Abstract
The emergence of variant Creutzfeld-Jakob disease, following on from the bovine spongiform encephalopathy (BSE) epidemic, led to concerns about the potential risk of iatrogenic transmission of disease by blood transfusion and the introduction of costly control measures to protect blood supplies. We previously reported preliminary data demonstrating the transmission of BSE and natural scrapie by blood transfusion in sheep. The final results of this experiment, reported here, give unexpectedly high transmission rates by transfusion of 36% for BSE and 43% for scrapie. A proportion of BSE-infected tranfusion recipients (3 of 8) survived for up to 7 years without showing clinical signs of disease. The majority of transmissions resulted from blood collected from donors at more than 50% of the estimated incubation period. The high transmission rates and relatively short and consistent incubation periods in clinically positive recipients suggest that infectivity titers in blood were substantial and/or that blood transfusion is an efficient method of transmission. This experiment has established the value of using sheep as a model for studying transmission of variant Creutzfeld-Jakob disease by blood products in humans.
Introduction
Transmissible spongiform encephalopathies (TSEs) are neurodegenerative diseases, which include Creutzfeld-Jakob disease (CJD) in man, scrapie in sheep and bovine spongiform encephalopathy (BSE) in cattle. A new variant of CJD (termed vCJD) was recognized in the United Kingdom in the mid-1990s, apparently as a result of transmission of BSE to humans.1 To date, there have been 166 cases of vCJD recorded in the United Kingdom, as well as several cases in other countries. Human TSEs are characterized by long asymptomatic incubation periods (usually several years), and there is no reliable test for detecting infection before the onset of clinical disease. It is not known how many people in the United Kingdom harbor vCJD, although estimates based on screening of tonsil and appendix samples suggest there could be up to 4000.2 These infected persons pose a risk of human-to-human transmission via blood transfusion or contaminated surgical instruments.
In patients with vCJD, there is widespread replication of the infectious agent and deposition of PrPSc (disease-associated form of prion protein) in lymphoreticular tissues, such as the tonsil, spleen, and lymph nodes, in contrast to sCJD, where lymphoreticular involvement is minimal.3 The fact that lymphocytes continuously recirculate between blood and lymphoreticular tissues strongly suggests that the blood of vCJD patients is probably infectious. Data from rodent TSE models had shown that the highest levels of infectivity in blood were associated with leukocytes and, to a lesser extent, plasma.4 As a result, costly control measures such as leukodepletion (filtration of blood and blood products to remove leukocytes) and importation of plasma were introduced to protect United Kingdom blood supplies, despite the limited data that were then available to judge the size of the risk and the efficacy of the control measures.
The potential for using sheep as a model for studying the risks of vCJD transmission by blood transfusion was highlighted by the similarity between the distribution of infectivity and PrPSc in sheep infected with TSEs and humans infected with vCJD.5-7 One factor limiting the successful transmission of TSEs by blood in rodent models was the small volumes of blood that could be injected. In contrast, the relative similarity in size of sheep and humans means that volumes of blood comparable with those used in human transfusion practice can be collected from and transfused into sheep. Using this model, we previously reported preliminary results showing that both BSE and natural scrapie could be transmitted between sheep by blood transfusion.8,9 Although scrapie is not thought to be transmissible to humans, it was included as a representative of infection acquired under field conditions, which may give different results to those obtained from experimentally infected animals. Our blood transfusion experiment in sheep is complete after 9 years, and this paper presents the full data from the study. The overall transmission rates for both scrapie and BSE are surprisingly high when factors such as the stage of infection and genetic background are taken into account, suggesting that blood transfusion represents an efficient route of transmission.
Methods
Donor and recipient sheep
The animal work was reviewed and approved by internal ethical review procedures at the Institute for Animal Health, United Kingdom, and carried out under the authority of Home Office Project Licences.
PrP genotypes of all sheep were confirmed by sequencing the coding region of the PrP gene10 and are represented by single letter amino acid code for codons 136, 154, and 171, which have been linked to scrapie susceptibility (eg, ARQ represents alanine, arginine, and glutamine, respectively, at codons 136, 154, and 171).
All donor sheep were from the Edinburgh NPU Cheviot flock, which has endemic natural scrapie. The recipient sheep (including scrapie negative control donors) were Cheviots derived from the DEFRA scrapie-free (DEFRA/SF) flock of New Zealand origin. Transfusion recipients and positive and negative controls were housed in a purpose-built isolation unit on a different site to the donors, with strict procedures in place to minimize the risk of cross-contamination between groups, as described.9 The sheep were scored at weekly intervals for clinical signs of TSEs and killed when they reached humane endpoints agreed with the Home Office. For experimentally inoculated animals (BSE donors, positive controls, and transfusion recipients), the incubation period (IP) in clinically positive sheep was defined as the period between the date of inoculation and the date of death. For scrapie-exposed donors, the IP in clinically positive sheep was defined as the age at death (ie, they were assumed to have become infected immediately after birth).
Blood collection and transfusion
Procedures for blood collection/transfusion were as previously described.9 Briefly, venous blood (450-500 mL = 1 unit) was collected into sterile collection bags (NBPI-Fresenius, Emmer-Compascuum, The Netherlands) containing citrate phosphate dextrose adenine solution as anticoagulant. From donors that were about to be killed, 2 units was collected just before postmortem, whereas from donors that were to be left alive, separate collections of 1 unit were made at least 28 days apart. However, for practical reasons, it was not always possible to collect 2 units of blood from every donor sheep. In most cases where 2 units of blood was obtained, one was transfused as whole blood (without leukodepletion) and the other was used to prepare a buffy coat fraction.
BSE blood transfusions
Fifteen sheep experimentally inoculated either orally (n = 14) or intracerebrally (n = 1) with 5 g or 0.05 g, respectively, of BSE-infected cattle brain homogenate were used as blood donors. The donor PrP genotypes were ARQ/ARQ (n = 3), ARQ/AHQ (n = 5), or AHQ/AHQ (n = 7), which are resistant to natural scrapie in the NPU flock but produce the shortest IPs after inoculation with BSE. Two sheep previously reported as donors9 were excluded from the study (along with their recipients) when regenotyping showed them to be ARQ/ARR and VRQ/AHQ, respectively, genotypes that result in relative resistance to oral infection with BSE.
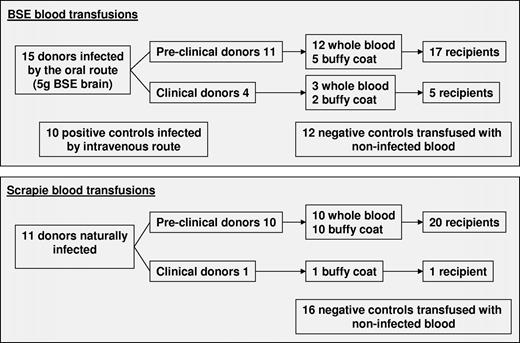
Eleven donor sheep provided blood for transfusion at the preclinical stage of infection. Eight of these were culled at the time of donation as part of a separate time course pathogenesis experiment. The remaining 3 preclinical donors went on to develop clinical signs of BSE, with respective IPs of 629, 761, and 2131 days after infection. Four sheep were used as blood donors once they had developed clinical signs of BSE at 561 to 671 days after infection. PrPSc deposits in brain and/or in peripheral tissues were confirmed in all clinically affected donors by immunohistochemistry (IHC). In 2 donors culled at the preclinical stage, sparse PrPSc deposits were found in only one tissue in each sheep: Peyer patch (58 × 81) and dorsal root ganglion (60 × 49). However, a negative result was obtained when the same tissues were immunostained in another laboratory. There were 15 ARQ/ARQ recipients of whole blood and 7 ARQ/ARQ recipients of buffy coat from BSE-infected donors. Figure 1 gives a summary of the experimental design, whereas details of the donor and recipient sheep are in Table 1.
Outcome of transfusions from BSE-exposed donor sheep
| Donor sheep details . | Recipient sheep details . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor sheep ID . | Donor genotype . | Clinical status at donation . | Percentage of actual or average incubation period at donation* . | Clinical outcome . | IHC result . | Incubation period, d . | Component transfused . | Recipient sheep ID . | Recipient PrP 168 codon genotype . | Clinical outcome . | IHC result . | Incubation period, d . |
| 58x51 | ARQ/ARQ | Preclinical | 12 | + | + | 2131 | WB | D529 | PP | +† | − | − |
| 60x49 | ARQ/ARQ | Preclinical | 22 | − | +/− | − | WB | D433 | PL | − | − | − |
| 44 | (DRG)‡ | WB | F14 | PL | − | − | − | |||||
| J2747 | ARQ/AHQ | Preclinical | 42 | − | − | − | BC | F182 | PP | − | − | − |
| 44 | WB | F181 | PP | − | − | − | ||||||
| 61x24 | ARQ/AHQ | Preclinical | 42 | − | − | − | BC | F238 | PP | +† | − | − |
| 43 | WB | F234 | PP | − | − | − | ||||||
| J2746 | AHQ/AHQ | Preclinical | 45 | − | − | − | WB | F19 | PP | + | + | 536 |
| J2559 | AHQ/AHQ | Preclinical | 51 | + | + | 629 | WB | D505 | PP | + | + | 610 |
| 58x81 | ARQ/AHQ | Preclinical | 61 | − | +/−(IPP)‡ | − | BC | D358 | PP | − | − | − |
| 58x28 | ARQ/AHQ | Preclinical | 61 | − | − | − | WB | D421 | PP | − | − | − |
| 61 | BC | D384 | PP | − | − | − | ||||||
| 58x27 | AHQ/AHQ | Preclinical | 61 | − | − | − | WB | D452 | PP | − | +§ | − |
| 61 | BC | D318 | PP | − | − | − | ||||||
| 58x39 | ARQ/AHQ | Preclinical | 62 | − | − | − | WB | D337 | PP | − | +‖ | − |
| 62 | WB | D386 | PP | − | − | − | ||||||
| J2499 | AHQ/AHQ | Preclinical | 86 | + | + | 761 | WB | D341 | PP | − | − | − |
| J2771 | AHQ/AHQ | Clinical | 100 | + | + | 561 | BC | G61 | PL | − | + | − |
| J2770 | AHQ/AHQ | Clinical | 100 | + | + | 589 | WB | G74 | PP | + | + | 594 |
| 60x69 | AHQ/AHQ | Clinical | 100 | + | + | 660 | WB | G78 | PP | + | + | 556 |
| BC | G49 | PP | + | + | 531 | |||||||
| D383 | ARQ/ARQ | Clinical | 100 | + | + | 671 | WB | G92 | PL | − | + | − |
| Donor sheep details . | Recipient sheep details . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor sheep ID . | Donor genotype . | Clinical status at donation . | Percentage of actual or average incubation period at donation* . | Clinical outcome . | IHC result . | Incubation period, d . | Component transfused . | Recipient sheep ID . | Recipient PrP 168 codon genotype . | Clinical outcome . | IHC result . | Incubation period, d . |
| 58x51 | ARQ/ARQ | Preclinical | 12 | + | + | 2131 | WB | D529 | PP | +† | − | − |
| 60x49 | ARQ/ARQ | Preclinical | 22 | − | +/− | − | WB | D433 | PL | − | − | − |
| 44 | (DRG)‡ | WB | F14 | PL | − | − | − | |||||
| J2747 | ARQ/AHQ | Preclinical | 42 | − | − | − | BC | F182 | PP | − | − | − |
| 44 | WB | F181 | PP | − | − | − | ||||||
| 61x24 | ARQ/AHQ | Preclinical | 42 | − | − | − | BC | F238 | PP | +† | − | − |
| 43 | WB | F234 | PP | − | − | − | ||||||
| J2746 | AHQ/AHQ | Preclinical | 45 | − | − | − | WB | F19 | PP | + | + | 536 |
| J2559 | AHQ/AHQ | Preclinical | 51 | + | + | 629 | WB | D505 | PP | + | + | 610 |
| 58x81 | ARQ/AHQ | Preclinical | 61 | − | +/−(IPP)‡ | − | BC | D358 | PP | − | − | − |
| 58x28 | ARQ/AHQ | Preclinical | 61 | − | − | − | WB | D421 | PP | − | − | − |
| 61 | BC | D384 | PP | − | − | − | ||||||
| 58x27 | AHQ/AHQ | Preclinical | 61 | − | − | − | WB | D452 | PP | − | +§ | − |
| 61 | BC | D318 | PP | − | − | − | ||||||
| 58x39 | ARQ/AHQ | Preclinical | 62 | − | − | − | WB | D337 | PP | − | +‖ | − |
| 62 | WB | D386 | PP | − | − | − | ||||||
| J2499 | AHQ/AHQ | Preclinical | 86 | + | + | 761 | WB | D341 | PP | − | − | − |
| J2771 | AHQ/AHQ | Clinical | 100 | + | + | 561 | BC | G61 | PL | − | + | − |
| J2770 | AHQ/AHQ | Clinical | 100 | + | + | 589 | WB | G74 | PP | + | + | 594 |
| 60x69 | AHQ/AHQ | Clinical | 100 | + | + | 660 | WB | G78 | PP | + | + | 556 |
| BC | G49 | PP | + | + | 531 | |||||||
| D383 | ARQ/ARQ | Clinical | 100 | + | + | 671 | WB | G92 | PL | − | + | − |
WB indicates whole blood; BC, buffy coat; DRG, dorsal root ganglion; IPP, ileal Peyer patch; +, positive; and −, negative.
Calculated from the days after infection at the time of donation, as a percentage either of the final incubation period (in sheep kept alive until the development of clinical signs) or of the average incubation period in orally infected donors (640 days), excluding the outlying incubation period of 2131 days (58x51).
No evidence of infection was found on postmortem examination of tissues from these clinical suspects; therefore, it is most likely they were clinically misdiagnosed.
These tissues were initially scored weakly positive by IHC, but the results were not reproducible in two laboratories and can therefore be considered as inconclusive.
This sheep died of unrelated causes (ie, without showing clinical signs of BSE) at 1139 days after transfusion but was positive by IHC.
This apparently healthy sheep was culled 3018 days after transfusion and found to be positive by IHC; however, further analysis suggested this was a case of ″atypical″ scrapie and therefore unlikely to be transfusion related.
Scrapie blood transfusions
The donors for this experiment were 10 VRQ/VRQ and one VRQ/ARQ Cheviot sheep from the Edinburgh NPU flock, where sheep of these genotypes show a disease incidence approaching 100%. Epidemiologic and pathologic evidence suggests that infection occurs around the time of birth. Blood collections were made from animals in 3 different age groups (200-250 days, 450-500 days, 700-850 days) to represent donors at different preclinical stages of disease, as well as from one clinical case. Seven donors were culled after developing clinical signs of scrapie at ages ranging from 1081 to 1556 days, and were confirmed positive by histopathology and IHC. Two donors were culled before the onset of clinical signs at 1197 and 1350 days of age, respectively, but PrPSc was detected in their tissues by IHC. Two donors died prematurely at 349 and 974 days of age: one was IHC negative, in the other the tissues were too decomposed to allow analysis. There were 21 recipients (all VRQ/VRQ PrP genotype) of blood from scrapie-exposed donors: 11 were transfused with buffy coat and 10 with whole blood. See Figure 1 for a summary of the experimental design and Table 2 for details of donor and recipient sheep.
Outcome of transfusions from scrapie-exposed donor sheep
| Donor sheep details . | Recipient sheep details . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor sheep ID . | Donor genotype . | Clinical status at donation . | Percentage of actual or average incubation period at donation* . | Clinical outcome . | IHC result . | Incubation period, d . | Component transfused . | Recipient sheep ID . | Clinical outcome . | IHC result . | Incubation period, d . |
| 67x42 | VRQ/VRQ | Preclinical | 17 | + | + | 1274 | BC | G247 | − | − | − |
| 19 | WB | G230 | − | − | − | ||||||
| 66x45 | VRQ/VRQ | Preclinical | 17 | − | − | − | WB | G267 | − | − | − |
| 19 | BC | G265 | − | − | − | ||||||
| 67x23 | VRQ/VRQ | Preclinical | 18 | + | + | 1207 | BC | G241 | − | − | − |
| 20 | WB | G228 | − | − | − | ||||||
| 65x13 | VRQ/VRQ | Preclinical | 28 | + | + | 1556 | WB | F275 | − | − | − |
| 30 | BC | F273 | − | − | − | ||||||
| 65x02 | VRQ/VRQ | Preclinical | 34 | − | + | − | WB | F310 | − | − | − |
| 37 | BC | F309 | + | + | 1101 | ||||||
| 65x03 | VRQ/VRQ | Preclinical | 34 | − | + | − | WB | F277 | + | + | 1138 |
| 37 | BC | F276 | +† | − | − | ||||||
| 61x75 | VRQ/ARQ | Preclinical | 53 | + | + | 1324 | BC | F149 | + | + | 782 |
| 57 | WB | F144 | + | + | 672 | ||||||
| 61x68 | VRQ/VRQ | Preclinical | 64 | + | + | 1113 | BC | F152 | + | + | 853 |
| 69 | WB | F153 | + | + | 660 | ||||||
| 61x66 | VRQ/VRQ | Preclinical | 62 | − | ND | − | WB | F286 | − | − | − |
| 64 | BC | F284 | − | − | − | ||||||
| 59x27 | VRQ/VRQ | Preclinical | 73 | + | + | 1137 | BC | F126 | + | + | 826 |
| 77 | WB | F141 | + | + | 575 | ||||||
| 59x28 | VRQ/VRQ | Clinical | 100 | + | + | 1081 | BC | F143 | + | + | 737 |
| Donor sheep details . | Recipient sheep details . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor sheep ID . | Donor genotype . | Clinical status at donation . | Percentage of actual or average incubation period at donation* . | Clinical outcome . | IHC result . | Incubation period, d . | Component transfused . | Recipient sheep ID . | Clinical outcome . | IHC result . | Incubation period, d . |
| 67x42 | VRQ/VRQ | Preclinical | 17 | + | + | 1274 | BC | G247 | − | − | − |
| 19 | WB | G230 | − | − | − | ||||||
| 66x45 | VRQ/VRQ | Preclinical | 17 | − | − | − | WB | G267 | − | − | − |
| 19 | BC | G265 | − | − | − | ||||||
| 67x23 | VRQ/VRQ | Preclinical | 18 | + | + | 1207 | BC | G241 | − | − | − |
| 20 | WB | G228 | − | − | − | ||||||
| 65x13 | VRQ/VRQ | Preclinical | 28 | + | + | 1556 | WB | F275 | − | − | − |
| 30 | BC | F273 | − | − | − | ||||||
| 65x02 | VRQ/VRQ | Preclinical | 34 | − | + | − | WB | F310 | − | − | − |
| 37 | BC | F309 | + | + | 1101 | ||||||
| 65x03 | VRQ/VRQ | Preclinical | 34 | − | + | − | WB | F277 | + | + | 1138 |
| 37 | BC | F276 | +† | − | − | ||||||
| 61x75 | VRQ/ARQ | Preclinical | 53 | + | + | 1324 | BC | F149 | + | + | 782 |
| 57 | WB | F144 | + | + | 672 | ||||||
| 61x68 | VRQ/VRQ | Preclinical | 64 | + | + | 1113 | BC | F152 | + | + | 853 |
| 69 | WB | F153 | + | + | 660 | ||||||
| 61x66 | VRQ/VRQ | Preclinical | 62 | − | ND | − | WB | F286 | − | − | − |
| 64 | BC | F284 | − | − | − | ||||||
| 59x27 | VRQ/VRQ | Preclinical | 73 | + | + | 1137 | BC | F126 | + | + | 826 |
| 77 | WB | F141 | + | + | 575 | ||||||
| 59x28 | VRQ/VRQ | Clinical | 100 | + | + | 1081 | BC | F143 | + | + | 737 |
+ indicates positive; and −, negative.
Calculated from the age at the time of donation, as a percentage either of the final incubation period (for sheep that survived until the development of clinical signs) or of the average incubation period (1296 days) for sheep that died or were culled before developing clinical signs.
No evidence of infection was found on postmortem examination of tissues from this clinical suspect; therefore, it is most likely it was clinically misdiagnosed.
Positive and negative controls
Seven ARQ/AHQ and 3 ARQ/ARQ sheep were infected intravenously with 0.2 g of the same BSE-infected cattle brain homogenate as given orally to the blood donors and served as positive controls. No positive controls were used in the scrapie transfusion experiment. As negative controls for the BSE transfusion experiment, 12 ARQ/ARQ recipients were given transfusions of whole blood (n = 6) or buffy coat (n = 6) from 7 uninfected donors (6 ARQ/AHQ, 1 ARQ/ARR). Two recipients died at 633 days and 1181 days after transfusion, respectively, and the remaining 10 recipients were culled between 2462 and 2586 days after transfusion. As negative controls for the scrapie experiment, 16 VRQ/VRQ sheep received either whole blood (n = 8) or buffy coat (n = 8) collected from 8 uninfected VRQ/VRQ donors. There were 2 intercurrent deaths at 397 days and 464 days after transfusion, and the other 14 animals were culled between 2052 and 2409 days after transfusion. None of the negative controls for the BSE or scrapie experiments showed clinical signs of TSEs, and all were IHC negative for PrPSc.
PrPSc detection by immunohistochemistry
Tissue samples from the brain, spleen, mesenteric lymph node, and palatine tonsil of the sheep under study were fixed in formaldehyde and processed according to standard procedures. Sections were immunolabeled for PrPSc detection by IHC with primary antibody R145, which recognizes the 222-226 amino acid sequence of ovine PrP,11 as described previously.12,13
Results
BSE transfusion experiment
A total of 5 transfusion recipients showed clinical signs of TSEs and were confirmed positive by IHC and/or Western blot (Table 1; Figure 2). These included 2 (F19 and D505) of 12 sheep transfused with whole blood from donors in the preclinical phase of infection (at 45% and 50% of estimated IP, respectively), as reported previously.8,9 Two of 3 recipients of whole blood and one of 2 recipients of buffy coat from donors clinically affected by BSE developed clinical BSE. The IPs in the 5 clinically positive recipient sheep ranged from 531 to 610 days after transfusion (mean ± standard deviation [SD] = 565 ± 35 days), and there was no obvious difference in the IPs of those that received blood from preclinical or clinical donors.
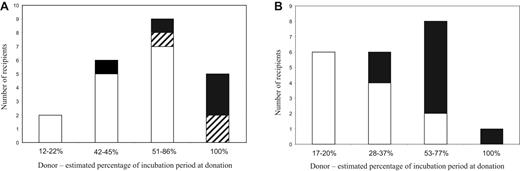
Outcome of transfusions as a function of the stage of disease incubation in the donor. (A) BSE-infected donors. (B) Scrapie-infected donors. For each stage of infection in the donor sheep, the number of uninfected (□), clinically positive/ IHC-positive (■), and clinically negative/IHC-positive ( ) recipients are shown.
) recipients are shown.
Outcome of transfusions as a function of the stage of disease incubation in the donor. (A) BSE-infected donors. (B) Scrapie-infected donors. For each stage of infection in the donor sheep, the number of uninfected (□), clinically positive/ IHC-positive (■), and clinically negative/IHC-positive ( ) recipients are shown.
) recipients are shown.
One recipient (D452) of whole blood from a preclinical donor died of unrelated causes at 1139 days after transfusion but had PrPSc-positive IHC labeling in brain and other tissues. One of 3 recipients of whole blood (G92) and one of 2 recipients of buffy coat (G61) from clinical donors showed weak PrPSc deposition in the brain and lymphoid tissues after being culled at 2003 and 2497 days after transfusion, respectively, in the absence of clinical signs. Full sequencing of the PrP gene of these sheep revealed that they carried an additional proline (P) to leucine (L) substitution at codon 168,14,15 which appears to be associated with the prolonged survival of these infected sheep. The polymorphism was also identified in 2 recipients of blood from a preclinical BSE-challenged donor, neither of which showed evidence of infection.
Taking the results for all 22 recipients of blood from BSE-exposed donors, 5 clinical cases and 3 sheep showing evidence of infection in the absence of clinical signs were identified, giving an overall transmission rate of 36%.
One recipient was culled for health reasons at 1444 days after transfusion, 2 were culled with suspected TSE clinical signs at 2480 and 2160 days after transfusion, respectively, and the remaining clinically negative sheep were culled between 2239 and 3068 days after transfusion. With one exception, examination of the tissues by IHC did not find evidence of infection. The exception (D337) was culled at 3018 days after transfusion and showed positive PrPSc labeling in the brain, but with a pattern distinct from that observed in other BSE-infected sheep. The brain PrPSc distribution involving major white matter tracts and sparing the dorsal motor nucleus of the vagus was similar to that of Nor98 (or “atypical” sheep scrapie) and therefore doubtful to be transfusion-related. No other sheep in the present study showed evidence of being infected with atypical scrapie.
Of the 10 sheep that were infected intravenously with BSE as positive controls, 8 developed clinical signs confirmed by IHC, with an average IP of 702 days (± 61 days, SD). The remaining 2 animals were culled at 2591 days after infection and, although not demonstrably clinically affected, IHC showed PrPSc deposition in the brains and lymphoid tissues of both animals. These 2 sheep were heterozygous (PL168) for the PrP polymorphism P168L, whereas the other 8 were homozygous (PP168).
The PrPSc profile obtained by IHC from BSE-positive recipients was the same as that found in the orally inoculated donors and in the positive controls.16 In addition, characteristic BSE glycoform patterns were obtained by Western blot analysis of PrPSc-positive donor and recipient sheep (data not shown),9 and inoculation of brain homogenates from infected donors and recipients into a panel of inbred mouse strains produced IPs and lesion profiles characteristic of BSE (data not shown). Taken together, these results confirm that the strain characteristics were not altered after transmission via blood.
Scrapie transfusion experiment
Four of 10 recipients of whole blood and 4 of 10 recipients of buffy coat from donors in the preclinical phase of scrapie infection developed clinical signs of scrapie, which were confirmed by positive IHC results. One sheep transfused with buffy coat from the single clinical donor was also clinically affected and IHC positive (Table 2; Figure 2). Four of these cases (F144, F153, F141, and F143) were reported previously.9 There were 4 intercurrent deaths at 354, 753, 1237, and 1615 days after transfusion, respectively, and the 8 remaining recipients were culled between 2329 and 2484 days after transfusion. These 12 animals were clinically negative at the time of death and showed no detectable PrPSc by IHC. Thus, 9 of 21 recipients of blood from scrapie-exposed sheep developed clinical scrapie, giving an overall transmission rate of 43%.
The majority of confirmed scrapie cases in recipients (n = 7) occurred in the groups that received transfusions from donors in the late preclinical (> 50% of estimated IP) or clinical phase of infection. Only 2 of 9 recipients in these groups remained free of infection. The other 2 positive recipients were in the group of 6 sheep that received transfusions from donors at 28% to 37% of estimated IP, and their IPs were much longer than the rest (1101 and 1138 days after transfusion compared with a range of 575-853 days in recipients of blood from donors at > 50% of estimated IP). No disease was confirmed in the 6 recipients that received blood from donors at less than or equal to 20% of estimated IP.
The PrPSc profile obtained from brains of donors and recipients highlighted some differences in terms of presence of vascular plaques or glia-associated PrPSc in donors but not in recipients, or vice versa (S.S., unpublished data, December 16, 2005). Such discrepancies were interpreted as presence of more than one natural scrapie strain in the flock of origin.
Discussion
The outcome of the blood transfusion experiments showed that 2 different TSE agents, scrapie and BSE, could be efficiently transmitted between sheep by blood transfusion, using volumes similar to those used in human transfusions. The overall transmission rates (percentage of all recipients that became infected) were 36% for BSE and 43% for scrapie. For BSE, the figure was much higher than anticipated because 3 of the 8 BSE-infected recipients survived for long periods without showing clinical signs, whereas all the scrapie-infected recipients identified by IHC were also clinically positive. The greater probability of subclinical infection in recipients of blood from BSE-exposed donors is largely the result of variability in the genetic susceptibility to infection among sheep used in the BSE experiment, which will be discussed in “Effect of genetic variation in susceptibility.” The results are consistent with the known facts about transmission of vCJD by blood transfusion in humans.17 Sixty-six patients known to have received labile blood products from 18 donors who subsequently developed vCJD were followed up in an ongoing study. Three of these recipients have been confirmed clinically and pathologically as vCJD cases, with intervals between transfusion and the development of clinical signs ranging from approximately 6.5 years to 8.5 years.18-20 Another patient, who died of unrelated causes 5 years after transfusion, showed PrPSc deposits in lymphoid tissues but not brain postmortem, and is thought to represent preclinical or subclinical infection.21 These 4 patients represent 6% of the total recipients, or 12.5% of recipients surviving longer than 5 years.
Various factors influence the transmission rate by transfusion in both sheep and humans, including: (1) the interval between blood donation and the onset of clinical signs in the donors, (2) genetic variation in susceptibility of donors and recipients, and (3) the blood component transfused.
Stage of incubation period of the donors at the time of blood donation
The effect of the stage of incubation can best be deduced from the results of the scrapie transfusion experiment because the PrP genotype of the sheep used (VRQ/VRQ) renders them almost 100% susceptible to natural and experimental infection.22 The stage of incubation of the donor has a strong influence on the probability of transmission to the recipient (Figure 2). When donations were made at less than or equal to 20% of the estimated IP, there was no disease transmission, whereas donations made at more than 50% of the estimated IP produced an 80% transmission rate, with a mean IP of 729 days (± 99, SD) in the recipients. Blood collected at 28% to 37% of the estimated IP transmitted infection at a lower rate of approximately 33%, and with longer IPs in the recipients of more than 1000 days. The data are consistent with a gradual increase in infectivity in the blood, from approximately 30% to 50% of IP until the clinical phase.
In the BSE transfusion experiment, the correlation between stage of infection and transmission is not clear-cut but shows the same general trend of increasing probability of transmission to recipients as infection progresses in the donors (Figure 2). Possible explanations for the lower transmission rates from preclinical BSE-infected blood donors compared with preclinical scrapie-infected donors include the following:
(a) Variation in susceptibility to infection of both donor and recipient sheep.
(b) Differences in the pathogenesis of natural scrapie and experimental BSE. VRQ/VRQ sheep naturally infected with scrapie have detectable PrPSc deposits in lymphoid tissues early after infection (ie, < 50% estimated IP).23,24 Time course studies of ARQ/ARQ sheep orally infected with BSE showed that PrPSc was not consistently detected in lymphoid tissues before at least 65% of the average IP.7 If infectivity in blood correlates with its presence in lymphoid tissues, this could explain the differences observed in the 2 transfusion experiments.
The probability of transmission from preclinical donors is of greatest relevance to the human situation. In the case of the 4 transfusion-related transmissions of vCJD, the donors developed clinical signs between 17 and 42 months after donation. The mean IP for vCJD has been estimated to be 16.7 years, with a lower 95% confidence interval of approximately 12.4 years.25 Therefore, it is probable that the transfusion-related vCJD cases resulted from donations made at least halfway through the IP, which is in agreement with the data from the sheep experiments. In vCJD cases, the timing of detectable lymphoid replication in the preclinical stages of disease is unknown; therefore, it is not clear whether the peripheral pathogenesis more closely resembles BSE or natural scrapie in sheep.
Effect of genetic variation in susceptibility
A small proportion of sheep with A136Q171/A136Q171 PrP genotypes do not die of infection after natural or experimental exposure to scrapie and BSE, or have very prolonged incubation periods.26-28 The reasons for this variability in response are not clearly understood, but it can be predicted to reduce infection rates in both donor and recipient sheep in the BSE transfusion experiment. The majority of preclinical donor sheep (8 of 11) in the BSE transfusion experiment were killed at, or shortly after, the time of donation, and none showed conclusive evidence of infection, although 2 transmitted infection to their respective transfusion recipients. It is potentially significant that donors that failed to transmit infection were heterozygous at PrP codon 154, whereas those that did transmit infection were homozygous. Thus, variable susceptibility to infection among the donor sheep may be the result of a protective effect of codon 154 heterozygosity to oral challenge with BSE, although more data are required to confirm this association.
A novel polymorphism, resulting in a proline to leucine substitution at codon 168 of the PrP gene, was identified in 4 BSE transfusion recipients and 2 positive control sheep inoculated intravenously with BSE.14 All 6 survived more than 2000 days without developing clinical signs of BSE, but on postmortem examination 4 showed PrPSc deposition in brain and lymphoid tissues. This suggests that the P168L polymorphism can protect against clinical disease but does not prevent infection by the intravenous route. This polymorphism has not been identified in the Edinburgh NPU Cheviots used as donors in the BSE experiment or in sheep with the VRQ/VRQ genotype.
Although the genetic basis of susceptibility to BSE infection in sheep and humans is not directly comparable, the variability in response to BSE found in ARQ/ARQ sheep provides a more realistic reflection of the situation with vCJD in the human population than the very uniform susceptibility of VRQ/VRQ sheep to scrapie infection. In addition, the survival of BSE-infected transfusion recipients for up to 7 years without clinical signs demonstrates that prolonged secondary incubation periods and/or a subclinical/“carrier” state are possible after transfusion in sheep. The existence of such subclinical or prolonged preclinical infection states in humans is recognized as one of the important factors influencing the probability of onward transmission, and thus the potential size of the vCJD epidemic.29 Susceptibility to human TSEs has been linked to codon 129 of the PrP gene, which can encode either methionine (M) or valine (V). Until recently, all clinical cases of vCJD (including the 3 transfusion-related cases) that have been tested have been homozygous for methionine at 129 (129MM). Interestingly, the “preclinical” patient thought to have been infected by transfusion was heterozygous (129MV).21 There is accumulating evidence to suggest that all human 129 genotypes may be susceptible to vCJD infection, with apparently greater likelihood of subclinical infection in 129MV and 129VV persons.30-32
Effect of blood component
The 4 transfusion-related vCJD infections occurred in patients who received transfusions of red cells that had not been leukodepleted. Leukodepletion was introduced in the United Kingdom in 1999 to control the risk of transmission of vCJD by blood transfusion because previous studies in rodents had shown that infectivity appeared to be concentrated in the buffy coat, which contains most of the blood leukocytes.4 Subsequently, leukodepletion of blood from scrapie-infected hamsters was shown to remove up to 72% of infectivity.33,34 In the sheep experiments, only whole blood and buffy coat were transfused because we were seeking to establish proof of principle of transmission of TSEs by blood transfusion, and assessing whether infectivity appeared to be concentrated in the buffy coat. The effect of leukodepletion was not investigated but is being addressed in a follow-up study, along with estimates of the distribution of infectivity among other blood components, including plasma, platelets, and red cells.
In our experiments, transmission rates did not appear to be significantly different in recipients receiving whole blood compared with recipients transfused with buffy coat. The number of sheep transfused with buffy coat in the BSE experiment was too small to allow statistical analysis. In the scrapie experiment, 5 of the positive recipients were transfused with buffy coat, and 4 with whole blood. The similarity in transmission rates for both components suggests that they contain approximately equivalent amounts of infectivity.
We have shown that, for sheep infected with scrapie and BSE, high transmission rates can be achieved using blood transfusion, particularly when donors are at more than 50% of incubation period. The results also revealed the possibility of prolonged incubation periods and/or subclinical infections in some recipients of BSE-infected blood, which is at least partly because of genetic variation in the sheep PrP gene. The suggestion of relatively high titers of infectivity in blood is perhaps surprising in view of the need for ultrasensitive methods of detection for PrPSc in blood.35,36 It may be that, in blood, infectivity is not closely correlated with levels of protease-resistant PrP, but comparative titrations of brain and blood-borne infectivity in sheep will be required to further define the relationship. The results of our sheep transfusion experiments are consistent with what is known about transfusion-associated vCJD transmission in humans, and support the use of sheep as an experimental model in which to study the risks associated with different blood products, the effectiveness of control measures, and the development of diagnostic and screening tests.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Calum McKenzie, Tony Smith, Richard Eynon, Emma Cartwright, Mhairi Baxter, and Dr Richard Lysons and colleagues for their excellent care of the sheep, technical assistance with blood collections/transfusions, clinical scoring, and postmortem tissue collection; Suzanne Beckett, Anne Coghill, Dawn Drummond, David Parnham, Aileen Boyle, and Irene McConnell for pathology and mouse transmission work; Paula Stewart for PrP genotyping; Hazel Baird, Lynne Fairlie, Ann Dunachie, and Maria Oliva for technical contribution to IHCs; and Professor C.J. Bostock for his advice and support for this project.
This work was supported by the U.K. Department of Health (project reference 1216713). The BSE challenged donor sheep were part of an experiment funded by the Department of Environment, Food, and Rural Affairs. The Scottish National Blood Transfusion Service supplied the blood packs used for blood collection and prepared the buffy coat fractions.
Authorship
Contribution: F.H. designed the study, performed transfusions and postmortems on recipient sheep, analyzed data, and wrote the paper; A.C. performed Western blots; S.M. performed Western blots and reviewed the report; J.F. coordinated collection of blood and postmortems on donor sheep; W.G. analyzed and interpreted PrP genotype data and reviewed the report; S.S. and L.G. examined tissues, interpreted IHC results, analyzed data, and reviewed the report; M.J. contibuted to the interpretation of IHC results and reviewed the report; and N.H. designed the study, analyzed data, and reviewed the report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fiona Houston, Division of Animal Production & Public Health, Faculty of Veterinary Medicine, University of Glasgow, Bearsden Road, Glasgow, G61 1QH, United Kingdom; e-mail: f.houston@vet.gla.ac.uk.