Abstract
Graft-versus-host disease (GVHD) is a serious complication of allogeneic bone marrow transplantation, and donor T cells are indispensable for GVHD. Current therapies have limited efficacy, selectivity, and high toxicities. We used a novel flow cytometry technique for the analysis of intracellular phosphorylation events in single cells in murine BMT models to identify and validate novel GVHD drug targets.1-7 This method circumvents the requirement for large numbers of purified cells, unlike western blots. We defined a signaling profile for alloactivated T cells in vivo and identified the phosphorylation of ERK1/2 and STAT-3 as important events during T-cell (allo)activation in GVHD. We establish that interference with STAT-3 phosphorylation can inhibit T-cell activation and proliferation in vitro and GVHD in vivo. This suggests that phospho-specific flow cytometry is useful for the identification of promising drug targets, and ERK1/2 and STAT-3 phosphorylation in alloactivated T cells may be important for GVHD.
Introduction
Analysis of in vivo T-cell signaling with Western blots is limited by requirements for large numbers of cells and the inability to access subpopulations. We used a flow cytometric method for measuring phosphorylated epitopes in single cells1-7 to establish a signaling profile for alloactivated T cells in vivo in murine graft-versus-host disease (GVHD) after allogeneic bone marrow transplantation. We identify and validate molecular targets important for GVHD pathobiology and demonstrate the importance of ERK1/2 and STAT-3 phosphorylation for T-cell alloactivation in vivo. Finally, we confirm that small molecule inhibitors of STAT-3 phosphorylation can attenuate T-cell activation, proliferation, and GVHD.
Methods
Bone marrow transplantation, transfer of CFSE-labeled T cells, and GVHD assessment were described previously8,9 and discussed in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). In vitro stimulation of T cells with cytokines, anti-CD3, anti-CD3 + CD28 antibody, or irradiated stimulator cells were previously described1,9 and further discussed in Document S1. All protocols were approved by the Institutional Animal Care and Use Committee of Memorial Sloan-Kettering Cancer Center.
Cell-surface staining, intracellular phospho-specific staining, flow cytometry, and data analysis were previously described2,4-6 and further discussed in Document S1. The T(X) population comparison metric was described previously10 and its use is further discussed in the Document S1. Finally, a list of phospho-specific antibodies and inhibitors used in this study can also be found in Document S1.
Results and discussion
We first validated the antibodies for this study (Figure S1A-C and accompanying text), and then examined subsets of splenic T cells in the normal mouse. When we divided CD4 T cells into naive (CD44loCD62Lhi), effector memory (CD44hiCD62Llo), and central memory (CD44hiCD62Lhi) and CD8 T cells into naive (CD44lo) and effector/memory (CD44hi) populations, we found that effector CD4 cells demonstrated increased phosphorylation of many proteins over naive CD4 cells, while CD4 central memory cells had further increases. Similar results were observed with CD8 cells (Figure S2). We also determined the total levels of many signaling proteins with nonphosphorylation-specific antibodies. This revealed similar but milder trends, and effector and memory T cells contain in many cases more total protein compared with naive cells, as reported in the literature.11
We stimulated splenic T cells with cytokines involved in activation and Th1 differentiation, namely IL-2, IL-7, IL-12, or IL-15 (Figure S3A), and observed highly specific phosphorylation of STAT-4 and STAT-5A in response, correlating with known receptor expression patterns on T-cell subpopulations. Some cytokines elicited an “all-or-none response,” where on exposure a subpopulation of cells strongly phosphorylated a signaling molecule, while the remainder showed no response. For example, only approximately 10% of CD4 T cells (∼10%) responded to IL-2 treatment (Figure S3B). Finally, we stimulated T cells with interferon-γ (IFNγ), a cytokine important in GVHD (Figure S3C). This revealed highly specific phosphorylation of STAT-1 only in naive T cells, again in agreement with the cell-surface expression of IFNγR2, which is not found on effector T cells.12
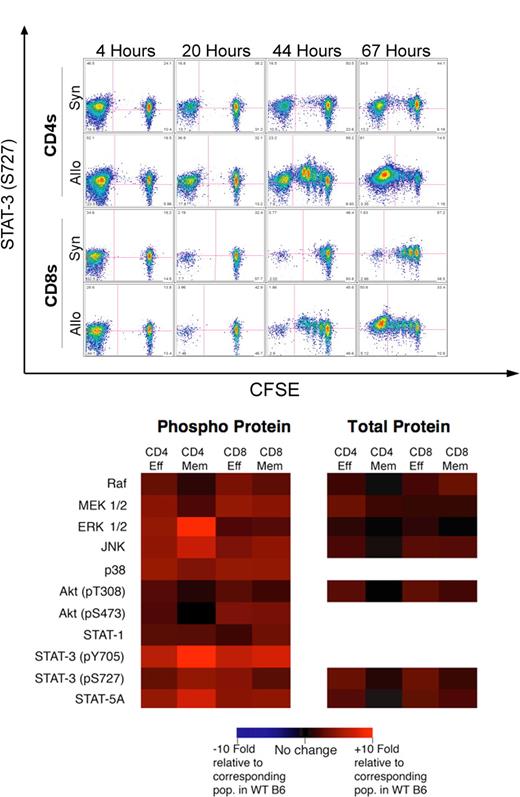
We next turned to mouse models of T-cell alloactivation and GVHD. We first infused CFSE-labeled B6 T cells into irradiated syngeneic B6 CD45.1 or allogeneic C3FeB6F1 hosts. In allogeneic recipients, CFSEhi cells represent homeostatically proliferating donor T cells or nondividing cells, while CFSElo cells represent fast-cycling, alloactivated donor T cells.8 Analysis of recipients at various time points revealed a significant increase in STAT-3 (pS727) phosphorylation in donor alloreactive T cells in the spleen (Figure 1A) at 44 and 67 hours after infusion (P < .01 vs CFSEhi cells in allogeneic or syngeneic recipients by T(X) testing10 ). Alloreactive T cells displayed increased STAT-3 (pS727) phosphorylation with each cell cycle, particularly at 44 hours, while homeostatically proliferating syngeneic T cells showed relatively little increase in STAT-3 (pS727) phosphorylation. Signaling profiles in the spleen, liver, and lymph nodes were similar (not shown). We observed similar results in the model B6 → BALB/c (Figure S4).
Donor alloreactive T cells in GVHD display a global increase in the phosphorylation of signal transduction proteins, including STAT-3 and ERK1/2. (A) A total of 107 CFSE-labeled purified B6 splenic T cells were infused into lethally irradiated B6 CD45.1 or C3FeB6F1 mice. Recipient spleens were analyzed at 4, 20, 44, and 67 hours for the proliferation of donor T cells and phosphorylation of selected intracellular signaling proteins. Bivariate plots demonstrating the proliferation with CFSE dilution versus the phosphorylation of STAT-3 (pS727) in both allogeneic and syngeneic settings are shown. Pink quadrant lines represent the limits of isotype control staining. These plots demonstrate an increase in the phosphorylation of STAT-3 with T-cell alloactivation (eg, at 44 hours) and proliferation, especially in the allogeneic setting. One of 3 independent experiments. (B) Lethally irradiated BALB/c mice (850 cGy) were transplanted with 5 × 106 B6 T cell–depleted bone marrow cells and 106 enriched B6 splenic T cells and harvested on day 14 after BMT. Donor T cells were analyzed by flow cytometry and levels of intracellular phosphorylated and total signal transduction proteins were compared with levels in the corresponding populations in nontransplanted B6 splenic T cells. All signal transduction proteins we examined demonstrated elevated levels of phosphorylation compared with levels in the corresponding naive B6 mouse. Naive donor T cells are not present at day 14 after BMT in T cell–replete transplants and were thus not analyzed. Populations were compared with corresponding populations in the normal B6 mouse. N = 8. One of 2 independent experiments.
Donor alloreactive T cells in GVHD display a global increase in the phosphorylation of signal transduction proteins, including STAT-3 and ERK1/2. (A) A total of 107 CFSE-labeled purified B6 splenic T cells were infused into lethally irradiated B6 CD45.1 or C3FeB6F1 mice. Recipient spleens were analyzed at 4, 20, 44, and 67 hours for the proliferation of donor T cells and phosphorylation of selected intracellular signaling proteins. Bivariate plots demonstrating the proliferation with CFSE dilution versus the phosphorylation of STAT-3 (pS727) in both allogeneic and syngeneic settings are shown. Pink quadrant lines represent the limits of isotype control staining. These plots demonstrate an increase in the phosphorylation of STAT-3 with T-cell alloactivation (eg, at 44 hours) and proliferation, especially in the allogeneic setting. One of 3 independent experiments. (B) Lethally irradiated BALB/c mice (850 cGy) were transplanted with 5 × 106 B6 T cell–depleted bone marrow cells and 106 enriched B6 splenic T cells and harvested on day 14 after BMT. Donor T cells were analyzed by flow cytometry and levels of intracellular phosphorylated and total signal transduction proteins were compared with levels in the corresponding populations in nontransplanted B6 splenic T cells. All signal transduction proteins we examined demonstrated elevated levels of phosphorylation compared with levels in the corresponding naive B6 mouse. Naive donor T cells are not present at day 14 after BMT in T cell–replete transplants and were thus not analyzed. Populations were compared with corresponding populations in the normal B6 mouse. N = 8. One of 2 independent experiments.
Increased STAT-3 phosphorylation with alloactivation and proliferation can be attributed to multiple factors. Activation of IL-2 or IL-15 receptor β-chains can trigger STAT-3 phosphorylation, as can the proinflammatory cytokine IL-6,13 which is also secreted by T cells. Indeed, IL-6 promoter polymorphisms have been implicated in GVHD.14 Finally, growth hormone, IGF-1, IFNγ, or TCR ligation15 can also activate STAT-3. Phosphorylated STAT-3 can regulate the transcription of CD25,16 in agreement with our studies demonstrating that alloreactive T cells (but not homeostatically proliferating T cells) up-regulate CD25.8
Next, we transplanted BALB/c mice with T cell–deleted bone marrow (TCD-BM) and induced GVHD with allogeneic B6 T cells. When we analyzed donor CD4 effector, central memory, and CD8 effector/memory cells in recipients on day 14 after transplantation, and compared their signaling to corresponding populations in nontransplanted B6 mice (Figure S4), we observed significantly more phosphorylation of nearly all proteins. Most notably, memory CD4 cells displayed nearly 10-fold increases in ERK 1/2 and STAT-3 (pY705) phosphorylation, potentially indicative of TCR and cytokine activity (Figure 1B). In addition, nearly all donor T cells demonstrated extensive p38 and JNK phosphorylation, potentially due to the proinflammatory cytokine environment. We also observed increased STAT-5A phosphorylation in donor T cells, which may reflect stimulation by IL-2, IL-7, and IL-15. Interestingly, STAT-1 phosphorylation (eg, due to IFNγ) was only modestly increased, which could be due to declining IFNγ levels or down-regulation of IFNγR2 on effector T cells.12
Having observed STAT-3 and ERK1/2 activation as prominent features of alloactivated T cells in this GVHD model, we next tested whether STAT-3 and ERK1/2 phosphorylation were required for T-cell (allo)activation. We inhibited ERK 1/2 phosphorylation with SL327, a selective inhibitor of MEK 1/2, the upstream MAPKK,17-20 and STAT-3 phosphorylation with cucurbitacin E and I, compounds that target upstream Janus kinases.21-24
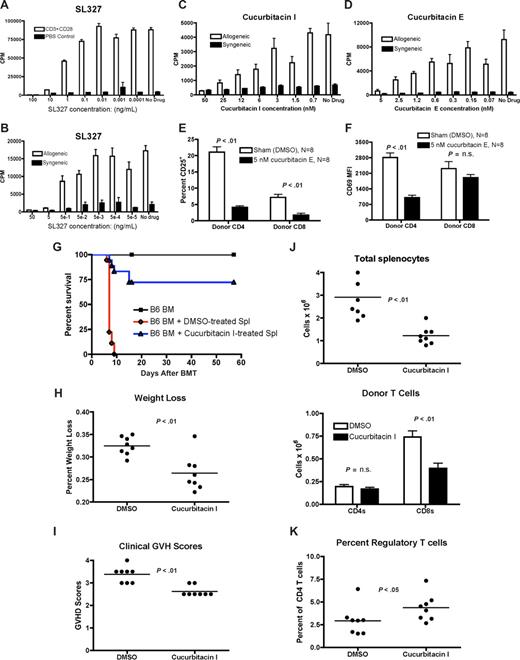
We first assessed whether inhibiting ERK 1/2 phosphorylation with SL327 could attenuate the proliferation of normal B6 splenic T cells in response to anti-CD3 + CD28 stimulation in vitro, and found a dose-dependent suppression of proliferation (Figure 2A). We evaluated the toxicity of SL327 and found that T cells were not significantly apoptotic at concentrations below 100 ng/mL (not shown). We also evaluated the effects of inhibiting ERK1/2 phosphorylation in allogeneic mixed leukocyte reactions (MLRs), which are more relevant to T-cell alloactivation during GVHD. SL327 again caused a dose-dependent suppression of T-cell proliferation (Figure 2B), and no significant toxicity was observed after 4 days of culture at concentrations below 50 ng/mL (not shown). This suggests that ERK1/2 phosphorylation is important for the activation or proliferation of T cells. While SL327 pharmacokinetics precluded treatment in vivo, the ERK1/2 inhibitor PD98059 is reported to attenuate murine cardiac allograft rejection, with decreased T-cell IFNγ and increased IL-4 secretion.25 This suggests the importance of ERK1/2 phosphorylation for T-cell alloreactivity in vivo.
SL327, a small-molecule inhibitor of ERK 1/2 phosphorylation attenuates T-cell alloactivation in vitro, and cucurbitacin I and cucurbitacin E, small-molecule inhibitors of STAT-3 phosphorylation, can attenuate T-cell alloactivation in vitro and GVHD in vivo. (A) A total of 105 RBC-lysed B6 splenic T cells were incubated for 24 hours with plate-bound anti-CD3 and anti-CD28 antibody (0.5 μg/mL concentration for both antibodies) in the presence of log-10 dilutions of SL327 (range: 100 ng to > 0.01 ng) and 3H thymidine incorporation was measured. One of 4 independent experiments. (B) A total of 105 RBC-lysed B6 splenic T cells (responders) were incubated with 105 irradiated and RBC-lysed B6 (syngeneic stimulators) or BALB/c splenic T cells (allogeneic stimulators) in a mixed leukocyte reaction. Log-10 dilutions of SL327 (range: 50 ng to > 0.05 ng) were added on day 0, and 3H thymidine was added on day 4 to measure proliferation. One of 4 independent experiments. (C) A total of 105 RBC-lysed B6 splenic T cells (responders) were incubated with 105 irradiated and RBC-lysed B6 (syngeneic stimulators) or BALB/c splenic T cells (allogeneic stimulators) in a mixed leukocyte reaction. Log-10 dilutions of cucurbitacin I (range: 50 nM to > 1 nM) were added on day 0, and 3H thymidine was added on day 4 to measure proliferation. One of 4 independent experiments. (D) Mixed leuckocyte reaction was performed as in panel A, with log-10 dilutions of cucurbitacin E (range: 5 nM to > 0.1 nM). Representative data from 1 of 4 independent experiments. (E) Whole B6 splenocytes (∼60-100 × 106 cells) were incubated with 5 nM cucurbitacin E or control (DMSO) for 1 hour at 37°C before adoptive transfer into lethally irradiated (850 cGy) BALB/c mice. Recipient spleens were analyzed at 24 hours for donor T cells and expression of CD25. Representative data from 1 of 4 independent experiments. (F) Experiment was performed as in panel A and median fluorescence intensity of CD69 was determined. N = 8 per group, combined data from 4 independent experiments. (G) B6 splenocytes (6 × 107 cells) were incubated with 5 nM cucurbitacin I or control (DMSO) for 1 hour at 37°C before adoptive transfer into lethally irradiated (850 cGy) BALB/c mice. Recipients also received 5 × 106 T cell–depleted B6 marrow, and were monitored and analyzed for survival, weight loss, and clinical signs of GVHD. Combined data from 2 independent experiments, with N = 20 per group for groups receiving splenocytes. (H,I) Mice were transplanted as in panel G and evaluated for weight loss from baseline and semiquantitative clinical GVHD scores on day 6 after allo-BMT (N = 8 per group). (J) Mice were transplanted and total splenic cellularity was assessed by hemacytometer on day 6. Cells were analyzed by flow cytometry, and donor CD4 and CD8 T cells were defined as H-2Kb–positive (N = 8 per group). (K) Mice were transplanted and total splenic cellularity was assessed by hemacytometer on day 6. Percentages of donor CD25+FoxP3+ regulatory T cells as a percentage of donor CD4 T cells was determined by intracellular staining and flow cytometry (N = 8 per group). Error bars in panels indicate standard error of the difference (SED).
SL327, a small-molecule inhibitor of ERK 1/2 phosphorylation attenuates T-cell alloactivation in vitro, and cucurbitacin I and cucurbitacin E, small-molecule inhibitors of STAT-3 phosphorylation, can attenuate T-cell alloactivation in vitro and GVHD in vivo. (A) A total of 105 RBC-lysed B6 splenic T cells were incubated for 24 hours with plate-bound anti-CD3 and anti-CD28 antibody (0.5 μg/mL concentration for both antibodies) in the presence of log-10 dilutions of SL327 (range: 100 ng to > 0.01 ng) and 3H thymidine incorporation was measured. One of 4 independent experiments. (B) A total of 105 RBC-lysed B6 splenic T cells (responders) were incubated with 105 irradiated and RBC-lysed B6 (syngeneic stimulators) or BALB/c splenic T cells (allogeneic stimulators) in a mixed leukocyte reaction. Log-10 dilutions of SL327 (range: 50 ng to > 0.05 ng) were added on day 0, and 3H thymidine was added on day 4 to measure proliferation. One of 4 independent experiments. (C) A total of 105 RBC-lysed B6 splenic T cells (responders) were incubated with 105 irradiated and RBC-lysed B6 (syngeneic stimulators) or BALB/c splenic T cells (allogeneic stimulators) in a mixed leukocyte reaction. Log-10 dilutions of cucurbitacin I (range: 50 nM to > 1 nM) were added on day 0, and 3H thymidine was added on day 4 to measure proliferation. One of 4 independent experiments. (D) Mixed leuckocyte reaction was performed as in panel A, with log-10 dilutions of cucurbitacin E (range: 5 nM to > 0.1 nM). Representative data from 1 of 4 independent experiments. (E) Whole B6 splenocytes (∼60-100 × 106 cells) were incubated with 5 nM cucurbitacin E or control (DMSO) for 1 hour at 37°C before adoptive transfer into lethally irradiated (850 cGy) BALB/c mice. Recipient spleens were analyzed at 24 hours for donor T cells and expression of CD25. Representative data from 1 of 4 independent experiments. (F) Experiment was performed as in panel A and median fluorescence intensity of CD69 was determined. N = 8 per group, combined data from 4 independent experiments. (G) B6 splenocytes (6 × 107 cells) were incubated with 5 nM cucurbitacin I or control (DMSO) for 1 hour at 37°C before adoptive transfer into lethally irradiated (850 cGy) BALB/c mice. Recipients also received 5 × 106 T cell–depleted B6 marrow, and were monitored and analyzed for survival, weight loss, and clinical signs of GVHD. Combined data from 2 independent experiments, with N = 20 per group for groups receiving splenocytes. (H,I) Mice were transplanted as in panel G and evaluated for weight loss from baseline and semiquantitative clinical GVHD scores on day 6 after allo-BMT (N = 8 per group). (J) Mice were transplanted and total splenic cellularity was assessed by hemacytometer on day 6. Cells were analyzed by flow cytometry, and donor CD4 and CD8 T cells were defined as H-2Kb–positive (N = 8 per group). (K) Mice were transplanted and total splenic cellularity was assessed by hemacytometer on day 6. Percentages of donor CD25+FoxP3+ regulatory T cells as a percentage of donor CD4 T cells was determined by intracellular staining and flow cytometry (N = 8 per group). Error bars in panels indicate standard error of the difference (SED).
Next we analyzed the importance of STAT-3 signaling for alloactivated T cells with the small-molecule inhibitors cucurbitacin I and E. In allogeneic MLRs, treatment resulted in a potent dose-dependent inhibition of proliferation in the range from 50 nM to approximately 5 nM for cucurbitacin I and from 5 nM to 0.5 nM for cucurbitacin E (Figure 2C,D). No significant toxicity was detected (not shown).
Although pharmacologic characteristics of cucurbitacin I and E precluded in vivo administration, we pretreated donor T cells ex vivo before adoptive transfer. Transient treatment with Cucurbitacin I inhibited T-cell proliferation and STAT-3 phosphorylation at up to 24 hours after a 1-hour incubation (Figure S5 and accompanying text.) This may suggest that Cucurbitacin I is taken up by naive T cells even in the absence of activating stimuli, and retained intracellularly at an active dose level for up to 24 hours.
We preincubated B6 splenocytes with cucurbitacin E or DMSO for 1 hour, washed and infused them into BALB/c recipients. Recipient spleens were analyzed at 24 hours for donor T-cell activation. Donor CD4 and CD8 T cells that had been pretreated with cucurbitacin E displayed reduced levels of the activation marker CD25 (Figure 2E), and donor CD4 T cells also showed attenuated levels of CD69 expression, another early marker of activation (Figure 2F). T cells were not significantly apoptotic (not shown). Similar results were obtained with cucurbitacin I (not shown).
Finally, we tested the importance of STAT-3 phosphorylation in GVHD. Donor B6 splenocytes were incubated for 1 hour with cucurbitacin I or DMSO, then washed and infused into irradiated BALB/c recipients with B6 TCD-BM. Recipients of cucurbitacin I–treated splenocytes had attenuated GVHD mortality (Figure 2G), less weight loss and GVHD morbidity on day 6 (Figure 2H,I), as well as decreased splenic cellularity and fewer donor CD8 T cells (Figure 2J).
Although numbers of donor CD4 T cells were similar between recipients of cucurbitacin I and DMSO-treated splenocytes, we observed a moderate (but significant) increase in the percentage of CD4+CD25+FoxP3+ regulatory T cells in recipients of cucurbitacin I–treated splenocytes (Figure 2K), which may also partially explain the decreased GVHD in these animals. We found negligible Th17 cells, and percentages of CD44+CD62L− effector Th1 cells were similar (data not shown).
Our results demonstrate the feasibility of phospho-specific flow cytometry for the in vivo analysis of intracellular signaling in a disease model. We detected increased ERK 1/2 and STAT-3 phosphorylation in alloreactive T cells in allo-BMT recipients with GVHD and showed that inhibiting ERK1/2 or STAT-3 activation can attenuate T-cell (allo)activation and GVHD. Development of STAT-3 or ERK 1/2 inhibitors suitable for administration in vivo could result in novel therapeutics for the prophylaxis and treatment of GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the Memorial Sloan-Kettering Cancer Center Research Animal Resources Center for excellent care.
This work was supported by grants HL69929, CA33049, CA023766, and CA107096 from the National Institutes of Health (NIH, Bethesda, MD), and by awards from the Leukemia & Lymphoma Society (White Plains, NY), the Ryan Gibson Foundation (Dallas, TX), the Emerald Foundation (New York, NY), the Byrne Fund (Etna, NH), and the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by Mr William H. Goodwin and Mrs Alice Goodwin, and the Commonwealth Foundation for Cancer Research (Richmond, VA) (M.R.M.v.d.B.). O.A. is the recipient of an Amy Strelzer Manasevit Scholar Award from the National Marrow Donor Program (NMDP, Minneapolis, MN) and the Marrow Foundation (Minneapolis, MN) and is supported by grant P20-CA103694 from the NIH. J.L.Z. is the recipient of a fellowship grant from the Lymphoma Research Foundation (New York, NY).
National Institutes of Health
Authorship
Contribution: S.X.L. designed and performed experiments, analyzed data, and wrote the paper; O.A., J.L., R.B., R.C.-G., X.W., and G.-J.G. designed and performed experiments and analyzed data; D.S., C.K., M.C., O.M.S., V.M.H., J.L.B., J.C.-P., J.L.Z., A.A.K., and A.C. performed experiments; G.A.-B. designed experiments; and M.R.M.v.d.B. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: S.L. has received honoraria from BD Biosciences. M.R.M.v.d.B. received reagents for this project from BD Biosciences. All other authors declare no competing financial interests.
Correspondence: Marcel R. M. van den Brink, Memorial Sloan-Kettering Cancer Center, Z-1419, Mailbox 111, 1275 York Avenue, New York, NY 10021; e-mail: vandenbm@mskcc.org.
References
Author notes
*O.A. and J.L. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal