Abstract
Finding that activated T cells control osteoclast (OCL) differentiation has revealed the importance of the interactions between immune and bone cells. Dendritic cells (DCs) are responsible for T-cell activation and share common precursors with OCLs. Here we show that DCs participate in bone resorption more directly than simply through T-cell activation. We show that, among the splenic DC subsets, the conventional DCs have the higher osteoclastogenic potential in vitro. We demonstrate that conventional DCs differentiate into functional OCLs in vivo when injected into osteopetrotic oc/oc mice defective in OCL resorptive function. Moreover, this differentiation involves the presence of activated CD4+ T cells controlling a high RANK-L expression by bone marrow stromal cells. Our results open new insights in the differentiation of OCLs and DCs and offer new basis for analyzing the relations between bone and immune systems.
Introduction
An increasing number of studies underline the interactions between the bone and immune systems and have led to the emergence of osteoimmunology.1,2 Excessive bone resorption is frequently associated with chronic infections and autoimmune and inflammatory diseases.3-6 The immune system plays a major role in this process, in particular through activated T cells, which secrete proinflammatory cytokines involved in osteoclastogenesis.7 However, less is known about the involvement of other immune cells in the control of bone resorption. Dendritic cells (DCs) also play an important role in autoimmune and inflammatory diseases.8 These cells derive from the same myeloid precursor as osteoclasts (OCLs), and both cell types are modulated by common factors, mainly by receptor activator of NF-κB ligand (RANK-L). RANK-L is essential for the differentiation of OCLs,9 the activity and survival of DCs.10 These data highlight a potential link between DCs and OCLs.
Cells from the myelomonocytic lineage, including DCs, display a high developmental and functional plasticity depending on local factors and stimuli experienced during their differentiation and maturation.11,12 Although they were considered to be terminally differentiated cells, recent studies have suggested that mature splenic DCs can be influenced by their microenvironment to undergo further differentiation. Splenic stromal cells induce mature DCs to differentiate into regulatory DCs, which differ from mature DCs by their phenotype, their cytokine secretion pattern, and their ability to inhibit T-cell proliferation.13 Moreover, DCs generated in vitro transdifferentiate into endothelial cells when cultured with tumor-conditioned media14 or into OCLs when cultured with osteoclastogenic factors.15,16 Although these in vitro studies revealed the capacity of DCs to transdifferentiate into other cell types under specific conditions, it is not clear yet whether this plasticity takes place in vivo.
Osteopetrosis is characterized by an impaired bone resorption because of the absence of OCL formation or activity.17 In the osteopetrotic oc/oc mouse, differentiated OCLs are present but are unable to resorb bone because of a deletion in the Tcirg1 gene encoding the a3 subunit of the vacuolar ATPase.18 The a3 protein is responsible for the acidification process necessary for the dissolution of the bone matrix leading to the formation of resorption lacunae. In the absence of a3 expression, the bone marrow of oc/oc mice is filled with numerous and disorganized trabeculae, and osteoclastogenesis is highly increased.19,20 The consequence of this severe osteopetrotic phenotype is a life span less than 3 weeks. Therefore, the oc/oc mouse provides an appropriate model to assess the in vivo capacity of wild-type precursor cells to give rise to functional OCLs.
To assess whether DCs have an osteoclastogenic potential, we purified them from normal mice and cultured them with RANK-L and macrophage-colony stimulating factor (M-CSF). We showed that this treatment allows DCs to differentiate efficiently into fully functional OCLs. However, each of the DC subsets had a different osteoclastogenic capacity, the most efficient being the conventional CD11chighMHC-II+ DCs (cDCs). We have therefore characterized, in more detail, the differentiation of cDCs into OCLs in vitro. This process was accompanied by an up-regulation of Nfatc1, a transcription factor essential for osteoclastogenesis, as shown for classic OCL precursors. We also evaluated this differentiation potential in vivo using the osteopetrotic oc/oc mouse deficient in OCL activity. We showed that splenic cDCs isolated from normal mice are able to home into the bone marrow and partially reverse the osteopetrotic phenotype of oc/oc mice by differentiating into functional OCLs. This differentiation is highly favored in oc/oc mice bone marrow because of the presence of inflammatory CD4+ T cells able to maintain a high RANK-L expression by bone marrow stromal cells.
This report is the first demonstration that, in vivo, cDCs can be influenced by the bone marrow cellular environment to participate to bone resorption through their differentiation into OCLs, and can be efficient in the treatment of a bone resorption disease.
Methods
Mice and treatment
oc/oc mice were genotyped as described,20 1 day after birth. CD11c-DTR/GFP mice21 were obtained from Département de Cryopréservation, Distribution, Typage et Archivage animal (Orléans, France). Animals were maintained in our animal facility in accordance with the general guidelines of the Direction des Services Vétérinaires. When indicated, oc/oc and control mice received total body irradiation of 3 Gy (nonlethal dose) 1 day after birth. On day 2 after birth, mice received intraperitoneal injection of either 50 μL phosphate-buffered saline (PBS; control oc/oc mice) or 2 to 15 × 106 splenic DCs purified from naive +/+ mice in 50 μL PBS. Animals injected with DCs from CD11c-DTR/GFP were irradiated and received an injection of 5 × 106 DCs together with diphtheria toxin (4 ng/g body weight). Animals were killed at the indicated day. Approval for the use of mice in this study was obtained from the animal facility committee from the Faculty of Medicine at the University of Nice Sophia Antipolis.
Purification of conventional splenic DCs and monocytes
cDCs were sorted from spleens of 5-week-old naive +/+ mice. After red blood cell lysis, splenocytes were depleted for T, B, NK cells, monocytes, and granulocytes, by treatment with monoclonal antibody mixture containing anti-CD3 (17A2), anti-CD19 (1D3), anti-CD49b (DX5), and anti-Ly6G/6C (RB6-8C5; BD Biosciences PharMingen, San Diego, CA) and separation with anti-rat Ig-coated magnetic beads (Dynal Biotech, Lake Success, NY). cDCs were obtained after CD11c+ enrichment of the remaining splenocytes by positive selection with CD11c microbeads on LS columns (Miltenyi Biotec, Auburn, CA). Enriched cells were labeled with APC-CD11c (HL3) and fluorescein isothiocyanate–IAb (AF6-120.1), and the CD11c+ DCs were sorted on a fluorescence-activated cell sorter (FACS)–Aria cell sorter (BD Biosciences, San Jose, CA) with high-purity sorting (100%). These purified cells were used for all in vivo experiments. Their phenotype was analyzed using PE-CD11b (M1/70), -CD86 (GL1), -CD80 (16-10A1), -CD40 (3/23), -B220 (RA3-6B2), -Ly6C (AL-21) (BD Biosciences PharMingen), or -F4/80 (ClA3-1; Invitrogen, Carlsbad, CA), by flow cytometry (FACSCanto; BD Biosciences). Bone marrow monocytes were purified on a FACSAria cell sorter after staining with anti-CD11b and -Ly6G/6C (RB6-8C5) antibodies (BD Biosciences PharMingen).
Purification and characterization of CD4+ T lymphocytes
Bone marrow or splenic CD3+ cells were enriched by magnetic cell sorting using biotinylated-CD3 (17A2) and streptavidin microbeads (Miltenyi Biotec). For polymerase chain reaction (PCR) analysis and coculture, CD4+ T cells were sorted from the bone marrow CD3+-enriched fraction after labeling with anti-CD4 antibody, on a FACSAria. For phenotyping, splenic CD3+-enriched cells were labeled with anti-CD4 and anti-CD62L (MEL-14) antibodies and analyzed on a FACSCanto.
Intracytoplasmic cytokine detection was performed according to the manufacturer's protocol (BD Biosciences). Briefly, 105 bone marrow CD3+-enriched cells were seeded in 96-well U-bottom plates and stimulated for 4 hours with 100 ng/mL phorbol-12-myristate-13-acetate and 1 μg/mL ionomycin. After 2 hours, brefeldin was added. Cells were labeled with an anti-CD4 antibody, fixed, permeabilized, labeled with anti–interferon-γ (IFN-γ) and anti–interleukin-17 (IL-17) antibodies (BD Biosciences), and analyzed by flow cytometry.
Purification of bone marrow stromal cells and coculture with T lymphocytes
For PCR analysis, bone marrow cells from femora were incubated with purified anti-CD45 (30-F11) and rabbit complement for 2 hours at 37° in minimum essential medium-α (MEM-α) supplemented with 5% fetal calf serum (HyClone; Thermo Fisher Scientific, Lafayette, CO) to remove hematopoietic cells. Cells were washed with PBS and allowed to attach for 3 hours on plastic culture dishes in the same MEM-α and gently washed. Attached cells represented marrow stromal cells and were used for RNA analysis.
For differentiation assays and coculture with T cells, calvaria of normal mice were crushed into pieces and seeded in a 6-cm dish for 10 days in MEM-α supplemented with 5% fetal calf serum and without ascorbic acid. Cells were then harvested by trypsin/ethylenediaminetetraacetic acid and seeded in 75-cm2 flasks in MEM-α supplemented with 5% fetal calf serum and without ascorbic acid. Once confluent, cells were seeded at 2 × 104 cells in 48-well plates in MEM-α supplemented with 5% fetal calf serum and cocultured with 2.5 × 104 DCs. Bone marrow-purified CD4+ T cells (5 × 105) and activated with 1 μg/mL phytohemagglutinin were added or not to the culture. After 7 days, 10 μg/mL ascorbic acid and 10−8 M 1,25(OH)2D3 were added for the last 3 days of culture. Cells were fixed and analyzed for tartrate-resistant acid phosphatase (TRAP) activity with the leukocyte acid phosphatase kit (Sigma-Aldrich, St Louis, MO).
PCR, OCL culture, and histologic analysis
See Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results
Splenic cDCs differentiate into functional OCLs in vitro
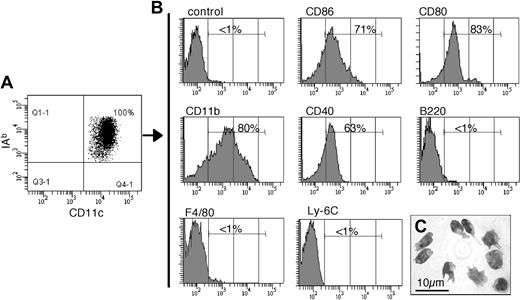
In vitro–generated DCs have been shown to form OCLs in the presence of RANK-L and M-CSF.15,16 However, the contribution of the different natural DC subsets has not been analyzed. Therefore, we analyzed the osteoclastogenic potential of the various splenic DC subsets. Our results show that cDCs differentiate into OCLs more efficiently than pDCs and that, among the cDC subsets, the CD4−CD8− subset contributes much more efficiently than others to this differentiation (Figure S1). Thus, we have further evaluated the formation of OCLs from cDCs purified from mouse spleen using CD11c, a specific cell surface marker of this population. To avoid any risk of contaminant cells, T, B, NK cells, granulocytes, and monocytes were depleted from splenocytes and CD11c+ cells were enriched from the remaining cells. Then, cDCs were purified from these CD11c+-enriched cells by FACS (Figure 1A). This procedure allows the recovery of pure CD11chigh DCs (100%).
Characterization of the sorted CD11c+ splenic DCs. Conventional CD11c+ cells were FACS sorted from the spleen of 5-week-old C57BL/6 normal mice. (A) Flow cytometric analysis revealed that they are pure (100%) and coexpress CD11c and the MHC-II protein IAb. (B) Phenotypic analysis of the sorted CD11c+ cells by flow cytometric analysis. Percentage of cells with fluorescence intensity over the dashed vertical lines, corresponding to the upper limit of control background staining, is indicated. (C) May-Grunwald-Giemsa staining of the sorted cells after cytospin revealed the typical phenotype of splenic cDCs.
Characterization of the sorted CD11c+ splenic DCs. Conventional CD11c+ cells were FACS sorted from the spleen of 5-week-old C57BL/6 normal mice. (A) Flow cytometric analysis revealed that they are pure (100%) and coexpress CD11c and the MHC-II protein IAb. (B) Phenotypic analysis of the sorted CD11c+ cells by flow cytometric analysis. Percentage of cells with fluorescence intensity over the dashed vertical lines, corresponding to the upper limit of control background staining, is indicated. (C) May-Grunwald-Giemsa staining of the sorted cells after cytospin revealed the typical phenotype of splenic cDCs.
Sorted CD11c+ cells are MHC-II+CD11b+, they are negative for F4/80, Ly-6C, and B220, and most of them express the costimulatory molecules CD80, CD86, and CD40 (Figure 1B). Therefore, this CD11c+ population does not correspond to monocytes (Ly-6C+CD11c−) or to macrophages (F4/80+). Moreover, sorted CD11c+ DCs display a typical dendritic morphology (Figure 1C). Furthermore, CD11c+ DCs purified from C57BL/6 mice induce the proliferation of naive CD4+ T cells from BALB/c mice, in a mixed lymphocyte reaction (data not shown). Taken together, this phenotype corresponds to the characteristic features of splenic CD11c+ cDCs previously described in the literature.13,24,25
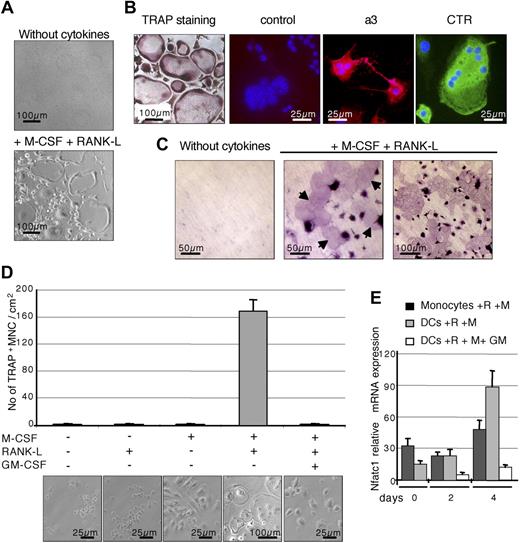
In the presence of the main osteoclastogenic factors RANK-L and M-CSF, purified splenic cDCs differentiated efficiently into multinucleated cells expressing the TRAP, the a3 subunit of the vacuolar proton pump, and the calcitonin receptor (Figure 2A,B), 3 markers typical of OCLs. Moreover, these cells generated resorption pits when cultured on dentine slices (Figure 2C), displaying the typical phenotype of functional OCLs.26,27 These results confirmed that cDCs have the potential to differentiate in vitro into functional OCLs in the presence of M-CSF and RANK-L. The differentiation process is highly efficient because, after 10 days of culture, 100% of cells were TRAP+, with more than 80% being multinucleated. In resorption assays, the surface of resorption pits corresponded to 40% to 50% of dentine slice surface (Figure 2C; and data not shown).
Differentiation of CD11c+ splenic DCs into functional OCLs in vitro. (A) Splenic DCs sorted from normal mice were cultured for 8 days either without cytokines or with 25 ng/mL M-CSF and 30 ng/mL RANK-L allowing their differentiation into multinucleated cells. (B) These differentiated cells were analyzed for their TRAP activity. The presence of the a3 protein (rhodamine) and the calcitonin receptor (fluorescein isothiocyanate) was also analyzed by immunofluorescence. Incubation with a preimmune serum served as negative control. Nuclei were stained with DAPI. (C) Resorption activity was assessed on dentine slices for cells cultured without cytokines or with M-CSF and RANK-L. Arrows indicate the presence of resorption lacunae with cytokine-treated cells. Equivalent results were found with DCs sorted from Balb/c and C57BL/6 mice. (D) Sorted splenic DCs were cultured for 8 days without cytokines or with various combinations of M-CSF (25 ng/mL), RANK-L (30 ng/mL), and GM-CSF (10 ng/mL). TRAP activity was analyzed and TRAP+ multinucleated cells (MNCs) were counted. The results are the mean plus or minus SD of 4 equivalent wells. (E) The kinetics of Nfatc1 expression was analyzed by real-time RT-PCR between days 0 and 4 of culture on cells treated with M-CSF (M) and RANK-L (R) and with our without GM-CSF (GM). The results are the mean plus or minus SD of 4 equivalent wells. All results are representative of 3 independent experiments. Images were captured using (panels A,D) an Axiovert 200 microscope (Carl Zeiss, Oberkochen, Germany) with a 10×/0.25 Ph1 or a 20×/0.3 Ph1 Var1 Plan objective (Carl Zeiss) and a JVC KY-F50 camera, (panel B) an Axioskop microscope (Carl Zeiss) with a 40×/1.30 oil objective (Carl Zeiss), an AxioCam HRc camera (Carl Zeiss), and Gel/Mount medium (Biomeda, Foster City, CA), and (panel C) an Axioskop microscope (Zeiss) with 20×/0.75 Fluar or 40×/0.75 Plan-Neofluar objectives. All images were acquired using AxioVision 4.5 software (Zeiss).
Differentiation of CD11c+ splenic DCs into functional OCLs in vitro. (A) Splenic DCs sorted from normal mice were cultured for 8 days either without cytokines or with 25 ng/mL M-CSF and 30 ng/mL RANK-L allowing their differentiation into multinucleated cells. (B) These differentiated cells were analyzed for their TRAP activity. The presence of the a3 protein (rhodamine) and the calcitonin receptor (fluorescein isothiocyanate) was also analyzed by immunofluorescence. Incubation with a preimmune serum served as negative control. Nuclei were stained with DAPI. (C) Resorption activity was assessed on dentine slices for cells cultured without cytokines or with M-CSF and RANK-L. Arrows indicate the presence of resorption lacunae with cytokine-treated cells. Equivalent results were found with DCs sorted from Balb/c and C57BL/6 mice. (D) Sorted splenic DCs were cultured for 8 days without cytokines or with various combinations of M-CSF (25 ng/mL), RANK-L (30 ng/mL), and GM-CSF (10 ng/mL). TRAP activity was analyzed and TRAP+ multinucleated cells (MNCs) were counted. The results are the mean plus or minus SD of 4 equivalent wells. (E) The kinetics of Nfatc1 expression was analyzed by real-time RT-PCR between days 0 and 4 of culture on cells treated with M-CSF (M) and RANK-L (R) and with our without GM-CSF (GM). The results are the mean plus or minus SD of 4 equivalent wells. All results are representative of 3 independent experiments. Images were captured using (panels A,D) an Axiovert 200 microscope (Carl Zeiss, Oberkochen, Germany) with a 10×/0.25 Ph1 or a 20×/0.3 Ph1 Var1 Plan objective (Carl Zeiss) and a JVC KY-F50 camera, (panel B) an Axioskop microscope (Carl Zeiss) with a 40×/1.30 oil objective (Carl Zeiss), an AxioCam HRc camera (Carl Zeiss), and Gel/Mount medium (Biomeda, Foster City, CA), and (panel C) an Axioskop microscope (Zeiss) with 20×/0.75 Fluar or 40×/0.75 Plan-Neofluar objectives. All images were acquired using AxioVision 4.5 software (Zeiss).
Differentiation of cDCs into OCLs is RANK-L and M-CSF dependent
Lineage bifurcation between DCs and OCLs from a common progenitor is reciprocally regulated by granulocyte-macrophage colony-stimulating factor (GM-CSF) and M-CSF.28 We therefore examined the effect of these cytokines on the DC-to-OCL differentiation. This differentiation requires the presence of both RANK-L and M-CSF (Figure 2D) and is inhibited by addition of GM-CSF (Figure 2D).
The negative effect of GM-CSF on differentiation OCLs from monocytes is, in part, mediated by a down-regulation of NFATc1, a RANK-L-induced factor essential for osteoclastogenesis.29,30 NFATc1−/− embryonic stem cells cannot differentiate into osteoclasts, and ectopic expression of NFATc1 induces osteoclastogenesis in the absence of RANK-L.30 Analysis of Nfatc1 expression by real-time reverse-transcribed PCR (RT-PCR)22 on monocytes and cDCs treated by RANK-L plus M-CSF showed that it was induced in DC-derived cells to a higher level than in monocyte-derived cells, and this induction was inhibited by GM-CSF (Figure 2E). Because NFATc1 is essential for osteoclastogenesis, this inhibition could explain the suppression by GM-CSF of DC-to-OCL differentiation, as reported for OCL differentiation from monocytes.29 In summary, our results show that splenic cDCs do behave as monocytic OCL precursors and that cDCs and monocytes probably share common signaling pathways.
Bone resorption activity is restored in cDC-treated oc/oc mice
These observations prompted us to examine whether splenic cDCs can generate OCLs in vivo. We have used the osteopetrotic oc/oc mouse as a model for detecting activity of OCLs derived from cDCs. These mice die before the age of 3 weeks because of their severe osteopetrotic phenotype. Their bone marrow is filled with numerous and disorganized trabeculae, and osteoclastogenesis is increased but leads to nonfunctional OCLs.19,20 Furthermore, they display a high expression of Rank-l and M-csf in the bone marrow compared with normal littermates (Figure S2). Thus, oc/oc mice are able to generate a high number of OCLs and provide an appropriate microenvironment to assess the in vivo capacity of DCs to give rise to OCLs.
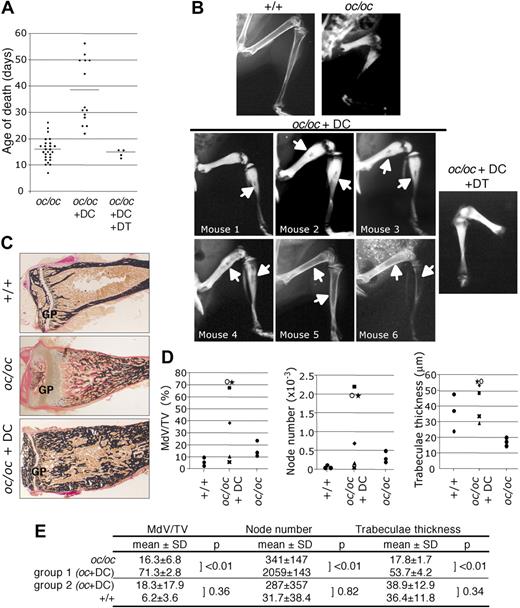
Two-day-old oc/oc mice were injected with FACS-purified cDCs from +/+ mice. Three independent experiments were performed using different numbers of DCs in irradiated or nonirradiated mice. In all experiments, the life span of DC-treated oc/oc mice increased at least 2- to 3-fold compared with PBS-treated oc/oc mice (Figure 3A), indicating an improvement of their phenotype. Radiologic analysis of DC-treated oc/oc mice that survived more than 1 month of age (n = 6) revealed the presence of a partial marrow cavity in long bones (Figure 3B) whatever the experimental conditions, whereas such a cavity was never observed in control oc/oc mice, indicating that a bone resorption activity has been restored in DC-treated mice. The cavity size was variable, the larger ones being observed in irradiated mice treated with 5 × 106 DCs (Figure 3B, mice nos. 5 and 6). In all experiments, no change in radiologic analysis was observed in DC-treated oc/oc mice that died before 1 month of age (n = 8) compared with PBS-treated oc/oc mice (not shown), even if their life span was increased. Thus, no histologic analysis of their bone phenotype was performed. This result is consistent with the observation that in oc/oc mice transplanted with hematopoietic stem cells, the first changes in bone structure observed radiographically were detected only 1 month after transplantation.31 No difference in survival and phenotype was observed between untreated oc/oc mice and oc/oc mice that have been irradiated and injected with PBS (data not shown), indicating that all these effects cannot be attributed to irradiation alone. Together, these data indicate that bone phenotype is restored in approximately 45% of DC-treated animals, the 55% remaining animals dying before any change in bone morphology can be observed. This efficiency is close to the one observed in therapeutic assays using various hematopoietic populations in newborn oc/oc mice.31-33 It can be explained by the fact that, during the first weeks after treatment, mice remain subjected to many deleterious factors such as hematologic defects, access to the mother for food, or nerve compression, that contribute to shorten their life span.
Partial reversion of the osteopetrotic phenotype in DC-treated oc/oc mice. (A) Distribution of the age of death of control oc/oc (n = 26), DC-treated oc/oc mice from 3 independent experiments (n = 14) and oc/oc treated with DCs purified from CD11c-DTR mice and diphtheria toxin (DT) (n = 4). (B) Radiographic analysis of normal mice, PBS-treated oc/oc mice, DC-treated oc/oc mice that survived more than 33 days (n = 6), and oc/oc mice treated with DCs from CD11c-DTR mice and DT. Arrows indicate the medullar cavity. (C) Histologic analysis of the tibia of normal littermates, control, and DC-treated oc/oc mice after van Gieson and von Kossa staining. GP indicates growth plate. The results are representative of the mean phenotype observed in 3 independent experiments. Images were captured using a DMLB microscope (Leica, Wetzlar, Germany), a 3CCD color video DXC-390 camera (Sony, Heerlen, The Netherlands) and the Osteomeasure Analysis System (Osteometrics, Atlanta, GA). (D) Bone morphometric analysis on +/+ mice (n = 3), PBS-treated oc/oc mice (n = 3), and DC-treated oc/oc mice (n = 6). The results are the distribution of the values measured for each animal. MdV/TV indicates mineralized volume/tissue volume. Correspondence with the radiographic analysis (C): ★ represents mouse 1; ■, mouse 2; ○, mouse 3; ♦, mouse 4; X, mouse 5; and ▲, mouse 6. (E) Statistical analysis of parameters from panel D. Parameters from control oc/oc mice were significantly different from those of DC-treated oc/oc mice group 1 (mice nos. 1-3) presenting a small bone marrow cavity (P < .01). A second group of DC-treated oc/oc mice, group 2 (mice nos. 4-6), presented a larger bone marrow cavity, and their histomorphometric parameters were not significantly different from normal +/+ mice (P > .05).
Partial reversion of the osteopetrotic phenotype in DC-treated oc/oc mice. (A) Distribution of the age of death of control oc/oc (n = 26), DC-treated oc/oc mice from 3 independent experiments (n = 14) and oc/oc treated with DCs purified from CD11c-DTR mice and diphtheria toxin (DT) (n = 4). (B) Radiographic analysis of normal mice, PBS-treated oc/oc mice, DC-treated oc/oc mice that survived more than 33 days (n = 6), and oc/oc mice treated with DCs from CD11c-DTR mice and DT. Arrows indicate the medullar cavity. (C) Histologic analysis of the tibia of normal littermates, control, and DC-treated oc/oc mice after van Gieson and von Kossa staining. GP indicates growth plate. The results are representative of the mean phenotype observed in 3 independent experiments. Images were captured using a DMLB microscope (Leica, Wetzlar, Germany), a 3CCD color video DXC-390 camera (Sony, Heerlen, The Netherlands) and the Osteomeasure Analysis System (Osteometrics, Atlanta, GA). (D) Bone morphometric analysis on +/+ mice (n = 3), PBS-treated oc/oc mice (n = 3), and DC-treated oc/oc mice (n = 6). The results are the distribution of the values measured for each animal. MdV/TV indicates mineralized volume/tissue volume. Correspondence with the radiographic analysis (C): ★ represents mouse 1; ■, mouse 2; ○, mouse 3; ♦, mouse 4; X, mouse 5; and ▲, mouse 6. (E) Statistical analysis of parameters from panel D. Parameters from control oc/oc mice were significantly different from those of DC-treated oc/oc mice group 1 (mice nos. 1-3) presenting a small bone marrow cavity (P < .01). A second group of DC-treated oc/oc mice, group 2 (mice nos. 4-6), presented a larger bone marrow cavity, and their histomorphometric parameters were not significantly different from normal +/+ mice (P > .05).
To clearly demonstrate the contribution of DCs to the restoration of the bone phenotype and to eliminate the possible involvement of nondendritic contaminant in the injected cells, we have also used cDCs from transgenic CD11c-DTR/GFP mice expressing diphtheria toxin receptor (DTR) under the control of the CD11c promoter. These mice have normal DC phenotype, but CD11c+ DCs are sensitive to diphtheria toxin.21 Furthermore, because murine cells do not express DTR, cells negative for CD11c expression are not affected by diphtheria toxin. Therefore, using this model, it is possible to specifically deplete CD11c+ DCs injected in oc/oc mice, without alteration of eventual CD11c− cells contaminating the cDC preparation. Experiments were performed using 5 × 106 purified DCs injected into irradiated oc/oc mice because these conditions gave the best bone phenotype recovery and mice survival. Injection of diphtheria toxin (4 ng/g body weight) in newborn mice was not lethal (not shown). In oc/oc mice injected with DCs purified from CD11c-DTR/GFP mice, administration of diphtheria toxin led to a complete depletion of injected DCs 48 hours after diphtheria toxin treatment (not shown). These mice (n = 4) displayed the same life span (Figure 3A) and the same bone phenotype (Figure 3B) as control oc/oc mice, revealing an absence of rescue of the osteopetrotic phenotype. These data confirmed that CD11c+ DCs are responsible for the restoration of bone resorption in oc/oc mice and excluded the contribution of CD11c− contaminants in this effect.
To confirm the partial restoration of bone phenotype in DC-treated oc/oc mice, histologic analyses were performed on tibia of mice that have survived more than 1 month (n = 6) and on control mice. In PBS-treated oc/oc mice, the growth plate was disorganized and large zones of unmineralized type I collagen matrix were observed below this plate (Figure 3C), as previously described.19,34 In DC-treated oc/oc mice, the growth plate was normal and a partial marrow cavity variable in size was observed (Figure 3C).
Bone histomorphometric analysis revealed that, in oc/oc mice, the bone tissue contained a mix of mineralized and unmineralized type I collagen and cartilage, making it difficult to clearly define the bone volume.23 Therefore, we measured the ratio between the mineralized volume and the tissue volume (MdV/TV). Histomorphometric values of DC-treated oc/oc mice evidenced the presence of 2 groups compared with control oc/oc mice (Figure 3D). Fifty percent of these mice (group 1, mice nos. 1-3) displayed a very small marrow cavity, and their MdV/TV ratio, number of nodes (reflecting the connectivity between trabeculae), and trabeculae thickness were dramatically increased compared with control oc/oc mice (Figure 3D,E), indicating that the bone matrix containing type I collagen has been mineralized.
Bone resorption and formation are tightly coupled through the activity of factors released from the bone matrix during bone resorption.35 This probably explains why the first consequence of a restoration of bone resorption in oc/oc mice is an increase in bone mineralization. Thus, our data reflect a partial restoration of the remodeling process through some resorption activity. In the 50% remaining DC-treated mice (group 2, mice nos. 4-6) displaying the larger bone marrow cavity, morphometric parameters were not significantly different from those of +/+ mice (Figure 3D,E), confirming a spectacular recovery of the bone morphology and a restoration of bone resorption activity.
Such a heterogeneity in the bone phenotype was also reported in oc/oc mice treated with in utero or neonatal hematopoietic stem cell transplantation.32,33 In addition to the radiation dose and the number of transplanted cells, this effect was attributed to interindividual variations and to the difficulty of intraperitoneal injection in newborn mice.33 Altogether, our data indicate that injection of cDCs allowed partial reversion of the osteopetrotic phenotype in oc/oc mice.
Splenic cDCs differentiate into functional OCLs in vivo
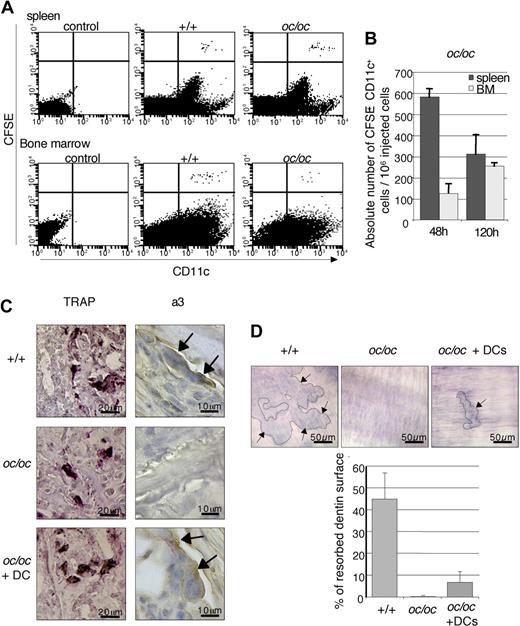
To assess whether injected cDCs directly contributed to the bone resorption activity, we first analyzed their potential to migrate into the bone marrow of oc/oc mice. Labeling cDCs with carboxyfluorescein succinimidyl ester (CFSE) before injection allowed us to analyze the distribution of CFSE+CD11c+ DCs in recipient spleen and bone marrow by flow cytometry. At 48 hours after injection, CFSE+CD11c+ DCs were detected in the spleen and bone marrow of +/+ mice, as previously described,36 but also of oc/oc mice (Figure 4A). Analysis of the absolute number of CFSE+CD11c+ DCs present in DC-treated oc/oc mice revealed that at 48 hours after injection, CFSE+CD11c+ DCs recovered from the bone marrow were 5-fold less abundant than those recovered from the spleen (Figure 4B). At 120 hours after injection, the number of CFSE+CD11c+ cells retained in the spleen decreased, whereas this population increased approximately 2-fold in the bone marrow, compared with 48 hours. These results demonstrate that cDCs injected intraperitonally are able to traffic and home into the bone marrow of +/+ and oc/oc mice.
Presence of active OCLs in DC-treated oc/oc mice. (A) Distribution of CFSE-labeled CD11c+ cDCs was analyzed by flow cytometry 48 hours after intraperitoneal injection into +/+ and oc/oc mice. (B) The absolute number of CFSE+CD11c+ DCs in the spleen and bone marrow (BM) of oc/oc mice was calculated by multiplication of the percentage of CFSE+CD11c+ DCs by the total number of resident leukocytes. Data are the mean plus or minus SD of the number of recovered CFSE+CD11c+ DCs per 106 injected cells (n = 4 to 6 per group) and are representative of 2 independent experiments. (C) Representative histologic analysis of the femora of +/+ mice, control, and DC-injected oc/oc mice. TRAP+ cells were detected in all mice, but a3-expressing cells were detected in +/+ and DC-treated oc/oc mice but not in control oc/oc mice. (D) Bone marrow cells from +/+ mice, control, and DC-treated oc/oc mice were cultured onto dentine slices, and their bone resorption activity was analyzed. Resorption lacunae (arrows) were detected in +/+ and DC-treated oc/oc mice but not in control oc/oc mice. Images were captured using an Axioskop microscope (Carl Zeiss) with a 20×/0.75 Fluar objective (Carl Zeiss), an AxioCam HRc camera (Carl Zeiss), and AxioVision 4.5 software (Zeiss).
Presence of active OCLs in DC-treated oc/oc mice. (A) Distribution of CFSE-labeled CD11c+ cDCs was analyzed by flow cytometry 48 hours after intraperitoneal injection into +/+ and oc/oc mice. (B) The absolute number of CFSE+CD11c+ DCs in the spleen and bone marrow (BM) of oc/oc mice was calculated by multiplication of the percentage of CFSE+CD11c+ DCs by the total number of resident leukocytes. Data are the mean plus or minus SD of the number of recovered CFSE+CD11c+ DCs per 106 injected cells (n = 4 to 6 per group) and are representative of 2 independent experiments. (C) Representative histologic analysis of the femora of +/+ mice, control, and DC-injected oc/oc mice. TRAP+ cells were detected in all mice, but a3-expressing cells were detected in +/+ and DC-treated oc/oc mice but not in control oc/oc mice. (D) Bone marrow cells from +/+ mice, control, and DC-treated oc/oc mice were cultured onto dentine slices, and their bone resorption activity was analyzed. Resorption lacunae (arrows) were detected in +/+ and DC-treated oc/oc mice but not in control oc/oc mice. Images were captured using an Axioskop microscope (Carl Zeiss) with a 20×/0.75 Fluar objective (Carl Zeiss), an AxioCam HRc camera (Carl Zeiss), and AxioVision 4.5 software (Zeiss).
Injected DCs were purified from +/+ mice; thus, OCLs deriving from these cells should express the a3 protein encoded by the Tcirg1 gene mutated in oc/oc mice, whereas OCLs derived from oc/oc precursors do not express this protein. Thus, we have analyzed the expression of a3 in the femur of oc/oc-treated mice by immunohistology. Multinucleated cells located at the bone surface and expressing TRAP and the a3 protein, thus corresponding to osteoclasts, were detected in the bone marrow of +/+ and DC-treated oc/oc mice, whereas in untreated oc/oc mice, only TRAP+ but not a3+ osteoclasts were detected, as expected (Figure 4C). However, a3 expression in OCLs from DC-treated oc/oc mice was lower than in +/+ mice, probably because of the fusion of injected a3+ DCs with a3− pre-OCLs from oc/oc mice. This result indicates that a3+ osteoclasts observed in DC-injected mice are indeed derived from the injected +/+ DCs.
Furthermore, bone marrow cells from DC-treated oc/oc mice cultured onto dentin slices with RANK-L and M-CSF are able to form a small number of resorption lacunae, whereas resorption pits were never observed with bone marrow cells derived from control oc/oc mice (Figure 4D). These data demonstrate that functional OCLs are recovered from DC-treated oc/oc mice.
Altogether, these results clearly show that active OCLs are present in oc/oc DC-treated mice and have differentiated from the injected cDCs, demonstrating that cDCs are able to differentiate into functional OCLs in vivo.
CD4+ T cells contribute to the DC differentiation into OCLs in vivo
Differentiation of DCs into OCLs was not detected in healthy mice (not shown), suggesting that this pathway requires a specific microenvironment. Indeed, oc/oc mice express high levels of Rank-l and M-csf in the bone marrow (Figure S2). High levels of osteoclastogenic factors are often associated with inflammation and are induced by activated CD4+ T cells.37 Moreover, Alnaeeli et al have recently reported that interactions of DCs with CD4+ T cells and foreign antigens allow T-cell activation and the differentiation of DCs into OCLs in vitro.16
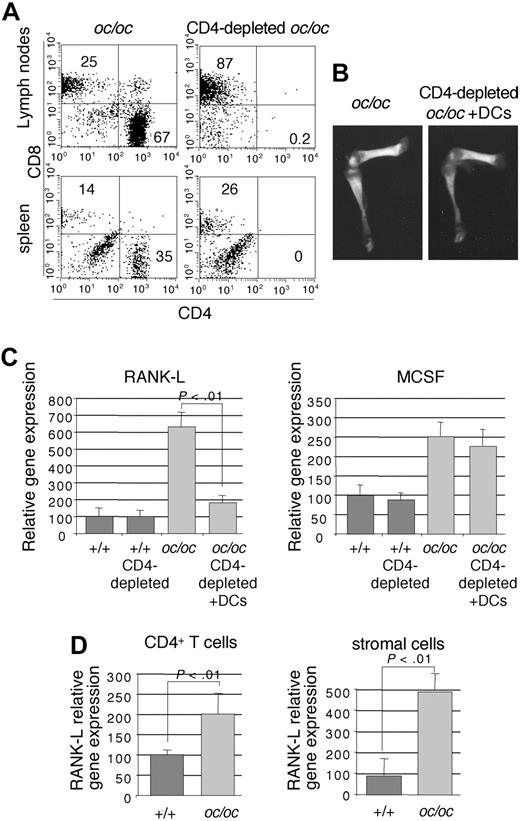
To assess whether CD4+ T cells are required for the in vivo DC-to-OCL differentiation, we have depleted CD4+ T cells in oc/oc mice by injecting an anti-CD4 antibody (Figure 5A). CD4+-depleted oc/oc mice injected with DCs did not survive longer than untreated oc/oc mice, whereas CD4+ T-cell depletion per se did not affect the survival of +/+ littermates (not shown). No radiologic difference was observed between control oc/oc mice and DC-injected CD4+-depleted oc/oc mice (Figure 5B), indicating that bone remodeling has not been restored in DC-injected oc/oc mice after CD4+ T-cell depletion, in contrast to undepleted DC-treated oc/oc mice (not shown). Altogether, these data indicate that CD4+ T cells participate in the in vivo differentiation of DCs into OCLs.
CD4+ T cells are required for the DC-to-OCL differentiation in oc/oc mice. (A) Flow cytometric analysis of the mesenteric lymph nodes and the spleen of control oc/oc mice and CD4-depleted and DC-injected oc/oc mice. (B) Representative radiography of the femur and tibia of control oc/oc mice and CD4-depleted and DC-injected oc/oc mice. (C) The expression of Rank-l and M-csf was analyzed by real-time RT-PCR in the bone marrow of normal mice, control oc/oc mice, and CD4-depleted and DC-injected oc/oc. The results are the mean plus or minus SD of 3 or 4 mice in each group. All results are representative of 2 independent experiments. (D) Real-time RT-PCR analysis of CD4+ T cells or stromal cells purified from the bone marrow of +/+ and oc/oc mice. The results are the mean plus or minus SD of 6 or 7 mice in each group.
CD4+ T cells are required for the DC-to-OCL differentiation in oc/oc mice. (A) Flow cytometric analysis of the mesenteric lymph nodes and the spleen of control oc/oc mice and CD4-depleted and DC-injected oc/oc mice. (B) Representative radiography of the femur and tibia of control oc/oc mice and CD4-depleted and DC-injected oc/oc mice. (C) The expression of Rank-l and M-csf was analyzed by real-time RT-PCR in the bone marrow of normal mice, control oc/oc mice, and CD4-depleted and DC-injected oc/oc. The results are the mean plus or minus SD of 3 or 4 mice in each group. All results are representative of 2 independent experiments. (D) Real-time RT-PCR analysis of CD4+ T cells or stromal cells purified from the bone marrow of +/+ and oc/oc mice. The results are the mean plus or minus SD of 6 or 7 mice in each group.
To assess whether CD4+ T cells are involved in the high expression of osteoclastogenic factors observed in the bone marrow of oc/oc mice, we have analyzed the effect of CD4+ T-cell depletion on this expression. CD4+ T-cell depletion induced a dramatic decrease of Rank-l expression in the bone marrow of oc/oc mice, which returned to the level observed in normal mice (Figure 5C), whereas M-csf mRNA levels were not affected. In normal mice, CD4+ T-cell depletion did not affect the expression of Rank-l and M-csf (Figure 5C). This result indicates that CD4+ T cells are involved in the increased Rank-l production observed in the bone marrow of oc/oc mice and that this high Rank-l expression provides a highly favorable environment required for DC-to-OCL differentiation.
RANK-L can be expressed by CD4+ T cells themselves or induced in stromal cells by inflammatory cytokines secreted by activated CD4+ T cells.37 Thus, to determine which cell subset is responsible for the high level of Rank-l in the bone marrow of oc/oc mice, we have purified CD4+ T cells and stromal cells from +/+ and oc/oc mice. Whereas Rank-l expression was doubled in CD4+ T cells from oc/oc mice, it was multiplied more than 5-fold in stromal cells from oc/oc mice, compared with +/+ mice (Figure 5D). These data suggest that, in the bone marrow of oc/oc mice, the main source of RANK-L is produced by stromal cells and controlled by CD4+ T cells.
Inflammatory CD4+ T cells support the DC-to-OCL differentiation through the stimulation of marrow stromal cells
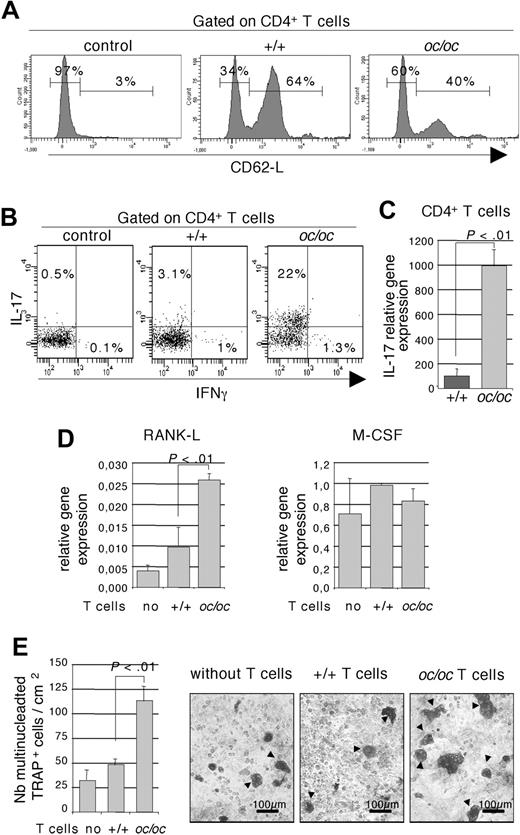
To understand the effect of CD4+ T cells from oc/oc mice on stromal cells, we have analyzed their activation and polarization status. Because the bone marrow is a reservoir for activated/memory T cells, even in normal mice,38,39 flow cytometric analysis of the activation status of CD4+ T cells has been performed on splenic cells. The majority of CD4+ T cells from oc/oc mice are CD62L−, thus corresponding to an activated phenotype (Figure 6A) in contrast with those from +/+ mice. Activated CD4+ T-cell polarization is characterized by their cytokine production, mainly IFN-γ for Th1, IL-4 for Th2, and IL-17 for Th17. Intracytoplasmic production of these cytokines was analyzed by flow cytometry on bone marrow CD4+ T cells. The percentage of IL-17-producing T cells greatly increased, whereas no significant change was observed in the percentage of IFN-γ–producing cells, in CD4+ T cells from oc/oc mice compared with +/+ mice (Figure 6B). IL-4 was not detected (not shown). We have previously shown that splenic CD4+ T cells from oc/oc mice secrete lower levels of IFN-γ than those from +/+ mice.20 This apparent discrepancy may reflect a difference in T-cell polarization between the spleen and the bone marrow of oc/oc mice, probably linked to the specific osteopetrotic bone microenvironment.
Inflammatory CD4+ T cells from oc/oc mice are responsible for a high RANK-L expression and an increased osteoclastogenesis. (A) Flow cytometric analysis of splenic CD4+ T cells from oc/oc and +/+ mice using the activation marker CD62L. An isotype control (control) is presented. The results are representative of at least 5 oc/oc and 5 +/+ mice. (B) Intracytoplasmic analysis of IL-17 and IFN-γ production by flow cytometry on CD3+ T cells purified from the bone marrow. Nonactivated T cells served as control. Data are gated on pooled CD4+ cells from 4 or 5 oc/oc or +/+ mice. All results are representative of 3 independent experiments. (C) Real-time RT-PCR analysis of CD4+ T cells purified from the bone marrow of +/+ and oc/oc mice. The results are the mean plus or minus SD of 5 mice in each group. (D) Osteoblasts from +/+ mice were cultivated alone or in the presence of CD4+ T cells purified from the bone marrow of +/+ and oc/oc mice. Expression of Rank-l and M-csf was analyzed in the osteoblasts by real-time RT-PCR. The results are the mean plus or minus SD of 3 wells and are representative of 3 independent experiments. (E) Osteoclasts were generated by coculture of DCs with osteoblasts from +/+ mice and with our without CD4+ T cells purified from the bone marrow of +/+ and oc/oc mice. A representative result is presented, and multinucleated TRAP+ osteoclasts (arrow) were enumerated. The results are the mean of 8 wells and are representative of 3 independent experiments. Images were captured using an Axiovert 200 microscope (Carl Zeiss) with a 10×/0.25 Ph1 Plan objective (Carl Zeiss), a JVC KY-F50 camera, and AxioVision 4.5 software (Zeiss).
Inflammatory CD4+ T cells from oc/oc mice are responsible for a high RANK-L expression and an increased osteoclastogenesis. (A) Flow cytometric analysis of splenic CD4+ T cells from oc/oc and +/+ mice using the activation marker CD62L. An isotype control (control) is presented. The results are representative of at least 5 oc/oc and 5 +/+ mice. (B) Intracytoplasmic analysis of IL-17 and IFN-γ production by flow cytometry on CD3+ T cells purified from the bone marrow. Nonactivated T cells served as control. Data are gated on pooled CD4+ cells from 4 or 5 oc/oc or +/+ mice. All results are representative of 3 independent experiments. (C) Real-time RT-PCR analysis of CD4+ T cells purified from the bone marrow of +/+ and oc/oc mice. The results are the mean plus or minus SD of 5 mice in each group. (D) Osteoblasts from +/+ mice were cultivated alone or in the presence of CD4+ T cells purified from the bone marrow of +/+ and oc/oc mice. Expression of Rank-l and M-csf was analyzed in the osteoblasts by real-time RT-PCR. The results are the mean plus or minus SD of 3 wells and are representative of 3 independent experiments. (E) Osteoclasts were generated by coculture of DCs with osteoblasts from +/+ mice and with our without CD4+ T cells purified from the bone marrow of +/+ and oc/oc mice. A representative result is presented, and multinucleated TRAP+ osteoclasts (arrow) were enumerated. The results are the mean of 8 wells and are representative of 3 independent experiments. Images were captured using an Axiovert 200 microscope (Carl Zeiss) with a 10×/0.25 Ph1 Plan objective (Carl Zeiss), a JVC KY-F50 camera, and AxioVision 4.5 software (Zeiss).
However, IL-17 level remained low in CD4+ T cells, probably the result of the young age of analyzed mice (15 days old). Therefore, we have confirmed our results by real-time PCR analysis. IL-17 expression was dramatically increased in CD4+ T cells from oc/oc compared with +/+ mice (Figure 6C). These data revealed that Th17 polarization is greatly augmented in oc/oc mice compared with +/+ mice.
IL-17 has been shown to induce RANK-L secretion in osteoblasts and Th17 cells contribute to osteoclastogenesis in inflammatory bone loss.40,41 To assess whether the increased RANK-L expression in stromal cells from oc/oc mice is indeed the result of CD4+ T cells, we have performed cocultures of osteoblasts from +/+ mice with CD4+ T cells from the bone marrow of oc/oc or normal mice (Figure 6D). After removing T cells, real-time PCR analysis was performed on osteoblasts. Rank-l expression was increased 2.5-fold in osteoblasts cocultured with T cells from oc/oc compared with +/+ mice, whereas M-csf expression remained unchanged (Figure 6D).
Finally, formation of OCLs was evaluated in cocultures between DCs and osteoblasts from +/+ mice and phytohemagglutinin-stimulated CD4+ T cells from the bone marrow of oc/oc or +/+ mice. No OCL was generated in the absence of T cells or in the presence of T cells from +/+ mice, and only few OCLs were generated in the presence of T cells from oc/oc mice (not shown). Thus, to increase the stimulation of stromal cells, ascorbic acid and vitamin D were added after 1 week of coculture. The number of OCLs observed without T cells and in the presence of +/+ T cells is not significantly different. Twice more OCLs were generated in the presence of oc/oc T cells compared with +/+ mice (Figure 6E).
Altogether, these results show that CD4+ T cells from oc/oc mice have an inflammatory phenotype and are able to support efficiently the DC-to-OCL differentiation by providing a RANK-L–enriched environment.
Discussion
Our most remarkable result is that injection of normal DCs partially reverted the osteopetrotic phenotype of oc/oc mice by restoring bone resorption activity in vivo. DCs and OCLs are closely related cells in terms of their monocytic origin and ability to respond to common regulatory factors, in particular RANK-L.42 However, contrary to what has been previously described,28 their differentiation is not mutually exclusive because in vitro, human monocyte-derived DCs and murine bone marrow–derived DCs can both give rise to OCLs.15,16 Our results extend these in vitro data by showing that, not only DCs generated in vitro, but also purified splenic DCs efficiently differentiate into OCLs in the presence of M-CSF and RANK-L. Furthermore, this report is the first demonstration that this differentiation can also take place in vivo.
Splenic DCs are composed of various subpopulations based on their phenotype and activity, and we showed that, among them, the CD4−CD8− cDC subset contributes much more efficiently than any other to osteoclastogenesis. Furthermore, DC maturation via activation of Toll-like receptors did not alter their osteoclastogenic potential. Moreover, cDCs are as efficient as monocytes for differentiating into OCLs. This differentiation from both cell types requires RANK-L plus M-CSF, and in response to these factors, both cell types up-regulate the expression of NFATc1, a master gene of osteoclastogenesis.30 Furthermore, GM-CSF completely inhibited the formation of OCLs from cDCs through a down-regulation of Nfatc1, as shown for monocytes.29 Altogether, these data address the question of whether DCs can represent genuine OCL precursors, independently of their maturation status.
DCs are dedicated to capture antigens, undergo maturation, and activate T cells, after which they are thought to undergo apoptosis.43 However, recent studies have shown that the fate of DCs can be influenced by their environment. Splenic stromal cells can drive mature splenic DCs to undergo further differentiation into a different DC subset with regulatory function.13 Moreover, DCs cultured with tumor cell–conditioned media acquire phenotypic properties of endothelial cells.14 Together with our results, these observations suggest that mature DCs are not necessarily terminally differentiated cells and underline their differentiation potential in response to growth and differentiation factor cocktails, in particular those driving osteoclastogenesis.
In agreement with these observations, the presence of OCL-like multinucleated giant cells has been reported in nonbone lesions of Langerhans cell histiocytosis, a pathology characterized by clonal proliferation and retention of Langerhans DCs.44 One hypothesis for the origin of these OCL-like cells has been the osteoclastic differentiation of Langerhans cells induced by a high level of inflammatory cytokines, RANK-L and M-CSF, observed in the lesions.44 Furthermore, immature monocyte-derived DCs give rise to OCLs when treated with RANK-L and M-CSF, and this in vitro differentiation is enhanced by cell-free rheumatoid arthritis synovial fluid enriched in inflammatory and osteoclastogenic factors.15
Indeed, RANK-L and M-CSF are both necessary to induce OCL formation from DCs in vitro, and high levels of RANK-L are required for this formation in vivo. In addition to its effect on OCL differentiation and activity, RANK-L is also an essential cytokine for the activity and survival of DCs both in vitro and in vivo.45 In particular, the longevity of mature DCs pretreated with RANK-L before subcutaneous injection is greatly enhanced in vivo.46 We have shown that injected DCs are able to traffic and home into the bone marrow of oc/oc mice. Thus, the RANK-L–enriched environment present in oc/oc mice bone marrow probably contributes to a higher DC survival in this organ. Furthermore, in oc/oc mice, osteoclastogenesis is greatly enhanced,20 and the particular cytokine profile with high expression of M-csf and Rank-l probably provides an ideal environment for efficient osteoclastogenesis from many potential precursors, including DCs.
Several studies have reported that high RANK-L expression is associated with abnormal T-cell activation leading to the induction of OCLs.47 T cells are probably not required for normal bone homeostasis as T cell–deficient mice have normal bone phenotype. However, abnormal systemic T-cell activation in CTLA4−/− or IL-2−/− mice induces an increased RANK-L–dependent osteoclastogenesis and bone loss.47,48 Our results indicate that inflammatory CD4+ T cells play a role in the differentiation of DCs through maintenance of a high level of RANK-L expression in vivo.
Activation status and Th17 polarization of CD4+ T cells are increased in oc/oc mice, but the reasons for this remain unknown. In addition to a3, the Tcirg1 gene mutated in oc/oc mice encodes another protein called TIRC7 and representing, in mouse, a marker of regulatory T-cell subsets.49 However, because expression of TIRC7 and distribution of TIRC7+ regulatory T cells are not altered in oc/oc mice (A.W., unpublished data, 2006), it is doubtful that the Tcirg1 mutation directly affects T-cell phenotype. More probably, this Th17 phenotype could result from the modifications of the bone microenvironment and cellular interactions resulting from osteopetrosis.
Th17 cells are characterized by IL-17 production and involved in autoimmune and inflammatory diseases.50 Th17 cells and IL-17 are present in inflamed tissues of patients presenting chronic inflammation, as rheumatoid arthritis.51,52 Th17 cells efficiently increase OCL formation41 and IL-17 activates osteoclastogenesis by stimulating RANK-L production by osteoblasts.40 These data are in good agreement with our results on oc/oc CD4+ T cells: they express high levels of RANK-L and are responsible for an increased expression of RANK-L by stromal cells. Moreover, in vitro, they induce the formation of OCLs more efficiently than those from +/+ mice. Increased production of IL-17 by oc/oc CD4+ T cells could probably contribute to their effect on stromal cells. Therefore, these inflammatory CD4+ T cells are very probably to contribute to the increased osteoclastogenesis observed in oc/oc mice and to the differentiation of DCs into OCLs.
In conclusion, these results suggest that differentiation of OCLs from DCs may represent an alternative OCL differentiation pathway induced by high levels of osteoclastogenic factors. They also revealed that inflammatory CD4+T cells are involved in this mechanism through their capacity to produce RANK-L and control its expression by bone marrow stromal cells.
The concept of osteoimmunology has emerged from studies dealing with the crosstalk between bone and immune systems. Until now, this crosstalk has been restricted to soluble factors secreted by activated T cells that induce bone resorption. Here we demonstrate that immune cells can directly differentiate into bone resorbing cells, opening thereby new perspectives both in the biology of DCs and in the relation between bone and immune systems. Furthermore, identification of DCs as OCL precursors has important consequences for the physiopathology and therapy of inflammatory pathologies coupled with bone destruction, such as periodontitis and rheumatoid arthritis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are indebted to Dr I. Mellman for critical comments on the manuscript. We thank M. Topi for animal care and genotyping. We are grateful to the Inspection Générale des Douanes Françaises for the kind gift of ivory samples. We also thank the Department of Radiology and the Department of Radiotherapy of the Center Antoine Lacassagne for animal radiography and irradiation and Dr A. Foussat (TxCell, Biot, France) for the kind gift of the depleting anti-CD4 antibody.
This work was supported by the Association Française contre les Myopathies, the Fondation pour la Recherche Médicale (A.W.), and Inserm (C.B.W.).
Authorship
Contribution: A.W. and C.B.-W. designed the research, performed experimental procedures, analyzed the data, and wrote the paper; A.M. performed in vivo experimental procedures and T-cell characterization; E.C. performed in vivo experimental procedures; R.D. and P.J. performed bone histomorphometric analysis and measurements; and G.F.C. participated in the discussion of the results.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claudine Blin-Wakkach, GEPITOS, Unite Mixte de Recherche 6235, Faculté de Médecine, 28 avenue de Valombrose, 06107 Nice Cedex 02, France; e-mail: blin@unice.fr.
References
Author notes
*G.F.C. and C.B.-W. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal