Abstract
Endoplasmic reticulum (ER) unfolded protein response (UPR) plays pivotal roles in both early B-cell development and plasma cell differentiation. As a major ER chaperone to mediate the UPR and a master chaperone for Toll-like receptors (TLRs), HSP90b1 (grp94, gp96) has long been implicated to facilitate the assembly of immunoglobulin. We hereby critically and comprehensively examine the roles of HSP90b1 in B-cell biology in vivo using B-cell–specific HSP90b1-null mice. We found that knockout B cells developed normally. There were no apparent problems with plasma cell differentiation, Ig assembly, class-switching, and Ig production. Strikingly, although both mutant conventional and innatelike B cells failed to compartmentalize properly due to loss of select but not all integrins, HSP90b1 was required for neither germinal center formation nor memory antibody responses in vivo. The only significant defect associated with HSP90b1 ablation in B cells was an attenuated antibody production in the context of TLR stimulation. Thus, our study has resolved the long-standing question regarding HSP90b1 in B-cell biology: HSP90b1 optimizes the function of B cells by chaperoning TLRs and integrins but not immunoglobulin. This study also has important implications in resolving the controversial roles of TLR in B-cell biology.
Introduction
Heat shock protein gp961 or grp94,2 encoded by hsp90b1,3 is an endoplasmic reticulum (ER) paralogue of the cytosolic HSP90. They share approximately 50% homology at the amino acid level with similar domain organizations consisting of the N-terminal ATP-binding domain, the charged middle domain, and the C-terminal homodimerization domain.3,4 HSP90b1 is believed to be one of the key downstream chaperones in the ER that mediates the ER unfolded protein response (UPR), which is crucial for maintaining protein homeostasis in the ER.5 Recent studies have highlighted the important link between normal B-cell function and the UPR. Genetic ablation of the UPR upstream transcription factor XBP-1 revealed its essential role for the differentiation of plasma cells.6-8 Induced more than 10-fold during B-cell activation,9 HSP90b1 has been widely reported to participate in Ig assembly by interacting with both Ig heavy chain (HC) and Ig light chain (LC).5,10,11 It may also assist in the formation of B-cell receptor (BCR) complexes through its association with Igα molecules.12 More recently, we found that HSP90b1 is a master chaperone for multiple TLRs including TLR1, TLR2, TLR4, TLR5, TLR6, TLR7, and TLR9,4,13 many of which have been implicated in regulating B-cell function during both physiologic14-16 and pathologic17-22 conditions.
The direct roles of HSP90b1 in B-cell biology, however, have not been studied in vivo. It is unclear for instance if HSP90b1 is indeed a critical chaperone for Ig or its role in Ig folding is redundant. We addressed this question by generating a B cell–specific HSP90b1-deficient mouse. We found that HSP90b1-null B cells were unable to proliferate in response to multiple TLR ligands and were defective in the expression/function of select integrins. Consistent with the loss of α4 and β2 integrins, HSP90b1-null conventional B cells did not accumulate efficiently in the lymph nodes, and innate-like B cells failed to compartmentalize properly. Despite these multiple defects, B cell–selective HSP90b1-deficient mice were able to mount a robust antigen-specific antibody response. HSP90b1 was dispensable for germinal center formation. The only significant defect associated with HSP90b1 ablation in B cells was an attenuated antibody production in the context of TLR stimulation. Such a defect was not due to the presumed roles of HSP90b1 in Ig assembly. Our study thus demonstrated that HSP90b1 plays more restricted roles in the function of B cells in vivo by chaperoning a limited set of client proteins including TLRs and integrins, but not Ig molecules. The fundamental function of B cells is thus independent of TLRs, integrins, and a major ER chaperone HSP90b1 in the UPR response.
Methods
Mice and genotyping
Conditional HSP90b1-deficient mice were described previously.13 Genotyping was performed by polymerase chain reaction (PCR) amplification of mouse tail genomic DNA to differentiate WT (561 bp) from floxed HSP90b1 allele (638 bp) (forward primer: 5′-TGCCAGAGACTACAATTCCCAGCA-3′; reverse primer: 5′-AAACACGAACT CACCAATCGTGCC-3′), to determine whether floxed HSP90b1 underwent successful cre-mediated recombination (440 bp; forward primer: 5′-AGCAAGGGCCA-AGCTACGCAACTG-3′; reverse primer: 5′-CAGGAAGGCTTCCC-CCGG-3′), to identify CD19-cre (715 bp; forward primer: 5′-AACCAGTCAACACCCTTCC-3′; reverse primer: 5′-TCAGCTACAC CAGAGACGG-3′), and to confirm the presence of WT CD19 locus (450 bp; forward primer: 5′-AACCAGTCAACACCCTTCC-3′; reverse primer: 5′-CCAGACTAGATACAGACCAG-3′). CD19cre mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Animal use was approved by the University of Connecticut Health Center Animal Care Committee.
Reagents
All TLR ligands were purchased from InvivoGen (San Diego, CA). Most Abs used for flow cytometry were obtained from BD Biosciences (Mountain View, CA) and eBioscience (San Diego, CA), except Ab against p-Erk, p-p38, and p-Syk (Cell Signaling Technology, Danvers, MA) and HSP90b1 Ab (Stressgen, Victoria, BC). All other chemicals were obtained from Sigma-Aldrich (St Louis, MO).
Flow cytometry, cell lines, and immunoprecipitation
Surface staining of cells and flow cytometry were done as described.13,23,24 B cells were purified from spleens using CD19-magnetic beads according to the manufacturer's protocol (Miltenyi Biotec, Auburn, CA). Purified murine splenic B cells were stimulated with LPS-free recombinant IL-4 and CD40 Ab for 3 days, followed by metabolic labeling with 35S-Met/Cys (Perkin Elmer, Boston, MA) for 30 minutes, and chased subsequently with unlabeled Met/Cys for 30 to 180 minutes. For some experiments, cells were labeled with 35S-Met/Cys for 4 hours without chasing with unlabeled Met/Cys. Cells were then lysed in radioimmunoprecipitation assay (RIPA) lysis buffer (0.01 M sodium phosphate, pH 7.2, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 2 mM AEBSF, 130 mM bestatin, 14 mM E-64, 0.3 mM aprotinin, and 1 mM leupeptin) and immunoprecipitated with κ chain Ab and 40 μL protein G–Sepharose beads and analyzed by SDS–polyacrylamide gel electrophoresis (PAGE) and autoradiography.
In vitro B-cell differentiation
Purified WT and KO splenic B cells were labeled with CFSE, and then stimulated with IL-4 and CD40 Ab for 5 days. This was followed by flow cytometric analysis of expression levels of cell surface CD44, IgM, IgD, and IgG1 by dividing cells.
Immunization and Ab detection
WT and KO mice were immunized in hind footpads with chicken OVA (50 μg/mouse) and LPS (5 μg/mouse) emulsified in IFA. For some experiments, mice were immunized intraperitoneally with LPS-free HSA (100 μg/mouse) coadsorbed on alum. TNP-Ficoll was administered at 50 μg per mouse via an intraperitoneal route. At different days after immunization, sera were collected for measuring Ag-specific Ab by enzyme-linked immunosorbent assay (ELISA).
Immunofluorescence
Cryosections of spleens (5 μm thick) were fixed with 4% paraformaldehyde, permeabilized with cold methanol, blocked and stained with HSP90b1 Ab (9G10), and costained with biotin-labeled PNA (Vector Laboratory, Burlingame, CA). Images of sections were taken under a fluorescent microscope (Zeiss, Chester, VA) and analyzed by AxioVision 4.4 software (Carl Zeiss Micro Imaging, Thornwood, NY).
Chemotaxis assay
Purified splenic B cells (3.5 × 105) in 100 μL RPMI/10% FCS were incubated for 2 hours at 37°C in the top chamber of 5-μm pore size Transwell, with the bottom chamber containing 600 μL RPMI/10% FCS with or without 10 to 100 ng/mL SDF-1 (PeproTech, Rocky Hill, NJ). Migrated cells in the lower chamber were enumerated by flow cytometer. The percentage of migrating cells was calculated by dividing the number of transmigrating cells by the number of input cells multiplied by 100.
In vivo B-cell migration assay
WT and KO splenocytes were labeled with high (5 μM) and low (0.5 μM) intensity of CFSE, respectively, and mixed at 1:1 ratio before injection. A total of 5 × 107 cells were injected via tail vein into each WT recipient. Two hours later, mice were killed. WT and KO donor cells in various lymphoid organs were analyzed by flow cytometry.
Statistical analysis
Error bars represent the standard error of the arithmetic mean (SEM). Student t test and ANOVA were used for statistical analysis. Values of P less than .05 were considered to represent statistically significant differences.
Results
Generation of a B cell–specific gp96-deficient mouse
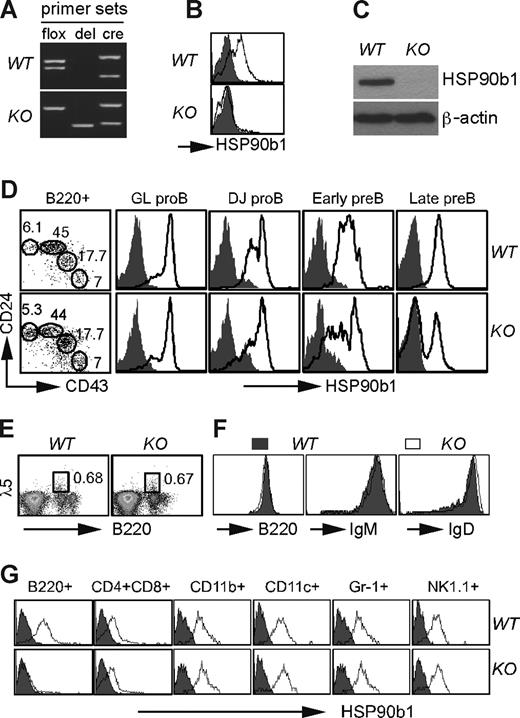
We crossed our recently described Hsp90b1flox/− mice13 with CD19cre mice25 to generate Hsp90b1flox/−CD19cre/WT (abbreviated as KO) mice (Figure 1). KO mice were developmentally normal and fertile. Cre-mediated recombination resulted in selective and efficient loss of HSP90b1 in mature B cells (Figure 1), without affecting the general hematopoiesis (data not shown). KO bone marrow B cells underwent a normal transition from B220+ germ-line (GL) pro-B cells (CD24−CD43high) and DJ pro-B cells (CD24lowCD43interm), to early pre-B cells (CD24highCD43low) and late pre-B cells (CD24highCD43−26,27 ; Figure 1D). This was expected because significant HSP90b1 reduction occurred efficiently only after late pre–B-cell stage (Figure 1D), coinciding with CD19 expression. There were no differences in the expression of the surrogate light chain28 of the pre-BCR complex on B cells between WT and KO mice (Figure 1E). Moreover, the expression levels of B220, IgM, and IgD were comparable, demonstrating that BCR assembly was not compromised in the absence of HSP90b1 (Figure 1F). HSP90b1 was not altered in mature T cells, natural killer (NK) cells, granulocytes, and macrophages (Mφs; Figure 1G). The fact that splenic B cells were normal in number despite the absence of HSP90b1 indicates that it was not essential for B-cell survival. Collectively, we have successfully generated a conditional B cell–specific HSP90b1-deficient mouse.
Generation of B cell–specific HSP90b1-deficient mice. (A) Genotype confirmation of KO and WT mice by PCR using allele-specific primers (flox indicates HSP90b1 gene floxed; del, HSP90b1 allele deleted; and cre, presence of cre gene). (B) Quantification of HSP90b1 in WT and KO splenic CD19+ cells by intracellular staining (open histogram indicates HSP90b1; shaded histogram, isotype control). (C) Immunoblot analysis of HSP90b1 using a HSP90b1 mAb in purified CD19+ splenocytes. (D) Quantification of HSP90b1 in different subset of B cells by intracellular staining (open histogram). (E) Comparative flow cytometric analysis of bone marrow cells for cell surface expression of B220 and λ5. Numbers on plots in panels D and E are the percentages of total cells in gate. (F) Phenotypic analysis of B220+ splenic B cells from KO and WT mice. Multiple experiments were conducted with similar findings. (G) Intracellular staining of HSP90b1 in WT and KO splenocytes. Various lineages were analyzed separately after gating with the indicated lineage-specific markers (open histogram indicates HSP90b1; shaded histogram, isotype control.
Generation of B cell–specific HSP90b1-deficient mice. (A) Genotype confirmation of KO and WT mice by PCR using allele-specific primers (flox indicates HSP90b1 gene floxed; del, HSP90b1 allele deleted; and cre, presence of cre gene). (B) Quantification of HSP90b1 in WT and KO splenic CD19+ cells by intracellular staining (open histogram indicates HSP90b1; shaded histogram, isotype control). (C) Immunoblot analysis of HSP90b1 using a HSP90b1 mAb in purified CD19+ splenocytes. (D) Quantification of HSP90b1 in different subset of B cells by intracellular staining (open histogram). (E) Comparative flow cytometric analysis of bone marrow cells for cell surface expression of B220 and λ5. Numbers on plots in panels D and E are the percentages of total cells in gate. (F) Phenotypic analysis of B220+ splenic B cells from KO and WT mice. Multiple experiments were conducted with similar findings. (G) Intracellular staining of HSP90b1 in WT and KO splenocytes. Various lineages were analyzed separately after gating with the indicated lineage-specific markers (open histogram indicates HSP90b1; shaded histogram, isotype control.
Inefficient migration/homing of HSP90b1-null B cells to lymph nodes
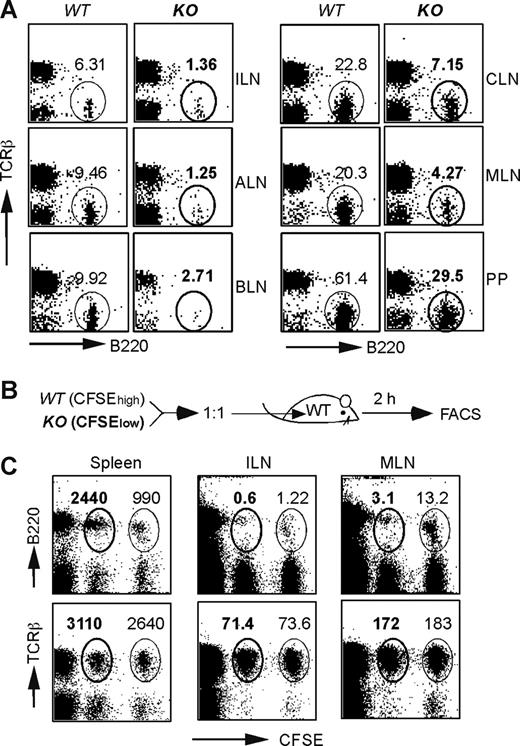
Although the cellularity and distribution of B cells were normal in the bone marrow and spleen in HSP90b1 KO mice (Figure 1 and data not shown), we observed significantly reduced B-cell numbers in the cervical, brachial, axillary, inguinal, and mesenteric LNs, as well as Peyer patches (PP; Figure 2A). To determine whether the reduced cellularity was due to the defect in migration, we performed the following migration experiment. WT and KO splenocytes were labeled with high- and low-intensity 5,6-carboxyfluorescein (CFSE), respectively, mixed at a 1:1 ratio, and then administered intravenously into WT recipients (Figure 2B). Two hours later, mice were killed. The ratios between WT and KO donor cells in various lymphoid organs were determined by flow cytometry (Figure 2C). We found that T cells from both WT and KO mice migrated normally to the spleen, inguinal LN (ILN), and mesenteric LN (MLN; Figure 2C bottom panels). By comparison, KO B cells migrated inefficiently to ILN and MLN, although the splenic enrichment of KO B cells was normal or even increased (Figure 2C top left panel).
Defective migration of HSP90b1 KO B cells to lymph nodes. (A) Phenotypic analysis of B- and T-cell distribution in LNs of WT and KO mice. Numbers indicate the percentage of gated cells over the total. (B) Schematic diagram of the in vivo migration experiment. (C) Phenotypic analysis of adoptively transferred B and T cells of WT (CFSEhigh) and KO (CFSElow) mice in WT recipient mice 2 hours after transfer. Numbers (× 103) represent the events of gated cells over the total number of live cells in each organ, including spleen (5 × 107), ILN (3 × 106), and MLN (7.5 × 106).
Defective migration of HSP90b1 KO B cells to lymph nodes. (A) Phenotypic analysis of B- and T-cell distribution in LNs of WT and KO mice. Numbers indicate the percentage of gated cells over the total. (B) Schematic diagram of the in vivo migration experiment. (C) Phenotypic analysis of adoptively transferred B and T cells of WT (CFSEhigh) and KO (CFSElow) mice in WT recipient mice 2 hours after transfer. Numbers (× 103) represent the events of gated cells over the total number of live cells in each organ, including spleen (5 × 107), ILN (3 × 106), and MLN (7.5 × 106).
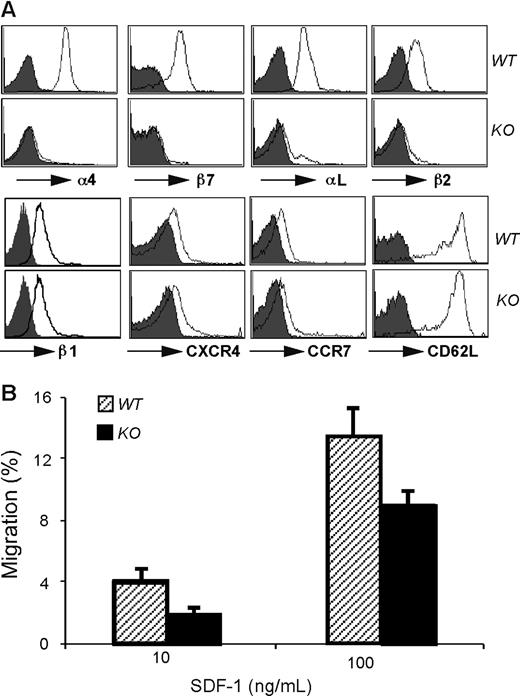
Transendothelial migration of B cells into lymph nodes is a multistep process, including L-selectin–dependent rolling,29 migration in response to the chemotaxic CCR7 and CXCR4 gradient,30 and finally the adherence to high endothelial venues (HEVs) and migration into B-cell follicles via the integrins αLβ2 (LFA-1)31 and α4β1.32-34 To probe the molecular basis for the migration defect, we compared the surface expression level of these molecules between WT and KO cells by flow cytometry. Although β1 integrin expression was normal on KO cells, we found that HSP90b1 KO B cells expressed very little α4, β2, β7, and αL on cell surfaces (Figure 3A), consistent with the roles of HSP90b1 as a critical chaperone for a subset of integrins.4 Indeed we found that HSP90b1 physically interacted with β2 integrins in WT cells (S. Jung and Z.L., unpublished observation, January 2007). The expression of CD62L, CCR7, and CXCR4 on KO B cells was uncompromised (Figure 3A). In addition, KO splenic B cells migrated efficiently in vitro in a Transwell migration assay in response to the CXCR4 ligand, CXCL12 (SDF-1; Figure 3B). We therefore concluded that the migration defect of KO B cells into LN was likely due to the defective adherence to HEVs, rather than problems in rolling or chemokine receptor–mediated signaling.
Selective integrin loss but normal expression of selectin and chemokine receptor by HSP90b1 KO B cells. (A) Phenotypic analysis of splenic B cells for the surface expression of indicated molecules (open histogram). Shaded histogram represents background stain with isotype control Ab. (B) In vitro migration assay of WT and KO B cells in response to SDF-1. P > .05. Error bars represent SD.
Selective integrin loss but normal expression of selectin and chemokine receptor by HSP90b1 KO B cells. (A) Phenotypic analysis of splenic B cells for the surface expression of indicated molecules (open histogram). Shaded histogram represents background stain with isotype control Ab. (B) In vitro migration assay of WT and KO B cells in response to SDF-1. P > .05. Error bars represent SD.
HSP90b1 ablation leads to defective compartmentalization of innate-like B cells
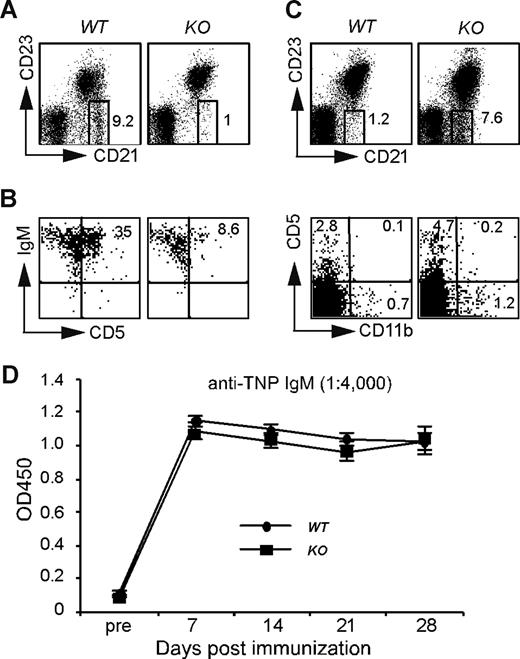
α4 and β2 integrins are also critically important for the retaining of marginal zone B (MZB) cells.35 We next studied the roles of HSP90b1 in this population and in other innate-like B cells including B1 cells. We found a significant reduction of splenic MZB cells (B220+CD21+CD23−; Figure 4A), and of peritoneal B1 cells (B220+IgM+CD5+Figure 4B) from KO mice. These data suggested that innate-like B cells were either developmentally compromised in the KO mice or defective in migration to the proper compartment. To differentiate these 2 possibilities, we enumerated blood MZB-like and B1-like cells. Blood B1 cells can be identified by surface phenotype of B220+CD5+CD11b−.36,37 We found that both cell populations were increased in the blood of KO mice (Figure 4C), indicating that CD19cre-driven HSP90b1 deletion in the late pre-B cells most likely does not lead to a developmental problem of innate-like B cells; rather, the reduction of splenic MZB cells and peritoneal B1 cells was due to a migration defect of innate-like B cells to their target tissues. To address this point further, we examined the function of innate-like B cells by immunizing mice with 50 μg TNP-Ficoll intraperitoneally to induce TI antibody (Ab) response against TNP. We found no difference in the production of TNP-specific IgM between WT and KO mice (Figure 4D).
HSP90b1 ablation leads to defective compartmentalization but uncompromised function of innate-like B cells. (A-C) Flow cytometric analysis of marginal zone B cells and B1 cells. Representative fluorescence-activated cell sorting (FACS) plots of WT and KO splenic marginal zone B cells (A), peritoneal B1 cells (B), and blood B220+CD21+CD23− and B220+CD5+CD11b− cells (C). Numbers on plots are percentages of gated populations. (D) Production of T cell–independent Ab by B cell–specific HSP90b1 KO mice. Anti-TNP IgM was measured before and after immunization with TNP-Ficoll (n = 6 in each group). Error bars represent SD.
HSP90b1 ablation leads to defective compartmentalization but uncompromised function of innate-like B cells. (A-C) Flow cytometric analysis of marginal zone B cells and B1 cells. Representative fluorescence-activated cell sorting (FACS) plots of WT and KO splenic marginal zone B cells (A), peritoneal B1 cells (B), and blood B220+CD21+CD23− and B220+CD5+CD11b− cells (C). Numbers on plots are percentages of gated populations. (D) Production of T cell–independent Ab by B cell–specific HSP90b1 KO mice. Anti-TNP IgM was measured before and after immunization with TNP-Ficoll (n = 6 in each group). Error bars represent SD.
Robust activation, Ab production, and plasma cell differentiation of HSP90b1 KO B cells in vitro
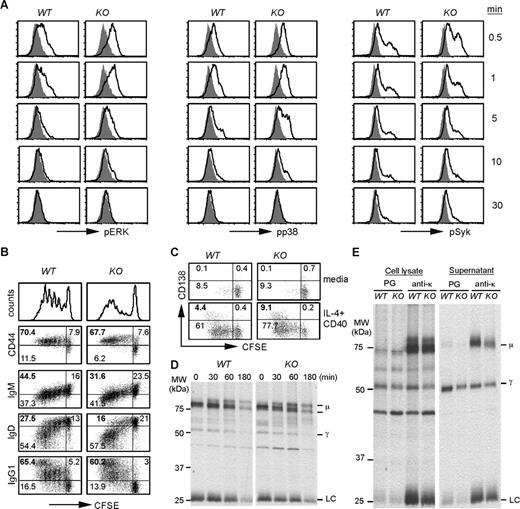
To understand the roles of HSP90b1 in assembly of BCR complexes functionally, we compared the activation of the downstream signaling molecules of WT and KO splenic B cells, including the phosphorylation of p38, Erk, and Syk, upon BCR ligation with anti-μ F(ab′)2. We found that both the kinetics and the magnitude of phosphorylation of p38, Erk, and Syk were comparable between WT and KO B cells (Figure 5A). Calcium flux also paralleled nicely between the 2 cell types (data not shown). To directly examine the impact of HSP90b1 loss on the functional capacity of KO B cells, we stimulated carboxyfluorescein succinimidyl ester (CFSE)–labeled WT and KO B cells with the combination of IL-4 and agonistic CD40 Ab for 5 days. We observed strong proliferation of both WT and KO B cells by CFSE dilution. Both cell types up-regulated CD44, down-regulated surface IgD and IgM, and successfully switched to IgG1 (Figure 5B). Based on CD138 expression, KO B cells differentiated to plasma cells as efficiently as WT cells (Figure 5C). Our demonstration of robust Ab production by HSP90b1 KO B cells calls the Ig-chaperone roles of HSP90b1 into question. We next performed a pulse-chase experiment to directly address the possible defect of Ig assembly, such as the interaction between heavy chain (HC) and light chain (LC) in the KO B cells. Purified B cells were stimulated with IL-4 and agonistic CD40 Ab for 3 days, then metabolically labeled with 35S-Met/Cys for 30 minutes, followed by chasing with a saturated amount of unlabeled Met/Cys for 30 to 180 minutes. Ig was immunoprecipitated with Ab against κ chain and resolved on a reducing SDS-PAGE (Figure 5D). We found that κ chain Ab was able to coimmunoprecipitate heavy chain (μ and γ chain) from both WT and KO cells; the ratio of HC to LC was similar between the 2 cells at all time points. To determine whether HSP90b1 loss affected the secretion of Ig, we immunoprecipitated both cell lysates and supernatant from 4-hour metabolically labeled B cells with protein G (binds to γ chain) or κ chain Ab followed by protein G. We again found no significant difference between WT and KO cells (Figure 5E). Taken together, we concluded that HSP90b1-deficient B cells retained their capacity to proliferate, differentiate, assemble, and secrete Ig.
Robust activation, Ab production, and plasma cell differentiation of HSP90b1 KO B cells in vitro. (A) BCR ligation triggers similar level of phosphorylation of downstream signaling molecules in WT and KO B cells. Splenic B cells from WT and KO mice were stimulated with anti-μ F(ab′)2 for 0 to 30 minutes, followed by intracellular staining and flow cytometric analysis of phosphorylation of Erk, p38, and Syk in B220+ populations. Shaded histograms represent unstimulated cells (time 0); open histograms indicate results after stimulation. (B) Purified WT and HSP90b1 KO B cells were labeled with CFSE, stimulated with CD40 Ab and IL-4 for 5 days, and analyzed by flow cytometry for cell surface CD44 and various Ig subclasses. Undivided (CFSEhigh) cells were indicated. Numbers represent the percentage of cells in each quadrant over the total number of cells. (C) Same as in panel B, except that cells were stimulated with media or IL-4 and CD40 for 4 days before analysis for plasma cell marker CD138. (D) Splenic CD19+ B cells were stimulated with IL-4 and agonistic CD40 Ab for 3 days, then metabolically labeled with 35S-Met/Cys for 30 minutes, and then chased with a saturated amount of unlabeled Met/Cys for 30 to 180 minutes. Ig was isolated by immunoprecipitation (IP) with Ab against κ chain, resolved on reducing SDS-PAGE, and visualized by autoradiography. (E) Same as in panel D, except that cells were metabolically labeled with 35S-Met/Cys for 4 hours followed immediately by IP with protein G (PG) or anti-κ Ab plus PG from both cell lysates and supernatant.
Robust activation, Ab production, and plasma cell differentiation of HSP90b1 KO B cells in vitro. (A) BCR ligation triggers similar level of phosphorylation of downstream signaling molecules in WT and KO B cells. Splenic B cells from WT and KO mice were stimulated with anti-μ F(ab′)2 for 0 to 30 minutes, followed by intracellular staining and flow cytometric analysis of phosphorylation of Erk, p38, and Syk in B220+ populations. Shaded histograms represent unstimulated cells (time 0); open histograms indicate results after stimulation. (B) Purified WT and HSP90b1 KO B cells were labeled with CFSE, stimulated with CD40 Ab and IL-4 for 5 days, and analyzed by flow cytometry for cell surface CD44 and various Ig subclasses. Undivided (CFSEhigh) cells were indicated. Numbers represent the percentage of cells in each quadrant over the total number of cells. (C) Same as in panel B, except that cells were stimulated with media or IL-4 and CD40 for 4 days before analysis for plasma cell marker CD138. (D) Splenic CD19+ B cells were stimulated with IL-4 and agonistic CD40 Ab for 3 days, then metabolically labeled with 35S-Met/Cys for 30 minutes, and then chased with a saturated amount of unlabeled Met/Cys for 30 to 180 minutes. Ig was isolated by immunoprecipitation (IP) with Ab against κ chain, resolved on reducing SDS-PAGE, and visualized by autoradiography. (E) Same as in panel D, except that cells were metabolically labeled with 35S-Met/Cys for 4 hours followed immediately by IP with protein G (PG) or anti-κ Ab plus PG from both cell lysates and supernatant.
Antigen-specific antibody production by B cell–selective HSP90b1 KO mice in vivo
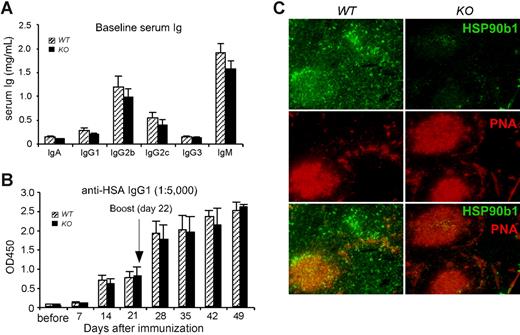
Consistent with the nonessential function of HSP90b1 in Ab production in vitro, the baseline serum level of Ab was only slightly reduced in KO mice (Figure 6A). We next asked the question whether KO mice had any defects in the production of Ab against T cell–dependent (TD) Ag in vivo. This is an important question to address in light of the increasing recognition of both ER UPR6 and TLRs14 in modulating B-cell function. We immunized WT and KO mice intraperitoneally with 100 μg LPS-free human serum albumin (HSA) in the presence of 2 mg type II helper T-cell (TH2)–polarizing adjuvant alum, followed by kinetic measurement of HSA-specific Ab in the sera. We found that KO mice generated similar levels of primary HSA-specific IgG1 (Figure 6B); no IgG2c against HSA was detected in either WT or KO mice (data not shown). We then boosted both WT and KO mice with HSA plus alum at day 22, and subsequently examined the secondary HSA-specific Ab response. Once again, no difference was noticed between WT and KO mice. Furthermore, we found a similar number of germinal centers (GCs) in the WT and KO spleen of immunized mice by staining with biotin-labeled peanut agglutinin (PNA) followed by streptavidin-phycoerythrin (PE; Figure 6C). Costaining with HSP90b1 Ab showed increased HSP90b1 expression by GC B cells in the WT spleen. Strikingly, despite little or no expression of HSP90b1 by KO cells, GC formation was unaltered. Our data thus demonstrate that HSP90b1 is dispensable for B-cell activation, GC formation, and Ab responses in vivo.
Efficient Ab production by B cell–specific HSP90b1 KO mice in vivo. (A) Baseline Ig level in the sera of 8- to 12-week-old WT and KO mice (n = 12 per group). P > .05 between WT and KO mice with all isotypes. (B) Mice were immunized with HSA plus alum and bled weekly for 3 times. Mice were then rechallenged with HSA and were bled weekly for 4 additional time points. Serum anti-HSA IgG1 was then quantified by ELISA (n = 4-6 mice per group). P > .05 at all time points. Error bars represent SEM. (C) Mice were killed 1 week after secondary immunization with HSA and alum; 5-μm-thick sections of spleens were stained with PNA (red) and HSP90b1 (green), and analyzed by fluorescence microscopy (200× magnification). Representative images were shown.
Efficient Ab production by B cell–specific HSP90b1 KO mice in vivo. (A) Baseline Ig level in the sera of 8- to 12-week-old WT and KO mice (n = 12 per group). P > .05 between WT and KO mice with all isotypes. (B) Mice were immunized with HSA plus alum and bled weekly for 3 times. Mice were then rechallenged with HSA and were bled weekly for 4 additional time points. Serum anti-HSA IgG1 was then quantified by ELISA (n = 4-6 mice per group). P > .05 at all time points. Error bars represent SEM. (C) Mice were killed 1 week after secondary immunization with HSA and alum; 5-μm-thick sections of spleens were stained with PNA (red) and HSP90b1 (green), and analyzed by fluorescence microscopy (200× magnification). Representative images were shown.
Attenuated Ab response after priming with Ag and a TLR4 ligand
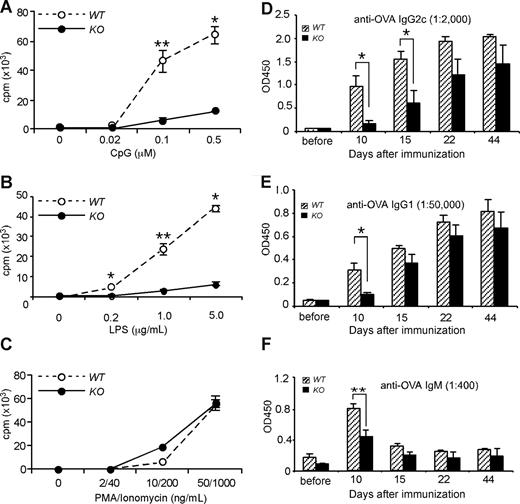
TLR signaling on B cells has been shown to augment Ab response in both physiologic14-16 and pathologic17-22 conditions. The B-cell compartments from our conditional HSP90b1 KO mice were unable to respond to TLR ligands, such as unmethylated CpG (Figure 7A) and LPS (Figure 7B) due to loss of the essential roles of HSP90b1 in folding TLR9 and TLR4, respectively.13 A proliferation defect was not observed when cells were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin (Figure 7C). All other hematopoietic cells expressed normal levels of HSP90b1 and were expected to respond normally to TLR stimulations (data not shown). The B cell–specific TLR defect, in conjunction with a lack of intrinsic B-cell problems (proliferation, differentiation, and Ab production) with our KO mice, allowed us to address the potential roles of direct TLR signaling on B-cell function. We immunized WT and KO mice with 50 μg chicken ovalbumin (OVA) in the presence of 5 μg of the TLR4 ligand LPS, emulsified in incomplete Freund adjuvant (IFA), followed by kinetic measurement of OVA-specific Ab (IgG2c, IgG1, and IgM) in the sera. We found that KO mice generated significantly less Ab in the early course of immunization (Figure 7D-F). Our finding is consistent with a study demonstrating the roles of B cell–intrinsic TLR in optimizing Ab responses.14
Lack of response to TLR stimulations and reduced Ab production by B cells from HSP90b1 KO mice in response to Ag and a TLR ligand. (A-C) Splenic B cells were purified using magnetic beads coated with CD19 Ab from WT and KO mice. Cells were stimulated at 105 cells per well in a 96-well round-bottom plate with CpG (A), LPS (B), and PMA/ionomycin (C) at the indicated concentrations for 38 hours followed by pulsing with 3H-thymidine for 10 hours. Proliferation (cpm) was indexed by 3H-thymidine incorporation. Four experiments were performed with the similar findings. *P < .05; **P < .01. (D-F) Mice were immunized with OVA plus LPS in IFA, followed by measurement of serum anti-OVA IgG2c (D), IgG1 (E), and IgM (F) by ELISA (n = 4-6 mice per group). *P < .05; **P < .01. Error bars represent SEM.
Lack of response to TLR stimulations and reduced Ab production by B cells from HSP90b1 KO mice in response to Ag and a TLR ligand. (A-C) Splenic B cells were purified using magnetic beads coated with CD19 Ab from WT and KO mice. Cells were stimulated at 105 cells per well in a 96-well round-bottom plate with CpG (A), LPS (B), and PMA/ionomycin (C) at the indicated concentrations for 38 hours followed by pulsing with 3H-thymidine for 10 hours. Proliferation (cpm) was indexed by 3H-thymidine incorporation. Four experiments were performed with the similar findings. *P < .05; **P < .01. (D-F) Mice were immunized with OVA plus LPS in IFA, followed by measurement of serum anti-OVA IgG2c (D), IgG1 (E), and IgM (F) by ELISA (n = 4-6 mice per group). *P < .05; **P < .01. Error bars represent SEM.
Discussion
As a major ER luminal chaperone, HSP90b1 has been shown to bind Ig10 and chaperone TLRs,4,13 both of which play important roles in B-cell functions. However, the in vivo roles of HSP90b1 in B-cell response and antibody production remain enigmatic. This is an important question to address in light of increasing recognition of both ER UPR6 and TLRs14 in modulating B-cell function. We have selectively deleted HSP90b1 from murine B cells using the cre-loxP system, and have performed a comprehensive functional study on HSP90b1-null B cells both in vivo and in vitro. We found that B cell–specific deletion of HSP90b1 abrogated their responsiveness to TLR ligands and induced the loss of selective integrins (β2 and α4 but not β1) on the cell surface, which are collectively sufficient to account for the observed phenotype associated with B cell–specific HSP90b1 loss. These B-cell phenotypes include poor migration of follicular B cells to LN, failure of B1 cells to dwell in the peritoneal cavity, loss of MZB cells in the spleen, and compromised Ab production to TD Ag when TLR ligand was used as an adjuvant. Unexpectedly, although Ig was among the first client proteins suggested to be chaperoned by HSP90b1,10 we found no intrinsic defect with KO B cells in terms of BCR expression, proximal signaling, Ig assembly and production, and plasma cell differentiation. Our study demonstrated that HSP90b1 is a specialized chaperone that plays restrictive roles in B-cell function in vivo. This work has significant implications in uncovering the precise effector molecules of UPR in B-cell biology in vivo and in resolving the current controversy over the physiologic function of TLRs in Ab response.14,38
CD19cre-mediated HSP90b1 deletion does not affect B-cell hematopoiesis, including B1- and B2-cell differentiation, indicating that HSP90b1 is not essential for B-cell development. Alternatively, HSP90b1 expression levels become substantially compromised only in the pre–B cells and mature B cells of KO mice, indicating that the fate of B1 and B2 cells might be determined before early pre–B-cell stage. A system to delete HSP90b1 from early lymphoid progenitors or hematopoietic stem cells should be useful in clarifying these 2 possibilities. Although B-cell development is largely unaffected by HSP90b1 deletion, the migration defect of KO B cells to lymph nodes and body cavities was profound, including a significant reduction of absolute B cells from all lymph nodes, loss of splenic MZB cells, and loss of peritoneal B1a cells. Transendothelial migration of B cells into lymph nodes is a multistep process, including L-selectin–dependent rolling,29 migration in response to CCR7 and CXCR4 gradient,30 and finally the adherence to HEVs and migration into B-cell follicles via the integrins αLβ2 (LFA-1)31 and α4β1.32-34 We found no defect with HSP90b1 KO cells in the expression of CD62L, or of the chemokine receptors CCR7 and CXCR4. The migration defect was thus most likely due to loss of α4 and β2 integrins. The integrity of these integrins has been shown to be critical for the retention of MZB cells in the splenic marginal zone.35 Of note, our study examined the long-term effect of multiple integrin (α4 and β2) loss on B-cell migration, which was not experimentally feasible previously. Further study is necessary to determine whether defect in migration of B1 cells and LN B cells in HSP90b1 KO mice is mediated solely by the loss of α4 and β2 integrins, or additionally by loss of other unknown client proteins.
Biochemical studies in myeloma cell lines, under cross-linking conditions, suggested that HSP90b1 binds to both Ig heavy chain and light chain.10 This study implicates that HSP90b1 plays a role in Ab production, although no studies have conclusively established the contribution of HSP90b1 in Ig assembly and production in nonmyeloma normal B cells. More importantly, no studies have examined the impact of HSP90b1 loss on Ab response in vivo. In this study, we showed that there was a normal level of baseline Ig in CD19cre96flox/− mice. Despite the migration problem of B1 cells, Ab against TI Ag was not compromised. Moreover, KO mice mounted a similar magnitude of TD Ab in response to antigen and non-TLR-ligand–containing adjuvant alum. Both GC formation and Ag-specific memory B-cell development proceeded normally without HSP90b1. No defects were seen related to in vivo plasma cell differentiation based on expression of Blimp1 and CD138 (data not shown). Consistent with our in vivo data, we demonstrated that KO B cells were able to transduce a similar degree of BCR signal in vitro. There was no apparent defect in KO B cells to proliferate, differentiate into plasma cells, or assemble and secrete Ig. Based on CFSE dilution, we found that B-cell differentiation occurred only after at least 1 round of cell division for both WT and KO cells. Thus there was no significant difference in B-cell responses between WT and KO mice, either qualitatively or quantitatively, arguing strongly that HSP90b1 is not a critical chaperone for Ig but is dispensable for intrinsic B-cell function.
Along with GRP78, HSP90b1 is one of the key molecular chaperones in mediating unfolded protein responses (UPRs). The integrity of UPR is essential for B-cell differentiation, evidenced by the complete failure of plasma cell differentiation in the absence of XBP-1, a key transcription factor responsible for initiating UPR.6,7,39 Normal antibody response in the absence of HSP90b1 suggests that either HSP90b1 plays no unique roles in B-cell function or its roles are redundant and can be compensated by other ER chaperones such as GRP7840 and GRP170.41 Both GRP78 and GRP170 can interact with Ig and both appear earlier in evolution than HSP90b1, indicating that they might play much broader and more fundamental functions in ER protein quality control than HSP90b1 does. At present, we could not rule out the possibility of these ER chaperones to compensate for the loss of HSP90b1 in Ig assembly. Interestingly, however, we found no compensatory increase of GRP78 in HSP90b1 KO B cells (data not shown).
In contrast, Ab responses (IgG1, IgM, and IgG2c) against a TD Ag in the presence of a TLR4 ligand LPS were significantly compromised in KO mice, particularly in the early time points after immunization. These findings indicate that the function of HSP90b1 has more to do with its roles in chaperoning TLRs rather than in Ig assembly and plasma cell differentiation. Our finding is consistent with a study demonstrating the roles of B cell–intrinsic TLR in optimizing Ab responses.14 Using natural protein Ag and defined TLR4 and TLR5 ligands in mice with a TLR defect restricted to B cells, it was found that TLR stimulation on B cells is critical for optimal IgM, IgG1, IgG2b, and IgG2c responses. More recently, it was found that TLR signaling on B cells promotes both Ag-specific primary IgM response and transient IgG memory response.42 Mechanistically, B cell–intrinsic TLR signalings have been attributed to augment IgG class-switch recombination.15,16
In summary, we have found that HSP90b1 has no essential roles in B-cell development, differentiation, and Ab production. The intrinsic function of HSP90b1 in B-cell biology relates primarily to its ability to chaperone TLRs and integrins but not Ig molecules. It is unclear, however, if Ig is simply not the bona fide client protein of HSP90b1 or the Ig-chaperoning function of HSP90b1 is redundant, which shall be resolved by future functional and proteomic studies into the complexities of the HSP90b1 chaperone-client protein network.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Yi Yang (now at The Kimmel Center for Biology and Medicine of the Skirball Institute, New York University) for establishing the initial colony of Hsp90b1floxCD19cre mice and members of our laboratory (Crystal Morales, Yi Yang, Matthew Staron, and Shuang Wu) for reviewing the manuscript.
This work was supported in part by the National Institutes of Health (Bethesda, MD) grant CA100191 and AI070603 (Z.L.). Z.L. is a clinical scholar of the Leukemia & Lymphoma Society (White Plains, NY).
National Institutes of Health
Authorship
Contribution: B.L. and Z.L. conceived the idea, designed the research, analyzed the data, and wrote the paper; B.L. performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zihai Li, MC 1601, Center for Immunotherapy of Cancer and Infectious Diseases, Department of Immunology, Neag Comprehensive Cancer Center, University of Connecticut School of Medicine, 263 Farmington Ave, Farmington, CT 06030-1601; e-mail: zli@up.uchc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal