquitously expressed autophagy genes that have been studied in mice using targeted knockout strategies lead to embryonic or perinatal lethality, precluding examination of the contribution of autophagy to postnatal cellular processes.50,51,61 In contrast, Ulk1 is not essential for murine survival. This may be because Ulk1 and Ulk2, which show greater than 50% overall homology, are functionally redundant in their ability to induce and carry out autophagy. The ability of Ulk1-deficient mice to survive beyond the newborn period when autophagy is critical for maintaining adequate supplies of amino acids and glucose in liver, heart, and skeletal muscle suggests either that Ulk1 is not required for this mechanism of autophagy or that its deficiency is compensated for by Ulk2. For example, in the RNAi screen performed in HEK293 cells,39 Ulk1, but not Ulk2, was shown to be critical for inducing autophagy in response to amino acid withdrawal. By contrast, in MEFs, which normally express detectable levels of Ulk2 (data not shown), we demonstrate that Ulk1 deficiency results in no significant defect in induction of autophagy in response to glucose withdrawal.
In summary, these findings demonstrate that Ulk1 plays a critical role in the targeted degradation of mitochondria and RNA-bound ribosomes in erythroid cells, but is not essential for induction of starvation-induced macroautophagy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the University of Pennsylvania Biomedical Imaging Core, especially Neelima Shah and Ray Meade, for technical assistance with electron microscopy; members of the Thompson laboratory for helpful discussions; and Mitch Weiss (Children's Hospital of Philadelphia) for providing peripheral blood from thalassemic mice.
This work was supported by the American, Lebanese, and Syrian Associated Charities (ALSAC) of St Jude Children's Research Hospital, Memphis, TN (P.A.N.) and grants from the American Society of Hematology (Washington, DC; M.K.), the Burroughs Wellcome Fund (Research Triangle, NC; M.K.), National Cancer Institute (Bethesda, MD; RO1 CA099179 and RO1 CA105463 to C.B.T), the National Heart, Lung and Blood Institute (Bethesda, MD; K08 HL084199 to M.K.), and the National Institute of Diabetes, Digestive and Kidney Diseases (Bethesda, MD; R21 DK074519 to P.A.N.).
National Institutes of Health
Authorship
Contribution: M.K. designed and performed research, analyzed data, performed statistical analysis, and drafted the paper; T.L. designed and performed research, analyzed data, and critically reviewed the paper; J.W., C.-Y.Y., F.Z., and J.Z. performed research; M.A.S. and P.A.N. designed and performed research; and C.B.T. designed research and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mondira Kundu, Abramson Family Cancer Research Institute, University of Pennsylvania, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: kundum@uphs.upenn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal