Abstract
The failure of engraftment in human cases of in utero hematopoietic cell transplantation (IUHCT) in which no immunodeficiency exists suggests the presence of an unrecognized fetal immune barrier. A similar barrier in murine IUHCT appears to be dependent on the chimerism level and is poorly explained by a lack of T-cell tolerance induction. Therefore, we studied the effect of the chimerism level on engraftment and host natural killer (NK)–cell education in a murine model of IUHCT. The dose of transplanted cells was found to exhibit a strong correlation with both the engraftment rate and chimerism level. More specifically, a threshold level of initial chimerism (> 1.8%) was identified that predicted durable engraftment for allogeneic IUHCT, whereas low initial chimerism (< 1.8%) predicted a loss of engraftment. NK cells taken from chimeras above the “chimerism threshold” displayed durable calibration of alloresponsive Ly49A receptors and tolerance to donor antigens. Depletion of recipient NK cells stabilized engraftment in low-level chimeras (< 1.8%). These studies illustrate the importance of the early chimerism threshold in predicting long-term engraftment and host NK-cell tolerance after in utero transplantation.
Introduction
Observations of “naturally” occurring hematopoietic chimerism, and of experimental chimeras produced by in utero hematopoietic stem cell transplantation (IUHCT), have demonstrated that allogeneic engraftment of the early gestational fetus with donor-specific tolerance is possible. However, except for a few cases, IUHCT has not been successful for the treatment of other congenital diseases in which no immunodeficiency exists. In the absence of immunosuppression, only microchimerism has been achieved in human fetal recipients or nonhuman primate models of IUHCT.1-6 These levels are too low for the correction of most diseases and have not been demonstrated to predict tolerance. The reasons for this failure are unclear and reflect a limited understanding of the engraftment barriers involved in clinical IUHCT.
One explanation is the existence of an unrecognized immunologic barrier to allogeneic engraftment within the early gestation fetus. This would explain the success of IUHCT in severe combined immunodeficiency (SCID) recipients and the success of immunosuppressive or immunoregulatory regimens. These regimens are capable of transforming subtherapeutic levels of initial chimerism after IUHCT to full donor chimerism by taking advantage of donor-specific tolerance established as a result of prenatal chimerism.7,8 However, these approaches have been ineffective in the setting of low-level initial chimerism (< 1.0%). This observation implies the existence of a “chimerism threshold” for prenatal tolerance to develop. With levels of chimerism above this threshold, both tolerance and stable chimerism can be predicted. Conversely, below this threshold, tolerance is not established and chimerism is lost.9,10 This concept is not unique to IUHCT as studies in postnatal mixed hematopoietic chimeras support the requirement for a minimum level of circulating tolerogen to sustain tolerance.11,12 A chimerism threshold would explain the inconsistent tolerance observed in low-level prenatal chimeras and improved tolerance in high-level chimeras. Surprisingly, no previous reports have thoroughly examined the requirement for a chimerism threshold in allogeneic IUHCT.
In the fetal transplant recipient, the presence of donor antigen during fetal lymphocyte development provides the impetus for prenatal tolerance to develop. Previously, we have reported durable T-cell tolerance in mice with extremely low levels of prenatal chimerism.13,14 Tolerant mice demonstrated mixed lymphocyte reaction (MLR) hyporesponsiveness, tolerance to donor-specific skin grafts, and deletion of alloreactive T-cell clones despite peripheral blood (PB) chimerism levels detectable only by polymerase chain reaction (< 0.1%). Thus, the loss of engraftment observed in prenatal chimeras with levels more than 0.1% is not explained by T cell–mediated rejection.
However, the developing innate immune system may also pose a significant barrier to allogeneic IUHCT.15 Fetal natural killer (NK) cells may require higher chimerism levels for the induction of tolerance; however, little is known about the fetal NK-cell response to allogeneic transplantation. The observation that umbilical cord blood contains NK cells with mature class I–responsive receptors suggests that NK-cell self-recognition occurs before birth in humans.16,17 Depending on the timing of transplantation, the presence of allogeneic donor cells might lead to host NK-cell education or activation. A better understanding of the critical relationships between chimerism levels and the fetal immune response to allogeneic IUHCT will facilitate the development of more effective prenatal transplantation strategies. Therefore, we studied the effect of the circulating chimerism level on the development of donor-specific allogeneic NK-cell tolerance in a murine model of IUHCT. The findings illustrate the existence of an NK-cell immune barrier to allogeneic transplantation that emerges during the early phases of host Ly49 receptor acquisition. The level of early chimerism critically determines the development of host NK-cell rejection or specific tolerance to the donor allograft.
Methods
Animals
Breeding stock for inbred strains of B6Ly5.2 (H2b, Ly5.2) and B6Ly5.1 (H2b, Ly5.1) were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in our colony. Balb/c (H2d, Ly5.2) mice were purchased from the Charles River Laboratories (Wilmington, MA). Animals were mated, and the females were checked for introital plugging daily. The day of plugging was defined as gestational day 0. All animals were housed in the Laboratory Animal Resource Center of the University of Wisconsin-Madison School of Medicine and Public Health, which is an American Association for Laboratory Animal Care–approved facility. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Wisconsin and followed guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Fetal liver harvest
Donor fetal liver light density mononuclear cells (LDMCs) were harvested from fetuses at 14 days postcoitum (dpc). Under isofluorane anesthesia and using sterile technique, a midline laparotomy was made in the pregnant dam and the uterus exposed. Fetuses were excised and exsanguinated in a Petri dish containing cold sterile phosphate-buffered saline (PBS; Invitrogen, Gaithersburg, MD). Fetal livers were individually dissected in a separate Petri dish and then dispersed into a single-cell suspension by gentle passage through a syringe over a 70-μm nylon mesh strainer. LDMCs were isolated from the sample using Ficoll density separation (Histopaque 1077; Sigma-Aldrich, St Louis, MO) after centrifugation at 900g for 15 minutes at 4°C. Cells were washed with sterile PBS before counting and assessing for viability using trypan blue exclusion.
In utero transplantation
Recipient fetal mice were injected at 14 dpc. A midline laparotomy was created in the recipient dam and the uterus exposed. Each fetus was transplanted with a single-cell suspension of LDMCs in 5 μL PBS by intraperitoneal injection through a translucent uterine wall using a 100-μm beveled glass micropipette. The abdomen was then closed with absorbable 5-0 suture. Pregnant dams were individually housed until delivery.
Determination of chimerism and lineage analysis
Recipient fetuses were allowed to progress to delivery and weaning at 3 weeks of age. At the time of weaning and at serial time points thereafter, PB samples were obtained via retro-orbital puncture. LDMCs were isolated using Ficoll density gradient separation. The following antibodies were used for determination of chimerism (purchased from BD PharMingen, San Diego, CA, unless otherwise specified): anti-Ly5 (30-F11; eBioscience, San Diego, CA) fluorescein isothiocyanate (FITC), anti–H-2Kd (SF1-1.1) phycoerythrin (PE), and anti-Ly5.2(104) FITC. Dead cells were excluded using propidium iodide (PI). Percentage nonerythroid donor chimerism was determined by either the percentage of Ly5.2+ or H-2Kd+ cells within the total (donor and host) Ly5+ gate in the congenic or allogeneic chimeras, respectively.
Recipient mice were killed at 1 year for analysis of marrow. The following directly conjugated monoclonal antibodies (mAbs) were purchased from BD PharMingen and used for determination of hematopoietic lineage: anti-CD3 FITC (145-2C11), anti-B220 FITC (RA3-6B2), anti-Gr-1 FITC (RB6-8C5), anti-Mac-1 FITC (M1/70), and anti-Ter-119 FITC (Ter-119). Cells were counterstained with anti-H-2Kb PE (AF6-88.5) or anti-H-2Kd PE and analyzed by flow cytometry. Dead cells were excluded by PI (Sigma-Aldrich). At least 10 000 gated donor or host cells were analyzed for each sample.
Analysis of NK-cell inhibitory receptor expression
LDMCs were separated from either PB or single-cell suspensions of splenocytes from biopsied chimeras and controls using a Ficoll gradient as described above. Samples for flow cytometry were standardized to 300 000 cells per tube. Each sample was stained with anti-Ly49A biotin (JR9-318) obtained from BD PharMingen. Samples were then counterstained with anti-NK1.1 PE (PK136) and anti-CD3 FITC (145-2C11). After incubation in the dark for 30 minutes at 4°C, samples were washed twice in PBS and stained with streptavidin-PE-Cy5 (BD PharMingen) for secondary labeling of primary biotinylated antibodies. After incubation in the dark for 30 minutes at 4°C, the samples were again washed twice in PBS. PI was added to each sample to exclude nonviable cells immediately before analysis with a FACSCalibur flow cytometer and CellQuest acquisition software (BD Biosciences, San Jose, CA). Using forward and side scatter properties, red blood cells, granulocytes, and macrophages were also excluded. Ly49A receptor expression was measured on 5000 gated CD3-NK1.1+ lymphocytes. The relative median fluorescence intensity (rMFI) was calculated by dividing the observed MFI of Ly49A receptor expressing cells from mixed chimeras or controls by the observed MFI for the same cell population harvested from naive B6.Ly5.2 mice. The quotient was then multiplied by 100 and expressed as a percentile.
NK-cell depletion
PB chimerism was determined at 3 weeks of age as previously described. Animals with low-level chimerism (0.4%-1.7%) were given weekly intraperitoneal injections of the purified monoclonal antibody PK136 (kindly provided by Dr Michael Brown, Virginia Lymphocyte Culture Center, University of Virginia, Charlottesville, VA) at 100 μg until 6 weeks of age, at which time the dose was increased to 200 μg. PB NK-cell fraction was assessed in each animal at baseline and weekly thereafter to ensure adequate depletion.
CD107a degranulation assay
LDMCs harvested from host splenocytes were suspended in RPMI (Sigma-Aldrich) containing 10% fetal bovine serum at a concentration of 106 cells/mL and mixed at an effector/target ratio of 1:1 with congenic (B6.Ly5.2, H2b), donor-specific allogeneic (Balb/c, H2d), and third-party allogeneic (C3H, H2k) splenocyte targets. Phorbol myristate acetate (1 mg; Tocris Bioscience, Ellisville, MO) and ionomycin (0.5 μg/mL; EMD Chemicals, Gibbstown, NJ) stimulation was used to determine maximum degranulation. PE-conjugated anti-CD107a mAb (0.125 μg, eBIO1D4B; eBioscience) was first added to each mixed sample. Samples were then incubated at 37°C in 5% CO2 for a total of 3 hours. Monensin (0.5 μg/mL; BioLegend, San Diego, CA) and brefeldin A (0.5 μg/mL, BD Golgi Plug; BD Biosciences) were added after 1 hour. After incubation, cells were washed twice with PBS and counterstained with anti-NK1.1 FITC and anti-CD3 peridinin chlorophyll protein. CD107a expression by effector CD3−/NK1.1+ cells was determined by multiparameter flow cytometry. Percentage specific CD107a expression for each effector/target ratio combination was calculated by subtraction of the spontaneous degranulation value obtained from control samples without targets.
Statistical analysis
Data are graphically represented as the mean of the respective group plus or minus SEM. Statistical comparisons between groups were performed using a 2-tailed Student t test for 2 samples assuming unequal variances. Calculation of Pearson correlation coefficients (r) and drawing of the best fit lines were performed using Microsoft Excel software (Redmond, WA).
Results
Hematopoietic chimerism after IUHCT correlates directly with the dose of transplanted cells
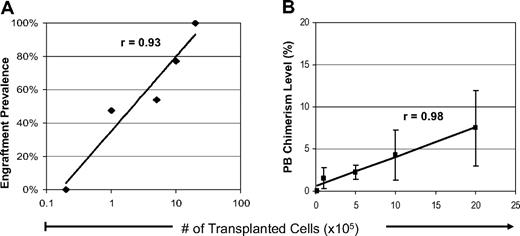
Given the highly competitive nature of the fetal environment, we hypothesized that delivering larger doses of cells would result in higher initial chimerism levels during the critical phases of fetal T- and NK-cell education. To evaluate this possibility, we transplanted serial doses of allogeneic Balb/c fetal liver LDMCs to 14 dpc B6 recipients. Of 124 injected fetuses, 68 (55%) survived to weaning at 3 weeks of age. Measurements of PB hematopoietic chimerism were obtained in surviving recipients. Figure 1 summarizes the PB chimerism (> 0.3%) at 3 weeks of age. As shown in Figure 1A, the engraftment prevalence in 3-week-old weanlings exhibited a strong correlation with the dose of transplanted cells (range, 0%-100%, r = 0.91). Chimerism was not detectable in any of the recipients at the lowest cell dose of 2 × 104 cells (108 cells/kg). Serial increases in cell dose resulted in a higher engraftment prevalence such that all of the surviving recipients of 2 × 106 cells (1010 cells/kg) were chimeric. Not surprisingly, Figure 1B illustrates that the level of PB chimerism at 3 weeks of age also correlated with the transplanted cell dose (range, 0.1%-7.5%; r = 0.98). As shown, PB chimerism could not be detected above background levels at 3 weeks of age in the recipients at the lowest dose of 2 × 104 cells and peaked at 7.5% in the recipients of 2 × 106 cells.
Initial chimerism in recipients of allogeneic IUHCT. Initial PB hematopoietic chimerism was measured at 3 weeks of age in B6Ly5.2 recipients of prenatal Balb/c donor fetal liver cell transplants. Recipients are grouped according to cell dose at transplantation (2 × 104-2 × 106 cells/fetus). (A) The engraftment prevalence (> 0.3%) at the initial PB analysis for each recipient group. (B) Chimerism level at initial PB analysis for each recipient group. Data points represent the mean value plus or minus standard error of the mean (SEM).
Initial chimerism in recipients of allogeneic IUHCT. Initial PB hematopoietic chimerism was measured at 3 weeks of age in B6Ly5.2 recipients of prenatal Balb/c donor fetal liver cell transplants. Recipients are grouped according to cell dose at transplantation (2 × 104-2 × 106 cells/fetus). (A) The engraftment prevalence (> 0.3%) at the initial PB analysis for each recipient group. (B) Chimerism level at initial PB analysis for each recipient group. Data points represent the mean value plus or minus standard error of the mean (SEM).
Higher donor cell dose correlates with durable long-term engraftment without graft-versus-host disease
If higher initial chimerism levels enhance the development of prenatal tolerance, then improved long-term engraftment should also be observed. To assess long-term engraftment, surviving recipients (58 of 68 weaned; 85% long-term survival rate) were followed through 12 months of age. No signs of graft-versus-host disease were observed in any of the mice. Serial body weights of chimeric recipients did not differ from that of age-matched nonchimeric controls (data not shown).
To assess the stability of the PB chimerism, we performed serial measurements in the surviving animals. As shown in Figure 2A,B, the dose of transplanted cells continued to exhibit a strong correlation with both the prevalence of engraftment (range, 0%-71%; r = 0.93) and level (range, 0.1%-10.5%; r = 0.98) of long-term PB chimerism.
Long-term analysis of allogeneic prenatal chimeras. (A) Engraftment prevalence at 6 months of age is plotted for each recipient group. Recipients are grouped according to cell dose at transplantation (2 × 104-2 × 106 cells/fetus). (B) PB chimerism level by cell dose in recipients at 6 months of age. Data points represent the mean value plus or minus SEM. (C) Surviving recipients who remained engrafted were killed at 1 year of age (n = 11), and a bone marrow lineage analysis for both donor and host hematopoiesis was performed (*P < .05). Data points represent the mean value plus or minus SEM.
Long-term analysis of allogeneic prenatal chimeras. (A) Engraftment prevalence at 6 months of age is plotted for each recipient group. Recipients are grouped according to cell dose at transplantation (2 × 104-2 × 106 cells/fetus). (B) PB chimerism level by cell dose in recipients at 6 months of age. Data points represent the mean value plus or minus SEM. (C) Surviving recipients who remained engrafted were killed at 1 year of age (n = 11), and a bone marrow lineage analysis for both donor and host hematopoiesis was performed (*P < .05). Data points represent the mean value plus or minus SEM.
To further demonstrate that the sustained engraftment observed in the higher transplantation dose chimeras was not a result of underlying graft-versus-host disease, a lineage analysis of marrow chimerism was performed at 12 months of age in recipients with detectable chimerism (initial chimerism level > 1.8%). As summarized in Figure 2C, balanced multilineage engraftment was observed in these stable long-term chimeras. Slightly lower levels of donor macrophages and B lymphocytes were seen compared with those cells of host origin (28.8% ± 4.6% and 10.8% ± 2.9% vs 40.6% ± 4.6%, and 21.6% ± 4.2, P < .05). However, despite these subtle differences, the lineage profile of donor hematopoiesis was quite similar to that of the host without evidence for a lymphocyte or other monocellular predominance.
Engraftment loss in low-level chimeras reveals a chimerism threshold in major histocompatibility complex (MHC) disparate but not MHC matched strain combinations
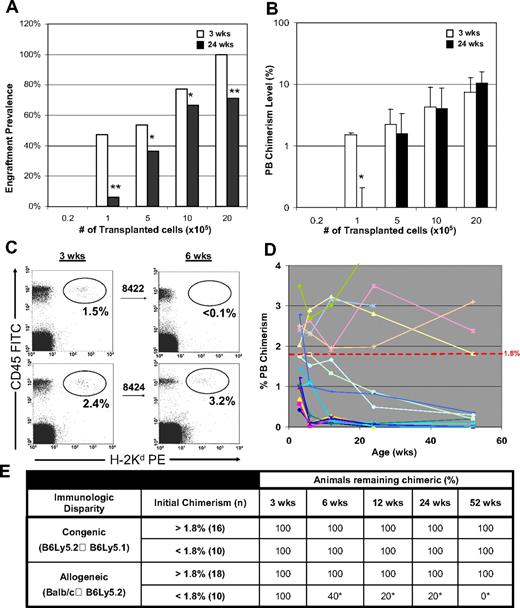
Interestingly, a subset of mice appeared to lose their engraftment over time. Figure 3A illustrates the engraftment loss for each group between short-term (3 weeks) and long-term (24 weeks) assessment points. Engraftment loss was most significant in the lower- level chimeric animals at a dose of 105 cells/fetus (48% → 6%, P < .001). The average level of PB chimerism in this group fell from 1.5% to levels that were not detectable (Figure 3B). The majority of chimeric mice that received higher cell doses remained chimeric without deterioration in the level of PB chimerism.
Evidence of chimerism threshold in allogeneic chimeras necessary for long-term engraftment. (A,B) The drop in the engraftment prevalence and chimerism level between 3 and 24 weeks of age for each group of prenatal chimeras is depicted (*P < .05, **P < .001). Data points for the chimerism level represent the mean value plus or minus SEM. (C) Sample flow cytometry dot-plots for donor chimerism assessment of 2 chimeric littermates (no. 8442 and no. 8424) with initial chimerism levels either above or below 1.8% at 3 and 6 weeks of age. (D) Plot of serial chimerism measurements in low-level chimeras (PB chimerism < 3.5%) illustrating significance of initial chimerism threshold in predicting the durability of long-term engraftment. Data for high-level chimeras (PB chimerism > 3.5%) are not plotted; however, all remained stable chimeras throughout the study period of 1 year. (E) Chimerism summary for congenic and allogeneic strain combinations demonstrating that the significance of the chimerism threshold is limited to the setting of immunologic disparity. Both allogeneic and congenic data are pooled from similar transplant dose cohorts. Prevalence of detectable chimerism throughout the study period is listed for animals either above or below 1.8% (*P < .01).
Evidence of chimerism threshold in allogeneic chimeras necessary for long-term engraftment. (A,B) The drop in the engraftment prevalence and chimerism level between 3 and 24 weeks of age for each group of prenatal chimeras is depicted (*P < .05, **P < .001). Data points for the chimerism level represent the mean value plus or minus SEM. (C) Sample flow cytometry dot-plots for donor chimerism assessment of 2 chimeric littermates (no. 8442 and no. 8424) with initial chimerism levels either above or below 1.8% at 3 and 6 weeks of age. (D) Plot of serial chimerism measurements in low-level chimeras (PB chimerism < 3.5%) illustrating significance of initial chimerism threshold in predicting the durability of long-term engraftment. Data for high-level chimeras (PB chimerism > 3.5%) are not plotted; however, all remained stable chimeras throughout the study period of 1 year. (E) Chimerism summary for congenic and allogeneic strain combinations demonstrating that the significance of the chimerism threshold is limited to the setting of immunologic disparity. Both allogeneic and congenic data are pooled from similar transplant dose cohorts. Prevalence of detectable chimerism throughout the study period is listed for animals either above or below 1.8% (*P < .01).
A closer analysis of the recipients that lost engraftment revealed them to all have an initial level of PB chimerism less than 1.8% regardless of the transplanted cell dose. Figure 3C provides an example of a chimerism assessment by flow cytometry performed on littermates whose initial PB chimerism was either above or below this “threshold” level. Note the clear loss of engraftment in no. 8422 (initial chimerism > 1.8%) compared with littermate no. 8424 (initial chimerism < 1.8%). To further illustrate this phenomenon, serial chimerism measurements from any recipient with initial PB chimerism less than 3.5% are plotted in Figure 3D. As shown, all recipients with an initial PB chimerism less than 1.8% (n = 10) exhibited an eventual drop to levels below the detectable range (< 0.3%). Conversely, despite minor fluctuations, the chimerism levels in recipients with an initial PB chimerism greater than the threshold level (n = 18) remained nearly identical to their initial level at one year of age. Deterioration in chimerism was seen after 12 months in only one animal with an initial PB chimerism above the threshold level (no. 8444, 2.77% → 0.35%).
Possible explanations for the existence of a chimerism threshold include: (1) limiting dilution engraftment of short-term rather than long-term repopulating hematopoietic progenitor cells; or (2) levels of circulating tolerogen below that needed to sustain immunologic tolerance within the allogeneic recipients. To distinguish between these 2 possibilities, parallel transplantation experiments were carried out between immunologically matched strains (B6Ly5.2 → B6Ly5.1) in transplantation doses identical to those in the allogeneic groups. Long-term engraftment stability among the recipient chimeras was compared with the fully allogeneic Balb/c → B6Ly5.2 strain combination and is summarized in Figure 3E. As shown, regardless of transplantation dose, all of the matched recipients remained chimeric despite initial chimerism levels below the threshold value of 1.8%. A lineage analysis of these animals at 6 months of age demonstrated balanced multilineage engraftment in all animals, regardless of initial PB chimerism level (data not shown). However, none of the allogeneic recipients with initial PB chimerism less than 1.8% remained chimeric. Of these 10 recipients, 8 of them lost their engraftment by 12 weeks. The remaining 2 recipients maintained very low levels of chimerism through 6 months of age and became undetectable thereafter. Statistically significant differences in engraftment within the allogeneic group were evident by 6 weeks of age. Thus, the existence of a chimerism threshold in immunologically disparate, but not in immunologically matched, strain combinations does not appear to be an artifact of the engraftment of short-term repopulating cells. Instead, the chimerism threshold appears to predict the stability of long-term prenatal engraftment in allogeneic recipients and probably the durability of donor-specific tolerance.
Chimerism threshold correlates with calibration of Ly49A expression by host NK cells
The maintenance of hematopoietic chimerism logically requires the induction of immunologic hyporesponsiveness by host immune cells. A potent allogeneic T-cell response has been demonstrated to occur just before birth in C57 mice.18-22 Although possible, T cell–mediated rejection of the transplanted cells should result in a loss of chimerism shortly after birth rather than weeks thereafter. Alternatively, competent class Ia NK-cell alloreactivity has not been demonstrated until after 3 weeks in B6 mice.23-27 Because the majority of engraftment loss occurred after 3 weeks of age, a lack of NK-cell tolerance may have led to rejection of the donor cells. Although no mechanism leading to tolerance has been identified, host NK-cell inhibitory Ly49 receptor calibration has been observed with stable long-term engraftment in mixed bone marrow chimeras.28-30 More specifically, several studies in both H2b/d mosaic mice and Balb/c → B6Ly5.2 mixed chimeras demonstrate a strong correlation between down-regulation of host Ly49A expression and the development of specific hyporesponsiveness to the H-2Dd ligand.31-35 Therefore, we studied the impact of the chimerism threshold on the calibration of Ly49A receptors on host NK cells at sequential time points after birth. The expression of Ly49A in B6 mice is responsive to the MHC class Ia antigen H-2Dd that is highly expressed on the donor Balb/c hematopoietic cells.36 If host NK-cell tolerance requires a threshold level of chimerism, then receptor calibration should exist only in the higher level chimeras (> 1.8%).
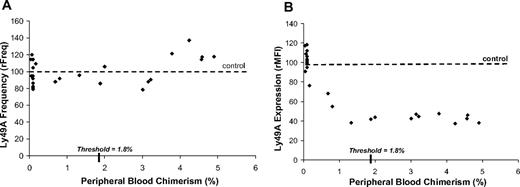
As shown in Figure 4A, analysis of the frequency Ly49A receptor-bearing NK cells in B6 recipients at 12 weeks of age was stable and showed no correlation with increasing PB chimerism levels (relative frequency [rFreq]; relative mean fluorescence intensity [rMFI]) (rFreq = 79%-140%, r = 0.2). Conversely, as shown in Figure 4B, recipient B6 NK cells demonstrated a down-regulation in the intensity of Ly49A receptor expression with increasing chimerism. Receptor down-regulation correlated well with increasing chimerism until the chimerism threshold was reached (rMFI = 38%-118%, r = −0.87). Higher levels of chimerism above the threshold value were not associated with further down-regulation in Ly49A expression (rMFI = 38%-47%). Thus, maximum receptor calibration was reached at the chimerism threshold and remained consistent in the stable chimeras. Furthermore, these findings suggest that Ly49A expression might be a useful gauge of host NK-cell tolerance in prenatal chimeras.
Ly49A receptor calibration in allogeneic recipients with chimerism levels above and below threshold. The expression of Ly49A on host NK cells was studied because B6 recipient mice are responsive to the MHC class Ia antigen H-2Dd that is highly expressed on the donor Balb/c hematopoietic cells. (A) Relationship between the frequency of Ly49A expression and the level of chimerism in B6 recipients. Y-axis values represent the frequency of receptor-bearing cells relative to reference values for age-matched noninjected controls (rFreq). (B) Relationship between the intensity Ly49A expression and the level of chimerism in B6 recipients. Y-axis represents the relative mean fluorescence intensity relative to naive animals (rMFI). Reference values for the frequency and intensity of Ly49A expression for naive animals is indicated by a dotted line.
Ly49A receptor calibration in allogeneic recipients with chimerism levels above and below threshold. The expression of Ly49A on host NK cells was studied because B6 recipient mice are responsive to the MHC class Ia antigen H-2Dd that is highly expressed on the donor Balb/c hematopoietic cells. (A) Relationship between the frequency of Ly49A expression and the level of chimerism in B6 recipients. Y-axis values represent the frequency of receptor-bearing cells relative to reference values for age-matched noninjected controls (rFreq). (B) Relationship between the intensity Ly49A expression and the level of chimerism in B6 recipients. Y-axis represents the relative mean fluorescence intensity relative to naive animals (rMFI). Reference values for the frequency and intensity of Ly49A expression for naive animals is indicated by a dotted line.
Host NK cells recognize subthreshold chimerism during acquisition of Ly49A receptors, but donor-specific NK-cell tolerance is only seen with sustained engraftment
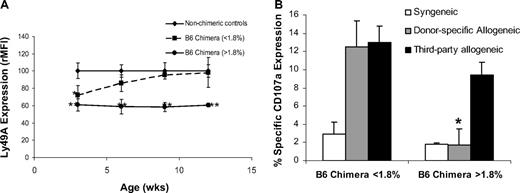
It remained unclear whether subthreshold chimerism was recognized by host NK cells during Ly49 receptor acquisition or whether a limited interaction with donor antigens prevented education of developing host NK cells. To sort between these possibilities, we tracked Ly49A expression in chimeric recipients and age-matched controls by serial analysis of PB from 3 to 12 weeks of age. As shown in Figure 5A, Ly49A expression was down-regulated relative to controls at 3 weeks of age in recipients both above and below the chimerism threshold. Subtle differences in receptor expression existed between the chimera groups at 3 weeks but were not statistically significant. Subsequently, chimeric recipients above the threshold level continued to display stable down-regulation of Ly49A and stable engraftment. However, subthreshold chimeras displayed only transient Ly49A down-regulation with expression increasing to control levels by 6 weeks of age and coinciding with engraftment loss. Thus, developing host NK cells recognize donor antigens regardless of initial chimerism level, but durable receptor calibration is only seen with sustained engraftment. The transient nature of receptor calibration and engraftment loss in subthreshold chimeras suggest that an inadequate degree of receptor-ligand interaction resulted in failed host NK-cell education during the period of receptor acquisition.
Kinetics of Ly49A calibration and development of tolerance in prenatal chimeras above and below chimerism threshold. (A) The relative intensity (rMFI) of Ly49A expression at 3, 6, 9, and 12 weeks is plotted for subthreshold chimeras (0.3%-1.8% PB chimerism) and chimeras above threshold (> 1.8% PB chimerism), and comparisons are made relative to nonchimeric littermate controls (< 0.3% PB chimerism; *P < .05, **P < .01). Data points represent the mean rMFI plus or minus SEM at each time point. (B) NK-cell tolerance as a function of CD107a cell surface expression is shown reported as percentage specific expression. Comparison is made between prenatal allogeneic chimeras above and below chimerism threshold in response to syngeneic (B6.Ly5.1), donor-specific allogeneic (Balb/c), and third-party allogeneic (C3H) target cells (*P < .05).
Kinetics of Ly49A calibration and development of tolerance in prenatal chimeras above and below chimerism threshold. (A) The relative intensity (rMFI) of Ly49A expression at 3, 6, 9, and 12 weeks is plotted for subthreshold chimeras (0.3%-1.8% PB chimerism) and chimeras above threshold (> 1.8% PB chimerism), and comparisons are made relative to nonchimeric littermate controls (< 0.3% PB chimerism; *P < .05, **P < .01). Data points represent the mean rMFI plus or minus SEM at each time point. (B) NK-cell tolerance as a function of CD107a cell surface expression is shown reported as percentage specific expression. Comparison is made between prenatal allogeneic chimeras above and below chimerism threshold in response to syngeneic (B6.Ly5.1), donor-specific allogeneic (Balb/c), and third-party allogeneic (C3H) target cells (*P < .05).
Although Ly49A receptor calibration does not directly explain NK-cell tolerance, it is probable that NK-cell tolerance did not develop in the subthreshold chimeras resulting in allograft rejection.32-35 To investigate the presence of NK-cell tolerance among prenatal allogeneic chimeras, we used a CD107a degranulation assay.37-39 As shown in Figure 5B, host NK cells from B6 chimeras below the chimerism threshold (< 1.8%) exhibited a significantly higher degranulation response to allogeneic donor-specific Balb/c (H2d) and third-party allogeneic C3H (H2k) targets compared with syngeneic targets (B6.Ly5.1, H2b). Conversely, chimeras above the threshold demonstrated a similar response to both donor and syngeneic targets yet exhibited a high level response to third party allogeneic targets. Therefore, donor-specific tolerance by host NK cells existed only in recipients above the chimerism threshold and correlated with durable Ly49A calibration.
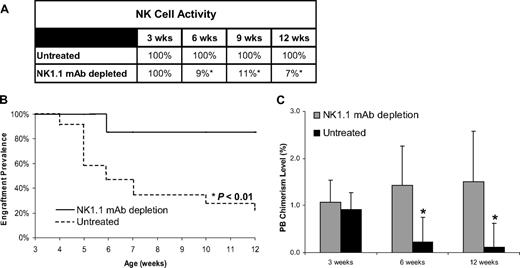
NK-cell depletion abrogates allograft rejection in subthreshold chimeras
NK-cell depletion has been shown to enhance long-term engraftment after MHC-mismatched hematopoietic cell transplantation, even in the setting of low-level chimerism.40 If the engraftment loss observed in subthreshold chimeras was the result of NK-cell rejection, then depletion of developing host NK cells should support sustained engraftment. To study this, confirmed subthreshold chimeras (0.4%-1.7%) were subjected to NK-cell depletion by weekly injection with mAb PK136 starting at weaning age of 3 weeks (4 weeks after transplant). Analysis of the PB in the NK cell–depleted group demonstrated a more than 90% reduction in the circulating NK-cell activity soon after PK136 treatment (Figure 6A). As shown in Figure 6B, depletion of NK cells in subthreshold chimeras prevented engraftment loss, whereas untreated subthreshold chimeras exhibited nearly universal engraftment loss (87% vs 20% engraftment prevalence at 12 weeks, P = .002). Remarkably, NK cell–depleted recipients with subthreshold chimerism levels exhibited stable chimerism over the study period in contrast to the rapid decay observed in the untreated controls (Figure 6C). Thus, the engraftment loss observed in the subthreshold chimeras resulted from the rejection of donor cells by alloreactive host NK cells. Collectively, these data unequivocally demonstrate the existence of a NK cell–mediated barrier to engraftment after allogeneic IUHCT that is highly dependent on the level of early chimerism.
Prevalence of engraftment and PB chimerism levels after NK-cell depletion in allogeneic recipients. (A) Level of circulating NK cells in control or NK1.1-depleted subthreshold chimeras expressed as a percentage of normal levels (nonchimeric controls). (B) Prevalence of engraftment is reported as a function of time for subthreshold prenatal allogeneic chimeras subject to mAb-mediated NK-cell depletion and chimerism-matched untreated subthreshold chimeras. (C) PB chimerism levels were measured at 3, 6, and 12 weeks of age in NK1.1-depleted and untreated chimeras. Data represent mean PB chimerism level of the group plus or minus SEM at each time point (*P < .05).
Prevalence of engraftment and PB chimerism levels after NK-cell depletion in allogeneic recipients. (A) Level of circulating NK cells in control or NK1.1-depleted subthreshold chimeras expressed as a percentage of normal levels (nonchimeric controls). (B) Prevalence of engraftment is reported as a function of time for subthreshold prenatal allogeneic chimeras subject to mAb-mediated NK-cell depletion and chimerism-matched untreated subthreshold chimeras. (C) PB chimerism levels were measured at 3, 6, and 12 weeks of age in NK1.1-depleted and untreated chimeras. Data represent mean PB chimerism level of the group plus or minus SEM at each time point (*P < .05).
Discussion
In the complexity of the developing hematopoietic and immunologic systems, it is probable that interplay between numerous mechanisms contributes to the stability of long-term engraftment after IUHCT. However, in various systems of postnatal hematopoietic cellular transplantation, higher cell doses have been consistently associated with higher levels of chimerism.41-44 Several explanations have been proposed for this relationship. The most simple of these is that an increased frequency of donor hematopoietic progenitors is available for competition with host cells for engraftment.45,46 However, if engraftment of age-matched foreign hematopoietic cells is governed solely by competition for available niches within the host hematopoietic microenvironment, then the levels of detectable chimerism should be continuous throughout the detectable range and no chimerism threshold should exist. Alternatively, long-term engraftment probably requires induction of immunologic tolerance and a minimum level of chimerism to maintain that tolerance. Below this level, an immune response by the host would cause graft rejection. This mechanism partially explains the threshold level of chimerism observed in these experiments.
The present study illustrates the impact of cell dose on the success of IUHCT as higher cell doses directly correlated with higher chimerism levels and engraftment rates. However, regardless of the initial cell dose, a PB chimerism level more than 1.8% is required to prevent graft rejection. Stable Ly49A receptor calibration and donor-specific NK-cell tolerance exist above the chimerism threshold and transient receptor calibration without NK-cell tolerance exist below the threshold. Although developing NK cells in subthreshold chimeras recognize the presence of donor antigen, graft rejection occurs during NK-cell maturation. Depletion of NK cells before graft rejection led to sustained engraftment in subthreshold chimeras. These findings define a critical relationship between the level of chimerism and the development of allospecific host NK-cell tolerance needed to sustain engraftment. Furthermore, 2 translationally relevant approaches are described to enhance the success of allogeneic IUHCT: increasing cell dose and host NK depletion.
One previous study has measured the dose-responsive nature of cell dose and chimerism level after allogeneic IUHCT. A report by Taylor et al compared the repopulating capacity of fetal liver and adult marrow hematopoietic cells in competitive cotransplantation experiments using allogeneic SCID fetal and adult recipients.47 The authors reported chimerism levels several-fold higher for cells of fetal liver origin compared with bone marrow cells. In addition, chimerism levels were higher with increasing doses of either donor cell type. However, PB chimerism measurements in immunodeficient recipients greatly overestimate stem cell engraftment because there is a competitive advantage for proliferating lymphocytes in SCID recipients, and a much lower chimerism level among nonlymphocyte populations was observed. This pattern is consistent with observations of “split chimerism” seen in clinical cases of IUHCT for SCID where extremely low levels of HSC engraftment may provide surprisingly high levels of relevant lymphocyte chimerism.
Other studies of IUHCT suggest the importance of a minimum level of chimerism needed to establish donor-specific tolerance. In a study by Hayashi et al, chimerism levels were enhanced in prenatal allogeneic chimeras with the use of donor lymphocyte infusion.8 However, the donor lymphocyte infusion was unsuccessful in recipients with initial PB chimerism levels less than 1%. Although this level is slightly lower than that reported in the present study (1.8%), the disparity probably results from the limited number of observations in their experiments. Further support for a chimerism threshold in this range comes from subsequent studies using postnatal booster transplantation strategies to enhance prenatally established chimerism. In 2 separate studies, prenatal chimeras were conditioned with either busulfan or nonmyeloblative irradiation before serial booster transplants.7,48 Both approaches were capable of converting relatively low-level chimerism to full donor chimerism. However, achieving high levels of chimerism was less reliable in the allogeneic recipients with initial PB chimerism levels less than 1% despite pretransplant conditioning. In this study, we used numerous observations to define a chimerism threshold of 1.8% needed to predict long-term engraftment in the absence of host conditioning. In addition, this is the first report to describe the importance of the initial PB chimerism level in the durable education of host alloreactive NK lymphocytes.
Although murine T and NK maturation are late gestational and postnatal events, respectively, human T and NK maturation occurs before birth. These differences must be considered when translating studies of prenatal transplantation. Regarding thymic development, single-positive T-cell phenotypes can be reliably detected in the B6 fetus by 17 dpc.21,49 Thymic maturation continues postnatally in newborn mice; however, naive B6 newborns do not accept fully allogeneic hematopoietic grafts without myeloablative or immunologic preparation.21,22 Conversely, phenotypically mature single-positive T cells can be reliably detected in the human thymus as early as 12 weeks' gestation,50,51 and functionally mature T cells have been identified in the fetal liver and spleen by 14 weeks' gestation.52-54 Thus, the critical phases of T-cell development that occur from 15 to 17 dpc in the B6 fetus correspond to 12 to 14 weeks' gestational age in the human fetus.
The gestational timing of human NK-cell maturation remains poorly defined. The earliest human and murine fetal NK cells express the heterodimeric NKG2A/CD94 inhibitory receptor.55,56 Recognition by NKG2A is restricted to the MHC class Ib antigens HLA-E in humans and Qa-1 in mice. Indirect recognition of class Ia molecules by this family of receptors is dependent on class Ib presentation of nonameric class Ia leader sequences.57,58 Studies of murine fetal NK cells have detailed the mechanistic regulation of early self-recognition through this system of class Ib–dependent presentation.23,59-61
Direct recognition of class Ia antigens by both human and murine NK cells occurs later in development. B6 NK cells do not begin to express mature class Ia receptors until several days after birth, and adult levels of Ly49 receptor expression are not observed until 6 weeks of age.26 An NK response to MHC-mismatched transplants is not demonstrable until approximately 3 weeks of age.62 Thus, a potential NK-cell–mediated barrier to class Ia-mismatched engraftment probably arises in the B6 mouse between 3 and 6 weeks after birth. Unlike the mouse, the human fetus may develop this potential long before term. Recent studies reveal NK cells possessing a mature repertoire of self-tolerant and alloreactive killer cell immunoglobulin-like receptors (KIR) present in human cord blood.17 Expression of KIR receptors by fetal NK cells could conceivably begin during the first or second trimester and may signal the acquisition of alloreactive potential. In a study of human fetal NK cells by Phillips et al,56 CD3−CD56+ cells were found at a low frequency within the fetal liver as early as 6 weeks' gestation. These fetal NK cells demonstrated a limited capacity for lysis of human tumor cell targets that increased later in gestation. However, KIR expression was not studied, and only tumor cell targets were used; thus, the alloreactive capacity of fetal NK cells was not directly assessed.
If early gestational human NK cells are capable of alloreactivity, an early chimerism threshold may be required to prevent rejection after clinical IUHCT. Subthreshold initial chimerism levels are probably given the relatively low cell doses transplanted in clinical cases of IUHCT compared with studies in mice. Subsequent booster transplants may paradoxically stimulate an immune response resulting in rejection of the graft. Further support for this hypothesis stems from reports of improved engraftment in immunologically normal human fetuses with the use of fetal donor sources.63,64 The higher competitive engraftment capacity of fetal stem cell sources may account for this relative success.
In conclusion, the current study demonstrates that transplantation of higher doses of donor hematopoietic cells can enhance chimerism after IUHCT. By establishing initial chimerism above a threshold level or depleting host NK cells, durable NK-cell tolerance to the allograft is achieved and engraftment is sustained. Further study is needed to characterize the timing of human fetal NK-cell maturation, and the critical receptor-ligand interactions essential to this educational process. A modified approach that incorporates this new information may then greatly expand the successful clinical application of IUHCT in the treatment of diseases in which no inherent immunodeficiency exists.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by a Faculty Research Fellowship Award from the American College of Surgeons (A.F.S.) and National Institutes of Health grants T32 AG02656 (E.T.D.) and R21 AI069882 (A.F.S.).
National Institutes of Health
Authorship
Contribution: E.T.D. performed research, collected, analyzed, and interpreted data, and prepared the manuscript; K.A.J. and D.R. performed research and collected data; and A.F.S. designed the research, collected, analyzed, and interpreted data, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Aimen F. Shaaban, Department of Surgery, 1600 JCP, 200 Hawkins Drive, Iowa City, IA 52242; e-mail: aimen-shaaban@uiowa.edu.