Abstract
The biochemical basis for complement acting directly on antigen-presenting cells to enhance their function in T-cell stimulation has been unclear. Here we present evidence that engagement of C3a receptor (C3aR) on the surface of dendritic cells (DCs) leads to alterations in the level of intracellular cyclic adenosine monophosphate (cAMP), a potent negative regulator of inflammatory cytokines. C3aR activation-induced depression of cAMP was associated with enhanced capacity of DCs for antigen uptake and T-cell stimulation. Conversely, C3aR-deficient DCs showed elevation of cAMP and impaired properties for antigen uptake and immune stimulation. Similarities in the phenotype of C3-deficient and C3aR-deficient DCs suggest that local production of C3 with extracellular metabolism to C3a is an important driver of DC alterations in cAMP. The finding of a link between complement and adaptive immune stimulation through cAMP offers new insight into how innate and adaptive immunity combine to generate efficient effector and memory responses.
Introduction
The complement system of protein components, receptors, and regulators not only plays a key role in innate immunity, but also bridges the innate and adaptive immune systems.1,2 Many complement components including C3 are produced mainly in the liver; however, extrahepatic synthesis of complement has been widely documented in many cells and tissues,3 including dendritic cells (DCs).4 C3 is the central component in the complement cascade and can be activated through 3 major pathways of complement activation. Cleavage of C3 generates a range of effector molecules and metabolites (eg, C3a, C5a, C3b, iC3b, and C3dg) that interact with their respective receptors (eg, C3aR, C5aR, CR1-CR4, CRIg) and lead to opsonophagocytosis, inflammation, and cell activation.5-7 The formation of the membrane attack complex (MAC, C5b-9) on cell surface causes the direct killing of pathogens and cell activation.1
In addition to its pivotal role in regulation of inflammation and host defense in innate immunity, complement also participates in regulating the adaptive immune response.8 Our understanding of the role for complement in the adaptive immune response has been mainly based on the induction of antibody responses. It has been proposed that B-cell recognition of nonself is dependent on C3-opsonized antigen binding to complement receptor 2 (CR2) on B cells and follicular dendritic cells (FDCs), thus augmenting the retention of antigen on FDCs and enhancing the direct activation of B cells. This facilitates the antigen-specific B-cell response in secondary lymphoid tissue.9,10 By contrast, our understanding of how complement regulates T-cell responses has only been appreciated recently.11 As the pathway for T-cell recognition of foreign antigens (Ags) is very different from that in B cells, the mechanism of complement-modulated T-cell recognition is likely to involve distinct molecular and cellular routes.
In general, T-cell activation is initiated through Ag presentation by antigen-presenting cells (APCs) to T cells. The initial interaction of T cells with APCs is mediated/regulated by costimulatory molecules that are expressed on APCs (eg, CD86, CD40).12,13 Therefore, the functional state of APCs is critical for stimulating T-cell responses.
Recent studies have indicated that complement-dependent modulation of APC function is an important mechanism by which complement regulates T-cell immunity. Thus, APCs prepared from C3−/− or factor B−/− or factor D−/− mice have reduced capacity to stimulate alloreactive T cells.4,14-16 In contrast, APCs from complement regulator (decay-accelerating factor)–deficient mice elicit augmented allospecific T-cell responses.15 In addition, DCs express complement receptors including C3aR, and C3 cleavage fragment C3a was detected in DC culture supernatant. Furthermore, DCs either from C3aR−/− mice or treated with C3aR antagonist (C3aRa) elicit defective T-cell priming against alloantigen expressed on the DCs.16 These results suggest that local production and activation of complement can up-regulate DC functional activity prior to encountering T cells by a C3aR-dependent mechanism. However, these studies were designed to assess the response of T cells stimulated by alloantigen, where the foreign antigen is synthesized and presented by donor APCs. Since most foreign antigens and autoantigens are taken up, processed, and presented by self-APCs, it is unclear if self-restricted T-cell recognition will depend on local production of C3 in the same way as for alloantigen. In addition, the molecular mechanism by which local production and activation of C3 participates in up-regulating APC function via C3aR is not defined. These questions are of wide importance in the context of viral infection, bacterial infection, cancer, and autoimmune disease, where therapeutic manipulation depends on identification of the critical signaling pathways contributing to the immune response.
In this study, using a model developed for examining the recognition of ovalbumin (OVA) Ag in the mouse, we investigated if DC Ag-presenting function is dependent on C3 and, more specifically, on the interaction of C3aR with the cleavage fragment of C3 (C3a). We also determined what aspect of DC function is affected by C3a-C3aR interaction. Furthermore, we explored several important intracellular signaling pathways by which triggering C3aR could modulate DC function, including cyclic adenosine monophosphate (cAMP), mitogen-activated protein kinase (MAPK), and phosphoinostitide 3-kinases (PI3K) pathways.
Methods
Reagents
Recombinant murine granulocyte macrophage–colony-stimulating factor (GM-CSF) was purchased from R&D Systems Europe (Abingdon, United Kingdom). CD11c microbeads were purchased from Miltenyi Biotec. Ltd UK (Surrey, United Kingdom). C3aRa was purchased from Merck Biosciences (Nottingham, United Kingdom). Human C3a was purchased from Fitzgerald Industries International (Concord, MA). OVA protein (grade VII) was purchased from Sigma-Aldrich (Poole, United Kingdom). FITC-OVA and BODIPY FL dye–conjugated OVA (DQ OVA) were purchased from Invitrogen (Paisley, United Kingdom). T-cell purification kits for CD4 and CD8 T cells were from StemCell Technologies (London, United Kingdom). Antibody reagents used in flow cytometry (ie, PE-conjugated rat anti–mouse MHC class II [I-A/I-E, M5/114.15.2], PE-conjugated rat anti–mouse CD40 [3/23], and PE-conjugated rat anti–mouse B7.2 [CD86, GL1]), and enzyme-linked immunosorbent assay (ELISA) kits used for measuring IFN-γ, IL-2, IL-12, TNF-α, IL-6, and IL-10 were purchased from BD Biosciences (Cowley, United Kingdom). Antibody reagents used in Western blot (ie, rabbit anti–mouse phospho-extracellular signal–regulated kinase [p-ERK1/2] [Thr202/Tyr204]; phospho-P38K [p-P38K] [Thy180/Tyr182]; and phospho-AKT [Ser473] antibodies; and rabbit anti–mouse ERK1/2, P38K, and AKT antibodies) were purchased from Cell Signaling Technology (Beverly, MA). 3H-thymidine was purchased from GE Healthcare UK Limited (Little Chalfont, United Kingdom).
Mice
Wild-type (WT) C57BL/6 (B6, H-2b) mice were purchased from Harlan UK (Bicester, United Kingdom). Homozygous C3−/− mice B6 were provided by Prof M. Carroll (Harvard) and were then backcrossed in our laboratory onto the B6 parental strain for 11 generations. C3aR−/− B6 mice have been backcrossed onto their parental strain for 8 generations and the WT littermates were used as control in all C3aR−/− DC studies. TCR transgenic OT1 and OT2 B6 mice were purchased from Charles River Laboratories (L'Arbresle, France). Male mice (6-7 weeks old) were used in all experiments. All animal procedures were carried out in accordance with the Animals Act in UK (Scientific Procedures, 1986), under project license no. PPL 70/5923.
Preparation of DCs
Bone marrow (BM) DCs were generated as described previously.16 On day 6, DCs were collected and enriched with CD11c microbeads. In some experiments, C3aR antagonist or C3a was added to DC culture medium every other day from the beginning of culture. In each experiment, DCs were prepared from 3 to 5 mice and pooled together for analysis. Six-day DCs were used in the assays of Ag uptake and Ag presentation, 7- or 8-day DCs were used in the assay of DC activation phenotype.
Preparation of T cells
CD4 or CD8 T cells were prepared from spleens of OT-2 or OT-1 TCR transgenic mice using the spin-Sep Enrichment Cocktail kit (StemCell Technologies). After separation, the purity of the T-cell preparation was routinely more than 95%, as determined by flow cytometry.
In vitro antigen-presentation assay
DCs were incubated with OVA protein (250 μg/mL) at 37°C for 40 minutes and then washed for 3 times. The OVA-loaded DCs were then cocultured with OT2 CD4 T cells or OT1 CD8 T cells in T-cell culture medium (RPMI 1640 containing 10% FCS, 50 μM β-mercaptoethanol, 100 μg/mL streptomycin). 3H-thymidine uptake was performed after 3 days of coculture. Cytokine secretion was measured by ELISA after 2 to 6 days of coculture.
Mice immunization
Mice were immunized by intraperitoneal injection of OVA (100 μg) or OVA-loaded DCs (prepared as in in vitro assay); 10 days or 60 days after immunization, mice were killed and splenocytes were prepared from each mouse. Splenocytes (2 × 105/well) were restimulated ex vivo with OVA. Cell proliferation and T-cell cytokines were determined by 3H-thymidine uptake and ELISA, respectively.
T-cell proliferation in vivo
Syngeneic mice were given CFSE-labeled OT2 CD4 T cells (2 × 106) and OVA-loaded DCs by intravenous injection. The control mice were only injected with CFSE-labeled OT2 CD4 T cells. Mice were killed on day 3 after injection, and cells from lymph nodes and spleen were used for the analysis of T-cell proliferation based on CFSE dilution using flow cytometry.
Antigen uptake
DCs were incubated with FITC-OVA at 37°C for 40 minutes and then washed thoroughly with cold phosphate-buffered saline (PBS). The uptake of FITC-OVA was assessed by flow cytometry (FACScan; Becton Dickinson, Oxford, United Kingdom). The results were expressed as mean fluorescence intensity (MFI). The fluorescence background (cells incubated with FITC-OVA at 0°C) was subtracted from the results.
Flow cytometry
Before staining with labeled antibodies, DCs were preincubated with Fc-blocking antibody. DCs were stained with PE-conjugated antibody or the appropriate isotype control antibody, at 4°C for 30 minutes. The cells were washed 3 times before being analyzed by flow cytometry.
Measurement of intracellular cAMP
DCs (2 × 106) were incubated with forskolin for 30 minutes. In some experiments, the cells were further incubated for 40 minutes in the presence or absence of C3a. The concentration of cAMP was determined in each sample using a Parameter cAMP assay kit (R&D Systems) according to the manufacturer's instructions.
Statistical analysis
The Student t test or analysis of variance (ANOVA) was used where appropriate to determine significant differences between samples. All experiments were repeated at least twice.
Results
C3−/− DCs have impaired Ag-presenting function in vitro
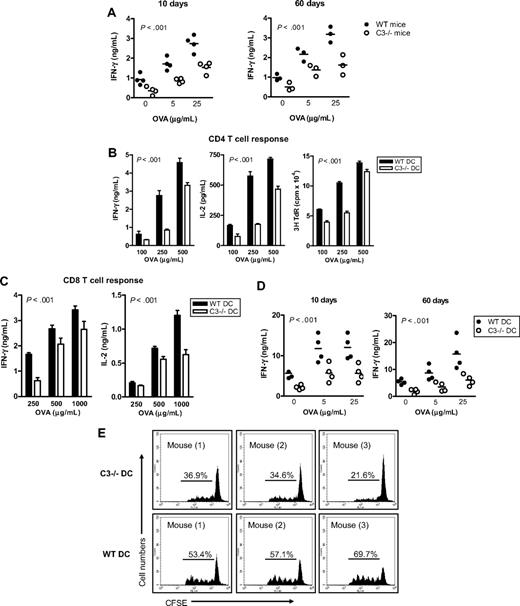
Before studying C3−/− DC Ag-presenting function, we investigated the specific T-cell response to exogenous Ag in C3−/− mice. We immunized WT and C3−/− mice with OVA protein and analyzed T-cell responses in these mice on day 10 (for measuring T-cell priming) and day 60 (for measuring memory T-cell response) after immunization, by restimulation ex vivo with various concentrations of OVA protein. T-cell priming and memory T-cell response, as measured by IFN-γ production (Figure 1A) and thymidine uptake (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), were significantly reduced in C3−/− mice compared with WT mice after immunization. Thus, sufficient T-cell priming and memory T-cell response against OVA Ag required the presence of C3.
C3−/− mice have impaired Ag-specific T-cell responses and C3−/− DCs have impaired Ag-presenting function. (A) C3−/− mice develop reduced specific T-cell response to OVA. WT and C3−/− mice were immunized by intraperitoneal injection with 100 μg OVA protein in incomplete Freund adjuvant. After 10 days and 60 days of immunization, mice were killed. Lymphocytes from lymph nodes and spleen were used for assessing T-cell reactivity by measuring cytokine production after restimulation ex vivo with OVA protein. Each dot represents a single animal and is shown as mean of 4 replicate wells of the ex vivo culture. A representative of 2 independent experiments is shown. (B,C) Ag presentation measured in vitro. BM DCs were prepared from WT and C3−/− mice and loaded with OVA protein at the indicated concentrations. The Ag-loaded DCs (104) were cocultured with naive OT2 CD4 T cells (105) (B) or OT1 CD8 T cells (105) (C). Specific T-cell responses were measured by IFN-γ and IL-2 production and thymidine uptake. All data are shown as mean plus or minus the standard error of the mean (SEM) (n = 4 for ELISA, or n = 8 for thymidine uptake). Data were analyzed by 2-way ANOVA. P values are for comparisons between WT DCs and C3−/− DCs. A representative of 4 independent experiments is shown. (D) OVA-specific T-cell responses measured ex vivo. OVA-loaded WT or C3−/− DCs were administered to WT syngeneic recipient mice by intraperitoneal injection (4 mice in each group). After 10 and 60 days of injection, splenocytes from these mice were restimulated ex vivo with OVA. Each dot represents a single animal and is shown as mean of 4 replicate wells of the ex vivo culture. All data were analyzed by 2-way ANOVA. A representative of 2 independent experiments is shown. (E) OVA-specific T-cell proliferation in vivo. OVA-loaded WT or C3−/− DCs and CFSE-labeled OT2 CD4 T cells were coinjected into syngeneic WT recipient mice (3 mice per group). After 3 days, CFSE+ cells from spleen and lymph nodes were gated to analyze cell division. Control mice received CFSE-labeled OT2 CD4 T cells only. A representative of 2 independent experiments is shown.
C3−/− mice have impaired Ag-specific T-cell responses and C3−/− DCs have impaired Ag-presenting function. (A) C3−/− mice develop reduced specific T-cell response to OVA. WT and C3−/− mice were immunized by intraperitoneal injection with 100 μg OVA protein in incomplete Freund adjuvant. After 10 days and 60 days of immunization, mice were killed. Lymphocytes from lymph nodes and spleen were used for assessing T-cell reactivity by measuring cytokine production after restimulation ex vivo with OVA protein. Each dot represents a single animal and is shown as mean of 4 replicate wells of the ex vivo culture. A representative of 2 independent experiments is shown. (B,C) Ag presentation measured in vitro. BM DCs were prepared from WT and C3−/− mice and loaded with OVA protein at the indicated concentrations. The Ag-loaded DCs (104) were cocultured with naive OT2 CD4 T cells (105) (B) or OT1 CD8 T cells (105) (C). Specific T-cell responses were measured by IFN-γ and IL-2 production and thymidine uptake. All data are shown as mean plus or minus the standard error of the mean (SEM) (n = 4 for ELISA, or n = 8 for thymidine uptake). Data were analyzed by 2-way ANOVA. P values are for comparisons between WT DCs and C3−/− DCs. A representative of 4 independent experiments is shown. (D) OVA-specific T-cell responses measured ex vivo. OVA-loaded WT or C3−/− DCs were administered to WT syngeneic recipient mice by intraperitoneal injection (4 mice in each group). After 10 and 60 days of injection, splenocytes from these mice were restimulated ex vivo with OVA. Each dot represents a single animal and is shown as mean of 4 replicate wells of the ex vivo culture. All data were analyzed by 2-way ANOVA. A representative of 2 independent experiments is shown. (E) OVA-specific T-cell proliferation in vivo. OVA-loaded WT or C3−/− DCs and CFSE-labeled OT2 CD4 T cells were coinjected into syngeneic WT recipient mice (3 mice per group). After 3 days, CFSE+ cells from spleen and lymph nodes were gated to analyze cell division. Control mice received CFSE-labeled OT2 CD4 T cells only. A representative of 2 independent experiments is shown.
Next, we determined whether Ag presentation in DCs through MHC class II to CD4 T cells is affected by the C3 status of DCs. DCs were prepared from WT and C3−/− mice and loaded with OVA, and then used to stimulate naive OVA-specific OT2 CD4 T cells. OVA-loaded C3−/− DCs stimulated significantly lower CD4 T-cell responses than OVA-loaded WT DCs, as measured by IFN-γ and IL-2 production and thymidine uptake in DC–T-cell cocultures (Figure 1B). These results demonstrate that DC presentation of exogenous Ag to CD4 T cells is dependent on DC production of C3.
Cross-presentation of exogenous Ag is a unique characteristic of professional APCs and allows the immune system to trigger a cell-killing response to extracellular pathogens or certain cancer cells. Therefore, we studied whether local production of C3 would have an impact on this function of DCs. WT and C3−/− DCs were loaded with OVA; instead of stimulating OT2 CD4 T cells, the OVA-loaded DCs were used to stimulate OVA-specific OT1 CD8 T cells that recognize the OVA peptide presented by MHC class I. OVA protein–loaded C3−/− DCs stimulated less effective CD8 T-cell responses than OVA-loaded WT DCs, in an Ag-dose–dependent manner (Figure 1C). Thus, DC cross-presentation of exogenous Ag to CD8 T cells also requires DC production of C3, suggesting that DC production of C3 is imperative for DC presentation of exogenous Ag through both MHC class II and MHC class I pathways.
C3−/− DCs have reduced capacity to induce OVA-specific T-cell response in vivo
To determine whether DC production of C3 would have an impact on specific T-cell priming in vivo, we performed 2 sets of experiments. In the first set, syngeneic WT recipient mice received an intraperitoneal injection of OVA-loaded WT or C3−/− DCs; control mice received PBS or no Ag-loaded WT or C3−/− DCs. On days 10 (for measuring T-cell priming) and 60 (for measuring memory T-cell response) after the injection, splenocytes were prepared from these mice and restimulated ex vivo with OVA. T-cell reactivity was significantly reduced in mice at 10 and 60 days after receiving OVA-loaded C3−/− DCs, as indicated by a lower level of IFN-γ in splenocytes (Figure 1D), compared with mice receiving OVA-loaded WT DCs. Control mice exhibited no specific T-cell reactivity (data not shown). In a second experiment, syngeneic WT recipient mice received an intravenous injection of carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled OT2 CD4 T cells and OVA-loaded WT or C3−/− DCs. On day 3 after the injection, cells from lymph nodes and spleen were used for the analysis of T-cell proliferation using flow cytometry. OVA-loaded C3−/− DCs induced weaker T-cell proliferation than Ag-loaded WT DCs (Figure 1E), indicating that C3−/− DCs were incompetent at generating OVA-specific T-cell response in vivo.
DCs with defective C3a-C3aR stimulation have impaired function in Ag presentation
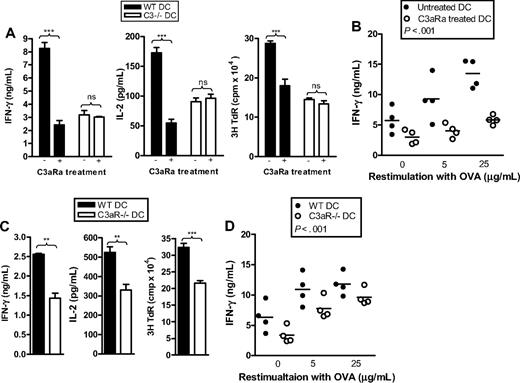
To understand the molecular pathway(s) by which complement activation modulates APC function, we investigated the role of C3aR activity on DCs. We initially treated DCs with C3a receptor antagonist (C3aRa) to block the C3a-C3aR interaction in DCs. C3aRa was added to the DC culture medium every other day from the beginning of BM cell culture for 6 days. C3aRa-treated DCs were then used for assessing their Ag-presenting function in vitro and in vivo. C3aRa-treated DCs elicited significantly reduced OVA-specific CD4 T-cell responses in vitro, as measured by IFN-γ and IL-2 production and thymidine uptake in DC–T-cell cocultures, and in vivo, as measured by IFN-γ production in splenocytes after ex vivo OVA restimulation (Figure 2A,B). The effect of C3aRa treatment on DC Ag-presenting function was most likely C3a specific, as C3aRa treatment in C3−/− DCs (which are incapable of generating C3a in culture) had no apparent effect on the T-cell responses (Figure 2A).
DCs with defective C3a-C3aR stimulation have impaired function in Ag presentation. (A,B) Effect of C3aRa treatment. C3aRa (50 nM) was added into DC culture medium every other day from the beginning of BM cell culture (from WT or C3−/− mice) for 6 days. DCs were then used for Ag-presentation assays in vitro and in vivo/ex vivo. (C,D) C3aR−/− DCs versus WT DCs. DCs were prepared from C3aR−/− mice and WT control mice, and then used for Ag-presentation assays in vitro and in vivo/ex vivo. (A,C) In vitro assays. Data are shown as mean plus or minus SEM (n = 4 for ELISA; n = 8 for thymidine uptake). Data were analyzed by Student t test. ***P < .001; **P < .003; NS, no significant difference. A representative of 3 independent experiments is shown. (B,D) In vivo/ex vivo assays. Each dot represents a single animal and is shown as mean of 4 replicate wells of the ex vivo culture. Data were analyzed by 2-way ANOVA. A representative of 2 independent experiments is shown.
DCs with defective C3a-C3aR stimulation have impaired function in Ag presentation. (A,B) Effect of C3aRa treatment. C3aRa (50 nM) was added into DC culture medium every other day from the beginning of BM cell culture (from WT or C3−/− mice) for 6 days. DCs were then used for Ag-presentation assays in vitro and in vivo/ex vivo. (C,D) C3aR−/− DCs versus WT DCs. DCs were prepared from C3aR−/− mice and WT control mice, and then used for Ag-presentation assays in vitro and in vivo/ex vivo. (A,C) In vitro assays. Data are shown as mean plus or minus SEM (n = 4 for ELISA; n = 8 for thymidine uptake). Data were analyzed by Student t test. ***P < .001; **P < .003; NS, no significant difference. A representative of 3 independent experiments is shown. (B,D) In vivo/ex vivo assays. Each dot represents a single animal and is shown as mean of 4 replicate wells of the ex vivo culture. Data were analyzed by 2-way ANOVA. A representative of 2 independent experiments is shown.
To further confirm the role of C3aR in modulating DC Ag- presenting function, we prepared DCs from C3aR−/− and WT mice and assessed their Ag-presenting function in vitro and in vivo. Similar results to C3aRa treatment were obtained. IFN-γ and IL-2 production and thymidine uptake in DC–T-cell cocultures were significantly lower in OT2 CD4 T cells stimulated by OVA-loaded C3aR−/− DCs than that stimulated by OVA-loaded WT DCs in vitro. IFN-γ production in splenocytes from mice that received OVA-loaded C3aR−/− DCs was significantly lower than in mice that received OVA-loaded WT DCs. (Figure 2C,D). Together, our data demonstrate that in the absence of C3a-C3aR interaction, the Ag-presenting function of DCs is impaired.
Ag uptake and surface expression of MHC class II and costimulatory molecules are reduced in C3−/− DCs and C3aR−/− DCs
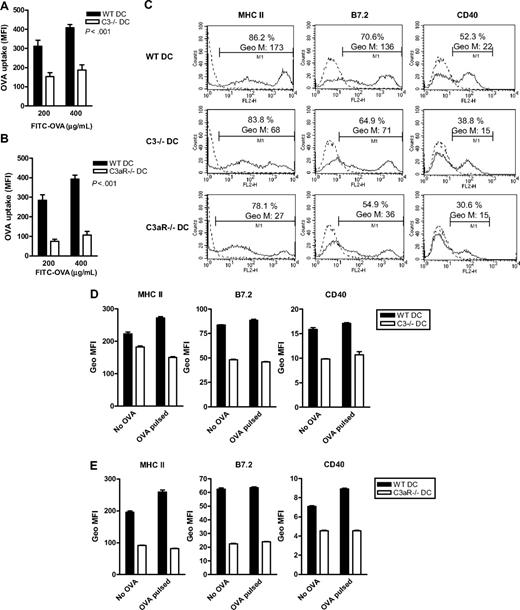
We have demonstrated that the specific T-cell response to exogenous Ag is dependent on the expression of C3 and C3aR in DCs. The next question we asked was what aspect of APC function is affected by C3. T-cell recognition of exogenous Ag depends on several properties of APCs, including Ag uptake, Ag processing, and surface molecule display. Therefore, we investigated if any of these properties were affected by DC expression of C3 and C3aR. To assess Ag uptake we used 6-day DCs, to assess DC activation phenotype we used 7- or 8-day DCs. We found that OVA uptake was reduced in C3−/− DCs and C3aR−/− DCs compared with WT DCs (Figure 3A,B). However, C3 seems not involved in Ag processing as we found that C3 did not enter the endocytic vesicles (where Ag is processed) by immuno-electron microscopy and the rate of OVA degradation was not altered in C3−/− DCs (Figure S2). These data indicate that impaired Ag uptake, rather than impaired Ag processing, may account for the lowered Ag-presenting function observed in C3−/− DCs and C3aR−/− DCs. In addition, we also found that surface expression of MHC class II and B7.2 (CD86) and CD40 was reduced in C3−/− DCs and C3aR−/− DCs, compared with WT DCs, irrespective of OVA loading (Figure 3C-E). This suggests that both reduced Ag uptake and lowered surface expression of MHC class II and costimulatory molecules contribute to the reduced capacity for T-cell stimulation in C3−/− DCs and C3aR−/− DCs.
Ag uptake and surface expression of MHC class II and costimulatory molecules are reduced in C3−/− DCs and C3aR−/− DCs. (A) Ag uptake in 6-day DCs prepared from C3−/− mice and WT mice. The data are shown as mean of triplicate samples. A representative of 5 independent experiments is shown. (B) Ag uptake in 6-day DCs prepared from C3aR−/− mice and WT mice. Data are shown as mean of triplicate samples. A representative of 3 independent experiments is shown. (C-E) Surface expression of MHC-II, CD40, and B7.2 in 7-day DCs by flow cytometry. (C) Representative histogram plots from WT DCs, C3−/− DCs, and C3aR−/− DCs. (D,E) Six-day DCs were incubated with or without OVA (250 μg/mL, 40 minutes) and washed with cold PBS. The washed DCs were further cultured for 24 hours and then used for the analysis. (D) C3−/− DCs versus WT DCs. (E) C3aR−/− DCs versus WT DCs. The data are shown as mean of duplicate samples. A representative of 3 independent experiments is shown.
Ag uptake and surface expression of MHC class II and costimulatory molecules are reduced in C3−/− DCs and C3aR−/− DCs. (A) Ag uptake in 6-day DCs prepared from C3−/− mice and WT mice. The data are shown as mean of triplicate samples. A representative of 5 independent experiments is shown. (B) Ag uptake in 6-day DCs prepared from C3aR−/− mice and WT mice. Data are shown as mean of triplicate samples. A representative of 3 independent experiments is shown. (C-E) Surface expression of MHC-II, CD40, and B7.2 in 7-day DCs by flow cytometry. (C) Representative histogram plots from WT DCs, C3−/− DCs, and C3aR−/− DCs. (D,E) Six-day DCs were incubated with or without OVA (250 μg/mL, 40 minutes) and washed with cold PBS. The washed DCs were further cultured for 24 hours and then used for the analysis. (D) C3−/− DCs versus WT DCs. (E) C3aR−/− DCs versus WT DCs. The data are shown as mean of duplicate samples. A representative of 3 independent experiments is shown.
C3a treatment enhances the capacity of DCs for Ag uptake and cell activation as well as their function in Ag presentation
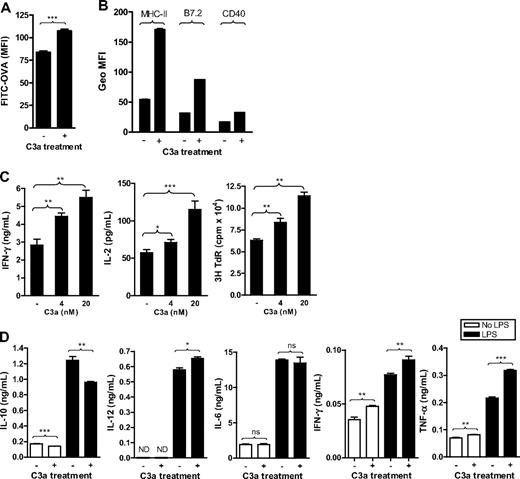
Besides the use of C3aRa and C3aR−/− mice, we examined the effect of C3aR agonist (C3a), to further investigate the role of C3a-C3aR interaction in DC function. As with C3aRa treatment, C3a was added in the DC culture medium from the beginning of BM cell culture. C3a-treated, 6-day DCs were then used for the assays of Ag uptake and Ag presentation, and C3a-treated, 7- or 8-day DCs were then used for the assays of surface molecule expression. Our results showed that C3a treatment enhanced Ag uptake and surface expression of MHC class II and costimulatory molecules (B7.2 and CD40) in WT DCs (Figure 4A,B). As expected, the treatment had no apparent effect in C3aR−/− DCs, either on Ag uptake or surface molecule expression (Figure S3), confirming that the effect of C3a treatment was C3aR specific. Coordinately, C3a treatment significantly up-regulated DC Ag- presenting functions in a dose-dependent manner (Figure 4C). Furthermore, C3a treatment clearly altered the IL-10, IFN-γ, TNF-α, and IL-12 production by DCs, with a decrease in IL-10 and an increase in IFN-γ, TNF-α, and IL-12 p70, although had no apparent effect on IL-6 production (Figure 4D). Overall, our results indicate a positive influence of C3a on DCs, in terms of activation phenotype, cytokine profile, and capacity for Ag uptake and Ag presentation. These findings are in agreement with results in receptor-blocked and receptor-deficient cells.
C3a treatment enhances the capacity of DCs for Ag uptake and cell activation as well as their function in Ag presentation. C3a (20 nM or the indicated concentration) was added into DC culture medium every 2 days from the beginning of BM cell culture. (A) Ag uptake performed in 6-day DCs. Data are shown as mean plus or minus SEM (n = 4). Data were analyzed by Student t test. (B) Surface expression of MHC class II (MHC-II), CD40, and B7.2 analyzed in 7-day DCs by flow cytometry. Data are shown as mean plus or minus SEM (n = 2). (C) Ag presentation performed in 6-day DCs. Data are shown as mean plus or minus SEM (n = 4 for ELISA; n = 8 for thymidine uptake). (D) Six-day DCs were further cultured for 24 hours in the presence or absence of LPS (0.5 μg/mL) as well as C3a; and the supernatants were used for cytokine measurement. Data are shown as mean plus or minus SEM (n = 4). Data were analyzed by Student t test. ***P < .001; **P < .005; *P < .05; NS, no significant difference. A representative of 3 independent experiments for all figures is shown.
C3a treatment enhances the capacity of DCs for Ag uptake and cell activation as well as their function in Ag presentation. C3a (20 nM or the indicated concentration) was added into DC culture medium every 2 days from the beginning of BM cell culture. (A) Ag uptake performed in 6-day DCs. Data are shown as mean plus or minus SEM (n = 4). Data were analyzed by Student t test. (B) Surface expression of MHC class II (MHC-II), CD40, and B7.2 analyzed in 7-day DCs by flow cytometry. Data are shown as mean plus or minus SEM (n = 2). (C) Ag presentation performed in 6-day DCs. Data are shown as mean plus or minus SEM (n = 4 for ELISA; n = 8 for thymidine uptake). (D) Six-day DCs were further cultured for 24 hours in the presence or absence of LPS (0.5 μg/mL) as well as C3a; and the supernatants were used for cytokine measurement. Data are shown as mean plus or minus SEM (n = 4). Data were analyzed by Student t test. ***P < .001; **P < .005; *P < .05; NS, no significant difference. A representative of 3 independent experiments for all figures is shown.
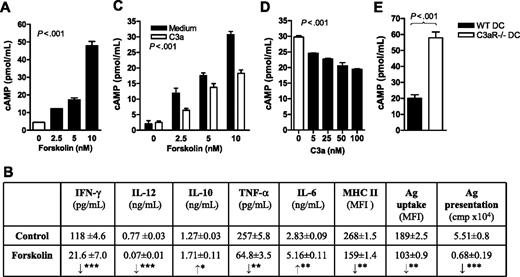
Engagement of C3aR on DCs mediates inhibition of the adenylate cyclase pathway
Having identified a putative role for C3aR in regulating DC function including Ag uptake and T-cell stimulation, we next examined the intracellular signaling pathways by which C3aR could elicit such effects. C3aR (like C5aR) is a G protein–coupled receptor (GPCR). A wide variety of ligands can use GPCRs to stimulate cells through multiple signaling pathways, among which cAMP pathway is one of the key pathways. Therefore we investigated whether engagement of C3aR could mediate this pathway in DCs. We first investigated the effect of intracellular cAMP on DC function and then determined if C3a stimulation or C3aR deficiency had impact on intracellular cAMP levels in DCs. Our results showed that intracellular cAMP was detectable in DCs and was increased after the stimulation by forskolin (a cAMP-elevating agent), in a forskolin dose-dependent manner (Figure 5A). In association with an elevation of cAMP, forskolin-treated DCs exhibited reduced Ag uptake, lowered surface expression of MHC class II and CD40, and had increased in IL-10 and IL-6, and decreased in IFN-γ, TNF-α, and IL-12. Furthermore, DC Ag-presenting function was significantly decreased after forskolin treatment (Figure 5B). Additionally, C3a stimulation reduced the forskolin-elevated cAMP levels in WT DCs (Figure 5C), in a C3a dose–dependent manner (Figure 5D). In contrast, C3aR−/− DCs exhibited about 3-fold elevation in cAMP compared with WT DCs (Figure 5E).
Critical role for intracellular cAMP in DC functions and effect of C3a treatment on DC intracellular cAMP level. (A) cAMP levels in 6-day DCs (2 × 106) after forskolin stimulation for 30 minutes at indicated concentrations. Data are shown as mean plus or minus SEM (n = 3). Data were analyzed by one-way ANOVA. (B) Effect of forskolin on cytokine secretion, surface expression of MHC class II, Ag uptake, and Ag presentation in DCs. Five-day DCs were further cultured for 24 hours in the presence or absence of forskolin (10 nM) and then used for Ag uptake and Ag-presentation assays. Six-day DCs were further cultured for 24 hours in the presence or absence of forskolin (10 nM). The cells were then used for surface expression of MHC class II assay, while the supernatants were used for cytokine ELISA. Data are shown as mean plus or minus SEM (n = 4 for cytokine secretion; n = 3 for Ag uptake; n = 2 for MHC II expression; and n = 8 for proliferation). Data were analyzed by Student t test. ***P < .001; **P < .005; *P < .05. (C) cAMP levels in 6-day DCs with or without C3a stimulation for 30 minutes in the presence of forskolin at indicated concentrations. Data are shown as mean plus or minus SEM (n = 3). Data were analyzed by 2-way ANOVA. P values are for comparisons between C3a and medium alone. (D) cAMP levels in 6-day DCs after C3a stimulation at indicated concentrations for 30 minutes in the presence of forskolin (10 nM). Data are shown as mean plus or minus SEM (n = 3). Data were analyzed by one-way ANOVA. (E) cAMP levels in 6-day WT DCs and C3aR−/− DCs after forskolin (10 nM) stimulation for 30 minutes. Data are shown as mean plus or minus SEM (n = 3). Data were analyzed by Student t test.
Critical role for intracellular cAMP in DC functions and effect of C3a treatment on DC intracellular cAMP level. (A) cAMP levels in 6-day DCs (2 × 106) after forskolin stimulation for 30 minutes at indicated concentrations. Data are shown as mean plus or minus SEM (n = 3). Data were analyzed by one-way ANOVA. (B) Effect of forskolin on cytokine secretion, surface expression of MHC class II, Ag uptake, and Ag presentation in DCs. Five-day DCs were further cultured for 24 hours in the presence or absence of forskolin (10 nM) and then used for Ag uptake and Ag-presentation assays. Six-day DCs were further cultured for 24 hours in the presence or absence of forskolin (10 nM). The cells were then used for surface expression of MHC class II assay, while the supernatants were used for cytokine ELISA. Data are shown as mean plus or minus SEM (n = 4 for cytokine secretion; n = 3 for Ag uptake; n = 2 for MHC II expression; and n = 8 for proliferation). Data were analyzed by Student t test. ***P < .001; **P < .005; *P < .05. (C) cAMP levels in 6-day DCs with or without C3a stimulation for 30 minutes in the presence of forskolin at indicated concentrations. Data are shown as mean plus or minus SEM (n = 3). Data were analyzed by 2-way ANOVA. P values are for comparisons between C3a and medium alone. (D) cAMP levels in 6-day DCs after C3a stimulation at indicated concentrations for 30 minutes in the presence of forskolin (10 nM). Data are shown as mean plus or minus SEM (n = 3). Data were analyzed by one-way ANOVA. (E) cAMP levels in 6-day WT DCs and C3aR−/− DCs after forskolin (10 nM) stimulation for 30 minutes. Data are shown as mean plus or minus SEM (n = 3). Data were analyzed by Student t test.
Thus, our results demonstrate a previously unrecognized link, namely that engagement of C3aR on DCs induces inhibition of cAMP production, consequently lifting the restraint of cAMP on DC activation and thereby up-regulating DC activation and Ag- presenting function.
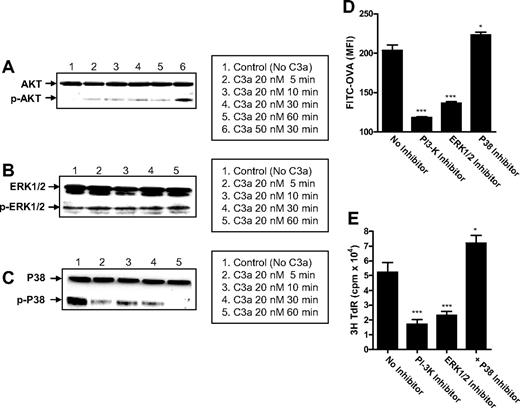
Differential effects of C3aR on phosphorylation pathways of signal transduction
In addition to cAMP, we investigated whether engagement of C3aR on DCs could have any effect on other important signaling pathways including MAPKs and PI3K pathways that can be mediated through the activation of GPCRs. We first determined the phosphorylation of AKT (a downstream effector of PI3K), and P38 and ERK1/2 (both members of MAPK family). Our results showed that after C3a stimulation, phosphorylated AKT (p-AKT) was clearly detected in DCs, which is more apparent with C3a stimulation at 50 nM (Figure 6A). The phosphorylated ERK1/2 (p-ERK1/2) was detectable in both C3a-stimulated and unstimulated DCs, with a slight increase after C3a stimulation (Figure 6B). Interestingly, a large amount of phosphorylated P38 (p-P38) was detectable in the unstimulated DCs but decreased in a time-related manner after C3a stimulation (Figure 6C). These results indicate that engagement of C3aR on DCs can induce the activation of PI3K/AKT pathway and possibly enhances the activation of MAPK/ERK1/2 pathway. In contrast, engagement of C3aR on DCs seems to provide an inhibitory signal for the activation of MAPK/P38 pathway.
Differential effects of C3aR on phosphorylation pathways of signal transduction. (A-C) Detection of AKT (PI3K), ERK1/2, and P38 (MAPK) phosphorylation in 6-day DCs (105 cells) after C3a stimulation, by Western blot analysis. In each blot the top row of bands corresponds to incubating membrane with appropriate total antibody and the bottom row of bands corresponds to incubating membrane with appropriate antiphospho antibody. A representative of 3 independent experiments is shown. (D,E) Effect of inhibition of AKT, ERK, and P38 pathways on Ag uptake and Ag presentation in DCs. Five-day DCs were further cultured for 24 hours in the presence or absence of the appropriate inhibitor (ie, wortmanin for AKT; U0126 for ERK; SB202190 for P38) and then were used for assays for Ag uptake and Ag presentation. Data are shown as mean plus or minus SEM (n = 4 for Ag uptake; n = 8 for proliferation). A representative of 2 independent experiments is shown.
Differential effects of C3aR on phosphorylation pathways of signal transduction. (A-C) Detection of AKT (PI3K), ERK1/2, and P38 (MAPK) phosphorylation in 6-day DCs (105 cells) after C3a stimulation, by Western blot analysis. In each blot the top row of bands corresponds to incubating membrane with appropriate total antibody and the bottom row of bands corresponds to incubating membrane with appropriate antiphospho antibody. A representative of 3 independent experiments is shown. (D,E) Effect of inhibition of AKT, ERK, and P38 pathways on Ag uptake and Ag presentation in DCs. Five-day DCs were further cultured for 24 hours in the presence or absence of the appropriate inhibitor (ie, wortmanin for AKT; U0126 for ERK; SB202190 for P38) and then were used for assays for Ag uptake and Ag presentation. Data are shown as mean plus or minus SEM (n = 4 for Ag uptake; n = 8 for proliferation). A representative of 2 independent experiments is shown.
We next investigated the effect of the activation of each of these pathways on DC functional activation (ie, cytokine secretion, Ag uptake, and Ag presentation) by using the pathway specific inhibitors (ie, Wortmanin [PI3K inhibitor], U0126 [ERK1/2 inhibitor], and SB202190 [P38 inhibitor]). Inhibition of any of these 3 pathways did not induce a typical anti-inflammatory or proinflammatory profile in DCs (Figure S4). However, the functional outcomes of DC activation were clearly modulated. Inhibition of PI3K and ERK1/2 in DCs resulted in decreased Ag uptake and Ag presentation. In contrast, inhibition of P38 resulted in increased Ag uptake and Ag presentation (Figure 6D,E). These results indicate that activation of PI3K and ERK1/2 in DCs mediates a positive regulation on Ag uptake and Ag presentation, while for the P38, a negative regulation on Ag uptake and their presentation.
By implication, our results suggest that in addition to inhibition of cAMP pathway, engagement of C3aR may also mediate the activation of PI3K and ERK pathways (positive modulators of DCs) and the inhibition of P38 pathway (a negative modulator of DCs), ultimately contributing to the up-regulation of DC function in Ag presentation.
Discussion
Our previous work identified an important effect of complement on the adaptive immune response, in particular the actions of locally produced C3/C3a on donor APCs prior to contacting allospecific T cells, which differs from recently published observations describing an action of C3a at the immune synapse between APC and T cells.17 The present study with ovalbumin not only extends our previous findings to the recognition of exogenous Ag, but also identifies several possible functions of DCs that could be influenced by complement, including their ability to stimulate naive T cells, cross-prime effector T cells, and elicit a T-cell memory phenotype. Importantly, our work identifies a biochemical basis for complement acting directly on APCs to enhance their function in T-cell stimulation.
DCs reside in peripheral tissues, particularly in the interstitial space at the site of encounter with pathogens and tissue stress factors, where it seems there may be insufficient access to complement components from the circulation, particularly for large molecules such as C3 (180 kDa).18 In this case, local synthesis of C3 by APCs and other local sources of complement components may be a critical factor for their function of Ag presentation. This is largely supported by our findings that DCs from C3−/− mice have impaired function of Ag presentation in vitro and reduced capacity to prime the Ag-specific T-cell response in vivo. Moreover, local production and activation of C3 appears sufficient for the action of C3a on DC receptor, since C3-deficient DCs as well as C3aR-deficient DCs had a defective cell phenotype including impaired Ag uptake and reduced capacity for Ag presentation. As DCs are surrounded by other cells capable of secreting complement into the local environment, including endothelial and epithelial cells, it is possible that these cells also contribute to complement-mediated modulation of APC function.
An important question arising from our observation on OVA uptake is how Ag uptake in DC is modulated by local production and activation of C3. Soluble proteins are efficiently internalized by macropinocytosis. This process is actin dependent, requires membrane ruffling, and results in the formation of large intracellular vacuoles.19 One of the unique features of DCs is their great capacity for constitutive macropinocytosis and Ag uptake in the fluid phase.20 It is possible that C3a engages C3aR, causing DC activation and cytoskeleton rearrangement,21 with consequent up-regulation of macropinocytosis. Several of our observations strongly support this explanation. First, C3aR deficiency (as well as C3 deficiency) led to reduced Ag uptake, whereas C3a stimulation increased Ag uptake, arguing in favor of a specific effect of C3a on its receptor, as opposed to an effect of the large cleavage fragment of C3 (C3b) on the opsonization of OVA. Second, triggering of the C3aR reduced intracellular cAMP, whereas C3aR deficiency increased intracellular cAMP. Previous studies have shown that intracellular cAMP is an important modulator of endocytosis through regulation of actin rearrangement; low cAMP level can enhance endocytosis,22,23 whereas high cAMP level can enhance exocytosis.24,25 Thus, reduced intracellular cAMP after triggering C3aR further suggests that up-regulation of Ag uptake is through C3a-mediated cell activation. Besides the small fragment, the large fragment of C3 (C3b) may regulate endocytosis through a receptor-mediated pathway as reported before on other APCs.26,27 However, murine DCs as well as human DCs do not express receptors for C3b (CR1 and CR2).16,28 Thus a role for C3b opsonization of Ag on Ag uptake is unlikely in our model. Furthermore, although DC express receptors for iC3b (CR3 and CR4), our previous study showed that DCs do not express factor I required for cleavage of C3b into iC3b, suggesting the iC3b would not be the major cleavage fragment of C3 generated in our DC cultures.16 It is therefore unlikely that Ag uptake mediated by this fragment would be significant in our model.
We explored the possibility that DC neo-synthesized C3 could be participating in Ag processing in DCs. Previous work on B cells and monocytes has suggested a protective role for C3 in proteolysis.29-31 C3b covalently bound to foreign Ag (tetanus toxin), when C3b-Ag is internalized, stabilizes the Ag in the endosomal/lysosomal compartment of the MHC class II pathway of the cells. These studies suggested that activated C3 fragment functions as a ‘chaperone’ in the MHC class II pathway. Although, in our model, no exogenous C3 was introduced, the synthesized C3 may transfer from the secretory pathway to the endocytic pathway before being secreted, through the potential mechanisms such as autophagy.32,33 Alternatively, the secreted C3 may be internalized and therefore DC-produced C3 could function as exogenous C3 and participate in Ag processing. However, our data showing that the rate of degradation of DQ-OVA was equal in WT and C3−/− DCs and C3 was found to be located exclusively in the DC cytosol and not in endocytic vesicles do not support a role for local production of C3 in Ag processing. Thus, enhanced T-cell priming in our study was likely to reflect increased uptake of Ag as well as increased surface expression of MHC and costimulatory molecules, rather than enhanced Ag processing.
A major finding in this study is a role for cAMP in mediating the effects of C3a on DC function. cAMP is a cyclic nucleotide that functions as an intracellular second messenger.34 Increasing evidence suggests that cAMP is not only an important physiologic mediator of the inflammatory process, but also plays a central role in regulating the immune response. Intracellular cAMP has inhibitory effects on IFN-γ, TNF-α, and IL-12 production, but has a stimulatory effect on IL-10 production in various cell types, including monocytes, macrophages, DCs, and T cells, and can modulate the activity of various cellular processes (ie, cell differentiation, endocytosis/exocytosis, ion channel conductivity).23,25,35-37 Many physiologic and pharmaceutical agents that alter cAMP levels, such as prostaglandin, histamine, and β2-adrenergic receptor agonist, are able to interact with differentiation processes of DCs via the G protein–adenylate cyclase pathway.38-42 However, a role for C3a-C3aR interaction in the regulation of cAMP levels in DCs has not been previously described. Our results show that C3a stimulation significantly reduced the intracellular levels of cAMP in DCs and enhanced DC activation and their Ag-presenting function, demonstrating that engagement of C3aR on DCs leads to inhibition of the adenylate cyclase pathway, consequently lifting the restraint of cAMP on DC activation and thus up-regulating DC activation and Ag-presenting function. Our experiments with forskolin confirmed that elevation of cAMP is likely to have a suppressive on DC function, including reduction of Ag uptake.
Cyclic AMP is synthesized from ATP by adenylate cyclase located at the cell membrane. Adenylate cyclase can be stimulated by G protein and by forskolin, as well as other class-specific substrates. Adenylate cyclase can be either activated or inhibited by G proteins coupled to membrane receptors and thus respond to hormonal or many other stimuli. Activation or inhibition of adenylate cyclase depends on the subunit of G proteins: Gαs protein-coupled receptors mediate the activation and Gαi protein-coupled receptors mediate inhibition.43 Previous studies have found that prostaglandins (I2 and E2) with anti-inflammatory properties, when binding to Gαs protein-coupled receptors (PGI2 receptor and PGE2 receptor), increase intracellular cAMP levels and subsequently inhibit proinflammatory cytokine production in DCs.38-40 In contrast to the observations on prostaglandins (I2 and E2), we found that the intracellular cAMP level in DCs was decreased after C3a stimulation, suggesting that C3a, as a potent inflammatory peptide, exerts its function through the Gαi protein-coupled C3aR and leads to decreased levels of intracellular cAMP.
Many of the cellular responses mediated by GPCRs not only involve the stimulation of conventional second-messenger–generating systems, such as cAMP, but also result from the functional integration of a complicated network of intracellular signaling pathways.43 Indeed, our results indicate that in addition to inhibition of the cAMP pathway, engagement of C3aR may also mediate the activation of PI3K and ERK pathways and the inhibition of the P38 pathway in DCs. In addition, several important signaling pathways have also been reported to be involved with C3aR in other types of cells, such as astrocytes, neutrophils, and macrophages.44-46
Based on our present findings together with previous observations on the importance of APC production of C3 in the adaptive immune response,4,14,16,47,48 we suggest the following model by which complement modulates APC function through C3aR signaling and subsequently regulates Ag specific T-cell responses. As illustrated in Figure 7, local production and activation of C3 generates extracellular C3a; binding of C3a to C3aR on DCs activates Gαi protein, which in turn inhibits the production of cAMP, and consequently modulates DC functional activation and results in the up-regulation of DC functions of Ag uptake and presentation. In addition, engagement of C3aR mediates inhibition of MAPK/P38 and activation of PI3K/AKT and MAPK/ERK1/2 pathways, which may contribute to the up-regulation of DC function in Ag presentation. This local C3aR-mediated process may be critical not only for handling of altered self under physiologic conditions, but also for mounting effective T-cell responses upon inflammatory stimulation or pathogen invasion of the local environment. In addition, this process could significantly contribute to both donor and recipient APC-induced alloreactive T-cell responses that are thought to trigger allograft rejection. Therefore, our findings offer new insight into the augmentation of adaptive immunity by complement activation and this could have relevance for vaccine design and therapeutic strategy.
Diagram showing proposed signaling pathways for C3aR-mediated up-regulation of DC function in Ag presentation. Local production and activation of C3 generates extracellular C3a; binding of C3a to C3aR on DCs activates Gαi protein, which in turn inhibits the production of cAMP, and consequently modulates DC functional activation and results in the up-regulation of DC functions of Ag uptake and presentation. In addition, engagement of C3aR mediates inhibition of MAPK/P38 and activation of PI3K/AKT and MAPK/ERK1/2 pathways, which may contribute to the up-regulation of DC function in Ag presentation.
Diagram showing proposed signaling pathways for C3aR-mediated up-regulation of DC function in Ag presentation. Local production and activation of C3 generates extracellular C3a; binding of C3a to C3aR on DCs activates Gαi protein, which in turn inhibits the production of cAMP, and consequently modulates DC functional activation and results in the up-regulation of DC functions of Ag uptake and presentation. In addition, engagement of C3aR mediates inhibition of MAPK/P38 and activation of PI3K/AKT and MAPK/ERK1/2 pathways, which may contribute to the up-regulation of DC function in Ag presentation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Professor M. Carroll for providing the C3 knockout mice. We thank Dr M.-B. Villiers for helpful scientific discussions.
This work was supported by the Medical Research Council of the United Kingdom.
Authorship
Contribution: W.Z. and K.L. designed the research and wrote the paper; K.L., K.A., Q.P., and N.W. performed research and analyzed data; B.L. provided C3aR-deficient mice and related information; A.N. and A.K. helped with research design; and S.S. helped with research design and manuscript discussion.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wuding Zhou, Complement Laboratory, MRC Centre for Transplantation, King's College London, 5th Floor Tower Wing, Guy's Hospital, Great Maze Pond, London SE1 9RT, United Kingdom; e-mail: wuding.zhou@kcl.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal