Abstract
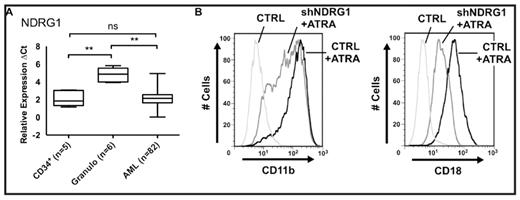
The N-myc down-regulated gene 1 (NDRG1) is a stressed induced protein whose expression is associated with growth arrest and differentiation of tumor cells. Although the exact function of NDRG1 protein remains unknown, various studies support its role as a suppressor of tumor metastasis. In prostate, colon and breast cancer its expression is associated with a better disease prognosis and patient survival. In hematopoietic cells, NDRG1 was identified in a differential display screen for differentiation-related genes in human myelomonocytic U937 cells. In the present study, we sought to investigate the role of NDRG1 in myeloid differentiation. To this end we first evaluated NDRG1 mRNA expression in acute myeloid leukemia (AML; n=82) patient samples as well as in CD34+ progenitor cells (n=5) and neutrophils (n=6) of healthy donors using quantitative real-time RT-PCR. We found significantly higher NDRG1 mRNA levels in granulocytes as compared to CD34+ (p=0.0043) or AML blast cells (p<0.0001), whereas no significant difference between CD34+ progenitor and AML blast cells was seen (Figure A). Moreover, we found that NDRG1 mRNA levels were increased in 4/5 APL patients upon ATRA therapy. In contrast, the closest relative of NDRG1, NDRG2, did not show significantly different expression in these primary cells, thus indicating a unique role for NDRG1 in granulocyte differentiation. Next we examined NDRG1 expression using quantitative RT-PCR and Western blotting in two different cell line models for ATRA-induced neutrophil differentiation. ATRA treatment of NB4 and HT93 acute promyelocytic leukemia (APL) cells induced NDRG1 mRNA 2.3- and 14.3- fold, respectively. Increased NDRG1 mRNA expression was paralleled by an increase of NDRG1 protein as well as a decrease in c-myc protein. Earlier reports described that NDRG1 is also suppressed by c-myc suggesting that down-regulation of c-myc in our cell line models allowed for an increase of NDRG1. In line with these observations, lentivirus-driven short hairpin (sh)RNA-mediated silencing of NDRG1 diminished ATRA-induced neutrophil differentiation of NB4 and U937 cells as measured by CD11b, CD11c and CD18 surface expression. In NB4 NDRG1 knockdown versus non-targeting shRNA expressing cells mean fluorescent intensities (MFI) for CD11b, CD11c and CD18 upon six days of ATRA-treatment were 99±17 vs 146±7, 20±2 vs 32±10 and 19±2 vs 45±6, respectively (Figure B). Similarly, U937 NDRG1 knockdown versus control cells displayed the following MFIs for CD11b and CD18 upon neutrophil differentiation: 61±1 vs 102±2 and 11±4 vs 33±13, respectively. In conclusion, we report here for the first time an association of low NDRG1 levels with an immature hematopoietic cell phenotype. Using RNAi technology we further provide evidence that NDRG1 is functionally involved in neutrophil maturation.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal