Abstract
Glucose is a major source of energy for living organisms, and its transport in vertebrates is a universally conserved property. Of all cell lineages, human erythrocytes express the highest level of the Glut1 glucose transporter with more than 200 000 molecules per cell. However, we recently reported that erythrocyte Glut1 expression is a specific trait of vitamin C–deficient mammalian species, comprising only higher primates, guinea pigs, and fruit bats. Here, we show that in all other tested mammalian species, Glut1 was transiently expressed in erythrocytes during the neonatal period. Glut1 was up-regulated during the erythroblast stage of erythroid differentiation and was present on the vast majority of murine red blood cells (RBCs) at birth. Notably though, Glut1 was not induced in adult mice undergoing anemia-induced erythropoiesis, and under these conditions, the up-regulation of a distinct transporter, Glut4, was responsible for an increased glucose transport. Sp3 and Sp1 transcriptions factors have been proposed to regulate Glut1 transcription, and we find that the concomitant repression of Glut1 and induction of Glut4 was associated with a significantly augmented Sp3/Sp1 ratio. Glucose transporter expression patterns in mice and human erythrocytes are therefore distinct. In mice, there is a postnatal switch from Glut1 to Glut4, with Glut4 further up-regulated under anemic conditions.
Introduction
Animals are heterotrophic, and most use sugar as their principal source of carbon. The facilitated diffusion of monosaccharides across the plasma membrane is mediated by specialized transporter molecules. The superfamily of related Glut sugar transporters comprises 14 identified isoforms in the human genome, all adopting a 12-membrane–spanning domain structure that delineate 6 extracellular loops.1 These Glut isoforms are characterized by a moderate binding affinity and a rapid rate of sugar transport.2 The first identified protein of this family, Glut1, is the main functional transporter of glucose in human erythrocytes and lymphocytes as well as the vast majority of transformed cell lines.1,3-5 Moreover, Glut1 also transports L-dehydroascorbic acid (DHA), the 2-electron oxidized intermediate of ascorbic acid, in various cell types.6-10
Of all cell lineages, the human erythrocyte expresses the highest level of the Glut1 transporter, harboring more than 200 000 molecules per cell. Moreover, in the context of the red cell membrane, Glut1 accounts for 10% of the total protein mass.5,11 We recently found that although both glucose and DHA are transported by Glut1 in human erythrocytes,12-14 there is a preferential uptake of DHA in these cells.15 The fact that Glut1 structure is conserved between mammals, birds, and fish indicates that the main features characterizing transport by this superfamily were established at an early phase of vertebrate evolution.10 However, our experiments revealed erythrocyte Glut1 to be unique to those few mammalian species unable to synthesize ascorbic acid from glucose15 (comprising humans, other higher primates, guinea pigs, and fruit bats), strongly suggesting that this feature constitutes a compensatory mechanism specific to mammals that are unable to synthesize vitamin C.
These data raised fundamental questions as to the manner via which glucose is transported into erythrocytes of vitamin C–synthesizing mammals. Our identification of Glut4 on mature erythrocytes of vitamin C–sufficient mice provided a major clue to glucose physiology in nonprimate erythrocytes.15 However, the relative expression and function of Glut1 and Glut4 transporters in physiologic and pathologic states of extensive erythropoiesis, such as during the neonatal period or under conditions of anemia, respectively, remain unknown. Moreover, the regulation of Glut1 and Glut4 during erythropoiesis has never been evaluated.
Here, we show that Glut1 is expressed in newborn erythrocytes of all mammals that we tested. Notably though, erythrocyte Glut1 expression in vitamin C–synthesizing mammals was lost during the neonatal period. This is in marked contrast with humans, where Glut1 is expressed at equivalently high levels in both neonates and adults. Using mice as a paradigm for vitamin C–synthesizing mammals, we determined that the loss of Glut1 from erythrocytes was not due to its shedding in exosomes. Rather, Glut1 and Glut4 expression during erythroblast development was inversely regulated. The induction of Glut4 was associated with a 4-fold increase in the relative ratio of the Sp3 to Sp1 zinc-finger transcription factors. Furthermore, anemia-induced erythropoiesis in adult mice did not result in the reappearance of Glut1; in contrast, it resulted in a significantly augmented Glut4 expression with a concomitant increase in glucose transport. Thus, the expression of distinct glucose transporters in nonhuman erythrocytes, regulated at the transcriptional level, characterizes different states of erythroid development and differentiation.
Methods
Induction of anemia
Anemia was induced in adult C57BL/6 mice with a baseline hematocrit of at least 35%, either by injection of phenylhydrazine or phlebotomy. For the former, mice were injected subcutaneously on days 0, 1, and 3 with 40 mg/kg phenylhydrazine hydrochloride solution in phosphate-buffered saline (PBS). In the case of phlebotomy, 500 μL blood was removed from the retro-orbital vein on days 0, 1, and 3. Hematocrits were measured in retroorbital samples on days 0, 3, and 6. Animals were killed on day 6 for splenocytes analysis. This study received Institutional Review Board approval for the use of animals from the Montpellier animal network (“Reseau Animalerie de Montpellier [RAM]”) and the Boisbonne large animal facility in Nantes, France.
Flow cytometry
Surface expression of CD71 and Ter119 was monitored by incubating cells with the appropriate fluorochrome-conjugated mAbs (Becton Dickinson, San Jose, CA) for 20 minutes on ice. Background fluorescence was measured using isotype-matched irrelevant antibodies. Surface Glut1 expression was monitored by binding to its ligand, the receptor-binding domain of a recombinant envelope glycoprotein from the human T lymphotrophic virus (HTLV)16 fused to the enhanced green fluorescent protein (EGFP) coding sequence (HRBDEGFP)17 as previously described.18,19 Cells were washed with PBS and analyzed on a FACSCalibur flow cytometer (Becton Dickinson). Data analyses were performed using CellQuest Pro (Becton Dickinson) and FlowJo (TreeStar, Ashland, OR) softwares.
Isolation of RBCs and RBC precursors
Red blood cells (RBCs) from various mammalian species (mouse, rat, cow, and dog) were obtained from heparinized blood samples in accordance with local animal facility institutional review board regulations. Murine neonates were decapitated, and blood was obtained directly in heparinized microhematocrit tubes. Older animals were bled from the retro-orbital vein.
For isolation of RBC precursors, freshly dissociated murine splenocytes were washed in PBS containing 2% fetal calf serum (FCS) and incubated on ice for 20 minutes with a rat anti-mouse Ter119 antibody (Becton Dickinson) at a 1:500 dilution. Cells were then incubated with anti-rat IgG microbeads (Dynal, Oslo, Norway) according to the manufacturer's instructions and positively selected by magnetic separation (Dynal). Cells were washed and immediately frozen as a dry pellet. Erythroid progenitors at different stages were fluorescence-activated cell sorter (FACS)–sorted following staining with Ter119 and CD71 as indicated (FACS ARIA; BD Biosciences).
To isolate Glut1+ and Glut1− erythroid precursors, splenocytes were first washed in PBS/2% FCS and incubated at 37°C for 30 minutes with the HRBD EGFP ligand, as described in “Flow cytometry.” Cells were then washed and stained with anti-Ter119 and CD71 mAbs prior to sorting. GFP+ and GFP− cells within the Ter119+/CD71med population were sorted on a FACSARIA (BD Biosciences).
Cell maturation and exosome isolation
Plasma was separated from RBCs by centrifugation (3000g for 5 minutes). RBCs were then washed 3 times in Ringer buffer and matured for 48 hours at 37°C in RPMI 1640 supplemented with 5 mM glutamine, 5 mM adenosine, 10 mM inosine, 3% exosome-free fetal calf serum, 50 U/mL penicillin, and 50 μg/mL streptomycin at a concentration of 30 μL packed cell volume per milliliter as previously described.20 Under conditions where the proteasome degradation pathway was inhibited, cells were cultured in the same maturation conditions in the presence of either 10 μM MG132 or 8 μM lactacystine (Calbiochem, San Diego, CA). Control experiments were performed in the presence of equivalent volumes of DMSO solvent. After 48 hours, cells were pelleted and the culture supernatant was centrifuged (20 000g for 20 minutes) to remove cellular debris. Exosomes were separated from the supernatant by ultracentrifugation (100 000g for 2 hours) and resuspended in PBS.
Quantitative analysis of mRNA levels
Total RNA was isolated from Ter119+ splenocytes or sorted cells using the GenElute Mammalian Total RNA Miniprep Kit (Sigma-Aldrich, St Louis, MO). cDNAs were prepared by reverse transcription and quantitative polymerase chain reaction (PCR) was performed using the Quantitect SYBR green PCR Master mix (Qiagen, Valencia, CA) with 2 μL cDNA in a final volume of 20 μL and the following primers at a final concentration of 500 nM. Primers for GAPDH were 5′-AACTTTGGCATTGTGGAAGG-3′ (forward) and 5′-ACACATTGGGGGTAGGAACA-3′ (reverse). Primers for Glut1 were 5′-GCTGTGCTTATGGGCTTCTC-3′ (forward) and 5′-CACATACATGGGCACAAAGC-3′. Primers for Glut4 were 5′-ACATACCTGACAGGGCAAGG-3′ (forward) and 5′-CGCCCTTAGTTGGTCAGAAG-3′ (reverse). Primers for actin were 5′-GAGACCTTCAACACCCCAGCC-3′(forward) and 5′-GGAGAGCATAGCCCTCGTAG-3′(reverse). Primers for SP1 were 5′-CTCTGGTGGGCAGTATGTTG-3′ (forward) and 5′-TTGGTTTGCACCTGGTATGA-3′(reverse). Primers for SP3 were 5′-ACGCTCAGCAGGTTCAGAT-3′ (forward) and 5′-AGCCACCAATTGCAACTCCC-3′ (reverse).
Amplification of Glut1, Glut4, actin, GAPDH, SP1, and SP3 cDNAs was performed using the LightCycler 2000 instrument (Roche, Indianapolis, IN). The cycling conditions comprised a denaturation step for 15 minutes at 95°C, followed by 40 cycles of denaturation (95°C for 15 seconds), annealing (59°C for 20 seconds), and extension (72°C for 15 seconds). After amplification, a melting curve analysis was performed with denaturation at 95°C for 5 seconds, then continuous fluorescence measurement from 70°C to 95°C at 0.1°C/second. Each sample was amplified in duplicate.
Glucose uptake
RBCs (10 × 106) were incubated in serum/glucose-free RPMI medium 1640 for 30 minutes in the presence or not of 100 μM cytochalasin B (CytB) or CytD. Cells were then washed and resuspended in 50 μL serum/glucose-free RPMI 1640 medium. Glucose uptake was initiated by addition of labeled 2-deoxy-D[1-3H]glucose (GE Healthcare, Little Chalfont, UK) to a final concentration of 0.5 μM (2 μCi [0.074 MBq]). Cells were incubated for 10 minutes at room temperature and washed in cold serum/glucose-free RPMI 1640 medium. Uptake was stopped by addition of ice-cold medium. Cells were then washed twice and solubilized in 500 μL 0.1% SDS. 3H incorporation in individual samples was counted by liquid scintillation.
Immunoblots
Nonboiled cell lysates were electrophoresed on SDS–10% acrylamide gels, transferred, and probed with the anti–carboxy-terminal polyclonal Glut1 antibody (1:10 000) kindly provided by A. Carruthers (University of Massachusetts, Worcester), the rabbit polyclonal anti-Glut4 antibody (Abcam, Cambridge, UK), the rabbit polyclonal anti-actin antibody (Sigma-Aldrich), the antitransferrin receptor (CD71) mAb raised against the cytoplasmic tail (Zymed Laboratories, South San Francisco, CA), or an antiactin antibody. Blots were then stained with a peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin, as indicated, and proteins were visualized using the ECLplus Kit (GE Healthcare). Band intensities were quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Results
Postnatal loss of erythrocyte Glut1 in mammals but not in humans
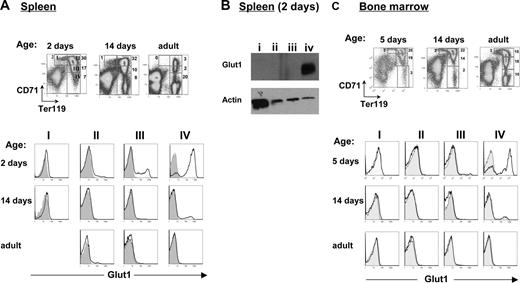
Erythrocyte sugar transport is significantly higher in humans than in other species, and this was long assumed to be due to a higher Glut1 density on human RBCs.21 However, we recently determined that the situation is more complex, as we could not detect Glut1 in RBCs isolated from adult mice even though it was readily detectable in newborn murine erythrocytes.15 Here, we have extended these data and found that in humans, Glut1 is expressed at high levels on both newborn and adult RBCs (Figure 1A), whereas in mice, it becomes detectable on less than 5% of erythrocytes by day 16 of life (Figure 1B). Notably, during the perinatal period, Glut1 was expressed at high levels on erythrocytes from all mammalian species which we tested, including mice, rats, cows, and dogs (Figure 1B). These species all shared the feature that erythrocyte Glut1 was lost before adulthood, albeit with slightly different kinetics.
Glut1 is highly expressed on erythrocytes of all mammalian species during the neonatal period. (A) Surface Glut1 expression was assessed on neonatal and adult human RBCs obtained from umbilical cord and peripheral blood samples, respectively, using an eGFP-tagged HTLV receptor-binding domain (HRBD) fusion protein17 that specifically binds this transporter.16 (B) Glut1 expression was monitored on murine, rat, bovine, and canine erythrocytes at the indicated day of life. Control stainings are presented as shaded histograms.
Glut1 is highly expressed on erythrocytes of all mammalian species during the neonatal period. (A) Surface Glut1 expression was assessed on neonatal and adult human RBCs obtained from umbilical cord and peripheral blood samples, respectively, using an eGFP-tagged HTLV receptor-binding domain (HRBD) fusion protein17 that specifically binds this transporter.16 (B) Glut1 expression was monitored on murine, rat, bovine, and canine erythrocytes at the indicated day of life. Control stainings are presented as shaded histograms.
Given the high level of erythropoiesis during the neonatal period, these data raised the question as to whether RBC Glut1 expression in nonhuman mammalian species is associated with a specific stage of erythroid differentiation. We therefore evaluated the expression of Glut1 in murine progenitors using the erythroid precursor classification generated by Lodish and colleagues,22 based on differential CD71 and Ter119 expression. These markers were used to distinguish successive stages of erythroid maturation; proerythroblasts, basophilic erythroblasts, polychromatic erythroblasts, and acidophilic erythroblasts, presented as regions I to IV, respectively, in Figure 2A (left panel). Notably, Glut1 was expressed in late erythroblast fractions in both spleen and bone marrow, with the highest percentage of surface Glut1+ cells in the late acidophilic erythroblast fraction (region IV; Figure 2A). This was also confirmed at the level of total protein: Glut1 was only detected in region IV by immunoblot analysis using a polyclonal anti-Glut1 antibody (Figure 2B). While the overall percentages of erythroid precursors in both the spleen and bone marrow (BM) decreased following birth, Ter119+ progenitors could be detected at all time points (Figure 2A,C top panels). Notably, however, Glut1 expression in the Ter119+CD71lo acidophilic erythroblasts (region IV) was only detected during the neonatal period with less than 5% of Glut1+ cells at 14 days of age, regardless of whether this parameter was evaluated in the spleen or BM. These data demonstrate that the loss of Glut1 expression is not related to changes in the specific stages of erythroid differentiation per se, at least under physiologic conditions of erythropoiesis.
Glut1 expression during erythroblast maturation is age-dependent. (A) Freshly isolated splenocytes obtained from 2-day-old, 14-day-old, and adult mice were stained with CD71 and Ter119 antibodies; density plots are shown. The percentages of cells in each region indicated in each plot, broadly correspond to proerythroblasts (region I), basophilic erythroblasts (region II), late basophilic/polychromatophilic erythroblasts (region III), and orthochromatophilic erythroblasts (region IV), as previously described.22 The relative expression of Glut1 in each of the 4 regions was determined by gating on the identified CD71/Ter119 populations. Shaded histograms show nonspecific staining. (B) At 2 days of age, splenic cells from regions I to IV were FACS-sorted as indicated in the dot plots in panel A. Total levels of Glut1 in each population were assessed by immunoblotting with a polyclonal anti-Glut1 antibody. Protein levels in each lane were controlled by staining with an antiactin antibody. (C) Erythroblast maturation in the bone marrows of 5-day-old, 14-day-old, and adult mice was assessed as described. The relative percentages of erythroid precursors in regions I to IV are indicated in dot plots showing CD71 staining as a function of Ter119. At each age, histograms showing surface Glut1 staining in the 4 regions are presented.
Glut1 expression during erythroblast maturation is age-dependent. (A) Freshly isolated splenocytes obtained from 2-day-old, 14-day-old, and adult mice were stained with CD71 and Ter119 antibodies; density plots are shown. The percentages of cells in each region indicated in each plot, broadly correspond to proerythroblasts (region I), basophilic erythroblasts (region II), late basophilic/polychromatophilic erythroblasts (region III), and orthochromatophilic erythroblasts (region IV), as previously described.22 The relative expression of Glut1 in each of the 4 regions was determined by gating on the identified CD71/Ter119 populations. Shaded histograms show nonspecific staining. (B) At 2 days of age, splenic cells from regions I to IV were FACS-sorted as indicated in the dot plots in panel A. Total levels of Glut1 in each population were assessed by immunoblotting with a polyclonal anti-Glut1 antibody. Protein levels in each lane were controlled by staining with an antiactin antibody. (C) Erythroblast maturation in the bone marrows of 5-day-old, 14-day-old, and adult mice was assessed as described. The relative percentages of erythroid precursors in regions I to IV are indicated in dot plots showing CD71 staining as a function of Ter119. At each age, histograms showing surface Glut1 staining in the 4 regions are presented.
Loss of Glut1 expression in murine erythrocytes is not due to sorting of the transporter into exosomes
The maturation of a reticulocyte to an erythrocyte is accompanied by the secretion of exosomes that originate from the fusion of multivesicular bodies with the plasma membrane. Proteins such as the transferrin receptor (CD71; TfR), which are highly expressed on reticulocytes (approximately 100 000 molecules per cell), are completely lost during maturation via the exosomal pathway.23,24 Moreover, during maturation of sheep reticulocytes, CytB binding, a measure of the presence of Glut-type glucose transporters, has been detected in exosomes.24 Therefore, we evaluated whether the loss of Glut1 was due to its shedding in exosomes during perinatal reticulocyte maturation.
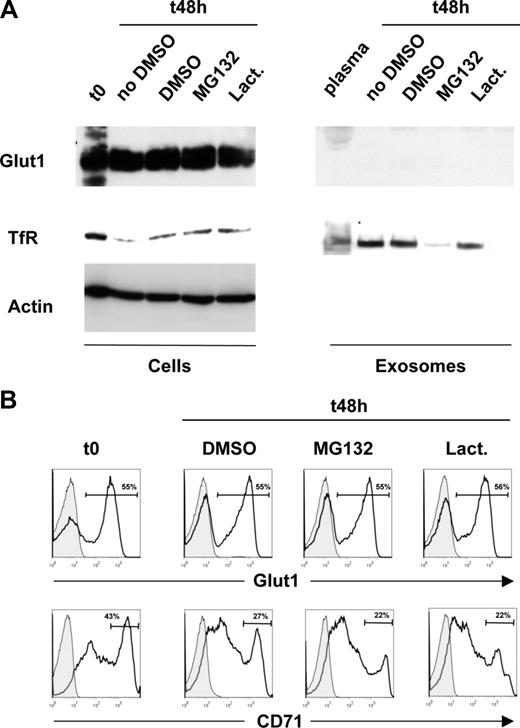
To test this hypothesis, RBCs were obtained from the peripheral circulation of 5-day-old C57BL/6 mice, a time point at which the percentage of reticulocytes is approximately 10%.25 Maturation was induced by ex vivo culture as previously described.23 As expected from previous studies,24 reticulocyte maturation was associated with a loss of total as well as surface TfR expression, but notably, neither total Glut1 levels nor surface Glut1 expression decreased (Figure 3A left panels,B). The presence of proteasome inhibitors MG132 or lactacystine also did not modulate Glut1 protein levels during reticulocyte maturation. Furthermore, Glut1 was not detectable in exosomes isolated from the plasma of neonatal mice or from exosomes obtained after ex vivo maturation of reticulocytes. In marked contrast, Western blot analyses demonstrated the presence of TfR in exosomes isolated under all of the aforementioned conditions (Figure 3A). As previously described, MG132 reduced TfR secretion into exosomes,26 but again, neither proteasome inhibitor modulated Glut1 levels. Altogether, these data demonstrate that in contrast to TfR, Glut1 is not released from reticulocytes via exosomes.
Glut1 expression is maintained during reticulocyte maturation and is not released in exosomes. (A) Red blood cells were obtained from 5-day-old mice (t0,) and reticulocyte maturation was induced by ex vivo culture (t48) in the absence or presence of the proteasome inhibitors MG132 and lactacystine (lact) or the DMSO solvent as a control. The levels of Glut1 and transferrin receptor (TfR) were monitored in all cell samples by immunoblot analysis using specific polyclonal and monoclonal antibodies, respectively (left panels). Exosomes from the plasma of 5-day-old mice as well as from 48-hour ex vivo–differentiated reticulocyte cultures described in panel A were obtained by differential centrifugation. Glut1 and TfR levels were monitored by immunoblot. (B) Surface Glut1 and TfR expression was monitored in freshly isolated RBCs from 5-day-old mice as well as following the 48-hour ex vivo maturation in the absence (DMSO) or presence of the proteasome inhibitors indicated. The percentage of Glut1+ and TfRhi cells is indicated in each histogram.
Glut1 expression is maintained during reticulocyte maturation and is not released in exosomes. (A) Red blood cells were obtained from 5-day-old mice (t0,) and reticulocyte maturation was induced by ex vivo culture (t48) in the absence or presence of the proteasome inhibitors MG132 and lactacystine (lact) or the DMSO solvent as a control. The levels of Glut1 and transferrin receptor (TfR) were monitored in all cell samples by immunoblot analysis using specific polyclonal and monoclonal antibodies, respectively (left panels). Exosomes from the plasma of 5-day-old mice as well as from 48-hour ex vivo–differentiated reticulocyte cultures described in panel A were obtained by differential centrifugation. Glut1 and TfR levels were monitored by immunoblot. (B) Surface Glut1 and TfR expression was monitored in freshly isolated RBCs from 5-day-old mice as well as following the 48-hour ex vivo maturation in the absence (DMSO) or presence of the proteasome inhibitors indicated. The percentage of Glut1+ and TfRhi cells is indicated in each histogram.
Anemia-induced erythropoiesis in adult mice is associated with an up-regulation of Glut4 but not Glut1
Thus far, the data presented indicate that Glut1 is expressed in murine erythrocytes during the neonatal period, and the loss of this transporter during postnatal life is not due to its shedding in exosomes. It was therefore important to determine whether erythrocyte Glut1 expression in mice is specifically associated with newborn erythropoiesis or, alternatively, is common to any physiologic state where an extensive erythropoiesis is induced.
We therefore subjected adult mice to a chemically induced hemolytic anemia by injection of phenylhydrazine (PHZ) or provoked anemia by serial phlebotomy. Adult mice controls have a hematocrit of at least 40% with a reticulocyte count less than 2%. Following induction of anemia, hematocrits fell sharply to 25% to 30% on day 6, with reticulocytes increasing to 60% of total RBCs, as previously described.22 The severity of the anemia was more extreme in PHZ-treated mice compared with those treated by phlebotomy. The spleen is the major site of hematopoiesis in mice, and the increased erythropoiesis was appreciated by a more pronounced splenomegaly in PHZ-treated animals after 6 days of treatment (Figure 4A). As shown in Figure 4B, the percentage of splenic Ter119+ erythroid precursors increased from 15% in control mice to 75% in PHZ-treated mice and 40% in mice undergoing phlebotomy. Notably though, despite the massive increase in both early and late erythroid progenitors, surface Glut1 expression was not detected on any of these populations (Figure 4B). Moreover, Glut1 was also not detected in peripheral RBCs of these anemic mice as assessed by immunoblot, despite high transporter levels in neonatal RBCs (Figure 4C).
Induction of anemia in adult mice does not result in the expression of Glut1 on differentiating erythroblasts. (A) The spleen of an adult mouse (control) is shown in comparison with those obtained from mice wherein anemia was induced either by phenylhydrazine treatment or phlebotomy of 0.5 mL blood on days 0, 1, and 3. Spleens were obtained from killed mice at day 6 after treatment. (B) Erythroblast differentiation was assessed in freshly isolated splenocytes obtained from control adult mice; 6 days following induction of anemia as indicated, differentiation was assessed by staining with CD71 and Ter119 antibodies. The percentages of cells in each region (regions I to IV) are indicated in the respective dot plot. The relative expression of Glut1 in each of the 4 regions was determined by gating on the identified CD71/Ter119 populations. Shaded histograms show nonspecific staining. (C) Glut1 expression in peripheral red blood cells from newborn mice, adult mice, and adult mice rendered anemic by phenylhydrazine (PHZ) treatment (mice numbered 1 and 2) was assessed by immunoblotting using an anti-Glut1 pAb. Protein loading was monitored by immunoblotting for actin. (D) Glut1 transcripts were assessed in Ter119+ splenic erythroid progenitors isolated from newborn, adult, and PHZ-treated anemic mice (numbered 4 to 6; day 6), as indicated, by qRT-PCR. cDNAs were amplified with primers specific for Glut1 and means plus SD of duplicate samples normalized to GAPDH are shown.
Induction of anemia in adult mice does not result in the expression of Glut1 on differentiating erythroblasts. (A) The spleen of an adult mouse (control) is shown in comparison with those obtained from mice wherein anemia was induced either by phenylhydrazine treatment or phlebotomy of 0.5 mL blood on days 0, 1, and 3. Spleens were obtained from killed mice at day 6 after treatment. (B) Erythroblast differentiation was assessed in freshly isolated splenocytes obtained from control adult mice; 6 days following induction of anemia as indicated, differentiation was assessed by staining with CD71 and Ter119 antibodies. The percentages of cells in each region (regions I to IV) are indicated in the respective dot plot. The relative expression of Glut1 in each of the 4 regions was determined by gating on the identified CD71/Ter119 populations. Shaded histograms show nonspecific staining. (C) Glut1 expression in peripheral red blood cells from newborn mice, adult mice, and adult mice rendered anemic by phenylhydrazine (PHZ) treatment (mice numbered 1 and 2) was assessed by immunoblotting using an anti-Glut1 pAb. Protein loading was monitored by immunoblotting for actin. (D) Glut1 transcripts were assessed in Ter119+ splenic erythroid progenitors isolated from newborn, adult, and PHZ-treated anemic mice (numbered 4 to 6; day 6), as indicated, by qRT-PCR. cDNAs were amplified with primers specific for Glut1 and means plus SD of duplicate samples normalized to GAPDH are shown.
Our inability to detect Glut1 in erythrocyte precursors or circulating RBCs in anemic mice using 2 independent reagents strongly suggests that this transporter is not expressed under these conditions. Nevertheless, it could be argued that Glut1 is present but that its conformation in conditions of anemia is modulated, precluding detection by these reagents. We therefore monitored Glut1 RNA levels in sorted Ter119+ erythroid precursors from spleens of anemic mice. Glut1 mRNA, as assessed by quantitative reverse transcriptase (qRT)–PCR, was at the limits of detection in these erythroid precursors and was not elevated compared with transcripts in control adult mice. Notably though, Glut1 mRNA levels were more than 30-fold higher in erythroid progenitors in neonates as compared with anemic mice (Figure 4D), consistent with the high protein expression detected in the former cells. The ensemble of these experiments demonstrates that Glut1 is specifically expressed on neonatal RBCs and is not induced under conditions of pathologic erythropoiesis in adult mice.
Erythropoiesis in neonatal mice is associated with an increased glucose uptake,15 likely due to the high levels of Glut1 on these cells. Intriguingly, we found that glucose uptake by peripheral RBCs from PHZ-anemic mice was significantly elevated as compared with control adult RBCs, as assessed by monitoring transport of the nonhydrolyzable 2-deoxy-D[1-3H]glucose (2-DG) analog (Figure 5A). This was somewhat surprising given the lack of detectable Glut1 on these cells. To assess whether the enhanced glucose transport was due to a Glut-type transporter, we treated these RBCs with CytB, a molecule that directly binds to the conserved sugar export site on this family of transporters.27,28 Importantly, glucose transport in RBCs from anemic mice was inhibited by greater than 90% in the presence of CytB (Figure 5B).
Increased glucose uptake in erythroblasts of adult anemic mice is associated with enhanced Glut4 expression. (A) Glucose uptake was assessed in peripheral RBCs isolated from 3 different control adult mice as well as 4 adult mice rendered anemic by PHZ treatment (day 6). Uptake of the nonhydrolyzable glucose analog 2-DG (0.5 μM [2 μCi; 0.074 MBq]) was assayed during a 10-minute uptake at room temperature. All data are presented as mean cpm plus SD of triplicate samples. (B) RBCs from 2 different anemic mice were pretreated in the absence or presence of the Glut inhibitor CytB or the related CytD molecule for 30 minutes. [3H]2-DG uptake was then assayed as in panel A. Relative uptakes are presented, with glucose and DHA uptake in nontreated human erythrocytes defined as 100%. (C) Glut4 protein levels were assessed in RBCs isolated from mice rendered anemic by either PHZ treatment or phlebotomy. Peripheral blood was obtained at day 3 (D3) and day 6 (D6) after the start of treatment; 2 samples are shown for each condition. RBCs from adult mice were used as a control, and protein loading in each sample was monitored by actin staining. The levels of Glut4 relative to actin in each sample were determined by comparison of the respective signals using ImageJ software.
Increased glucose uptake in erythroblasts of adult anemic mice is associated with enhanced Glut4 expression. (A) Glucose uptake was assessed in peripheral RBCs isolated from 3 different control adult mice as well as 4 adult mice rendered anemic by PHZ treatment (day 6). Uptake of the nonhydrolyzable glucose analog 2-DG (0.5 μM [2 μCi; 0.074 MBq]) was assayed during a 10-minute uptake at room temperature. All data are presented as mean cpm plus SD of triplicate samples. (B) RBCs from 2 different anemic mice were pretreated in the absence or presence of the Glut inhibitor CytB or the related CytD molecule for 30 minutes. [3H]2-DG uptake was then assayed as in panel A. Relative uptakes are presented, with glucose and DHA uptake in nontreated human erythrocytes defined as 100%. (C) Glut4 protein levels were assessed in RBCs isolated from mice rendered anemic by either PHZ treatment or phlebotomy. Peripheral blood was obtained at day 3 (D3) and day 6 (D6) after the start of treatment; 2 samples are shown for each condition. RBCs from adult mice were used as a control, and protein loading in each sample was monitored by actin staining. The levels of Glut4 relative to actin in each sample were determined by comparison of the respective signals using ImageJ software.
These data strongly supported a role for another Glut family member in the augmented glucose transport observed in the RBCs of anemic mice. Based on previous observations,15 we next monitored Glut4 protein levels in RBCs of control adult mice compared with anemic mice. Erythrocyte Glut4 protein levels were significantly increased in anemic mice, with an augmentation of 6-fold by day 6 following induction of anemia. Moreover, Glut4 levels were higher in the PHZ-treated animals compared with the phlebotomized mice, with the former displaying a much more severe anemia (Figure 4A; data not shown). Thus, outside the neonatal period, murine erythropoiesis is associated with an increased expression of the Glut4 glucose transporter.
Regulation of Glut1 and Glut4 expression in murine erythrocytes is associated with an altered Sp3/Sp1 profile
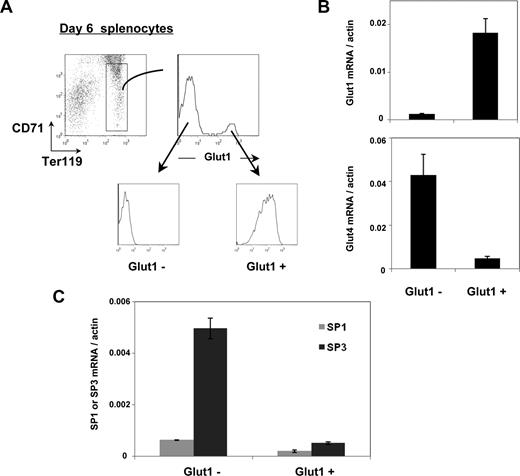
The data presented here suggested that expression of Glut1 and Glut4 are conversely regulated. To gain additional insights into the relative expression of these 2 glucose transporters, we isolated Ter119+/CD71med/lo erythroid precursors from neonatal (6-day-old) mice. Within this population, cells harboring Glut1 at their surface were distinguished from the Glut1− population (Figure 6A). Glut1 mRNA in these 2 populations, as assessed by qRT-PCR, tightly corresponded with protein expression; the relative level of Glut1 mRNA was 15-fold higher in the Glut1+ erythroid precursors than in the Glut1− precursors. Even more striking, the presence of Glut4 transcripts was inversely proportional to that of Glut1, with a 9-fold higher expression in Glut1− precursors (Figure 6B).
Inverse relationship between Glut1 and Glut4 expression during neonatal erythropoiesis is associated with a decreased SP3/SP1 ratio. (A) Ter119+/CD71lo erythroblasts isolated from splenocytes of 6-day-old mice are indicated in the dot plot and surface Glut1 expression was assessed as shown in the histogram. Glut1+ and Glut1− erythroblasts were FACS-sorted and the Glut1 profiles of the 2 sorted populations are shown. (B) Glut1 and Glut4 mRNA levels in the sorted Glut1+ and Glut1− erythroblasts were assessed by qRT-PCR using the respective primers and normalized to actin. Means plus SD are shown. (C) Expression of the Sp1 and Sp3 zinc-finger transcription factors was assessed in the Glut1− and Glut1+ erythroblast subsets by qRT-PCR and normalized to actin. Means plus SD are shown.
Inverse relationship between Glut1 and Glut4 expression during neonatal erythropoiesis is associated with a decreased SP3/SP1 ratio. (A) Ter119+/CD71lo erythroblasts isolated from splenocytes of 6-day-old mice are indicated in the dot plot and surface Glut1 expression was assessed as shown in the histogram. Glut1+ and Glut1− erythroblasts were FACS-sorted and the Glut1 profiles of the 2 sorted populations are shown. (B) Glut1 and Glut4 mRNA levels in the sorted Glut1+ and Glut1− erythroblasts were assessed by qRT-PCR using the respective primers and normalized to actin. Means plus SD are shown. (C) Expression of the Sp1 and Sp3 zinc-finger transcription factors was assessed in the Glut1− and Glut1+ erythroblast subsets by qRT-PCR and normalized to actin. Means plus SD are shown.
Previously published research has shown that in muscle cells, the Sp3 zinc-finger transcription factor directly binds to the Glut1 proximal promoter, inhibiting its transcription.29 Moreover, expression of the related Sp1 transcription factor enhances expression of Glut1 in muscle cells30 and Glut4 in adipocytes.31 While no studies assessing the regulation of Glut1 in murine erythrocytes have been reported, the implication of these factors in nonerythroid cells led us to assess their expression in erythroid precursor subsets. Sp3 was detected at only very low levels in Glut1+ erythroid precursors but was highly expressed (at 10-fold higher levels) in Glut1− progenitors (Figure 6C). Variations in the Sp3/Sp1 ratio have been proposed to regulate Glut1 expression during myogenesis, with higher ratios resulting in a suppression of Glut1 promoter activity.29 Indeed, the Sp3/Sp1 ratio was 4-fold higher in Glut1− than Glut1+ precursors (Figure 6C). Changes in Sp3/Sp1 are therefore tightly correlated with the glucose transporter phenotype of murine erythrocytes.
Discussion
The present study demonstrates that erythrocytes from humans and most nonprimate mammalian species can be distinguished on the basis of their glucose transporter expression profile. We recently found that only erythrocytes of vitamin C–defective mammals maintain Glut1 expression on RBC erythrocytes.15 Here, we show that in other mammalian species, Glut1 is expressed during the perinatal period but is rapidly lost following birth. Using mice as a paradigm for nonprimate mammals, we find that Glut1 is up-regulated during perinatal erythropoiesis, but Glut4 rapidly becomes the sole glucose transporter. This switch is not related to a decrease in postnatal erythropoiesis per se, as massive anemia-mediated erythropoiesis in adult mice resulted in enhanced Glut4 expression without any induction of Glut1. This switch was associated with changes in the expression of Sp1 and Sp3 transcription factors: Glut1− erythroblasts express a 10-fold higher level of the inhibitory Sp3 factor compared with Glut1+ erythroblasts. Thus, there is a distinct regulation of Glut1 and Glut4 glucose transporters in human and murine RBCs.
In this study, we find that more than 90% of all circulating erythrocytes in newborn mice express Glut1. At birth, the vast majority of murine erythrocytes are generated from definitive erythroid progenitors in the fetal liver. While primitive erythroblasts continue to circulate, they account for less than 1 in 1000 erythroid lineage cells.32,33 Glut1 does not appear to be differentially regulated in primitive and definitive erythroblasts, as there was no correlation between the absence or presence of betaH1 globin,32,34 a marker of the former, and Glut1 (A.M.-H., unpublished observations, March 2008). Thus, expression of the Glut1 glucose transporter is a characteristic of those erythrocytes that differentiated in the fetal liver.
Notably though, the loss of Glut1 on erythrocytes of all the vitamin C–producing mammals assessed here was extremely rapid. In mice, less than 30% and 5% of erythrocytes harbored Glut1 at days 9 and 16 of life, respectively. Several nonexclusive possibilities can account for this dramatic change. One possibility that we evaluated is that Glut1 is shed during postnatal maturation. This hypothesis is excluded by our finding that neither total nor cell-surface Glut1 expression changed upon neonatal RBC maturation, even though this process resulted in decreased transferrin receptor levels. Moreover, while the TfR, known to be shed during reticulocyte maturation, was present at high levels in exosomes, Glut1 protein was not detected.
The rapid loss of Glut1+ erythrocytes from the circulation may be due to 2 related phenomena: their rapid death after birth and/or the dilution of fetal liver-derived Glut1+ erythrocytes with Glut1− erythrocytes formed in other organs. Notably, after birth, the site of definitive erythropoiesis shifts from the fetal liver to the BM with extensive erythropoiesis in the spleen induced in response to high erythropoietic requirements. Indeed, we found that the percentage of splenic erythroid progenitors decreased with age, from 55% at birth to 25% in adults, while the percentage of BM erythroid precursors remained stable over time, at approximately 50% (Figure 2). Nevertheless, cell death alone is unlikely to account for the massive decrease in Glut1+ erythrocytes by day 13 of life, as it has been shown that the life span of fetal erythrocytes, while lower than that of adult RBCs, rapidly approaches adult levels by birth.35 It is therefore much more probable that the loss of fetal liver–derived Glut1+ RBCs is due to their dilution with postnatally differentiated Glut1− RBCs, concordant with a blood volume that massively expands from 0.1 mL in neonates to 1.5 mL in adult mice.35
Regardless of the process(es) accounting for the postnatal decrease in Glut1+ RBCs, our data indicate that glucose transporter expression is distinctly regulated during fetal and postnatal life. However, stress-induced erythropoiesis in the adult spleen shares common features with fetal liver erythropoiesis, allowing a rapid reaction to increasing erythropoietic needs.36,37 As such, we were interested in assessing whether an extensive splenic erythropoiesis in adult mice would be associated with an up-regulation of Glut1 as observed in fetal liver–derived erythroblasts. Surprisingly, Glut1 was not detected on the high number of erythroblasts differentiating in response to anemia (Figure 4). Anemia-induced erythropoiesis did result in a significant increase in glucose uptake by erythroid-lineage cells, but this phenomenon was associated with a 6-fold augmentation in the Glut4 glucose transporter. Thus, Glut4 is a central postnatal erythroid glucose transporter in mice under both physiologic and pathologic conditions of erythropoiesis.
While both Glut1 and Glut4 transport glucose with high affinity, their properties of transport differ. Glucose transport under equilibrium exchange conditions show a K(m) for Glut4 which is 3- to 12-fold lower than that of Glut138-40 but under zero-trans conditions, Glut4 has a higher K(m) than Glut1.41-43 Glut1 and Glut4 also differ in that only glucose transport mediated by Glut1 demonstrates an accelerated exchange with a Vmax that is 9-fold higher under conditions of equilibrated exchange as compared with zero-trans influx.43 Nonetheless, it is important to note that it is not clear how these in vitro characteristics of glucose transport modulate the in vivo physiologic properties of RBCs expressing one or another of these transporters.
The role of glucose transporters in the architecture of the erythrocyte also needs to be considered. Glut1 associates with stomatin,44-46 a cholesterol-binding, structural/scaffolding protein that forms large oligomeric complexes associated with cholesterol-rich membrane domains (reviewed in Salzer et al47 ). Moreover, the interaction of this lipid raft–associated membrane protein with Glut1 regulates the distinctive transport properties of Glut1 in human erythrocytes.15,48 While a deficiency in stomatin is not in itself responsible for the physiology of overhydrated stomatocytosis,49 its absence in this erythrocyte disorder may condition the differential recruitment of channel and transporter glycoproteins to structured membrane microdomains. Glut4 has not been reported to interact with stomatin, but it has been shown to interact with another stomatin-like protein that is highly expressed in RBCs, flotillin-1.50-52 Altogether, these data point to the formation of distinct transporter complexes in human and murine RBCs, complexes that constitute a significant percentage of the membrane mass. Thus, this parameter needs to be considered in comparative intraspecies erythroid studies.
The regulation of the Glut4 transporter in erythroid lineages cells is completely unknown. However, it is notable that insulin, regulating cell-surface Glut4 localization in adipose tissue and muscle cells,53-56 has long been known to be important in erythroid differentiation and, more specifically, in late stages of differentiation.57-59 Thus, it will be of interest to determine whether the actions of insulin in erythropoiesis are related to its role in the regulation of Glut4 expression and translocation. It was nonetheless surprising to identify Glut4 as responsible for glucose uptake in murine erythrocytes because this transporter is generally found in intracellular compartments and is exocytosed to the plasma membrane only following insulin stimulation. While the targeting signals within Glut4 that regulate its subcellular localization have still not been clearly identified,60 a recent study has shown the importance of Rab31, a Rab5 subfamily GTPase, in Glut4 trafficking.61 At least in humans, mature erythrocytes express functional insulin receptors, as demonstrated by increased calcium flux and tyrosine phosphorylation in response to insulin.62-64 It remains to be determined whether Glut4 function in murine RBCs is altered by this stimulus.
As this report and our precedent study are the first to identify Glut4 in erythrocytes,15 the relative regulation of Glut1 and Glut4 in this cell lineage has not been previously evaluated. However, Zorzano and colleagues have shown inversely proportional changes in Glut1 and Glut4 expression in rat heart, skeletal muscle, and brown adipose tissue.65 During the postnatal period, the kinetics of these changes were strikingly similar to that reported here, with Glut1 decreasing spectacularly by 20 days of age in heart muscle. In their model, Glut1 expression during the perinatal period was shown to be positively regulated by the Sp1 transcription factor.30 In contrast, Sp3, which directly binds to the Glut1 proximal promoter, has been reported to block Glut1 transcriptional activity in muscle and nonmuscle cells, with this inhibitory effect being dominant over the stimulatory effect of Sp1.29 Our observation that Sp3 is expressed at 10-fold lower levels in Glut1+ than in Glut1−/Glut4+ erythroblasts is compatible with the rat cardiac model. On the basis of these data, it is tempting to speculate that the absence of Sp1 and/or Sp3 transcription factors during murine erythropoiesis will negatively affect glucose uptake, resulting in a pathologic erythropoiesis. In support of this notion, it has recently been shown that Sp3−/− cells are specifically deficient in erythroid lineage differentiation as monitored in murine repopulation experiments,66 and Sp1/Sp3 compound heterozygous mutant embryos show an anemia resulting from an impaired maturation of erythrocytes.67 It will therefore be important to assess glucose transporter expression and function in these genetic conditions.
In conclusion, glucose transporter expression distinguishes human erythrocytes from those of other mammalian species. In humans, Glut1 is expressed on erythrocytes throughout life whereas in mice, the Glut4 transporter plays a critical role in physiologic BM-localized erythropoiesis as well as in pathologicsplenic erythropoiesis. Differences in RBC glucose transporter expression in mice and humans need to be considered in genetic modification experiments affecting erythroid lineage cells; the specificity of the expressed Glut may modulate the consequences of genetic mutations affecting this cell lineage. The ramifications of these distinct glucose transporters on erythrocyte physiology and function await further investigation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to all the members of our laboratories for their careful and enthusiastic input throughout the course of this study. We thank Margot Coville and Philippe Moullier (Nantes) as well as the Tissot family for generously making animal samples available to us. We are indebted to the staff at Clinique St Roch for their precious assistance, and J. Palis for sharing his insights on fetal erythropoiesis. Flow cytometry and animal experiments were made possible by the MRI-RIO imaging platform (Infrastrutures en Biologie Sante et Agronomie (IBISA), Languedoc-Roussillon) and T&TA core facilities, respectively. The expertise of Cedric Mongellaz in cell sorting is greatly appreciated.
A.M.-H. was supported by successive grants from the French Ministry of Education and ARC. L.B. was supported by a grant from the French Ministry of Education. C.J., M.S., and N.T are all supported by INSERM. This work was funded by grants from the European Community (contract LSHC-CT-2005-018914 “ATTACK”), Sidaction, Agence Nationale de Recherche sur le Sida (ANRS), Fondation de France, Association Française Contre les Myopathies (AFM), and ARC.
Authorship
Contribution: A.M.-H. was the principal participant designing and performing research, analyzed data, and contributed to the writing of the manuscript; L.B. designed and performed research, and analyzed data; M.B.-C. designed and performed flow cytometry experiments; C.J. designed mouse experiments and performed research; M.V. designed research and analyzed data; M.S. designed research, analyzed data, and contributed to the writing of the manuscript; and N.T. was responsible for the overall study, designed research, and contributed to the writing of the manuscript;
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Naomi Taylor, Institut de Génétique Moléculaire de Montpellier, 1919 Route de Mende, 34293 Montpellier, Cedex 5, France; e-mail: taylor@igmm.cnrs.fr.



![Figure 5. Increased glucose uptake in erythroblasts of adult anemic mice is associated with enhanced Glut4 expression. (A) Glucose uptake was assessed in peripheral RBCs isolated from 3 different control adult mice as well as 4 adult mice rendered anemic by PHZ treatment (day 6). Uptake of the nonhydrolyzable glucose analog 2-DG (0.5 μM [2 μCi; 0.074 MBq]) was assayed during a 10-minute uptake at room temperature. All data are presented as mean cpm plus SD of triplicate samples. (B) RBCs from 2 different anemic mice were pretreated in the absence or presence of the Glut inhibitor CytB or the related CytD molecule for 30 minutes. [3H]2-DG uptake was then assayed as in panel A. Relative uptakes are presented, with glucose and DHA uptake in nontreated human erythrocytes defined as 100%. (C) Glut4 protein levels were assessed in RBCs isolated from mice rendered anemic by either PHZ treatment or phlebotomy. Peripheral blood was obtained at day 3 (D3) and day 6 (D6) after the start of treatment; 2 samples are shown for each condition. RBCs from adult mice were used as a control, and protein loading in each sample was monitored by actin staining. The levels of Glut4 relative to actin in each sample were determined by comparison of the respective signals using ImageJ software.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2008-05-159269/7/m_zh80230827400005.jpeg?Expires=1769173532&Signature=IpWPr0YWUQf7x3wY-OY5skPcNATM1Xqp9FhzMs~FsqMmVqb9itjPFW5ArAp8kMwIlMeV-ce6b0Nv-iL9-RniytjeXe~Yr~1yrmJ2-JKSAgV4wsND5fLMlgl~1VADRpLzGSb609-o2UdKfjO1uRdF-J~rYiVO2a0og99Puc-7r4EWV2D4GAoRWRQSJARv0OgM3tMX~3kT2fw5vhvuW1C9Ydd4KtUndDbzB8-Wf5HERYDKWyqhdhHvwwveZIIBZGbxntWguSNK8nlgHBzli4WYEck6U-zqe~gpBMxKDwXIKlfeJtPpL4ptLTq8eIbMt1IvMwEK-aLZ20KMQiij1k-v-Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal