Abstract
Dendritic cells (DCs) express many endocytic receptors that deliver antigens for major histocompatibility class (MHC) I and II presentation to CD8+ and CD4+ T cells, respectively. Here, we show that targeting Epstein-Barr virus (EBV) nuclear antigen 1 (EBNA1) to one of them, the human multilectin DEC-205 receptor, in the presence of the DC maturation stimulus poly(I:C), expanded EBNA1-specific CD4+ and CD8+ memory T cells, and these lymphocytes could control the outgrowth of autologous EBV-infected B cells in vitro. In addition, using a novel mouse model with reconstituted human immune system components, we demonstrated that vaccination with αDEC-205-EBNA1 antibodies primed EBNA1-specific IFN-γ–secreting T cells and also induced anti-EBNA1 antibodies in a subset of immunized mice. Because EBNA1 is the one EBV antigen that is expressed in all proliferating cells infected with this virus, our data suggest that DEC-205 targeting should be explored as a vaccination approach against symptomatic primary EBV infection and against EBV-associated malignancies.
Introduction
Epstein-Barr virus (EBV) is a ubiquitous human γ-herpesvirus that latently infects B cells and establishes chronic infection in more than 90% of the adult population. Although infection with EBV during adolescence can lead to infectious mononucleosis (IM), the vast majority of infected people acquires and harbors EBV as a benign lifelong infection, which is controlled by strong T-cell immunity. However, in a small subset of infected individuals, EBV latency programs with different viral antigen expression patterns are associated with malignancies such as Hodgkin and Burkitt lymphomas as well as nasopharyngeal carcinoma (NPC).1 The nuclear antigen 1 (EBNA1) is the one EBV antigen that is expressed in all of these EBV-associated tumors as well as in EBV-positive proliferating cells in healthy carriers.2 EBNA1 is crucial for viral persistence, because it initiates viral DNA replication and anchors the circular viral episome to the mitotic chromosomes during cell division, thereby ensuring the survival of the viral genome in proliferating cells. Thus, even in the absence of all other EBV proteins, such as in Burkitt lymphoma, EBNA1 expression must be maintained, and from an immune surveillance point of view, EBNA1 should be a critical target of protective immunity. Indeed, EBNA1 is consistently recognized by Th1-type CD4+ T cells,3-6 and can elicit CD8+ T-cell responses7-9 in healthy EBV carriers. These T cells that recognize mostly epitopes in the C-terminal domain of EBNA1 can target EBV-transformed B cells and prevent their outgrowth in vitro.10 While EBNA1-specific T-cell responses can also be detected in peripheral blood of NPC patients,11 they are greatly diminished in patients with EBV-associated non-Hodgkin lymphoma in the context of HIV infection,12 EBV-associated Hodgkin disease (K. N. Heller, F.A., P. Steinherz, C. Postlook, A. Chadburn, K. Kelly, C.M., manuscript submitted) and endemic Burkitt lymphomas (Ann Moormann, Case Western Reserve University, Cleveland, OH, personal communication April 2008), thus making EBNA1, and specifically its C-terminal domain, a logical target for vaccine development against all EBV-associated malignancies
A promising cell type, to which EBNA1 could be targeted for vaccine design, is dendritic cells (DCs). These sentinels of the immune system have an exceptional T-cell stimulatory capacity, which includes their ability to efficiently process antigens, and present them on both major histocompatibility class (MHC) I and class II molecules in combination with T-cell costimulatory molecules.13 DCs are also crucial for initiating protective innate and adaptive immune responses against bacterial and viral pathogens in vivo,14,15 which further supports targeting DCs for therapeutic vaccination. However, many current DC-targeted immunization approaches use individualized culture, antigen loading, and activation of DCs in vitro for adoptive transfer.16 A more recent strategy that circumvents the analysis of ex vivo DCs is to target antigens to DCs in vivo by incorporating specific microbial or tumor antigens into antibodies against DC endocytic receptors, and inject them with suitable adjuvant formulations.13
One such receptor, DEC-205, is an endocytic type I transmembrane multilectin protein that delivers antigens to late endosomes or lysosomes, which leads to degradation and presentation of antigens on MHC class II molecules.17,18 DEC-205 can mediate antigen cross-presentation on MHC class I molecules, which results in CD8+ T-cell stimulation.19,20 In mice, in vivo targeting of antigens to DEC-205 enhances the efficiency of antigen presentation to both CD4+ and CD8+ T cells by approximately 100-fold.18,19 DEC-205 is also expressed on human monocyte-derived DCs as well as on DCs in the T-cell areas of human lymph nodes and spleen, where they are ideally positioned to stimulate T cells.21 In addition, coupling of the vaccine antigen HIV gag to an antihuman DEC-205 antibody led to efficient cross-presentation on MHC class I molecules to cultured CD8+ T cells.22 To test the hypothesis that antigen targeting to DEC-205 would also elicit protective T-cell responses in humans, we decided to target a dominant CD4+ T-cell antigen—in our case the immunogenic C-terminal domain of EBNA1 (amino acids [aas] 400-641)—to the DEC-205 endocytic receptor as a first step to develop this strategy for vaccine development against EBV-associated malignancies.
We will show that the targeting of EBNA1 to DEC-205 expands protective EBNA1-specific T cells in vitro and primes EBNA1-specific T cells and antibody responses in vivo. αDEC-205-EBNA1–loaded DCs are consistently more efficient in expanding IFN-γ–secreting EBNA1-specific CD4+ as well as CD8+ T cells compared with isotype control-EBNA1–loaded DCs. In addition, the DEC-205–targeted fusion antibody stimulates EBNA1-specific T cells more efficiently from bulk peripheral blood mononuclear cell (PBMC) cultures, and these expanded T cells are able to control outgrowth of autologous EBV-transformed B cells. Furthermore, we can detect EBNA1-specific IFN-γ–secreting T cells as well as anti-EBNA1 antibodies after intraperitoneal αDEC-205-EBNA1 vaccination of mice with reconstituted human immune system components, whereas the isotype antibody coupled to EBNA1 does not elicit any responses in vivo. Collectively, these data suggest that targeting EBNA1 to the DEC-205 endocytic receptor expands protective T-cell responses against this crucial viral antigen, and should be further explored for vaccine development against EBV-associated malignancies.
Methods
Cell lines
The research study was approved under IRB protocol number CMU-0478. 293T cells were cultured in Dulbecco modified Eagle medium (DMEM) plus 10% fetal calf serum (FCS) 2 mg/mL gentamycin. The generation of CHO-NEO cells was described previously,23,24 and CHO-huDEC205 cells were generated similarly. In brief, a cDNA fragment encoding mature human DEC-205 (huDEC-205) was cloned from the human thymic cDNA library purchased from Clontech (Mountain View, CA) by polymerase chain reaction (PCR) and sequenced. Sequences of a signal peptide and a V5 epitope tag were inserted into the N-terminal side of mature huDEC-205 cDNA (GenBank accession no. AY68209125 ) and subsequently into the pCMV mammalian expression vector (Clontech). Then, CHO-huDEC-205 cells were generated by transfection with pCMV-huDEC-205 with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) followed by selection of G418 (1.5 mg/mL) in culture with DMEM medium supplemented with 10% FCS and 1× GIBCO antibiotic-antimycotic solution (Invitrogen).
Cloning and production of fusion EBNA1 mAbs
Antihuman DEC-205 and the IgG2b isotype control antibody to mouse I-Ak were cloned from total RNA from the MG38.226 and 10-2.16 (TIB93; ATCC, Manassas, VA) hybridomas, respectively, as described earlier.22 cDNA coding for EBNA1 amino acids 400 to 641 followed by a 6His tag was cloned in frame into the COOH terminus of the anti–DEC-205 and the control mAb heavy chains. Unconjugated anti–DEC-205 mAb produced by stably transfected 293T cells was purified on Protein G columns (GE Healthcare, Little Chalfont, United Kingdom). Fusion mAbs were produced by transient transfection (calcium phosphate) in 293T cells, purified on high-performance nickel sepharose columns (GE Healthcare), and characterized by SDS–polyacrylamide gel electrophoresis (PAGE) and Western blotting using anti-EBNA1 (1H4; a gift from Dr Friedrich Grässer, University of Saarland, Homburg, Germany). The fusion antibodies were tested for binding to CHO cells transfected to express the human DEC-205 receptor, and to human monocyte-derived immature DCs by flow cytometry using a phycoerythrin-conjugated goat α-mouse IgG secondary antibody (Biosource, Camarillo, CA).
Peptide libraries
Overlapping peptides of 12- to 22-aa length (average of 15-aa length) with 11-aa overlap were designed for the EBNA1400-641 sequence of the B95.8 EBV strain27 and the MP1 sequence of the influenza A/PR8/34 strain28 using the peptide generator tool of the HIV Sequence Database at the Los Alamos National Laboratory (http://www.hiv.lanl.gov/content/sequence/PEPTGEN/peptgen.html29 ), and synthesized as previously described.27
Preparation of human monocyte-derived dendritic cells and T cells
PBMCs were isolated from leukocyte concentrates (New York Blood Center, New York, NY) by density-gradient centrifugation on Ficoll/Hypaque. CD14+ cells were isolated from PBMCs by positive magnetic cell separation (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) and cultured for 5 days in RPMI1640, 2% plasma from healthy laboratory donors, gentamicin, IL-4, and granulocyte-macrophage colony-stimulating factor (GM-CSF) to generate immature DCs according to standard protocols.30 The CD14− cells were frozen for later use as a source of bulk T cells.
Immunofluorescence
Human spleen sections were prepared for immunofluorescence as described previously.21 The sections were stained with unconjugated antihuman DEC-205 as a positive control and with the indicated fusion antibodies. T-cell areas were identified using an antibody against CD3 (clone SK7, IgG1; BD Biosciences, San Jose, CA). After staining with the primary antibodies, sections were stained with Alexa Fluor 488 or Alexa Fluor 568 conjugated antimouse subclass-specific secondary antibodies (Molecular Probes, Eugene, OR). Slides were analyzed on an inverted Olympus AX70 microscope (Melville, NY) running MetaMorph software version 6.3r6 (Universal Imaging Corporation, West Chester, PA).
Murine splenic tissue was frozen in OCT compound (Sakura Finetechnical, Tokyo, Japan); 5-μm sections were prepared using a Microm cryostat (Microm Laborgerate, Walldorf, Germany) and fixed in acetone. After rehydration in phosphate-buffered saline (PBS), the sections were blocked with the Zymed endogenous avidin/biotin blocking kit (Invitrogen) and stained with the primary antibodies: αDEC-205 (clone MG38; Biolegend, San Diego, CA), αCD11c (3.9; Biolegend), and αHLA class II (hybridoma IVA12; ATCC). After washing with PBS, the sections were stained with Alexa Fluor 555–conjugated streptavidin (Invitrogen) and fluorescein-conjugated streptavidin using the Vector MOM Fluorescein Kit (Vector Labs, Burlingame, CA). Slides were analyzed as described in the previous paragraph.
Expansion of EBNA1-specific T cells in DC/T-cell cocultures and PBMCs
Immature monocyte-derived DCs at day 5 of culture in GM-CSF and IL-4 were pulsed with medium, EBNA1 peptides (1 μg/mL), αDEC-205-EBNA1, or control Ig-EBNA1 at 1 μg/mL with 25 μg/mL polyinosinic-polycytidylic acid (poly(I:C); Invivogen, San Diego, CA) as a DC maturation stimulus. Forty-eight hours later, autologous CD14− cells were thawed and cultured with the matured DCs at a DC/T ratio of 1:100 in RPMI1640 with 5% pooled human serum (PHS) and gentamicin. The cocultures were incubated for 9 days and then an aliquot of each sample was restimulated with EBNA1 peptides (1 μg/mL per peptide) in the presence of brefeldin A for 6 hours to block cytokine secretion. The cells were fixed, permeabilized with 0.1% saponin, and stained with the fluorochrome-conjugated antibodies: αCD8-fluorescein isothiocyanate (FITC; clone SK1; BD Biosciences), αIFN-γ-phycoerythrin (PE; 4S.B3; eBioscience, San Diego, CA), αCD3-PE-Cy5.5 (S4.1; Invitrogen), and αCD4-allophycocyanin (APC; RPA-T4; BD Biosciences). Fluorescence-activated cell sorting (FACS) analysis was done using a BD Biosciences LSR II with Diva software. The remainder of the cells that were not used for intracellular cytokine staining were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and restimulated with EBNA1 peptides (1 μg/mL per peptide) for an additional 6 days. Proliferation of T cells was evaluated by CFSE dilution and staining with the fluorochrome-conjugated antibodies: αCD8-PE (SK1; BD Biosciences), αCD3-PECy5.5, and αCD4-APC. Cells were then analyzed on a BD Biosciences LSR II using Diva software.
To check for the expansion of EBNA1-specific T cells from PBMCs, freshly prepared PBMCs were plated at a density of 2 × 106 cells/well in 48-well plates in RPMI1640 with 5% PHS, gentamicin, and 10 U/mL IL-2, and stimulated with medium, αDEC-205-EBNA1, or control Ig-EBNA1 at 5 μg/mL with 25 μg/mL poly(I:C) as a maturation stimulus for the blood DCs. IFN-γ secretion on day 9 and CFSE dilution on day 15 were evaluated as described in the previous paragraph.
EBV infection of B cells in the presence of expanded EBNA1-specific T cells
Freshly isolated bulk PBMCs were stimulated with medium, 5 μg/mL αDEC-205-EBNA1, or isotype-EBNA1 in combination with 25 μg/mL poly(I:C) and 10 U/mL IL-2. After 9 days, the cells were restimulated and expanded with EBNA1 peptides and 10 U/mL IL-2 for an additional 6 days. Autologous CD19+ B cells were purified from PBMCs by positive magnetic cell separation (Miltenyi Biotech, Bergisch-Gladbach, Germany), infected with EBV overnight using filtered supernatants of the EBV+ B95-8 marmoset cell line, washed, and added into the expanded T-cell cultures at a B/T-cell ratio of 2:1; supplemented with 10 U IL-2, 10 ng/mL IL-7, and 10 ng/mL IL-15. After 16 days of coculture, the cells were fixed and stained with αCD19-APC (HIB19; BD Biosciences), αCD23-PE (M-L233; BD Biosciences) and analyzed by FACS to determine the percentage of EBV-infected B cells.
Preparation of humanized mice and αDEC-205-EBNA1 vaccination
NOD/LtSz-scid IL2Rγnull (NOG) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and raised under pathogen-free conditions. Human fetal liver was obtained from Advanced Bioscience Resources (Alameda, CA). The tissue was minced and treated with 2 mg/mL collagenase D (Roche Diagnostics, Indianapolis, IN) in Hanks balanced salt solution with CaCl2/MgCl2 for 30 minutes at room temperature followed by filtering through 70-μm nylon cell strainers (BD Biosciences). CD34+ human hematopoietic stem cells (HSCs) were isolated using the Direct CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec). NOG mice (2 to 5 days old) were irradiated with 100 cGy and injected intrahepatically with 1 to 3 × 105 CD34+ HSCs 6 hours after irradiation. The mice were bled 10 to 12 weeks after engraftment and peripheral lymphocytes were analyzed by FACS as described previously to check for the reconstitution of the human immune system.31,32 To test the in vivo efficacy of αDEC-205 targeting, humanized NOG mice were injected intraperitoneally with PBS control, 5 μg αDEC-205-EBNA1, or isotype-EBNA1 with 50 μg poly(I:C) as an adjuvant; the animals were boosted with the same dose of antibodies and adjuvant a month later. One to 3 months after boost, the mice were killed, and EBNA1-specific T- and B-cell responses were analyzed in splenocytes and plasma using an IFN-γ enzyme-linked immunosorbent spot (ELISPOT) and EBNA1-specific enzyme-linked immunosorbent assay (ELISA) assays, respectively.
IFN-γ ELISPOT and EBNA1 Ig ELISA
ELISPOT assays for IFN-γ were performed as described previously.4 Bulk splenocytes from vaccinated mice were seeded at 2 × 105/well and stimulated in triplicate with either EBNA1 peptides or an influenza matrix protein 1 (MP1) peptide library for 18 hours. Spots were counted with an ELISPOT reader (Autoimmun Diagnostika, Strassberg, Germany). For the detection of EBNA1-specific IgM and IgG antibodies, sera from vaccinated mice were tested using a commercially available EBNA1 Ig ELISA kit (Diamedix, Miami, FL). As suggested in the manufacturer's instructions, an index value of 1.10 or higher was used as proof of human EBNA1-specific Ig.
Statistical analysis
Statistical analyses were performed with the paired 2-tailed Student t test. The P value of significant differences is reported. Plotted data represent mean plus standard deviation (SD), unless otherwise stated.
Results
Production of the αDEC-205-EBNA1 fusion antibody
To target the immunogenic C-terminal domain of EBNA1 (aas 400-641) to the DEC-205 endocytic receptor, we cloned a cDNA encoding for this domain with a C-terminal 6His tag to the carboxyl terminus of the heavy chain of the antihuman DEC-205 antibody MG38.226 (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). A fusion with the heavy chain of the isotype-matched antibody TIB93, specific for the mouse MHC class II molecule H2-Ak, was also engineered as a control. The fusion mAbs were produced by transient transfections into 293T cells and purified on nickel-sepharose columns. The purified mAbs were characterized by silver staining (Figure S1B) and by Western blot analysis using an anti-EBNA1 antibody after SDS-PAGE (Figure S1C). To assess their biologic activity, the fusion antibodies were tested for binding to CHO cells transfected to express the human DEC-205 receptor as well as human monocyte-derived immature DCs (Figure S2A). In addition, both the αDEC-205-EBNA1 and the isotype-EBNA1 fusion Abs were used to stain frozen human spleen sections. Whereas the isotype control failed to stain, the αDEC-205-EBNA1 fusion protein was comparable with the unconjugated αDEC-205 Ab (Figure S2B), and stained DCs in the T- and B-cell zones of human splenic white pulp.21 Although localization of DCs has not been typically observed in B-cell zones of murine spleens, scattered CD11c+ and DEC-205+ cells are readily detected in human splenic B-cell areas21,33 where they may be involved in generating T-cell help for the strong humoral immune responses generated upon DEC-205–targeted vaccination.34 In conclusion, our αDEC-205-EBNA1 fusion mAb recognizes DEC-205 expressed by cells, including DCs in situ.
Expansion of EBNA1-specific T cells by αDEC-205-EBNA1–loaded DCs
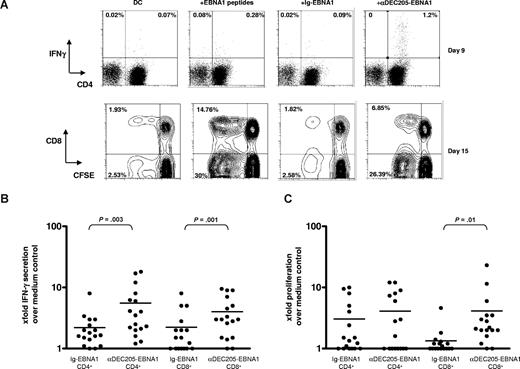
As most people have protective immunity against EBV,10 we used monocyte-derived DCs from healthy blood donors to evaluate their ability in expanding EBNA1-specific memory T cells after loading with αDEC-205-EBNA1. The DCs were pulsed with the indicated antibodies or an EBNA1 peptide library as a positive control. After being matured with the TLR3/MDA-5 ligand poly(I:C), which has been explored for in vivo use in humans and could therefore be used as an adjuvant for clinical vaccination,35 the DCs were cocultured with autologous PBMCs and EBNA1-specific T-cell expansion was monitored by both IFN-γ production on day 9 (Figure 1A top panel) as well as by proliferation on day 15 (Figure 1A bottom panel) after restimulation with the EBNA1 peptide library on day 9. In 11 of the 17 individuals we studied, the frequency of IFN-γ+ CD4+ and/or CD8+ T cells expanded by αDEC-205-EBNA1–pulsed DCs and specific for EBNA1400-641 peptides was more than twice the number of T cells expanded as a result of isotype-EBNA1 stimulation (Figure 1B). In addition, the frequency of CD3+ IFN-γ+ EBNA1-specific T cells expanded by αDEC-205-EBNA1 targeting ranged from 0.5% to 12.5%, whereas the expansion achieved by isotype-EBNA1 targeting only rarely exceeded 2%. Although we were able to detect both CD8+ and CD4+ IFN-γ+ EBNA1-specific T cells, the greater proportion of the responding cells were of the CD4+ kind, and more healthy volunteers expanded EBNA1-specific CD4+ T cells upon αDEC-205-EBNA1 stimulation than in response to isotype-EBNA1. To reveal additional subdominant T-cell responses against EBNA1, the already expanded T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and restimulated with an EBNA1 peptide library for 6 more days. The proliferation of the responding cells was measured by monitoring CFSE dilution. Once again, we observed that T cells cocultured with αDEC-205-EBNA1–pulsed DCs proliferated more vigorously in response to EBNA1 peptide restimulation compared with T cells cocultured with isotype-EBNA1–pulsed DCs (Figure 1C). Among the 17 individuals who we studied, the mean of the frequency of EBNA1-specific CFSE-low T cells was 42% in the αDEC-205-EBNA1 group compared with 29% in the isotype-EBNA1 stimulations (data not shown). This difference was statistically significant (P value of .002). Whereas we observed predominantly IFN-γ–secreting CD4+ T cells after 9 days of coculture with αDEC-205-EBNA1–pulsed DCs, restimulation with the EBNA1 peptide library expanded EBNA1-specific CD8+ T cells in addition. For EBNA1-specific CD4+ T cells, the second round of expansion, however, diminished differences between αDEC-205-EBNA1 and isotype-EBNA1–mediated T-cell stimulation, which both supported antigen-specific T-cell expansion to high levels at day 15. In conclusion, among most of the 17 blood donors we processed, αDEC-205-EBNA1 was approximately 2-fold more efficient as vaccine antigen formulation than the isotype-EBNA1 in expanding EBNA1-specific T cells.
Induction of EBNA1-specific T-cell responses by αDEC-205-EBNA1 targeting to DCs. (A) PBMCs from blood donors were stimulated with mature autologous monocyte-derived DCs that were pulsed with medium, EBNA1 peptides, 1 μg/mL αDEC-205-EBNA1, or control Ig-EBNA1 using poly (I:C) as an adjuvant. After 9 days, frequencies of IFN-γ+ CD3+ T cells were determined by intracellular staining after EBNA1 peptide restimulation (top row). After a second round of stimulation with an EBNA1 peptide library on day 9, proliferation was evaluated by CFSE dilution on day 15 (bottom row). Representative data of 17 experiments are shown. Percentages on plots are of total cells within respective gates. Summary of the EBNA1-specific T-cell responses for 17 donors evaluated (B) by IFN-γ+ secretion on day 9 and (C) by CFSE dilution on day 15. Horizontal lines represent medians.
Induction of EBNA1-specific T-cell responses by αDEC-205-EBNA1 targeting to DCs. (A) PBMCs from blood donors were stimulated with mature autologous monocyte-derived DCs that were pulsed with medium, EBNA1 peptides, 1 μg/mL αDEC-205-EBNA1, or control Ig-EBNA1 using poly (I:C) as an adjuvant. After 9 days, frequencies of IFN-γ+ CD3+ T cells were determined by intracellular staining after EBNA1 peptide restimulation (top row). After a second round of stimulation with an EBNA1 peptide library on day 9, proliferation was evaluated by CFSE dilution on day 15 (bottom row). Representative data of 17 experiments are shown. Percentages on plots are of total cells within respective gates. Summary of the EBNA1-specific T-cell responses for 17 donors evaluated (B) by IFN-γ+ secretion on day 9 and (C) by CFSE dilution on day 15. Horizontal lines represent medians.
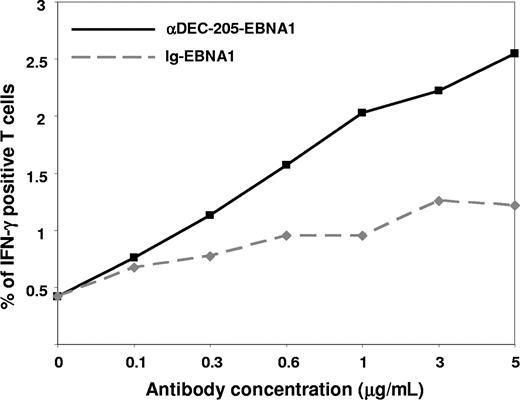
To further characterize the efficacy of targeting EBNA1 to DEC-205, we pulsed monocyte-derived DCs with titrated doses of the αDEC-205-EBNA1 and isotype-EBNA1 antibodies. Consistent with the results in Figure 1, DCs that took up EBNA1 via DEC-205 at an antibody concentration of 1 μg/mL expanded antigen-specific T cells to more than 2-fold higher frequencies compared with control isotype-EBNA1–targeted DCs (Figure 2; P = .006 across all concentrations). More importantly, we found that DCs loaded with 0.3 μg/mL αDEC-205-EBNA1 expanded IFN-γ–secreting T cells just as efficiently as DCs that were pulsed with 3 μg/mL isotype-EBNA1. Thus, DEC-205–targeted EBNA1 expanded T cells at 10-fold lower concentrations than antigen without targeting.
Dose-dependent response to αDEC-205-EBNA1 targeting. Monocyte-derived DCs were pulsed with increasing concentrations of αDEC-205-EBNA1 (■) or Ig-EBNA1 (♦) control antibodies using poly(I:C) as a maturation stimulus. PBMCs from the same donor were stimulated with the antigen-loaded and matured DCs for 9 days, and frequencies of EBNA1-specific IFN-γ+ CD3+ T cells were determined by intracellular staining after EBNA1 peptide restimulation. One of 2 evaluated donors is shown.
Dose-dependent response to αDEC-205-EBNA1 targeting. Monocyte-derived DCs were pulsed with increasing concentrations of αDEC-205-EBNA1 (■) or Ig-EBNA1 (♦) control antibodies using poly(I:C) as a maturation stimulus. PBMCs from the same donor were stimulated with the antigen-loaded and matured DCs for 9 days, and frequencies of EBNA1-specific IFN-γ+ CD3+ T cells were determined by intracellular staining after EBNA1 peptide restimulation. One of 2 evaluated donors is shown.
Expansion of EBNA1-specific T cells by αDEC-205-EBNA1 addition to bulk PBMCs
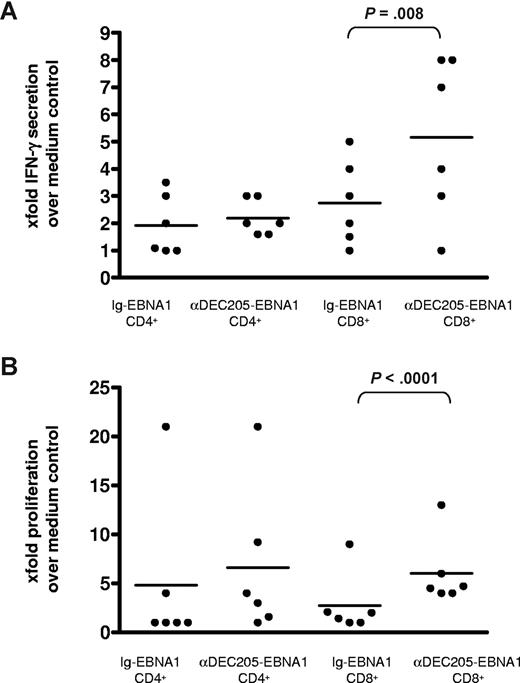
Having established that loading of monocyte-derived DCs with EBNA1 via the DEC-205 receptor mediates antigen presentation to T cells, we next incubated our vaccine construct with bulk PBMCs, in which the infrequent subset of BDCA3+ myeloid DCs expresses the highest levels of DEC-205.36 αDEC-205-EBNA1 was added to freshly isolated PBMCs with poly(I:C) as a DC maturation stimulus, and EBNA1-specific T-cell expansion was monitored as described for Figure 1. The frequency of IFN-γ+ CD3+ T cells ranged from 0.25% to 0.7% in the αDEC-205-EBNA1–stimulated PBMCs, whereas it was between 0.15% and 0.3% for the isotype-EBNA1 group. Among the 6 tested PBMC donors, 4 of them had at least 2 times more EBNA1-specific IFN-γ–secreting CD8+ and/or CD4+ cells when stimulated with αDEC-205-EBNA1 compared with the isotype-EBNA1 stimulation (Figure 3A). Just as we had observed in the DC/T-cell cocultures, upon restimulation with an EBNA1 peptide library, CD8+ T cells from αDEC-205-EBNA1–stimulated PBMCs proliferated much more vigorously than the isotype-EBNA1 control–stimulated cultures (Figure 3B). The mean of the frequency of EBNA1-specific CFSE-low T cells was 30% in the αDEC-205-EBNA1 group compared with 18% in the isotype-EBNA1 (data not shown); the difference was statistically significant (P value of .001). These findings indicate that DEC-205–targeted EBNA1 is presented on MHC class I molecules in bulk PBMC cultures, leading to EBNA1-specific CD8+ T-cell expansion.
αDEC-205-EBNA1 in combination with poly(I:C) induces EBNA1-specific T-cell responses in bulk PBMCs. PBMCs from blood donors were pulsed with medium, 5 μg/mL αDEC-205-EBNA1, or isotype-EBNA1 using poly(I:C) as an adjuvant and with 10 U/mL IL-2. EBNA1-specific (A) IFN-γ production on day 9 and (B) proliferation by T cells on day 15 were determined as in Figure 1 for 6 different donors. Horizontal lines represent medians.
αDEC-205-EBNA1 in combination with poly(I:C) induces EBNA1-specific T-cell responses in bulk PBMCs. PBMCs from blood donors were pulsed with medium, 5 μg/mL αDEC-205-EBNA1, or isotype-EBNA1 using poly(I:C) as an adjuvant and with 10 U/mL IL-2. EBNA1-specific (A) IFN-γ production on day 9 and (B) proliferation by T cells on day 15 were determined as in Figure 1 for 6 different donors. Horizontal lines represent medians.
Protection against EBV infection of B cells upon αDEC-205-EBNA1–mediated T-cell expansion
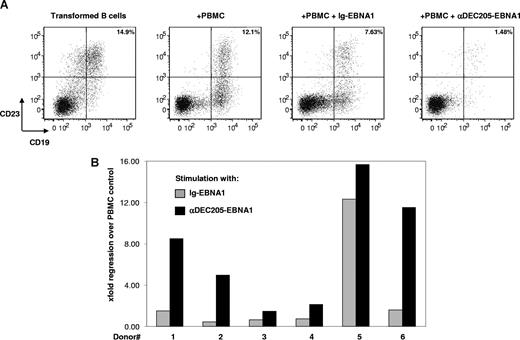
To test whether these expanded EBNA1-specific T cells were of protective value, we tested their capacity to restrict EBV infection of B cell in vitro. B cells, latently infected by EBV, express the activation marker CD23; thus the outgrowth of infected cells can be monitored by flow cytometric analysis of CD19+ CD23+ double-positive cells. To assess whether memory T cells expanded from αDEC-205-EBNA1–pulsed bulk PBMCs could mediate the regression of autologous EBV-infected B cells, we cocultured the stimulated PBMCs with the infected B cells at an effector to target ratio of 1:2 for 16 days. In the absence of T cells, there was outgrowth of 15% to 25% EBV-infected B cells. While addition of T cells alone caused regression of EBV infection, stimulation of the PBMCs with αDEC-205-EBNA1 led to significantly elevated regression compared with isotype-EBNA1 control stimulation (Figure 4). Thus, by targeting EBNA1 to the DEC-205 receptor, we could achieve expansion of protective EBNA1-specific memory T cells that were able to significantly reduce EBV infection of autologous B cells.
PBMCs primed by αDEC-205-EBNA1 control EBV infection. Bulk PBMCs were pulsed with medium, 5 μg/mL αDEC-205-EBNA1, or control Ig-EBNA1 in combination with poly(I:C) and 10 U/mL IL-2. After 9 days, the cells were restimulated and expanded with an EBNA1 peptide library for an additional 6 days at which point autologous EBV-infected B cells were added into the culture and supplemented with 10 U/mL IL-2, 10 ng/mL IL-7, and 10 ng/mL IL-15. (A) After 16 days of coculture, outgrowth of EBV-infected B cells was assessed by flow cytometric analysis of CD19+ CD23+ double-positive cells. Representative data of 6 experiments are shown. Percentages on plots are of total cells within respective gates. (B) Summary of EBV-infected B-cell regression for 6 different donors.
PBMCs primed by αDEC-205-EBNA1 control EBV infection. Bulk PBMCs were pulsed with medium, 5 μg/mL αDEC-205-EBNA1, or control Ig-EBNA1 in combination with poly(I:C) and 10 U/mL IL-2. After 9 days, the cells were restimulated and expanded with an EBNA1 peptide library for an additional 6 days at which point autologous EBV-infected B cells were added into the culture and supplemented with 10 U/mL IL-2, 10 ng/mL IL-7, and 10 ng/mL IL-15. (A) After 16 days of coculture, outgrowth of EBV-infected B cells was assessed by flow cytometric analysis of CD19+ CD23+ double-positive cells. Representative data of 6 experiments are shown. Percentages on plots are of total cells within respective gates. (B) Summary of EBV-infected B-cell regression for 6 different donors.
In vivo priming of EBNA1-specific T cells and antibodies by αDEC-205-EBNA1
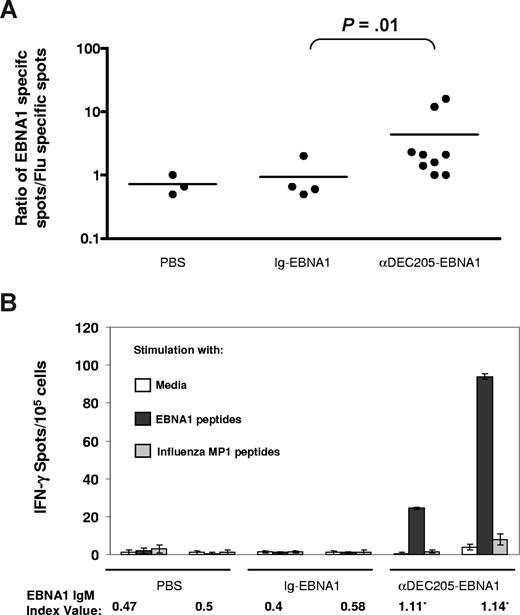
In mice, in vivo targeting of antigens to DEC-205 enhances the efficiency of antigen presentation to both CD4+ and CD8+ T cells.18,19 The ability of αDEC-205-EBNA1 to expand antigen-specific T cells in vitro encouraged us to test the potential of DEC-205 targeting in vivo to initiate immune responses. For this purpose, we used the NOD-scid IL2Rγnull (NOG) immunodeficient mouse reconstituted with human immune system components. Upon injection with human CD34+ hematopoietic stem cells, NOG mice develop human B, T, natural killer (NK), and myeloid cells including dendritic cells, and thus the mice represent a promising model to study human immune responses in vivo37 (data not shown). Using fluorescent immunohistochemistry, we could demonstrate that the spleens of these mice contain DEC-205+ MHC II+ CD11c+ cells with dendritic cell morphology in proximity to CD3+ T cells (Figure S3 and data not shown). Next, we immunized reconstituted NOG mice intraperitoneally with αDEC-205-EBNA1 or isotype-EBNA1 antibodies plus poly(I:C) as a DC maturation stimulus, followed by a booster injection a month later. We tested the efficacy of the vaccine using an IFN-γ ELISPOT assay to compare splenic EBNA1 peptide-specific responses with nonspecific responses against an influenza matrix protein 1 (MP1) peptide library. Although we did not observe any EBNA1-specific IFN-γ secretion one month after the boost, when the animals were killed 2 months after the booster injection, we detected a statistically significant difference in EBNA1-specific T-cell responses between the αDEC-205-EBNA1–vaccinated animals and isotype-EBNA1–injected controls by IFN-γ ELISPOT assays (Figure 5A; P = .01). In 2 of the vaccinated animals with the highest EBNA1-specific T-cell responses (> 20 IFN-γ spots per 105 splenocytes), we also detected anti-EBNA1 IgM, but not IgG, antibodies (Figure 5B and data not shown). Therefore, targeting viral antigens to the human DEC-205 receptor in vivo has the potential to prime both human T- and B-cell responses.
Induction of primary T- and B-cell responses via αDEC-205-EBNA1 vaccination in immunodeficient mice reconstituted with human immune system components. Reconstituted mice were vaccinated intraperitoneally with PBS, Ig-EBNA1, or αDEC-205-EBNA1 using poly(I:C) as an adjuvant and boosted a month later. Two months after boost, bulk splenocytes were harvested, stimulated with either EBNA1 peptides or an influenza matrix protein 1 peptide library, and subjected to an IFN-γ ELISPOT assay. (A) Ratio of EBNA1-specific IFN-γ spots to nonspecific spots against the influenza peptides summarized for each vaccine group. Horizontal lines represent medians.(B) IFN-γ spots/105 splenocytes and EBNA1 IgM levels shown for 2 animals from each group. An index value of 1.1 or higher confirms the presence of anti-EBNA1 antibodies. Error bars represent SD.
Induction of primary T- and B-cell responses via αDEC-205-EBNA1 vaccination in immunodeficient mice reconstituted with human immune system components. Reconstituted mice were vaccinated intraperitoneally with PBS, Ig-EBNA1, or αDEC-205-EBNA1 using poly(I:C) as an adjuvant and boosted a month later. Two months after boost, bulk splenocytes were harvested, stimulated with either EBNA1 peptides or an influenza matrix protein 1 peptide library, and subjected to an IFN-γ ELISPOT assay. (A) Ratio of EBNA1-specific IFN-γ spots to nonspecific spots against the influenza peptides summarized for each vaccine group. Horizontal lines represent medians.(B) IFN-γ spots/105 splenocytes and EBNA1 IgM levels shown for 2 animals from each group. An index value of 1.1 or higher confirms the presence of anti-EBNA1 antibodies. Error bars represent SD.
Discussion
Targeted in vivo delivery of antigens to DCs constitutes a promising new avenue of vaccination. Although this approach has been validated mainly in mice,13 we show here that targeting EBNA1 to human DCs via DEC-205 in the presence of a DC maturation stimulus is capable of expanding protective EBNA1-specific CD4+ and CD8+ memory T cells. Targeting in bulk PBMCs with αDEC-205-EBNA1 antibody addition was also efficient in expanding antigen-specific T cells, thus negating the need for in vitro DC preparations. Furthermore, these expanded lymphocytes could control the outgrowth of autologous EBV-infected B cells. In addition, we demonstrate that αDEC-205-EBNA1 antibody vaccination of NOD-scid IL2Rγnull mice with reconstituted human immune system components can prime EBNA1-specific human T- and B-cell responses. EBV infection of human B cells followed by induction of primary HLA-restricted EBV-specific human T cells has been previously reported in 2 mouse models of human immune system reconstitution.38,39 Hence, we suggest that DEC-205-EBNA1 fusion antibody vaccination was also able to induce primary human T- and, in a subset of mice, B-cell responses in NOD-scid IL2Rγnull mice with reconstituted human immune system components. Whether prior vaccination with αDEC-205-EBNA1 will affect the outcome of EBV infection and the accompanying T-cell responses will be interesting to study and the topic of future research in our laboratory. We observed that the frequency and breadth of the primary immune responses in this mouse model of the human immune system were modest and slow to develop compared with mouse DEC-205–targeted vaccines tested in C57BL/6 and BALB/c mice. This may be due to diminished secondary lymphoid organ and germinal center formation in these and similarly reconstituted mice,40,41 and comparable observations have been reported by other groups using ovalbumin,42 tetanus toxoid,39 and Haemophilus influenzae type b (HIB) conjugate vaccines43 as well as in the context of HIV infection.32,44 Nevertheless, our results indicate that directing EBV antigens to DEC-205 with poly(I:C) as an adjuvant may be a feasible vaccine strategy in humans for the induction of primary immune responses as well as for the expansion of protective antigen-specific CD4+ and CD8+ memory T cells against EBV-associated diseases.
Induction of primary immune responses as well as the expansion of preexisting memory T cells specific for EBNA1 can especially benefit Hodgkin and Burkitt lymphoma patients who have deficiencies in their EBNA1-specific T-cell responses (K. N. Heller, F. A., P. Steinherz, C. Postlook, A. Chadburn, K. Kelly, C.M., manuscript submitted; Ann Moormann, Case Western Reserve University, written communication, April 2008). Moreover, HIV-infected individuals who develop EBV-associated CNS or non-Hodgkin lymphomas were also found to have decreased EBV-specific CD4+ T-cell responses.12,45 In the case of EBV-associated non-Hodgkin lymphoma, this loss was particularly pronounced for EBNA1-specific CD4+ T cells, but affected CD4+ T-cell responses to the lytic EBV antigen BZLF1 to a much lesser extent.12 Although frequencies of EBV-specific T cells in peripheral blood of NPC patients were found to be similar to healthy controls,46 the same study also found that a great majority of these patients did not have detectable EBNA1-specific CD8+ T cells.11 Because boosting preexisting EBNA1-specific CD4+ and CD8+ T cells via DEC-205 targeting leads to better control of EBV infection (Figure 4), our vaccine strategy may benefit patients with all types of EBV-associated malignancies by either correcting for a selective loss of EBNA1-specific CD4+ T-cell responses, as seen in EBV-associated lymphomas, or increasing the generally low CD8+ T-cell response to this antigen.
To avoid symptomatic primary EBV infection, and the increased risk after IM to develop Hodgkin disease (HD) as well as the autoimmune disease multiple sclerosis,47,48 vaccines to induce neutralizing antibodies and T-cell responses are under development. Along these lines, a phase 1/2 clinical trial with recombinant EBV major surface glycoprotein gp350 as the vaccine antigen was successful in eliciting neutralizing antibodies. Although this vaccine seemed to diminish the incidence of IM, its efficacy in preventing virus infection and/or EBV-associated diseases remains unknown.49,50 It seems unlikely that sterilizing immunity against EBV can be achieved via vaccination because 1 to 10 virus particles are enough for the establishment of lifelong γ-herpesvirus infections,51,52 and reinfection of healthy EBV carriers with new EBV strains has been documented.53,54 Thus, more recent immunotherapeutic approaches against primary EBV infection have concentrated on inducing T-cell responses to aid in asymptomatic seroconversion, rather than preventing infection, and to target EBV-associated malignancies. Along these lines, a single CD8+ T-cell peptide epitope–based vaccine for IM has been tested in a phase 1 clinical trial. Peptide-specific responses against the injected epitope were detected in the vaccinated individuals, but the insufficient number of recipients precluded assessment of vaccine efficacy.55 Therefore, no vaccine that completely prevents symptomatic EBV infection has been developed, and we propose to evaluate DEC-205–mediated EBV antigen targeting for induction of protective immune responses against IM.
In addition to vaccination against symptomatic EBV seroconversion, there is a need for therapeutic vaccination against EBV-associated malignancies. One clinically successful passive immunization approach against EBV-positive tumors is the adoptive transfer of antigen-specific T cells. EBV-specific T cells from seropositive individuals can be expanded in vitro by coincubation with autologous EBV-transformed B-lymphoblastoid cell lines (LCLs), and infusion of these expanded lymphocytes into the recipients of hematopoietic stem cell or solid organ transplants has been successful in the treatment of EBV-induced posttransplantation lymphoproliferative disease (PTLD).56 However, the same approach has resulted in limited responses to other EBV-positive malignancies such as Hodgkin disease (HD) and nasopharyngeal carcinoma (NPC). This is, in part, because HD and NPC, unlike PTLD, express only 3 viral proteins (EBNA1, LMP1, and LMP2), but LCLs tend to expand T cells reactive against the immunodominant EBNA3 antigens present in PTLD. To overcome this challenge, various recombinant viruses expressing T-cell epitopes from EBNA1, LMP1, and LMP2 have been used to infect PBMCs to expand T cells specific for these 3 viral antigens. These approaches have resulted in expansion of CD8+ T cells specific for EBNA1 and LMPs in vitro, and they were able to control outgrowth of LMP-expressing tumors in mice57-59 ; however, the effectiveness of this strategy in a clinical setting has not been evaluated. On the other hand, DCs pulsed with CD8 T-cell epitopes of LMP2 were injected into NPC patients and, although LMP2-specific CD8+ cytotoxic T lymphocytes (CTLs) were detected, these LMP2-specific responses were too weak and transient to achieve clinical success against the tumors.60 Thus, vaccine strategies that aim to expand only protective EBV-specific CD8+ T-cell responses have not proven sufficient as therapies against EBV-associated malignancies.
Because CD4+ T cells are necessary to maintain CD8+ T-cell immunity and can target infected cells directly,5,6,61 more recent vaccine attempts have focused on inducing both CD4+ and CD8 T+ cells. In addition, stimulation of CD4+ CTLs against EBV-infected tumors might be crucial for Burkitt lymphoma where EBNA1 is the only viral protein expressed and MHC class I antigen presentation is severely impaired.62,63 To achieve simultaneous stimulation of both CD4+ and CD8+ T cells against EBV antigens, several recombinant viral vaccines have been developed to target dendritic cells, as these cells are the most potent antigen-presenting cells in the initiation of antiviral immune responses. Furthermore, DCs can expand EBV-specific CD8+ T cells more efficiently than LCLs64 and they can prime protective immune responses in vitro.65 DCs infected in vitro with a recombinant adenovirus expressing the EBV LMP2 antigen were able to expand LMP2-specific CD4+ and CD8+ CTLs that could kill NPC cells.66 A modified vaccinia virus vector encoding a fusion between the CD4+ T-cell epitope-rich C-terminal domain of EBNA1 and full-length LMP2 protein has also been used to infect DCs in vitro, and the infected DCs were able to reactivate both LMP2-specific CD8+ and EBNA1-specific CD4+ memory T cells from seropositive donors.67 Although these approaches have been successful in eliciting tumor-specific T cells, generation of DCs in vitro is labor intensive, has to be performed for every patient individually, and might be limited by the DC number that can be derived. In addition, there are safety concerns over the use of vaccinia and adenoviral vector–based vaccines in humans. In contrast, we propose that in vivo targeting of viral or tumor antigens directly to the endocytic receptor DEC-205, using an antibody-based vaccine, may be safer and more effective. Another advantage of DEC-205 targeting is that this receptor is expressed on many DCs in the T-cell areas of human lymphoid tissues, and this positioning would allow the DEC-205–targeted vaccine to efficiently select specific clones of T cells that circulate from the blood through lymphoid organs. Therefore, the expansion of protective CD4+ and CD8+ T-cell responses against EBNA1 in vitro and the priming of EBNA1-specific human T-cell responses in vivo by the αDEC-205-EBNA1 hybrid antibody suggest that targeted EBV antigen delivery to DEC-205 in vivo should be explored for preventive vaccination against symptomatic primary EBV infection as well as for therapeutic immunization against EBV-associated malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Arnold and Mabel Beckman Foundation (Irvine, CA), the Alexandrine and Alexander Sinsheimer Foundation (New York, NY), the Burroughs Wellcome Fund (Research Triangle Park, NC), the Dana Foundation Neuroimmunology program (New York, NY), the Starr Foundation, the National Cancer Institute (Bethesda, MD; R01CA108609 and R01CA101741), the National Institute of Allergy and Infectious Diseases (Bethesda, MD; RFP-NIH-NIAID-DAIDS-BAA-06-19), the Foundation for the National Institutes of Health Grand Challenges in Global Health grant nos. CA108609 and CA101741 (C.M.), and an Institutional Clinical and Translational Science Award to the Rockefeller University Hospital
National Institutes of Health
Authorship
Contribution: C.G., T.S., F.B., M.P., and F.A. performed research; C.T. and C.G.P. contributed vital new reagents; and C.G., R.M.S., and C.M. designed research and wrote the paper.
Conflict-of-interest disclosure: R.M.S. is a consultant to Celldex Therapeutics (Phillipsburg, NJ), which is developing human DEC-205–based vaccines. The remaining authors declare no competing financial interests.
Correspondence: Christian Münz, Laboratory of Viral Immunobiology, The Rockefeller University, 1230 York Ave, New York, NY 10065; e-mail: munzc@rockefeller.edu.