Abstract
The advancement of gene therapy has been slowed, in part, by inefficient transduction of targeted cells and poor long-term engraftment of genetically modified cells. Thus, the ability to select for a desired population of cells within a recipient would be of great benefit for improving gene therapy. Proposed strategies for in vivo cell selection using drug resistance genes have had disappointing outcomes and/or require highly genotoxic medications to be effective. We hypothesized that resistance to purine analogs, a well-tolerated, relatively low-toxicity class of medications, could be provided to cells using interfering RNA against hypoxanthine phosphoribosyl transferase. Using a lentiviral vector, we found that interfering RNA-mediated purine analog resistance (iPAR) provided relative resistance to 6-thioguanine (6TG) in murine hematopoietic cells compared with control- and untransduced cells. iPAR attenuated 6TG-induced G2/M checkpoint activation, cell-cycle arrest, and apoptosis. Furthermore, in recipients of transplanted bone marrow cells with iPAR, treatment with 6TG resulted in increased percentages of transduced peripheral blood cells and hematopoietic progenitor cells in the bone marrow. Secondary transplantations resulted in higher hematopoietic contributions from 6TG-treated primary recipients relative to phosphate-buffered saline-treated recipients. These findings indicate that iPAR/6TG can be used for in vivo hematopoietic progenitor cell selection.
Introduction
Gene therapy has long been touted for its potential to cure a variety of congenital diseases.1,2 Indeed, for a small group of patients, this has come to fruition.3-7 Nonetheless, gene therapy trials are still small in number because of several problems that have prevented larger-scale application of current technology. In addition to the widely publicized malignant side effects in the trials involving correction of X-linked severe combined immunodeficiency,8 inefficient transduction, poor long-term expression, and engraftment failure of ex vivo manipulated cells have slowed the practical advancement of gene therapy.2,9-11
Newer, self-inactivating (SIN), HIV-based lentiviral vectors have recently been explored as a means to avoid some of the pitfalls noted in the previous paragraph.12 Lentiviruses do not require cells to be in cycle,13,14 thus allowing for transduction during relatively short-term ex vivo culture.15,16 Furthermore, SIN vectors reduce the risk of replication competent recombination15 and may reduce the risk of aberrantly activating coding sequences, including proto-oncogenes, near the integration site.17,18 Despite these advances, problems remain with the ability to transduce sufficient numbers of cells, as well as the ability to achieve long-term engraftment and stable gene expression.
Therefore, the ability to select for or amplify a population of cells that has been modified to express a gene of interest might enhance the effectiveness of gene therapy.9-11,19 Several different approaches to programmed drug resistance have been evaluated: ectopic expression of the multidrug resistance 1 gene product (MDR1),20 cytidine deaminase (CD),21 glutathione S-transferase (GST),22 or cytosolic 5′-nucleotidase I (cN-I)23 ; or expression of mutated forms of dihydrofolate reductase (Δ-DHFR)24 or O6-methylguanine-DNA-methyltransferase (Δ-MGMT).25 Concerns with these strategies remain, though, because of a lack of effectiveness in selecting hematopoietic stem cells (HSCs) in vivo (Δ-DHFR, cN-I), potential development of multiple-drug resistant leukemia (MDR1),26 or the use of highly genotoxic, carcinogenic drugs for selection (GST, MDR1, Δ-MGMT).11 Thus, the development of an effective, nontoxic strategy of programmed drug resistance could have tremendous impact in the advancement of genetic medicine.
6-Thioguanine (6TG) and 6-mercaptopurine (6MP) are purine analogs that have been used extensively in the treatment of leukemia for decades.27 Myelosuppression is the primary side effect of purine analogs, although a subset of patients experience hepatic toxicity from 6TG.28 Purine analogs are taken orally, are very well tolerated for long periods of time, and can be titrated to the desired level of hematopoietic toxicity. 6TG and 6MP must be metabolized intracellularly into their active metabolites by hypoxanthine-guanine phosphoribosyl transferase (HPRT). These metabolites are incorporated into DNA.27 The presence of these fraudulent nucleotides results in activation of the DNA mis-match repair system (MMR), which leads to G2/M checkpoint activation and apoptosis.29
We hypothesized that knockdown of HPRT using shRNA would provide cellular resistance to purine analogs and thus provide a means to select for a desired population of cells. Using lentiviral vectors, we demonstrate that treatment with 6TG provides a selective advantage for cells which have interfering RNA mediated purine analog resistance (iPAR) in a murine hematopoietic cell line in vitro and in murine primary bone marrow (BM) cells ex vivo. This advantage stems from these cells' ability to escape the G2/M checkpoint and avoid apoptosis that is induced by 6TG. We further show that iPAR in hematopoietic progenitor cells (HPCs) allows for the selection of these cells and their progeny in sublethally irradiated, 6TG-treated recipient mice. These data suggest that knockdown of HPRT and treatment with 6TG may be a safe and effective way to enhance therapeutic gene delivery.
Methods
Cell culture
The murine pro-B cell line, FL5.12,30 was seeded at 0.5 to 1 × 105 cells/mL in RPMI supplemented with 10% fetal bovine serum (FBS) and 15% WEHI3 conditioned media (as a source of interleukin-3 [IL-3]). Cells were reseeded every 48 to 72 hours in fresh media. Primary BM cells were cultured at 105 cells/mL in Iscove modified Dulbecco medium supplemented with 10% FBS, 0.1% bovine serum albumin (BSA; Sigma-Aldrich, St Louis, MO) and the following cytokines: 100 ng/mL human stem cell factor (a gift from Dr Chris Hogan), 100 ng/mL human IL-11 (PeproTech, Rocky Hill, NJ), 1.5 ng/mL murine IL-3 (PeproTech), and 100 ng/mL human IL-6 (PeproTech). BM cells were replated in fresh cytokine containing media every 72 to 96 hours. 6TG, 6MP, and cisplatinum (Sigma-Aldrich) were used at the indicated concentrations.
Lentivirus production and transduction
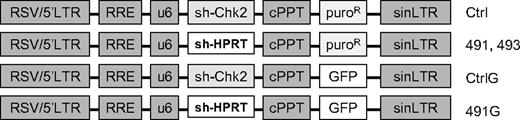
The pLKO.1 lentiviral constructs with shRNA directed against murine HPRT (TRCN0000110490-494; Figure 1) were purchased from Open Biosystems (Huntsville, AL). The sequences of the hairpins are provided in supplemental material (available on the Blood website; see the Supplemental Materials link at the top of the online article). To construct similar lentivirus vectors with expression of green fluorescent protein (GFP), the puromycin resistance gene was replaced with the GFP gene. A control vector (Ctrl) with a hairpin directed against human Chk2 was used to control against nonspecific effects of the engagement of shRNA machinery. The Ctrl shRNA efficiently knocks down Chk2 in human cells, but not in murine cells. Virus containing media was prepared by transient transfection of 293FT cells with experimental constructs and packaging vectors coding for VSV-G, Gag, Pol, and Rev (generously provided by Dr Yosef Raefeli). FL5.12 or primary BM cells were cultured overnight in virus containing media with 8 μg/mL polybrene (Sigma-Aldrich). Transduction of primary BM cells was performed in plates coated with RetroNectin (Takara, Kyoto, Japan). Where indicated, transduced cells were selected in 2.5 μg/mL puromycin.
Schematic diagram of plasmids. LTR indicates long-terminal repeat; RRE, Rev response element; U6, human U6 promoter; cPPT, central polypurine tract; puroR, puromycin resistance; GFP, green fluorescent protein; sh-Chk2, short hairpin directed against human Chk2; sh-HPRT, short hairpin directed against HPRT.
Schematic diagram of plasmids. LTR indicates long-terminal repeat; RRE, Rev response element; U6, human U6 promoter; cPPT, central polypurine tract; puroR, puromycin resistance; GFP, green fluorescent protein; sh-Chk2, short hairpin directed against human Chk2; sh-HPRT, short hairpin directed against HPRT.
Cell counting, apoptosis assessment, and cell-cycle analysis
Cultured FL5.12 cells were diluted in 2 μg/mL propidium iodide (PI) and live cell concentrations and percentages of GFP+ cells were determined using a Cell Lab QuantaSC MPL (Beckman Coulter, Fullerton, CA) flow cytometer. For assessment of apoptosis, FL5.12 cells were stained with fluorescein isothiocyanate-linked annexin V (Invitrogen, Carlsbad, CA) and 5 μg/mL PI and analyzed with the Cell Lab QuantaSC MPL. For cell-cycle analysis, FL5.12 cells were fixed in cold ethanol overnight, stained with 20 μg/mL PI, and analyzed using a Cytomics FC500 flow cytometer (Beckman Coulter). Cell sorting for GFP+ cells was performed on a MoFlo cell sorter (Dako North America, Carpinteria, CA).
Mice
C57BL/6J mice were originally purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were housed in sterile micro-isolators in the Center for Comparative Medicine at the University of Colorado Denver. The Animal Care and Use Committee of the University of Colorado Denver approved all mouse experiments.
Bone marrow transplantation and 6TG treatment of mice
Whole BM was harvested from tibiae and femora of donor mice and transduced overnight in virus containing media with cytokines as described in “Cell culture.” The next day, recipient mice were sublethally irradiated with 450 cGy (RS2000; Rad Source, Alpharetta, GA). After the recipients were sedated with tribromoethanol (Sigma-Aldrich), the BM was transplanted via the retro-orbital venous sinus. The animals were left unmanipulated for 4 weeks to allow reconstitution of hematopoiesis, after which time peripheral blood was obtained from the lateral tail vein for analysis by flow cytometry. Recipient mice were treated with intraperitoneal 6TG as described. Secondary recipients were irradiated with 500 cGy and transplanted with 2 × 106 nucleated, PI− BM cells. Secondary engraftment was defined as chimerism greater than 3 SDs above the mean level of background detection of GFP in the peripheral blood Gr1+ population.31
Flow cytometry and complete blood counts
Peripheral blood was collected from the lateral tail vein in heparinized microfuge tubes at the indicated time points. Complete blood counts were performed on a Cell-Dyn 1700 (Abbott Laboratories, Abbott Park, IL). Antibodies were purchased from BD Biosciences (San Jose, CA) except as noted. The remaining blood was treated with hemolytic buffer and stained with antibodies directed against B220 and Gr1 (eBioscience, San Diego, CA) and conjugated to R-phycoerythrin (PE) and PE-cyanine 7 (PE-Cy7), respectively. Stained peripheral blood was analyzed on a Cell Lab QuantaSC MPL. BM from killed animals was harvested from tibiae and femora and hemolyzed. The number of live nucleated cells was determined using PI exclusion and flow cytometry. The BM cells were then labeled with lineage antibodies against Gr1, B220, Mac1, Ter119, CD4, and CD8 linked to PE, as well as Flk2-PE (where indicated), allophycocyanin (APC)–conjugated c-Kit, and PE-Cy7-conjugated Sca1. Alternatively, whole BM was stained with PE-linked lineage antibodies and CD48-PE, CD150-APC (BioLegend, San Diego, CA), and biotin-linked CD244.2, followed by secondary staining with streptavidin-linked PE-Cy7. These cells were analyzed using a Cyan ADP Analyzer (Dako North America).
Statistical and flow cytometric analyses
Results were analyzed using GraphPad Prism 4 (GraphPad Software, San Diego, CA). Unpaired t tests were used to determine statistical significance between 2 groups. Analysis of variance was used to determine statistical significance between groups with repeated measures over time or between multiple groups, where indicated. Fisher exact test was used to determine statistical significance between groups where indicated. Except where indicated, data are representative of at least 3 separate experiments. Mean values from triplicate samples are depicted, with error bars indicating the SEM. Error bars are plotted on all graphs but are sometimes obscured because of narrow SEM. Flow cytometry data were analyzed using CXP (Beckman Coulter) or Summit (Dako North America) software.
Results
Lentiviral expression of short hairpin RNA directed against HPRT results in efficient knockdown of HPRT protein levels and provides relative resistance to 6TG in vitro
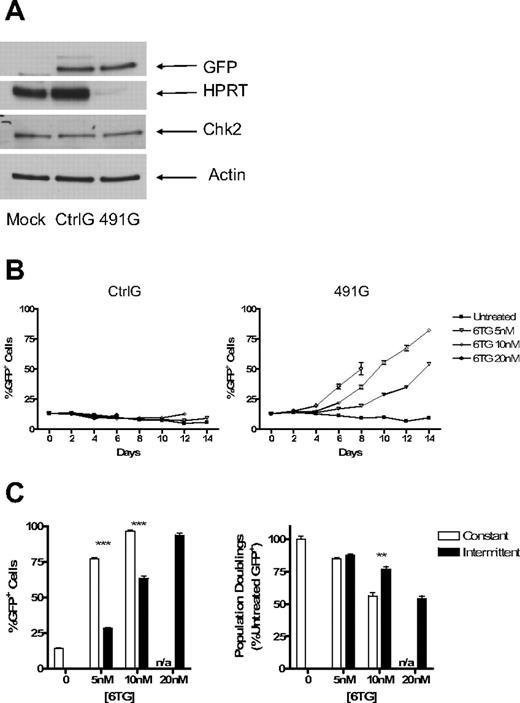
FL5.12 cells were transduced with lentiviral vectors expressing 5 different hairpin sequences targeting murine HPRT and selected in puromycin. After 5 days of selection, cell lysates were analyzed by Western blotting. With each of the constructs tested, there was significant knockdown of HPRT protein levels compared with untransduced and Ctrl-transduced cells. Constructs 491 and 493 demonstrated the most effective knockdown (Figure 2A).
Knockdown of HPRT provides hematopoietic cells with resistance to 6TG. Fl5.12 cells were transduced with control (Ctrl) and experimental constructs (490-494) with sequences coding for shRNA against HPRT. The cells were then selected in puromycin for 5 days. (A) Whole cell lysates were subject to Western blot analysis using antibodies against HPRT (ab10479; Abcam, Cambridge, MA) and actin (sc-1616; Santa Cruz Biotechnology, Santa Cruz, CA). (B) Proliferating cells were cultured at an initial concentration of 5 × 104 cells/mL in the presence of 6TG at 10, 20, 40, 60, and 100 nM or no drug (UT). The concentration of live cells after 96 hours is depicted as a percentage of Ctrl UT live cells (P < .001; ANOVA). (C) Transduced FL5.12 cells were plated at 105 cells/mL in the presence of 5 nM and 10 nM 6TG or no drug. Cells were replated at 105 cells/mL in fresh media with or without 6TG every 48 hours. Population doublings are plotted against time (P < .001 for 491 [6TG 5 nM] compared with Ctrl [6TG 5 nM]: ANOVA). Population doubling was calculated by taking the log2 of the cumulative expansion. Cumulative expansion was calculated by multiplying the interval expansion (measured cell concentration/seeded concentration) by the cumulative expansion. (D) Ctrl- and 491-transduced cells were cultured with cisplatinum or no drug. Cells were counted after 48 hours. The number of population doublings is depicted as a percentage of untreated Ctrl-transduced cells' population doublings. ***P < .001.
Knockdown of HPRT provides hematopoietic cells with resistance to 6TG. Fl5.12 cells were transduced with control (Ctrl) and experimental constructs (490-494) with sequences coding for shRNA against HPRT. The cells were then selected in puromycin for 5 days. (A) Whole cell lysates were subject to Western blot analysis using antibodies against HPRT (ab10479; Abcam, Cambridge, MA) and actin (sc-1616; Santa Cruz Biotechnology, Santa Cruz, CA). (B) Proliferating cells were cultured at an initial concentration of 5 × 104 cells/mL in the presence of 6TG at 10, 20, 40, 60, and 100 nM or no drug (UT). The concentration of live cells after 96 hours is depicted as a percentage of Ctrl UT live cells (P < .001; ANOVA). (C) Transduced FL5.12 cells were plated at 105 cells/mL in the presence of 5 nM and 10 nM 6TG or no drug. Cells were replated at 105 cells/mL in fresh media with or without 6TG every 48 hours. Population doublings are plotted against time (P < .001 for 491 [6TG 5 nM] compared with Ctrl [6TG 5 nM]: ANOVA). Population doubling was calculated by taking the log2 of the cumulative expansion. Cumulative expansion was calculated by multiplying the interval expansion (measured cell concentration/seeded concentration) by the cumulative expansion. (D) Ctrl- and 491-transduced cells were cultured with cisplatinum or no drug. Cells were counted after 48 hours. The number of population doublings is depicted as a percentage of untreated Ctrl-transduced cells' population doublings. ***P < .001.
To determine whether knockdown of HPRT (HPRTkd) provides resistance to purine analogs, transduced and puromycin selected cells were cultured in the presence of 6TG at concentrations of 10 to 100 nM or no drug. Ctrl-transduced cells had diminished numbers of live cells in a dose-dependent fashion after 96 hours of treatment; construct 491- and construct 493–transduced cells were significantly resistant to the toxic effects of 6TG (Figure 2B). Similar results were found using 6MP for both constructs 491 and 493 (Figure S1). In a longer-term experiment, Ctrl-transduced cells demonstrated significantly slowed proliferation in the presence of 6TG at 5 nM. At a higher dose of 10 nM, the toxicity of 6TG was more pronounced and eventually prevented proliferation altogether (Figure 2C). Notably, cells transduced with construct 491 demonstrated relative resistance to 6TG compared with Ctrl-transduced cells; construct 491–transduced cells continued to proliferate at near-normal rates in 5 nM of 6TG. Furthermore, construct 491–transduced cells continued to proliferate for as long as 30 days at the higher dose of 6TG, indicating that the relative resistance to 6TG is stable over many population doublings (Figure 2C). Like 6TG, cisplatinum requires the MMR system for cytotoxicity.32 To determine whether knockdown of HPRT had unintended, off-target effects impairing MMR, transduced cells were also treated with cisplatinum at 1 μM. Both Ctrl- and 491-transduced cells stopped proliferating after 2 days in the presence of cisplatinum (Figure 2D), indicating that MMR remained intact.
The puromycin resistance gene was then replaced with the enhanced GFP gene so that the relevant population of cells (HPRTkd/GFP+) could be monitored by flow cytometry. Expression of GFP was confirmed by Western blotting as well as flow cytometry (Figure 3A; and data not shown). HPRTkd/GFP+ cells did not have any selective advantage when left untreated, indicating that there is no unanticipated advantage or disadvantage conferred to FL5.12 cells by knockdown of HPRT (Figure 3B). However, in the presence of 6TG at multiple doses, HPRTkd/GFP+ cells had a selective advantage, whereas Ctrl/GFP+ cells had no such advantage (Figure 3B,C). The protection from 6TG toxicity is not absolute, as HPRTkd/GFP+ cells stopped proliferating after 8 days at the highest concentration of 6TG tested (20 nM). HPRTkd/GFP+ cells did not have a selective advantage in the presence of cisplatinum, again indicating the specificity of drug resistance provided by knockdown of HPRT (not shown).
Knockdown of HPRT allows for selection of marked cells in mixed cultures of transduced and untransduced cells. The puromycin resistance gene was replaced with the gene for GFP in the Ctrl and 491 plasmids, resulting in plasmids CtrlG and 491G. Fl5.12 cells were transduced and sorted for GFP+ cells. (A) Western blot analysis of cell lysates from sorted GFP+ cells and untransduced cells with antibodies against HPRT, Chk2 (sc-9064; Santa Cruz Biotechnology), GFP (Clontech, Mountain View, CA), and actin. (B) GFP+ cells were mixed with untransduced cells at a ratio of 1:10. The mixed cultures were then subject to treatment with 6TG or no drug. The percentage of GFP+ cells is plotted against time (P < .001 for 6TG 5 and 10 nM compared with untreated; ANOVA). (C) The same mixed cultures of 491G transduced and untransduced cells were subjected to either constant or intermittent treatment with 6TG or no drug. Intermittent treatment consisted of 48 hours of exposure to 6TG alternating with 96 hours with no drug. The percentage of GFP+ cells after 16 days of treatment is shown on the left, whereas the number of population doublings of GFP+ cells is graphed as a percentage of the number of population doublings of untreated GFP+ cells on the right. n/a indicates constant treatment with 20 nM 6TG resulted in cell death by 10 days, even in cells with knockdown of HPRT. **P < .01; ***P < .001.
Knockdown of HPRT allows for selection of marked cells in mixed cultures of transduced and untransduced cells. The puromycin resistance gene was replaced with the gene for GFP in the Ctrl and 491 plasmids, resulting in plasmids CtrlG and 491G. Fl5.12 cells were transduced and sorted for GFP+ cells. (A) Western blot analysis of cell lysates from sorted GFP+ cells and untransduced cells with antibodies against HPRT, Chk2 (sc-9064; Santa Cruz Biotechnology), GFP (Clontech, Mountain View, CA), and actin. (B) GFP+ cells were mixed with untransduced cells at a ratio of 1:10. The mixed cultures were then subject to treatment with 6TG or no drug. The percentage of GFP+ cells is plotted against time (P < .001 for 6TG 5 and 10 nM compared with untreated; ANOVA). (C) The same mixed cultures of 491G transduced and untransduced cells were subjected to either constant or intermittent treatment with 6TG or no drug. Intermittent treatment consisted of 48 hours of exposure to 6TG alternating with 96 hours with no drug. The percentage of GFP+ cells after 16 days of treatment is shown on the left, whereas the number of population doublings of GFP+ cells is graphed as a percentage of the number of population doublings of untreated GFP+ cells on the right. n/a indicates constant treatment with 20 nM 6TG resulted in cell death by 10 days, even in cells with knockdown of HPRT. **P < .01; ***P < .001.
Because continuous treatment with 6TG attenuated population doubling rates even in cells with iPAR, we sought to determine whether intermittent treatment may be less toxic, but similarly effective, in selection of HPRTkd/GFP+ cells. Therefore, an intermittent dosing strategy was compared with continuous treatment. Whereas continuous treatment resulted in more rapid increases in the percentage of HPRTkd/GFP cells (Figure 3C left), intermittent therapy resulted in significantly more population doublings of HPRTkd/GFP+ at higher doses (Figure 3C right). Thus, intermittent 6TG treatment promotes efficient selection and expansion of HPRTkd cells in vitro.
Knockdown of HPRT attenuates 6TG-induced G2/M checkpoint arrest and apoptosis
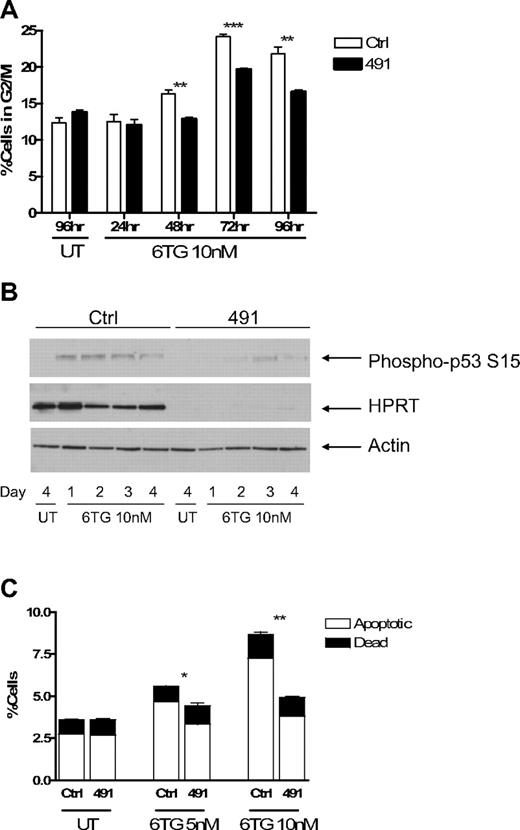
Treatment of cells with 6TG has been shown to result in arrest at the G2/M phase of the cell cycle.29 To determine how HPRTkd FL5.12 cells were able to continue to proliferate in the presence of 6TG, we performed cell cycle analysis using PI staining. Ctrl-transduced cells accumulated at G2/M in the presence of 6TG, and this accumulation was attenuated in HPRTkd cells (Figures 4A, S2A). As expected, knockdown of HPRT had no consistent effect on the accumulation of cells at G2/M on treatment with cisplatinum (Figure S2B). Treatment of cells with 6TG activates DNA damage checkpoints, including p53, which mediate the cytotoxic effects of the drug.29,33 Western blot analysis of cell lysates obtained at 24-hour intervals demonstrates diminished serine 15 phosphorylation of p53, indicative of reduced p53 activation, in HPRTkd cells compared with Ctrl-transduced cells (Figure 4B).
Cells with impaired function of HPRT escape G2/M checkpoint arrest and apoptosis induced by 6TG. Fl5.12 cells transduced with Ctrl or 491 were cultured in the presence of 10 nM 6TG or no drug (UT). (A) At the indicated time points, a fraction of the culture was fixed, stained with PI, and analyzed by flow cytometry. The percentage of cells in the G2/M phase of the cell cycle (as defined by 4N DNA content) is graphed. **P < .01. ***P < .001. (B) Whole cell lysates from the indicated time points were subject to Western blot analysis with antibodies against p53 phosphorylated at serine 15 (P-p53; ab 9284; Cell Signaling Technology, Danvers, MA), HPRT, and actin. (C) After 3 days of treatment, a fraction of the culture was stained with fluorescein isothiocyanate-linked annexin V and PI and analyzed by flow cytometry. The percentages of apoptotic (annexin V+-PI−) and dead (annexin V+-PI+) cells are graphed. *P < .05. **P < .01.
Cells with impaired function of HPRT escape G2/M checkpoint arrest and apoptosis induced by 6TG. Fl5.12 cells transduced with Ctrl or 491 were cultured in the presence of 10 nM 6TG or no drug (UT). (A) At the indicated time points, a fraction of the culture was fixed, stained with PI, and analyzed by flow cytometry. The percentage of cells in the G2/M phase of the cell cycle (as defined by 4N DNA content) is graphed. **P < .01. ***P < .001. (B) Whole cell lysates from the indicated time points were subject to Western blot analysis with antibodies against p53 phosphorylated at serine 15 (P-p53; ab 9284; Cell Signaling Technology, Danvers, MA), HPRT, and actin. (C) After 3 days of treatment, a fraction of the culture was stained with fluorescein isothiocyanate-linked annexin V and PI and analyzed by flow cytometry. The percentages of apoptotic (annexin V+-PI−) and dead (annexin V+-PI+) cells are graphed. *P < .05. **P < .01.
6TG treatment also leads to apoptosis.29 Knockdown of HPRT results in less apoptosis after treatment with 6TG compared with Ctrl-transduced cells (Figures 4C, S2C). Knockdown of HPRT did not have any effect on the extent of apoptosis in the absence of IL-3, or in the presence of cisplatinum, compared with control cells (Figure S2D), highlighting the specificity of HPRT knockdown for protection from 6TG. Taken together, these data suggest that knockdown of HPRT allows cells to escape activation of the G2/M checkpoint and induction of apoptosis induced by 6TG.
Knockdown of HPRT in primary hematopoietic cells provides resistance to 6TG
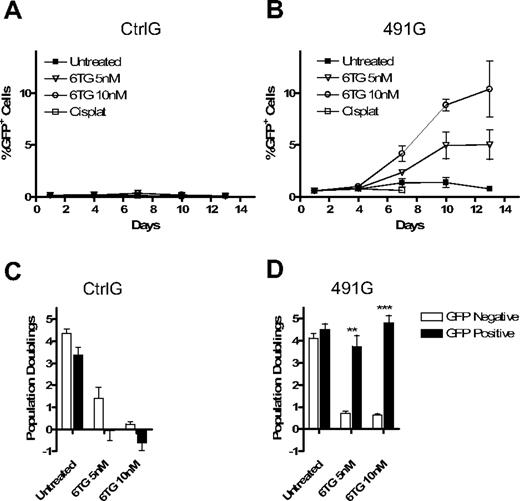
To determine whether primary HPCs could similarly be provided with resistance to 6TG by knockdown of HPRT, BM cells were harvested from wild-type mice and transduced with CtrlG or with 491G. Four days after transduction, the cultures were subjected to treatment with 6TG. Treatment with 6TG resulted in an increase in the percentage of HPRTkd/GFP+ cells, whereas CtrlG cells were not provided a similar advantage (Figure 5A,B). As with hematopoietic cell lines, knockdown of HPRT did not confer a survival or proliferative advantage to untreated cells or to cisplatinum-treated cells. Importantly, HPRTkd/GFP+ cells not only survived in the presence of 6TG, they continued to proliferate at rates similar to untransduced, untreated cells, whereas CtrlG-transduced cells proliferated poorly in the presence of 6TG (Figure 5C,D).
Knockdown of HPRT in primary BM cells provides resistance to 6TG and promotes selection of transduced cells. Primary BM cells were harvested from donor mice and transduced with CtrlG and 491G vectors overnight. The cells were cultured with cytokines in the presence of 6TG (5 nM and 10 nM), cisplatinum (1 μM), or no drug. Cells were counted, analyzed by flow cytometry, and replated every 72 hours. (A,B) The percentage of GFP+ cells is plotted against time for CtrlG (A) and 491G (B) transduced cells (P < .001; ANOVA). (C,D) The number of population doublings of GFP+ and GFP− cells for each condition was calculated and is represented for CtrlG (C) and 491G (D) transduced cells. **P < .01. ***P < .001.
Knockdown of HPRT in primary BM cells provides resistance to 6TG and promotes selection of transduced cells. Primary BM cells were harvested from donor mice and transduced with CtrlG and 491G vectors overnight. The cells were cultured with cytokines in the presence of 6TG (5 nM and 10 nM), cisplatinum (1 μM), or no drug. Cells were counted, analyzed by flow cytometry, and replated every 72 hours. (A,B) The percentage of GFP+ cells is plotted against time for CtrlG (A) and 491G (B) transduced cells (P < .001; ANOVA). (C,D) The number of population doublings of GFP+ and GFP− cells for each condition was calculated and is represented for CtrlG (C) and 491G (D) transduced cells. **P < .01. ***P < .001.
6TG can be used to select for HPRTkd/GFP+ cells in vivo
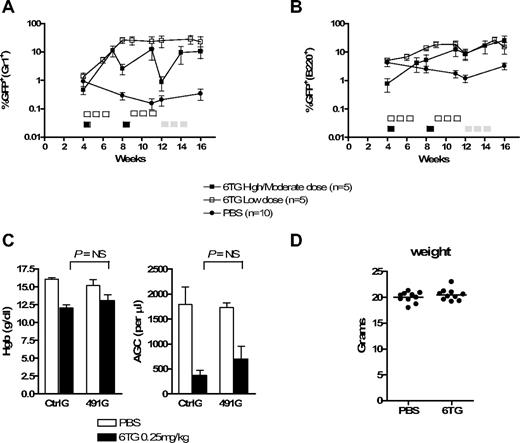
We next asked whether HPRT knockdown could be used to select for cells in murine recipients of gene-transduced BM cells. In 2 different experiments, donor BM was transduced with CtrlG or 491G viral media with low transduction efficiency, such that a small fraction of cells expressed the shRNA and GFP, followed by transplantation into sublethally irradiated recipients. Four weeks later, peripheral blood was analyzed for GFP expression in myeloid (Gr1+) and B-lymphoid (B220+) cells (Figure 6A,B), at which point approximately 1% of peripheral blood Gr1+ cells were GFP+ for both CtrlG and 491G groups. The mice were then subjected to treatment with 6TG or phosphate-buffered saline (PBS).
HPRT knockdown and 6TG treatment potently select for gene-modified hematopoietic progenitor cells in mice. Primary BM cells were harvested from donor mice, transduced with CtrlG and 491G vectors, and transplanted into recipient mice. Peripheral blood was periodically analyzed by flow cytometry. (A,B) The percentage of GFP+ cells within the Gr1+ (A) and B220+ (B) peripheral blood cell populations from recipients of 491G-transduced BM is plotted versus time. Note log scale. Five-day courses of treatment are indicated by □ (0.25 mg/kg per day), ■ (2 mg/kg per day), or  (0.5 mg/kg per day). The number of 6TG-treated mice at weeks 14 and 16 in the high/moderate dosing experiment is 4 and 3, respectively, because of toxicity-related deaths. (C) Complete blood counts from mice in the low-dose experiment were performed at day 22 of cycle 2 (week 12). The hemoglobin concentration and absolute granulocyte counts (AGC) are depicted. AGC was calculated using the total white blood cell count multiplied by the fraction of Gr1+ cells as determined by flow cytometry. NS indicates not significant. (D) The weights of all PBS- and 6TG-treated mice were determined at day 22 of cycle 2 (week 12) in the low-dose experiment.
(0.5 mg/kg per day). The number of 6TG-treated mice at weeks 14 and 16 in the high/moderate dosing experiment is 4 and 3, respectively, because of toxicity-related deaths. (C) Complete blood counts from mice in the low-dose experiment were performed at day 22 of cycle 2 (week 12). The hemoglobin concentration and absolute granulocyte counts (AGC) are depicted. AGC was calculated using the total white blood cell count multiplied by the fraction of Gr1+ cells as determined by flow cytometry. NS indicates not significant. (D) The weights of all PBS- and 6TG-treated mice were determined at day 22 of cycle 2 (week 12) in the low-dose experiment.
HPRT knockdown and 6TG treatment potently select for gene-modified hematopoietic progenitor cells in mice. Primary BM cells were harvested from donor mice, transduced with CtrlG and 491G vectors, and transplanted into recipient mice. Peripheral blood was periodically analyzed by flow cytometry. (A,B) The percentage of GFP+ cells within the Gr1+ (A) and B220+ (B) peripheral blood cell populations from recipients of 491G-transduced BM is plotted versus time. Note log scale. Five-day courses of treatment are indicated by □ (0.25 mg/kg per day), ■ (2 mg/kg per day), or  (0.5 mg/kg per day). The number of 6TG-treated mice at weeks 14 and 16 in the high/moderate dosing experiment is 4 and 3, respectively, because of toxicity-related deaths. (C) Complete blood counts from mice in the low-dose experiment were performed at day 22 of cycle 2 (week 12). The hemoglobin concentration and absolute granulocyte counts (AGC) are depicted. AGC was calculated using the total white blood cell count multiplied by the fraction of Gr1+ cells as determined by flow cytometry. NS indicates not significant. (D) The weights of all PBS- and 6TG-treated mice were determined at day 22 of cycle 2 (week 12) in the low-dose experiment.
(0.5 mg/kg per day). The number of 6TG-treated mice at weeks 14 and 16 in the high/moderate dosing experiment is 4 and 3, respectively, because of toxicity-related deaths. (C) Complete blood counts from mice in the low-dose experiment were performed at day 22 of cycle 2 (week 12). The hemoglobin concentration and absolute granulocyte counts (AGC) are depicted. AGC was calculated using the total white blood cell count multiplied by the fraction of Gr1+ cells as determined by flow cytometry. NS indicates not significant. (D) The weights of all PBS- and 6TG-treated mice were determined at day 22 of cycle 2 (week 12) in the low-dose experiment.
Based on our in vitro data demonstrating optimal selection using high, intermittent dosing of 6TG, mice were initially treated with a high-dose/short course of 6TG: 2 mg/kg per day for 5 days (cycle 1, days 1-5), followed by 3 weeks of rest (days 6-28). The high-dose/short course was then repeated (cycle 2). Using this strategy, 4 of the 5 6TG-treated recipients of 491G-transduced BM had dramatic increases in the percentages of GFP+ peripheral blood myeloid and lymphoid cells (Figures 6A,B and S3A). The single nonresponder had near background levels of GFP+ myeloid cells 4 weeks after transplantation (before the initiation of treatment with 6TG), suggesting transplantation of insufficient numbers of transduced HSCs. Notably, the most dramatic increases were on day 21 of cycles 1 and 2. Analysis at day 28 of these cycles also demonstrated higher than initial GFP+ peripheral blood leukocytes, but with far less dramatic changes over baseline, resulting in a saw-tooth pattern when plotted over time. This suggested selection at a committed hematopoietic progenitor cell level, rather than at the level of the HSC. Thus, we altered the treatment regimen of these animals to provide more prolonged exposure to 6TG, which requires cells to be in cycle for its cytotoxic effects. Beginning at 12 weeks after transplantation, the recipients were treated with a moderate dose of 6TG: 0.5 mg/kg per day for 15 days during a 3-week period (days 1-5, 8-12, and 15-19 of cycle 3). We again saw an increase in the percentage of GFP+ myeloid and lymphoid cells. However, this moderate dosing strategy had considerable hematotoxicity and treatment-related mortality (not shown). Nonetheless, in this experiment, the increase in GFP+ peripheral blood cells was prolonged and significant in the myeloid (P = .01; analysis of variance [ANOVA]) and lymphoid (P = .04; ANOVA) compartments for the duration of the experiment for 2 and 3 of the mice, respectively (Figure S3B; and not shown). None of the 6TG-treated recipients of CtrlG-transduced BM or the PBS-treated recipients of CtrlG- or 491G-transduced BM demonstrated consistent or substantial increases in GFP+ peripheral blood cells (Figures 6A,B, S3, and S4).
In a second experiment, 6TG dosing was reduced to eliminate treatment-related toxicity and determine whether cell selection could occur at a more clinically relevant dose. 6TG was given at 0.25 mg/kg per day for 15 days over a 3-week period (days 1-5, 8-12, 15-19), followed by 2 weeks of no treatment (days 20-35). The cycle was then repeated. Using this dosing strategy, we found that treatment of recipients of 491G-transduced BM resulted in significant increases in the percentages of GFP+ peripheral blood myeloid (P = .01; ANOVA) and lymphoid (P < .001; ANOVA) cells in all 5 recipients (Figures 6A,B and S3B). In one of the 6TG-treated recipients, the selection was transient, and by day 28 of the second cycle, the percentage of GFP+ cells approached background levels, again suggesting selection at a short-term hematopoietic progenitor level. Notably, in this recipient, GFP was undetectable in the myeloid lineage before the initiation of 6TG treatment. However, in the remaining 6TG-treated mice, selection was sustained for the 5 weeks after the last dose of 6TG (4 months after transplantation). This dosing strategy did result in moderate myelosuppression (Figure 6C) but was otherwise well tolerated by the recipients, as determined by their general appearance over the duration of the experiment and their weight at day 22 of the second course of therapy (Figure 6D).
In total, 9 of 10 6TG-treated recipients of 491G-transduced BM had greater than 5-fold increases in GFP+ peripheral blood myeloid cells, at least transiently, compared with 0 of 10 PBS-treated recipients of the same BM (P < .001, Fisher exact test; data from individual mice are provided in Figure S3).
6TG allows for cell selection at the level of early hematopoietic progenitors
Practical application of in vivo cell selection for treatment of genetic disease necessitates selection of HSC that will provide hematopoiesis for the lifetime of the recipient. Thus, cell selection using 6TG would require that this drug have effects on the proliferation and/or survival at the level of HSCs. As an initial test of the effects of 6TG on phenotypic HSCs, we treated mice with PBS or 6TG at the high and moderate dosing levels and measured the percentage and number of HSCs in the BM, as determined by flow cytometry. We found that the frequency and number of HSCs were significantly reduced in the 6TG-treated animals, compared with PBS-treated animals (Figures S5, S6; data not shown).
We next sought to determine whether long-term hematopoietic stem cells (LT-HSCs) were selected using HPRTkd and 6TG. LT-HSCs have been variably defined using phenotypic and functional assays.34 LT-HSCs are highly enriched in the lineage negative, c-Kit+, Sca-1+ subset of the bone marrow.35 Bone marrow cells from 3 recipients of 491G BM, which were treated with PBS or 6TG at the high/moderate doses, were harvested at day 28 of the third cycle (16 weeks after transplantation). The percentages of GFP+ Kit+Sca+Lin− (KSL) cells were determined by flow cytometry. The 6TG-treated recipients of 491G-transduced BM had variable, but substantially increased, percentages of GFP+ KSLs compared with the PBS-treated recipients of 491G-transduced BM (2.6%, 52.1%, and 76% vs 0.16%, 0.40%, and 0.78%).
However, although the KSL subset is enriched for HSCs, it also includes short-term progenitors. Furthermore, we wanted to analyze BM for hematopoietic progenitors in a more steady state, after normal hematopoiesis was no longer impaired by 6TG treatment. Therefore, we analyzed BM from recipients of 491G-transduced BM that had been previously treated with PBS or low-dose 6TG, 5 weeks after the last dose (16 weeks after transplantation). In this experiment, we used 2 sets of cell surface markers that identify populations of cells more highly enriched for HSC activity and for multipotent progenitor cells (MPP; Figure S5).36,37 In 3 of 5 mice, the percentages and number of GFP+ HSCs were considerably higher in the 6TG-treated recipients, compared with the PBS-treated recipients (Table 1). In 4 of 5 mice, the percentages and number of GFP+ MPP were considerably higher in the 6TG-treated recipients. There were no consistent differences in the percentages of GFP+ HSCs or MPP in recipients of CtrlG BM, regardless of treatment with PBS or 6TG (not shown).
Knockdown of HPRT and treatment with 6TG allows for selection of primitive hematopoietic progenitor cells in primary recipients
| Treatment by animal ID . | GFP + HPC, no. (%)* . | ||
|---|---|---|---|
| HSC (KSL-F) . | HSC (SLAM) . | MPP . | |
| PBS | |||
| 988 | 0 (0) | 18 (1.72) | 9 (0.26) |
| 989 | 0 (0) | 5 (0.23) | 0 (0) |
| 992 | 19 (1.38) | 0 (0) | 5 (0.25) |
| 993 | 12 (0.41) | 0 (0) | 0 (0) |
| 995 | 13 (0.68) | 0 (0) | 21 (0.26) |
| Mean | 9 (0.49) | 5 (0.39) | 7 (0.16) |
| 6TG | |||
| 990 | 180 (14.51) | 64 (6.94) | 2238 (54.08) |
| 991 | 235 (16.67) | 738 (34.13) | 522 (10.99) |
| 994 | 69 (1.87) | 11 (0.58) | 336 (9.20) |
| 996 | 0 (0) | 21 (0.88) | 0 (0) |
| 997 | 923 (44.85) | 358 (23.16) | 358 (8.27) |
| Mean | 281 (15.58) | 238 (13.14) | 691 (16.5) |
| Treatment by animal ID . | GFP + HPC, no. (%)* . | ||
|---|---|---|---|
| HSC (KSL-F) . | HSC (SLAM) . | MPP . | |
| PBS | |||
| 988 | 0 (0) | 18 (1.72) | 9 (0.26) |
| 989 | 0 (0) | 5 (0.23) | 0 (0) |
| 992 | 19 (1.38) | 0 (0) | 5 (0.25) |
| 993 | 12 (0.41) | 0 (0) | 0 (0) |
| 995 | 13 (0.68) | 0 (0) | 21 (0.26) |
| Mean | 9 (0.49) | 5 (0.39) | 7 (0.16) |
| 6TG | |||
| 990 | 180 (14.51) | 64 (6.94) | 2238 (54.08) |
| 991 | 235 (16.67) | 738 (34.13) | 522 (10.99) |
| 994 | 69 (1.87) | 11 (0.58) | 336 (9.20) |
| 996 | 0 (0) | 21 (0.88) | 0 (0) |
| 997 | 923 (44.85) | 358 (23.16) | 358 (8.27) |
| Mean | 281 (15.58) | 238 (13.14) | 691 (16.5) |
Flow cytometric data from BM of PBS- and 6TG-treated primary recipients of 491G-transduced BM. GFP indicates green fluorescent protein; HPC, hematopoietic progenitor cell; HSC, hematopoietic stem cell; MPP, multipotent progenitor cell.
Background levels of HSCs and MPP were 0%.
Knockdown of HPRT and treatment with 6TG allows for selection of primitive hematopoietic cells in secondary recipients
| Donor treatmentby animal ID . | Mean GFP%, 4 wk* . | Mean GFP%, 8 wk (n) . | ||||
|---|---|---|---|---|---|---|
| WBC . | Gr1 . | B220 . | WBC . | Gr1 . | B220 . | |
| Background | 0.08% | 0.02% | 0.03% | 0.05% | 0.02% | 0.02% |
| PBS | ||||||
| 988 | 0.09% | 0.01% | 0.06% | 0.07% (3) | 0.04% (3) | 0.02% (3) |
| 989 | 0.09% | 0.09% | 0.05% | 0.08% (3) | 0.05% (2) | 0.04% (2) |
| 992 | ||||||
| 993 | 0.12% | 0.01% | 0.23% | 0.08% (2) | 0.05% (2) | 0.03% (2) |
| 995 | 0.08% | 0.09% | 0.02% | 0.06% (2) | 0.03 (2) | 0.02% (2) |
| Mean | 0.10% | 0.05% | 0.09% | 0.07% | 0.04% | 0.03% |
| 6TG | ||||||
| 990 | 0.90% | 0.08% | 3.17% | 0.14% (1) | 0.02% (1) | 0.49% (1) |
| 991 | 7.03% | 12.28% | 3.25% | 0.41% (3) | 1.15% (3) | 0.39% (3) |
| 994 | 0.29% | 0.11% | 0.58% | 0.08% (2) | 0.03% (2) | 0.09% (2) |
| 996 | ||||||
| 997 | 10.20% | 13.93% | 1.86% | 0.77% (3) | 2.49% (3) | 2.61% (3) |
| Mean | 4.61% | 6.60% | 2.22% | 0.35% | 0.92% | 0.90% |
| Donor treatmentby animal ID . | Mean GFP%, 4 wk* . | Mean GFP%, 8 wk (n) . | ||||
|---|---|---|---|---|---|---|
| WBC . | Gr1 . | B220 . | WBC . | Gr1 . | B220 . | |
| Background | 0.08% | 0.02% | 0.03% | 0.05% | 0.02% | 0.02% |
| PBS | ||||||
| 988 | 0.09% | 0.01% | 0.06% | 0.07% (3) | 0.04% (3) | 0.02% (3) |
| 989 | 0.09% | 0.09% | 0.05% | 0.08% (3) | 0.05% (2) | 0.04% (2) |
| 992 | ||||||
| 993 | 0.12% | 0.01% | 0.23% | 0.08% (2) | 0.05% (2) | 0.03% (2) |
| 995 | 0.08% | 0.09% | 0.02% | 0.06% (2) | 0.03 (2) | 0.02% (2) |
| Mean | 0.10% | 0.05% | 0.09% | 0.07% | 0.04% | 0.03% |
| 6TG | ||||||
| 990 | 0.90% | 0.08% | 3.17% | 0.14% (1) | 0.02% (1) | 0.49% (1) |
| 991 | 7.03% | 12.28% | 3.25% | 0.41% (3) | 1.15% (3) | 0.39% (3) |
| 994 | 0.29% | 0.11% | 0.58% | 0.08% (2) | 0.03% (2) | 0.09% (2) |
| 996 | ||||||
| 997 | 10.20% | 13.93% | 1.86% | 0.77% (3) | 2.49% (3) | 2.61% (3) |
| Mean | 4.61% | 6.60% | 2.22% | 0.35% | 0.92% | 0.90% |
Flow cytometric data from peripheral blood of secondary recipients of BM from PBS- and 6TG-treated donors.
n = 3 secondary recipients per group.
The most stringent measure of LT-HSC activity is multilineage repopulation of secondary transplantation recipients. Therefore, we performed secondary transplantations with whole BM from the animals treated with PBS or the low dose of 6TG. Four and 8 weeks later, peripheral blood cells were analyzed by flow cytometry. At 4 weeks after secondary transplantation, 12 of 12 recipients of BM from 6TG-treated donors were engrafted with transduced BM, compared with 1 of 12 recipients of BM from PBS-treated donors (P < .001, Fisher exact; Table 2, Figure S7). The extent of engraftment was highly variable, but the recipients of 2 of 4 6TG-treated donors' BM (991 and 997) demonstrated substantial and prolonged secondary engraftment. Not surprisingly, these 2 donors had the highest fractions of GFP+ phenotypic HSCs.
Together with sustained selection for HPRT knockdown in the myeloid lineage up to 4 months after transplantation, these data suggest that HPRT knockdown can select for gene transduced cells in both MPP and long-term HSC compartments.
Discussion
The presented data demonstrate that knockdown of HPRT using lentivirus-expressed shRNA provides hematopoietic cells with protection from the toxic effects of purine antimetabolites and attenuates 6TG-induced G2/M checkpoint activation, cell cycle arrest, and apoptosis. Furthermore, we have demonstrated that drug resistance can be provided to primary hematopoietic cells using shRNA, allowing for the strong selection of transduced cells, both in vitro and in vivo. Importantly, the duration, multilineage potential, and ability to enhance secondary transplantation indicate that in vivo selection occurs at the level of primitive hematopoietic progenitor cells and suggests that it may occur at the LT-HSC level.
The use of programmed drug resistance in HPCs was proposed and validated in seminal studies using Δ-DHFR.24 Since then, multiple strategies have been proposed and tested in animal models.9,10,19 Several of these strategies have been successful in selecting for transduced cells in animal models20,21,24,25 ; clinical trials have been reported using the MDR1 gene38 and are completed or underway with Δ-MGMT.39 Nonetheless, each of these strategies has its limitations and potential dangers. We therefore hypothesized that the use of iPAR may be an effective and safe method for in vivo cell selection. The use of lentivirus-coded iPAR has several distinct advantages for delivery of programmed drug resistance.
One advantage of iPAR is that the sequence required to knockdown HPRT includes only a hairpin of 21 base pair (bp) sense and antisense stems and a 6-bp loop. These 48 bp are far fewer than the hundreds or thousands of base pairs required to engender resistance to other drugs. For example, the MDR1 gene is approximately 4400 bp.40 The small shRNA cassette makes subsequent modification of the plasmid much simpler and efficient, allowing the introduction of larger genes and complex regulatory elements for therapeutic replacement.10 Because the capacity of retroviruses and lentiviruses is limited, this benefit would be particularly advantageous for the replacement of large genes, such as that for factor VIII.
Knockdown of HPRT provides drug resistance to a small class of drugs that are very well tolerated. These purine analogs have been used for decades and are routinely titrated to desired hematopoietic toxicity in leukemia trials. Although there has been some suggestion that antimetabolite therapy may enhance the risk of treatment-related leukemia, this is in the context of concomitant administration of highly genotoxic medications.41 For patients with rheumatoid arthritis and inflammatory bowel diseases who are treated with purine analogs in the absence of other genotoxic medications, an increased risk of malignancy has been suggested, but this association is controversial and does not preclude the use of these medications for these patients.42 In contrast, alkylating and DNA-damaging agents, which are used in conjunction with Δ-MGMT and MDR1, present an undisputed high carcinogenic risk.43,44
Nonetheless, as with any gene therapy strategy, careful assessment of potential toxicities is necessary. Purine analogs must be used cautiously as nonhematologic side effects can occur. For example, hepatotoxicity will need to be considered in future investigations. Our experiments were also not designed to examine the important issues of transgene silencing and the possibility of selection of cells that are not only resistant to purine analogs, but also those with advantageous insertion sites, which might lead to oligoclonal hematopoiesis or malignant transformation.
Although iPAR would probably be as effective using γ-retroviral expression, we chose to deliver the shRNA and marker gene using a SIN HIV-based lentiviral vector. Lentiviruses have been demonstrated to transduce nondividing cells,14 which may allow for less ex vivo manipulation of transplanted cells and thus reduce the risk of losing critical stem cell qualities and graft failure. Indeed, lentiviruses have been shown to effectively transduce murine HSCs with only 1 hour of transduction time.16 Inherent lentiviral properties and the inclusion of the SIN LTR in the plasmid may reduce the risk of insertional mutagenesis leading to leukemia, although the mechanisms of leukemogenesis resulting from retroviral integration are not yet fully understood.15,17,18
Our data corroborate previous data that indicate that simultaneous gene expression and gene knockdown using RNA interference are possible,45 as well as data demonstrating that long-term, in vivo expression of shRNA in HSCs can be achieved.46 HSC function can be defined most simply by the ability to provide long-term lymphomyeloid hematopoiesis in recipient mice.34 The duration of selection in primary recipients was at least 16 weeks in 7 of 10 primary recipients, meeting this elementary definition. Flow cytometric data using 2 different strategies of HSC identification confirmed selection in populations of cells highly enriched for HSC activity. However, the most stringent definition of HSC function is the ability to contribute multilineage hematopoiesis in secondarily transplanted recipients. In our secondary transplantation experiments, we found enhanced engraftment from a subset of the 6TG-treated donors compared with the PBS-treated donors, suggesting that selection with iPAR and 6TG can occur at the true HSC level. This and the fact that PBS-treated recipients of 491G-transduced BM maintained stable, albeit low, levels of transduced peripheral blood cells (Figure S3) indicate that potential toxic effects from shRNA expression47,48 are not sufficient to impair HSC function enough to lead to extinction of transduced HSCs.
The primary transplantation experiments described were designed to model those diseases for which in vivo cell selection may be most beneficial. For example, HSCs from patients with Fanconi anemia are reduced in number and quality49 and are particularly sensitive to ex vivo manipulation.50 Thus, we purposefully transduced limited numbers of cells that were not primed with 5-fluorouracil, with relatively short exposure to viral particles before transplantation. Despite very low levels of transduced peripheral blood cells in recipients before treatment (∼1% of myeloid cells), we observed dramatic increases in transduced HSCs in the periphery when recipients were transplanted with cells that were provided with iPAR and were treated with 6TG. For most of the mice, this also translated into larger numbers of transduced HPCs in the bone marrow as well. The fact that a few of the 6TG-treated recipients only demonstrated evidence of short-term HPC selection may be because of suboptimal transduction, a suboptimal selection strategy, or the need for continuous selection for the duration of the experiment.
In conclusion, we have proposed a novel strategy for providing cells with drug resistance using shRNA against HPRT. Our data indicate that shRNA-mediated purine analog resistance is a powerful strategy of cell selection in vitro and in vivo. Future experiments will explore more directly the minimal HSC dose and optimal 6TG doses required for long-term selection in mice. Because other strategies of in vivo cell selection have had promising data from murine experiments but failed in human trials, this strategy will need to be carefully validated in large-animal and human xenograft models before considering trials in human patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Brent Eley/Brian Hicks Research Award from the Brent Eley Foundation (C.C.P.) and by the National Institutes of Health grant RO1 CA109657 (J.D.).
National Institutes of Health
Authorship
Contribution: C.C.P. designed and performed experiments, interpreted data, and wrote the manuscript. J.D. designed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James DeGregori, University of Colorado Denver, Mail Stop 8101, RC1 South Tower, Room 9401, 12801 East 17th Avenue, PO Box 6511, Aurora, CO 80045; e-mail: James.DeGregori@UCHSC.edu; or Christopher Porter, University of Colorado Denver, RC1 South Tower, Room 9401, 12801 East 17th Avenue, PO Box 6511, Aurora, CO 80045; e-mail: Chris.Porter@UCHSC.edu.

![Figure 2. Knockdown of HPRT provides hematopoietic cells with resistance to 6TG. Fl5.12 cells were transduced with control (Ctrl) and experimental constructs (490-494) with sequences coding for shRNA against HPRT. The cells were then selected in puromycin for 5 days. (A) Whole cell lysates were subject to Western blot analysis using antibodies against HPRT (ab10479; Abcam, Cambridge, MA) and actin (sc-1616; Santa Cruz Biotechnology, Santa Cruz, CA). (B) Proliferating cells were cultured at an initial concentration of 5 × 104 cells/mL in the presence of 6TG at 10, 20, 40, 60, and 100 nM or no drug (UT). The concentration of live cells after 96 hours is depicted as a percentage of Ctrl UT live cells (P < .001; ANOVA). (C) Transduced FL5.12 cells were plated at 105 cells/mL in the presence of 5 nM and 10 nM 6TG or no drug. Cells were replated at 105 cells/mL in fresh media with or without 6TG every 48 hours. Population doublings are plotted against time (P < .001 for 491 [6TG 5 nM] compared with Ctrl [6TG 5 nM]: ANOVA). Population doubling was calculated by taking the log2 of the cumulative expansion. Cumulative expansion was calculated by multiplying the interval expansion (measured cell concentration/seeded concentration) by the cumulative expansion. (D) Ctrl- and 491-transduced cells were cultured with cisplatinum or no drug. Cells were counted after 48 hours. The number of population doublings is depicted as a percentage of untreated Ctrl-transduced cells' population doublings. ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2008-03-146571/7/m_zh80180824080002.jpeg?Expires=1769081863&Signature=dr-CFnFjaYfwNGoDGt9aDL6FbuZBfDW0CejZDa0i7mLgV88a6KtZZ4dtxZbKeLmZ180IzN1w0BYdZeMOGVFKSuzjSL7OIUice8nSF11jkWKaqqoG3M1kIYdomP-WOCTgPA5bLqWde6756x7shBc3lehfuxBRJPPDmhn3L6zb3jJmH2nas1YmwvF3UW0iKexRgygntXDDalZPLD4ATH1eoIl7enHTMadIKy1-5qtqegz1otmhkLZj2Xe2rwHBbamVx176pjmAYaZK3DJiAdvgiu8XbmYiBVXJILePbg3ppDn9mcmke47hqd-bOesSqD0jjjptmxpt8Z~eW9npkrBCMw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)